Abstract
Capacitative calcium entry (CCE) through store-operated channels (SOCs) has been shown to contribute to the rise in intracellular calcium concentration ([Ca2+]i) and mediate pulmonary artery smooth muscle contraction. CCE is activated as a result of depletion of intracellular Ca2+ stores but there is a great deal of controversy surrounding the underlying signal that active CCE and the molecular makeup of SOCs. The discovery of canonical subgroup of transient receptor potential channels (TRPC) and recent identification of stromal-interacting molecule 1 (STIM1) protein have opened a door to the study of the identity of SOCs and the signal that activates these channels. Among all the TRPC channels, TRPC1 is widely studied in many cell types and shown to be part of SOCs components, whereas STIM1 protein is found to act as a Ca2+ sensor in the intracellular Ca2+ stores and activates SOCs. However, there is very little evidence for the roles of TRPC1 and STIM1 in the contribution of CCE in pulmonary artery. This chapter outlines the roles of TRPC1 and STIM1 in pulmonary artery smooth muscle cells and discusses our recent findings that TRPC1 and STIM1 are functionally interact with each other to mediate CCE in these cells. We also propose a model for the molecular makeup of SOCs formed by TRPC1 and STIM1 in pulmonary artery.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Capacitative Ca2+ entry (CCE) or store-operated Ca2+ entry was first proposed by Putney in 1986: Depletion of intracellular Ca2+ stores leads to the activation of Ca2+ entry from the extracellular space to reload the stores.1 The stores serve as a capacitor, and much as in an electrical circuit, a capacitor must be charged before current can flow through it.1 CCE can be activated by any procedure that causes depletion of Ca2+ from the intracellular Ca2+ stores. This includes agonists activating receptors coupled to the inositol 1,4,5-trisphosphate (IP3) signaling pathway, intracellular dialysis of high concentrations of Ca2+ chelators Ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid (EGTA) or 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA), thereby preventing store refilling by chelating Ca2+ that leaks from the stores or using agents that inhibit the sarcoplasmic reticulum (SR)/endoplasmic reticulum Ca2+-ATPase (adenosine triphosphatase) (SERCA), such as cyclopiazonic acid (CPA) or thapsigargin.2 The concept of CCE was first confirmed by electrophysiological evidence in mast cells, in which store depletion caused activation of a Ca2+ current, so-called Ca2+-release activated Ca2+ current (I CRAC).3 I CRAC is voltage independent, inwardly rectifying, and highly selective to Ca2+ and has a very low unitary conductance of 0.02 pS. It is now accepted that I CRAC is a predominant Ca2+ entry pathway in nonexcitable cells, but it is not the only store-operated current.2 Many studies have reported a variety of store-operated currents with a range of channel conductances (>1 pS), along with varying Ca2+ selectivity in both nonexcitable and excitable cells.2
Unlike nonexcitable cells, Ca2+ entry in excitable cells such as vascular smooth muscle cells is generally accomplished by voltage-operated Ca2+ channels (VOCCs) or receptor-operated channels (ROCs). However, increasing evidence has shown that Ca2+ entry through store-operated nonselective cation channels (SOCs) contributes to the rise in [Ca2+]i and responsible for vascular smooth muscle contraction.4 To date, there are only a few studies that showed the recording of store-operated currents in vascular smooth muscle cells. In cell-attached patches of cultured aorta smooth muscle cells, a 3-pS cation-conducting channel activated by thapsigargin or the cell-permeant Ca2+ chelator BAPTA-AM (acetoxymethyl ester) was reported by Trepakova et al.5 In cell-attached patches of cultured human pulmonary artery smooth muscle cells (PASMCs), Golovina et al.6 reported a 5.4-pS Ca2+-permeable channel that was activated by CPA. In rabbit portal vein cells, CPA, caffeine, BAPTA-AM, or calmodulin antagonist W-7 activated SOCs with a single-channel conductance of 2.1 pS.7 Following this observation, Saleh et al.8,9 also recorded 2.6- and 1.9-pS SOC in coronary artery and mesenteric artery smooth muscle cells, respectively. It is not clear whether the single-channel events described in these studies were indeed due to SOCs as opposed to another Ca2+ entry pathway because Ca2+ release from the stores may activate other nonselective cation channels; even in cells loaded with BAPTA-AM, it has not always been shown that sufficient BAPTA has accumulated in the cytosol to suppress the rise in intracellular Ca2+ following store depletion.2 Perhaps the more convincing evidence for the existence of SOCs in vascular smooth muscle cells comes from the whole-cell recording of rat PASMCs10 and human saphenous vein cells.11 In the former study, a CPA-induced inward current was recorded when cells were internally dialyzed with 10 mM EGTA or 10 mM BAPTA. The latter study showed that thapsigargin activated lanthanum-sensitive currents in cells dialyzed with 40 mM EGTA. However, the single-channel conductance for SOCs has not been reported in these studies. Thus, the electrophysiological evidence for SOCs in vascular smooth muscle cells remains to be elucidated.
2 Molecular Composition and Molecular Signals that Underlie Store-Operated Channels in Vascular Smooth Muscle
Over the past decade, CCE has gained a significant amount of attention in vascular smooth muscle research.5-15 However, the molecular composition of SOCs and the intracellular signals that activate these channels remain unclear. The discovery of mammalian homologues of Drosophila transient receptor potential (TRP) gene that encode 28 nonselective cation channel proteins with varying permeability to Ca2+ has led to a plethora of studies on the possible role of TRP channels underlying SOCs and ROCs. Since 1995, increasing evidence indicated members of the canonical subgroup of transient receptor potential nonselective cation channel (TRPC) constitute tetramers of both ROCs and SOCs.2,16,17 Based on the structural and functional similarities, members of the TRPC family are divided into four subgroups: TRPC1, TRPC2, TRPC3/6/7, and TRPC4/5. These TRPC members have all been suggested as components of SOCs, but there is also evidence that they are components of ROCs.16 This could be explained by the findings that different TRPC subunits can associate to form heterotetramers in different cell types. In general, TRPC1, 4, and 5 are sensitive to store depletion and interact with each other to function as SOCs, whereas TRPC3, 6, and 7 can interact with each other and function as ROCs that are gated by G protein-phospholipase C and diacylglycerol.16 TRPC2 is a pseudogene in humans.
Several studies have confirmed the existence of TRPC channels in various vascular preparations.4,17 However, there is little evidence that TRPC channels function as SOCs in vascular smooth muscle cells. Using inhibitory antibodies, antisense, and small-interfering RNA (siRNA) methods, several studies have presented evidence for TRPC1 as an essential component for SOCs in vascular smooth muscle cells from the aorta,18,19 cerebral artery,20 mesenteric artery,8 coronary artery,9,21 and portal vein.7,9 Interestingly, TRPC1 and TRPC5 have been shown to colocalize and associate with one another in the rabbit pial arteriole,22 and antibodies directed against TRPC1 and TRPC5 were shown to inhibit store-operated currents in mesenteric artery,9 suggesting that TRPC1/TRPC5 may form heterotetramers in vascular smooth muscle. TRPC1, 5, and 6 were suggested to form SOCs in rabbit coronary artery, and TRPC1, 5, and 7 were suggested to form SOCs in rabbit portal vein.9 This finding goes against the original principle that TRPC1, 4, or 5 and TRPC3, 6, or 7 can only interact with each other within the group to form heterotetramers and function as ion channels.23,24 However, it has also been proposed that functioning cation channels may be composed of subunits from both of these groups.25-27 Thus, comparison of the proposed heterotetrameric structures in expression systems with native SOCs is required to precisely identify the molecular makeup of endogenous SOCs.
While the molecular identity of SOCs is incomplete, the molecular signals that activate these channels also remain unresolved. Many studies have been performed since 1990 to examine the molecular signals that activate SOCs.2 These include (1) the generation of a “Ca2+ influx factor” from the SR after store depletion, which induces activation of Ca2+-independent phospholipase A2, leading to the activation of SOCs; (2) conformational coupling of the SR IP3 receptors with SOCs on the cell membrane; and (3) fusion of vesicles containing SOCs in the cell membrane, leading to the increase in channel number in the membrane. However, none of these hypotheses is unequivocally accepted for the activation of SOCs.2 The discovery of STIM1 (stromal interacting molecule 1) has opened a new direction toward the search for a molecular intermediate involved in the activation of SOCs. STIM1 was found to act as a sensor within the stores28,29 and may play a role in the plasma membrane29,30 to activate I CRAC. To date, there is little information on the role of STIM1 in vascular smooth muscle cells. STIM1 messenger RNA (mRNA) was shown to be expressed in cultured human coronary artery smooth muscle cells,31 mouse aorta smooth muscle cells,32 and human saphenous vein cells,11 and siRNA targeting STIM1 resulted in reduction of Ca2+ entry and whole-cell current activated by CPA or thapsigargin.11,31,33 These results suggest an important role of STIM1 in mediating CCE in vascular smooth muscle cells. Interestingly, In human embryonic kidney 293 (HEK293) cells, STIM1 was found to bind to TRPC1, TRPC4, and TRPC5 and directly regulate these channels, whereas the regulation of TRPC3 and TRPC6 by STIM1 was mediated by STIM1-dependent heteromultimerization of TRPC3 with TRPC1 and TRPC6 with TRPC4.34 Therefore, the notion that STIM1 interacts with various TRPC channels to mediate CCE cells has emerged as an important focus in vascular research.
3 Role of TRPC1 as an Essential Component for Store-Operated Channels in Pulmonary Artery
The TRPC channels, except TRPC2 have been found in pulmonary vascular preparations.10,14,35-37 However, there is little evidence for the functional significance of these channels in pulmonary vascular research. Because of the relatively abundant expression of TRPC1 in PASMCs, the functional role of TRPC1 has been widely studied compared to other TRPC channels. There is increasing evidence that TRPC1 functions as SOCs in pulmonary arteries. In human PASMCs, CCE is enhanced in proliferative cells,38 and this is associated with an increase in TRPC1 expression in these cells.6 In addition, TRPC1 gene expression and CCE were significantly reduced in human PASMCs treated with TRPC1 antisense.39 Overexpression of human TRPC1 in rat pulmonary arteries enhanced the contractile responses to CPA.40 Knockdown of TRPC1 protein with siRNA inhibited cation influx caused by thapsigargin in rat PASMCs,41 further supports that TRPC1 is an important molecular candidate for SOCs in PASMCs. Our study in mouse PASMCs also suggested that TRPC1 is an essential component of SOCs in these cells.42 We found that CPA caused an increase in nifedipine-insensitive transient and sustained rise in [Ca2+]i and an increase in Mn2+ quench of fura-2 fluorescence (Fig. 8.1). These increases in [Ca2+]i and Mn2+ quench rate were due to CCE because they were activated by store depletion and blocked by SKF-96365, Ni2+, La3+, and Gd3+,42 a characteristic property of SOCs in many tissues, including pulmonary arteries.10,12,14,15,36 We have also shown the expression of endogenous TRPC1 mRNA and protein in mouse PASMCs. Furthermore, the increase in dihydropyridine-insensitive sustained rise in [Ca2+]i and the increase in Mn2+ quench rate caused by CPA were both inhibited by antibody raised against an extracellular epitope of TRPC1, suggesting an important role of TRPC1 in the contribution of CCE in mouse PASMCs.
TRPC1 mediates CCE in mouse PASMCs. (a) When applied in Ca2+-free solution, depletion of intracellular stores with 10 μM CPA transiently elevated the fura-2 fluorescence ratio, indicating Ca2+ release from the intracellular stores (arrow). Readdition of 2 mM Ca2+ in the continued presence of CPA caused a transient followed by a sustained increase in the fluorescence ratio. The transient but not the sustained component was reduced by 10 μM nifedipine (Nif). (b) Depletion of intracellular Ca2+ stores with 10 μM CPA increased the rate of Mn2+ quench of fura-2 fluorescence in the presence of 10 μM nifedipine. (c) RT-PCR (reverse-transcription polymerase chain reaction) products from cultured mouse PASMCs amplified using primers for mouse TRPC1 (516 bp) and β-actin (498 bp). (d) TRPC1 protein and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were detected in cultured mouse PASMCs using Western blot analysis. A negative control was performed by preincubating TRPC1 antibody with the antigen peptide. (e) TRPC1 antibody (1:100) inhibited the CPA-induced sustained but not transient increase in fura-2 fluorescence ratio in the presence of 10 μM nifedipine. (f) TRPC1 antibody (1:100) inhibited the increase in Mn2+ quench of fura-2 fluorescence caused by 10 μM CPA in the presence of 10 μM nifedipine. Modified with permission42
4 STIM1 Mediates Capacitative Ca2+ Entry in Pulmonary Artery Smooth Muscle
STIM1 mRNA and protein were found to express in rat PASMCs.37 However, the functional role of STIM1 in the activation of CCE in PASMCs remains unknown. We have studied the functional role of STIM1 in mouse PASMCs using the siRNA approach and overexpression methods.42 We have shown that endogenous STIM1 mRNA and protein express in mouse PASMCs, and the expression level of STIM1 protein was reduced by siRNA knockdown of STIM1 mRNA (Fig. 8.2). We found that siRNA knockdown of STIM1 protein reduced the dihydropyridine-insensitive transient and sustained rise in [Ca2+]i and cation influx activated by store depletion, and these responses to store depletion were enhanced in cells overexpressing STIM1. These data provide the first functional evidence that endogenous STIM1 contributes to CCE in PASMCs.
STIM1 mediates CCE in mouse PASMCs. (a) RT-PCR products from cultured mouse PASMCs amplified using primers for mouse STIM1 (473 bp) and β-actin (498 bp). (b) STIM1 protein and GAPDH were detected in mouse PASMCs (nontransfected) and in PASMCs transfected with 200 nM scrambled siRNA (negative control). The expression of STIM1 but not GAPDH was reduced significantly in cells transfected with 200 nM STIM1 siRNA. (c) siRNA knockdown of STIM1 reduced the CPA-induced transient and sustained increase in fura-2 fluorescence ratio in the presence of 10 μM nifedipine. (d) siRNA knockdown of STIM1 reduced the increase in Mn2+ quench of fura-2 fluorescence caused by 10 μM CPA in the presence of 10 μM nifedipine. (e) Overexpression of STIM1 enhanced the increase in CPA-induced transient and the sustained rise in fura-2 fluorescence ratio in the presence of 10 μM nifedipine. Inset STIM1 protein and GAPDH were detected in noninfected mouse PASMCs and in PASMCs infected with adenovirus containing green fluorescent protein (Ad-GFP). The expression of STIM1 increased markedly in cells infected with STIM1-GFP-adenovirus (Ad-GFP-STIM1). (f) Overexpression of STIM1 enhanced the increase in Mn2+ quench of fura-2 fluorescence caused by 10 μM CPA in the presence of 10 μM nifedipine. Modified with permission42
5 Functional Interaction of TRPC1 and STIM1 in Pulmonary Artery Smooth Muscle
So far, there is little evidence for the functional interaction between TRPC1 and STIM1 in vascular smooth muscle cells. Although TRPC1 was shown to be an essential component of SOCs in PASMCs,39 aorta,18,19 cerebral artery,20 mesenteric artery,8 and coronary artery21 smooth muscle cells and STIM1 was found to mediate CCE in human airway smooth muscle cells,33 cultured human coronary artery cells,31 and mouse aorta smooth muscle cells,32 none of these studies showed that STIM1 is functionally linked to TRPC1 in mediating CCE in the same cell. Only one study suggested STIM1 may interact with TRPC1, which was reported by Li et al.11 in cultured human saphenous vein cells. They showed that thapsigargin-induced rise in [Ca2+]i was inhibited by STIM1 antibody and STIM1 siRNA. On the other hand, they also showed that thapsigargin-induced rise in [Ca2+]i was inhibited by TRPC1 antibody. However, they did not test whether STIM1 is functionally associated with TRPC1. To address this question, we have studied the effects of TRPC1 antibody in cells overexpressing STIM1.42 We found that overexpression of STIM1 resulted in an increase in the dihydropyridine-insensitive transient and sustained rise in [Ca2+]i and the Mn2+ quench rate caused by CPA (Fig. 8.3). These responses were reduced in cells treated with TRPC1 antibody, suggesting a functional association of STIM1 and TRPC1 to mediate CCE in mouse PASMCs.
STIM1 associates with TRPC1 to mediate CCE in mouse PASMCs. (a) In cultured mouse PASMCs overexpressed with STIM1, TRPC1 antibody (1:100) reduced the CPA-induced transient and sustained increase in the fura-2 fluorescence ratio in the presence of 10 μM nifedipine. (b) In cultured mouse PASMCs overexpressing STIM1, TRPC1 antibody (1:100) inhibited the increase in Mn2+ quench of fura-2 fluorescence caused by 10 μM CPA in the presence of 10 μM nifedipine. Modified with permission42
More interestingly, we found that STIM1 coimmunoprecipitates TRPC1, and the precipitation level of TRPC1 was increased during store depletion (Fig. 8.4). Therefore, SOCs may consist of a molecular complex composed of TRPC1 and STIM1 in mouse PASMCs, and when the intracellular Ca2+ stores are depleted, STIM1 that resides in the cytosol may be recruited to the cell membrane and interacts with more TRPC1 to enhance CCE. This molecular complex has not previously been described in any vascular smooth muscle preparation, including PASMCs. Although STIM1 has been shown to coimmunoprecipitate with TRPC1 in cultured human saphenous vein cells, the possibility that the association between TRPC1 and STIM1 maybe increased after store depletion in these cells was not examined.11
TRPC1 interacts with STIM1 to form SOCs in mouse PASMCs. STIM1 coimmunoprecipitates TRPC1 in cultured mouse PASMCs in the absence and presence of store depletion. STIM1 was first immunoprecipitated (IP) with EXBIO STIM1 antibody (10 μg), and the blot was subsequently probed with BD Biosciences STIM1 antibody (WB, 1:100). The blot was then probed for co-IP of TRPC1 expression using TRPC1 antibody (WB, 1:100, Alomone). Modified with permission42
6 Summary and Conclusions
Our study in mouse PASMCs confirmed a novel functional interaction between STIM1 and TRPC1, in which TRPC1 mediates CCE through activation of STIM1.42 This is supported by the evidence that endogenous TRPC1 (Fig. 8.1) and STIM1 (Fig. 8.2) mediate CCE in mouse PASMCs. Overexpression of STIM1 increased CCE, and this increase in CCE was significantly reduced by TRPC1 antibody (Fig. 8.3). Moreover, STIM1 coimmunoprecipitates TRPC1, and store depletion enhances the association of STIM1 and TRPC1 in mouse PASMCs (Fig. 8.4). These data provide strong evidence for a functional link between STIM1 and TRPC1 to mediate CCE.
Another interesting finding is that the dihydropyridine-insensitive transient rise in [Ca2+]i caused by CPA was not affected by TRPC1 antibody but was significantly reduced in PASMCs transfected with STIM1 siRNA (Figs. 8.1 and 8.2). Therefore, it is likely that other TRPC channels may heteromultimerize with TRPC1 and STIM1 to function as SOCs in mouse PASMCs. It is also possible that the dihydropyridine-insensitive transient rise in [Ca2+]i may be mediated by Orai1, which has been shown to be a pore-forming subunit of the calcium release-activated calcium (CRAC) channel in nonexcitable cells.43,44 Coexpression of Orai1 and STIM1 was found to cause a significant gain in CRAC channel function, suggesting that STIM1 interacts with Orai1 to cause CCE.45,46 On the other hand, overexpression of Orai1 in HEK cells was found to interact with the store depletion-insensitive channels TRPC3 and TRPC6 and confer store depletion sensitivity to these channels.47 Furthermore, TRPC1 was shown to form a complex with STIM1 and Orai1 to activate SOCs in human salivary gland cells.48 Thus, future studies on whether other TRPC channels or Orai1 interact with STIM1 and mediate the dihydropyridine-insensitive transient rise in [Ca2+]i in mouse PASMCs are warranted.
Based on our study in mouse PASMCs and other studies in nonexcitable cells,42,45,48 we have proposed a model for the molecular makeup of SOCs activated by STIM1 and other Ca2+ entry pathways activated by store depletion in PASMCs (Fig. 8.5). Depletion of intracellular Ca2+ stores causes Ca2+ release from the SR, activation of the Ca2+-activated chloride channel, leading to chloride efflux and membrane depolarization, which causes activation of VOCCs. Store depletion also causes activation of SOCs, leading to Na+ and Ca2+ entry. When the stores are depleted, STIM1 in the SR senses the depletion of Ca2+, coalesces, and moves to the cell membrane to interact with STIM1 that resides in the membrane. The STIM1 complex then activates the opening of SOCs in the cell membrane. SOCs may be composed of α- and β-subunits. TRPC1 channels possess six transmembrane-spanning regions, which may serve as the α-subunit, either as a homotetramer or a heterotetramer with other TRPC channels. Orai1 is composed of four transmembrane-spanning regions that may serve as the β-subunit. Orai1 may act as a transducer of STIM1 signals, leading to the activation of nonselective cation TRPC1 channels and Ca2+ and Na+ entry.
A plausible model for molecular makeup of store-operated channels activated by STIM1 in mouse PASMCs. Abbreviations: Clca Ca2+-activated chloride channel; CPA cyclopiazonic acid; DAG diacylglycerol; IP 3 inositol 1,4,5-trisphosphate; PLC phospholipase C; TG thapsigargin; VOCC voltage-operated Ca2+ channel
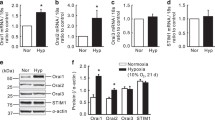
To date, the study of CCE has become one of the important areas for pulmonary vascular research because of its implication in hypoxic pulmonary vasoconstriction (HPV). Pulmonary vasoconstriction in response to hypoxia is an important protective mechanism that diverts blood flow away from hypoxic alveoli into better-ventilated regions of the lung. This acute hypoxic pressor response is a unique physiological process that distinguishes pulmonary from the systemic circulation, which usually dilates in response to hypoxia. Evidence that hypoxia causes a sustained rise in [Ca2+]i through activation of CCE in PASMCs,15,37 intact pulmonary arteries,49 and isolated lungs50 confirms a significant role of CCE in HPV. Interestingly, expression of TRPC1 and TRPC6 was significantly elevated in chronic hypoxia, and TRPC1 was found to mediate CCE in rat PASMCs,41 suggesting a potential role of the TRPC1 channel in HPV. As discussed in this chapter, STIM1 was found to interact with TRPC1 to mediate CCE in mouse PASMCs.42 This may serve as an important model for future studies of the mechanisms underlying HPV.
References
Putney JW Jr (1986) A model for receptor-regulated calcium entry. Cell Calcium 7:1-12
Parekh AB, Putney JW Jr (2005) Store-operated calcium channels. Physiol Rev 85:757-810
Hoth M, Penner R (1992) Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355:353-356
Leung FP, Yung LM, Yao X, Laher I, Huang Y (2007) Store-operated calcium entry in vascular smooth muscle. Br J Pharmacol 153:846-857
Trepakova ES, Gericke M, Hirakawa Y, Weisbrod RM, CohenRA, Bolotina VM (2001) The properties of a native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells. J Biol Chem 276:7782-7790
Golovina VA, Platoshyn O, Bailey CL et al (2001) Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol 280:H746-H755
Albert AP, Large WA (2002) A Ca2+-permeable non-selective cation channel activated by depletion of internal Ca2+ stores in single rabbit portal vein myocytes. J Physiol 538:717-728
Saleh SN, Albert AP, Peppiatt CM, Large WA (2006) Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes. J Physiol 577:479-495
Saleh SN, Albert AP, Peppiatt-Wildman CM, Large WA (2008) Diverse properties of store-operated TRPC channels activated by protein kinase C in vascular myocytes. J Physiol 586:2463-2476
Ng LC, Gurney AM (2001) Store-operated channels mediate Ca2+ influx and contraction in rat pulmonary artery. Circ Res 89:923-929
Li J, Sukumar P, Milligan CJ et al (2008) Interactions, functions, and independence of plasma membrane STIM1 and TRPC1 in vascular smooth muscle cells. Circ Res 103:e97-e104
Wilson SM, Mason HS, Smith GD et al (2002) Comparative capacitative calcium entry mechanisms in canine pulmonary and renal arterial smooth muscle cells. J Physiol 543:917-931
Weirich J, Dumont L, Fleckenstein-Grun G (2005) Contribution of capacitative and non-capacitative Ca2+-entry to M3-receptor-mediated contraction of porcine coronary smooth muscle. Cell Calcium 38:457-467
McElroy SP, Gurney AM, Drummond RM (2008) Pharmacological profile of store-operated Ca2+ entry in intrapulmonary artery smooth muscle cells. Eur J Pharmacol 584:10-20
Ng LC, Kyle BD, Lennox AR, Shen XM, Hatton WJ, Hume JR (2008) Cell culture alters Ca2+ entry pathways activated by store-depletion or hypoxia in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 294:C313-C323
Pedersen SF, Owsianik G, Nilius B (2005) TRP channels: an overview. Cell Calcium 38:233-252
Albert AP, Saleh SN, Peppiatt-Wildman CM, Large WA (2007) Multiple activation mechanisms of store-operated TRPC channels in smooth muscle cells. J Physiol 583:25-36
Xu SZ, Beech DJ (2001) TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ Res 88:84-87
Brueggemann LI, Markun DR, Henderson KK, Cribbs LL, Byron KL (2006) Pharmacological and electrophysiological characterization of store-operated currents and capacitative Ca2+ entry in vascular smooth muscle cells. J Pharmacol Exp Ther 317:488-499
Bergdahl A, Gomez MF, Wihlborg AK et al (2005) Plasticity of TRPC expression in arterial smooth muscle: correlation with store-operated Ca2+ entry. Am J Physiol Cell Physiol 288:C872-C880
Takahashi Y, Watanabe H, Murakami M et al (2007) Involvement of transient receptor potential canonical 1 (TRPC1) in angiotensin II-induced vascular smooth muscle cell hypertrophy. Atherosclerosis 195:287-926
Xu SZ, Boulay G, Flemming R, Beech DJ (2006) E3-targeted anti-TRPC5 antibody inhibits store-operated calcium entry in freshly isolated pial arterioles. Am J Physiol Heart Circ Physiol 291:H2653-H2669
Goel M, Sinkins WG, Schilling WP (2002) Selective association of TRPC channel subunits in rat brain synaptosomes. J Biol Chem 277:48303-48310
Hofmann T, Schaefer M, Schultz G, Gudermann T (2002) Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci U S A 99:7461-7466
Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE (2003) Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem 278:39014-39019
Liu X, Bandyopadhyay BC, Singh BB, Groschner K, Ambudkar IS (2005) Molecular analysis of a store-operated and 2-acetyl-sn-glycerol-sensitive non-selective cation channel. Heteromeric assembly of TRPC1-TRPC3. J Biol Chem 280:21600-21606
Poteser M, Graziani A, Rosker C et al (2006) TRPC3 and TRPC4 associate to form redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heterotetrameric channels in endothelial cells. J Biol Chem 281:13588-13595
Roos J, DiGregorio PJ, Yeromin AV et al (2005) STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169:435-445
Zhang SL, Yu Y, Roos J et al (2005) STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437:902-905
Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL (2006) STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci U S A 103:4040-4045
Takahashi Y, Watanabe H, Murakami M et al (2007) Functional role of stromal interaction molecule 1 (STIM1) in vascular smooth muscle cells. Biochem Biophys Res Commun 361:934-940
Dietrich A, Kalwa H, Storch U et al (2007) Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflügers Arch 455:465-477
Peel SE, Liu B, Hall IP (2006) A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir Res 7:119-126
Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S (2007) STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol 9:636-645
Walker RL, Hume JR, Horowitz B (2001) Differential expression and alternative splicing of TRP channel genes in smooth muscles. Am J Physiol Cell Physiol 280:C1184-C1192
Wang J, Shimoda LA, Sylvester JT (2003) Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 286:L848-L858
Lu W, Wang J, Shimoda LA, Sylvester JT (2008) Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 295:L104-L113
Golovina VA (1999) Cell proliferation is associated with enhanced capacitative Ca2+ entry in human arterial myocytes. Am J Physiol Cell Physiol 277:C343-C349
Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX-J (2002) Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 283:L144-L155
Kunichika N, Yu Y, Remillard CV, Platoshyn O, Zhang S, Yuan JX-J (2004) Overexpression of TRPCs enhances pulmonary vasoconstriction induced by capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 287:L962-L969
Lin MJ, Leung GP, Zhang WM et al (2004) Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95:496-505
Ng LC, McCormack MD, Airey JA et al (2009) TRPC1 and STIM1 mediate capacitative calcium entry in mouse pulmonary artery smooth muscle cells. J Physiol 587:2429-2442
Feske S, Gwack Y, Prakriya M et al (2006) A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441:179-185
Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG (2006) Orai1 is an essential pore subunit of the CRAC channel. Nature 443:230-233
Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL (2006) Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem 281:20661-20665
Mercer JC, Dehaven WI, Smyth JT et al (2006) Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor. Stim1. J Biol Chem 281:24979-24990
Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L (2007) Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A 104:4682-4687
Cheng KT, Liu X, Ong HL, Ambudkar IS (2008) Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J Biol Chem 283:12935-12940
Robertson TP, Hague D, Aaronson PI, Ward JP (2000) Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol 525:669-680
Weigand L, Foxson J, Wang J, Shimoda LA, Sylvester JT (2005) Inhibition of hypoxic pulmonary vasoconstriction by antagonists of store-operated Ca2+ and nonselective cation channels. Am J Physiol Lung Cell Mol Physiol 289:L5-L13
Acknowledgments
Our work was supported by National Institutes of Health grants HL 49254 (Joseph R. Hume), P20RR15581 from the National Center for Research Resources (Joseph R. Hume), and an American Heart Association Scientist Development Grant (Lih Chyuan Ng).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Humana Press, a part of Springer Science+Business Media, LLC
About this paper
Cite this paper
Ng, L.C., Airey, J.A., Hume, J.R. (2010). The Contribution of TRPC1 and STIM1 to Capacitative Ca2+ Entry in Pulmonary Artery. In: Yuan, JJ., Ward, J. (eds) Membrane Receptors, Channels and Transporters in Pulmonary Circulation. Advances in Experimental Medicine and Biology, vol 661. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-60761-500-2_8
Download citation
DOI: https://doi.org/10.1007/978-1-60761-500-2_8
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-60761-499-9
Online ISBN: 978-1-60761-500-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)