Abstract
Hepatocellular carcinoma (HCC) is currently the fifth most common tumor worldwide and is expected to continue to increase in incidence over the next couple of decades. The majority of patients with HCC have cirrhosis of the liver, with chronic hepatitis B and C as the major etiological agents. Despite advances in technology, the prognosis of patients with HCC has shown little improvement over time likely due to the fact that most patients are diagnosed at advanced stages. HCC meets the criteria established by the Word Health Organization for performing surveillance in those at risk for developing this tumor, i.e., patients with cirrhosis of the liver. The objective of surveillance is to use a relatively simple and inexpensive test in a large number of individuals to determine if they are likely or unlikely to have cancer, with an overall goal of reducing morbidity and mortality from the cancer. Alpha-fetoprotein and liver ultrasound are the most widely utilized surveillance tests but their performance is not optimal. There is an urgent need for new surveillance tests. In this chapter we will review the criteria and the current and newer tests for the surveillance of HCC.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Key Words
11.1 Introduction
There have been little improvements in the overall survival of hepatocellular carcinoma (HCC) over the last decades primarily due to patients being diagnosed at advanced stages. One of the most important aspects of HCC is that it commonly occurs in patients with chronic liver disease, which also complicates the treatment of these patients. However, this important fact should be taken advantage in devising a strategy for the early detection of this tumor. In this chapter we will review the criteria for the screening or surveillance for HCC, the tests that are utilized, and new tests that may lead to better outcomes.
11.2 Screening/Surveillance for Hepatocellular Carcinoma
The decision to screen an at-risk population for cancer is based on well-established criteria ( 1 ). The objective of screening is the use of a relatively simple and inexpensive test in a large number of individuals to determine whether they are likely or unlikely to have the cancer for which they are being screened ( 2 ). Screening is the one-time application of a test that allows detection of a disease at a stage where curative intervention may achieve the goal of reducing morbidity and mortality. Surveillance is the continuous monitoring of disease occurrence (using the screening test) within an at-risk population to accomplish the same goals of screening.
Criteria have been developed, first promoted by the World Health Organization, to assess the benefits of screening for a specific disease ( 4 ): (a) the disease in question should be an important health problem; its significance may be defined by disease burden, including morbidity and mortality. (b) There should be an identifiable target population. (c) Treatment of occult disease (i.e., disease diagnosed before the symptoms appear) should offer advantages compared with the treatment of symptomatic disease. (d) A screening test should be affordable and provide benefits justifying its cost. (e) The test must be acceptable to the target population and to health-care professionals. (f) There must be standardized recall procedures. (g) Screening tests must achieve an acceptable level of accuracy in the population undergoing screening. (h) Surveillance should reduce mortality from the disease. We will evaluate the rationale for the surveillance of patients with HCC based on these criteria.
11.2.1 The Disease in Question Should Be an Important Health Problem; Its Significance May Be Defined by Disease Burden, Including Morbidity and Mortality
HCC is the fifth most common tumor worldwide, with an incidence rate that is similar to the death rate. In the 2007 annual report to the nation on the status of cancer, liver cancer was the 13th most common tumor in the United States and it had the largest increase in incidence of all solid tumors when 1995 and 2004 were compared ( 3 ). The incidence of HCC has been rising in both Europe and the United States, largely due to the growing prevalence of hepatitis C cirrhosis ( 4–7 ). A molecular clock study indicated that the epidemic of hepatitis C (HCV) in the United States started in the 1960s and peaked in the late 1980s ( 8 ). Because of the lag time between the onset of infection and the development of cirrhosis, the authors postulate that the incidence of HCV-related HCC will continue to increase over the next 20 years. HCC is the third most common cause of cancer-related deaths worldwide resulting in over 500,000 deaths per year. In the United States HCC is the eighth most common cause of cancer-related death at 8.5 deaths per 100,000 but has the largest increase in mortality of all solid tumors when comparing 2004 to 1995 ( 3 ). Despite advances in technology and available treatments, there have been little improvements overall due to the fact that most patients are diagnosed at advanced stages ( 9, 10 ). Together with the increasing incidence, it may lead to a significant health burden.
11.2.2 There Should Be an Identifiable Target Population
Cirrhosis has been recognized as the most important risk factor for the development of HCC ( 11 ). Table 1 shows the incidence rates for those with HCV, hepatitis B (HBV), and alcoholic-related cirrhosis ( 12 ). This table shows that HCV and HBV are the major etiological agents that lead to the development of HCC while alcohol does increase the risk to a lesser degree. HCV-associated cirrhosis is the causative agent that has been largely responsible for the increase in incidence of HCC in the United States ( 13 ). However, HBV is the leading cause of HCC worldwide, particularly in Asia and Africa ( 14 ). Recently, an association between non-alcoholic liver disease and HCC has been made ( 15 ), but there are no prospective cohort studies evaluating the natural history of non-alcoholic fatty liver disease. Other etiologies of chronic liver disease such as hemochromatosis, primary biliary cirrhosis, autoimmune hepatitis, and alpha-1 antitrypsin deficiency are less common causes of chronic liver disease with prevalence rates in patients with HCC between 1 and 8% ( 16–18 ). Furthermore, improvements in the survival of patients with cirrhosis due to better specialty care may further increase the number of individuals at risk for developing HCC ( 19 ). At the present time, patients with cirrhosis, regardless of the etiology, should undergo surveillance for HCC ( 20 ).
Even though the annual risk of developing HCC among patients with cirrhosis is between 2 and 7%, not every patient with cirrhosis will develop this tumor. Male gender, older age, obesity, alcohol and tobacco consumption, and diabetes are factors associated with an increased risk of HCC ( 21–25 ). In patients with chronic HBV infection, a baseline HBV DNA level of greater than 100,000 copies/mL increases the risk of HCC 10-fold ( 26 ). This biological gradient of HCC risk in relation to HBV DNA levels suggests that persistent viral replication increases the risk of HCC. A prospective cohort study of patients with cirrhosis found that prothrombin activity <75% of baseline, age >55, platelet count <75 mm3, and HCV were independent risk factors for developing HCC ( 27 ). They stratified patients into a high-risk group (presence of these factors) and into a low-risk group (absence of risk factors), and the 5-year cumulative incidence of HCC was 30% for the high-risk group and 4% for the low-risk group ( p < 0.0001). Further studies should be performed to determine if stratification according to risk factors is beneficial for delineating a sub-group of patients with cirrhosis that may be at a higher risk of developing HCC in whom more aggressive surveillance can be applied.
11.2.3 Treatment of Occult Disease Should Offer Advantages Compared with the Treatment of Symptomatic Disease
The effectiveness of the treatments for HCC will depend on the stage at the time of diagnosis. For early-stage tumors, surgical resection has provided 5-year survival rates of 70% in carefully selected patients with preserved hepatic function, no evidence of portal hypertension, and single small asymptomatic tumors (<5 cm in maximal diameter) ( 20 ). Liver transplantation is the preferred method of treatment for patients not amenable to surgical resection but for those restricted to the Milan criteria (single nodule <5 cm or <3 nodules each <3 cm in diameter) ( 28 ). The 5-year survival reported for liver transplantation is >70% ( 29 ). Ablative treatments, specifically percutaneous ethanol injection and radiofrequency ablation, have 5-year survival rates similar to hepatic resection ( 30 ). Therefore, therapies currently exist for patients with early-stage HCC, and an efficacious surveillance program is critical for the identification of HCC at these early stages.
11.2.4 A Screening Test Should Be Affordable and Provide Benefits Justifying Its Cost
The standard threshold for cost-effectiveness of a medical test or procedure has been determined to be a maximal of $50,000 per quality-adjusted life year (QALY). Economic models studying the benefits of surveillance programs in HCC have been performed. Surveillance with biannual alpha-fetoprotein (AFP) and ultrasonography in Child class A cirrhotics increase the mean life expectancy with cost-effectiveness ratios between $26,000 and $55,000 per QALY ( 31 ). When a similar analysis was performed in HCV cirrhotics, the cost-utility ratio was $26,689 per QALY ( 32 ). Another study evaluating the cost-effectiveness of biannual AFP and ultrasound in HCV Child class A cirrhosis showed a cost-effective ratio of $33,083 per QALY ( 33 ). Therefore, screening with ultrasound and AFP has been shown to be cost-effective in compensated cirrhotics even though the performance of these tests is not the best.
11.2.5 The Test Must Be Acceptable to the Target Population and to Health-Care Professionals
Surveillance for HCC seems to be acceptable to patients with cirrhosis. Such data come indirectly from cohort studies showing that only about 3–18% of cirrhotic patients were noncompliant with surveillance using ultrasound and AFP ( 11 ), which compares favorably with the 67% noncompliance rate seen with using colonoscopy for colon cancer screening ( 34 ). HCC surveillance also seems to be well accepted by physicians. In a national survey of 554 members of the American Association for the Study of Liver Disease, 84% of respondents indicated that they routinely screened patients with cirrhosis for HCC using AFP and ultrasound ( 35 ).
11.2.6 There Must Be Standardized Recall Procedures
A recent consensus conference offered guidelines on how to investigate abnormalities of the commonly used screening tests (AFP and ultrasound) in patients with cirrhosis ( 20 ). CT scan, MRI, and contrast-enhanced ultrasound are the major diagnostic modalities used to establish the diagnosis of HCC without the need for a histopathological examination. The main imaging characteristic for HCC is the finding of arterial enhancement of the lesion followed by washout of contrast in the delayed venous phases ( 36 ). A recent study has validated the American Association for the Study of Liver Disease guidelines for the diagnostic evaluation of an abnormal surveillance test ( 37 ). Therefore, appropriate recall modalities do exist to evaluate abnormal surveillance tests.
11.2.7 Screening Tests Must Achieve an Acceptable Level of Accuracy in the Population Undergoing Screening
Ultrasound and AFP have been recommended as the primary radiologic screening test for HCC ( 20 ). US is inexpensive, non-invasive, and widely available, which makes it an attractive surveillance test. There have been no randomized controlled trials in patients with cirrhosis to date assessing the efficacy of US as a surveillance test. The performance of ultrasound has been evaluated primarily in cohort studies as shown in Table 2 ( 38–46 ). The sensitivity for the detection of early-stage HCC ranges from 25 to 100%, while the specificity ranges from 82 to 100%. The high degree of operator dependence, differences in the equipment, body habitus, and the lack of evidence by randomized trials are significant limitations of US as a surveillance test for HCC.
AFP has been the most widely utilized serologic test to screen for HCC. The operating characteristics of AFP are dependent on the cutoff level chosen to support the diagnosis of HCC. At higher cutoff levels, the test is more specific for HCC but at a cost of decreased sensitivity; at low cutoff levels conversely, AFP becomes increasingly sensitive but with a higher rate of false positives ( 47 ). In a case–control study using 170 patients with HCC, about 60% of the patients had advanced HCC, and 170 matched patients without HCC demonstrated that the optimal cutoff was 20 ng/mL via receiver operating curve analysis ( 48 ). Therefore, a level greater than 20 ng/mL is the most commonly used cutoff in clinical practice to trigger a recall test for the diagnosis of HCC. Even at the optimal cutoff level in this study, the sensitivity was only 60% while the specificity was 90%. A recent systematic review of five studies evaluating AFP in patients with hepatitis C cirrhosis showed sensitivities ranging from 41 to 65% and specificity ranging from 80 to 94% ( 49 ). In addition, serum AFP values are frequently elevated among patients with chronic hepatitis C with advanced hepatic fibrosis even in the absence of HCC, with levels declining after antiviral therapy ( 50 ). AFP alone is insufficient for the surveillance for HCC among patients with cirrhosis. In hepatitis B carriers, the combination of ultrasound and AFP increased the sensitivity of HCC detection when compared to either test alone, increasing from 71% with ultrasound alone to 79% when ultrasound and AFP were used together ( 51 ). Chronic elevations of AFP have also been shown to increase the risk of developing HCC among patients with cirrhosis ( 52 ) and in hepatitis B carriers ( 53 ). While better tests are needed to improve the detection of early-stage HCC, AFP offers benefits in the surveillance of patients with cirrhosis leading to diagnosis in about half of the patients with HCC and determining their risk of developing this tumor.
11.3 Efficacy of Surveillance
As previously indicated, the goal of a surveillance program is for the tests to reduce overall mortality. The most reliable method to evaluate the efficacy of ultrasound and AFP for HCC surveillance would be a randomized controlled trial. There have been two large randomized controlled trials conducted in China using ultrasound and AFP among patients with chronic hepatitis B ( 54, 55 ). In both trials, surveillance was conducted every 6 months and compared to patients who did not receive any routine screening. The first study evaluated 17,920 patients that are carriers of the hepatitis B virus who were randomized to surveillance (n = 8,109) or no surveillance (n = 9,711) and then followed for an average of 14.4 months ( 54 ). Of the patients randomized to the surveillance group, 38 patients developed HCC of whom 29 (76.3%) were detected at early stages, whereas 18 patients developed HCC in the no-surveillance group, of whom none were detected at an early stage (p < 0.01). A higher proportion of patients in the surveillance group met criteria for surgical therapy, with 24 patients having surgical resection in the surveillance group compared to zero patients in the no-screening group (p < 0.05). Accordingly, the 1-year and 2-year survival rates for the surveillance group were 88.1 and 77.5%, respectively, compared to a 0% survival rate at 1 year for the no-screening group. The authors concluded that surveillance reduces HCC-associated mortality. The second randomized controlled trial evaluated 19,200 hepatitis B carriers who were randomized to surveillance (n = 9,757) and no surveillance (n = 9,443) ( 55 ). A total of 86 patients developed HCC in the surveillance group, of which 45% were early stage, compared to 67 patients with HCC in the no-surveillance group, of which none were early stage. Table 3 summarizes the results. The mortality rate of patients undergoing surveillance was significantly lower than the control group (83.2 vs. 131.5 per 100,000, p < 0.01), with a hazard ratio of 0.63 (95% CI 0.41–0.98). These results demonstrate that the strategy of surveillance with US and AFP among patients with chronic hepatitis B reduces overall mortality. However, it is unclear if all the patients in these two studies had the same risk of developing HCC, given the low rate of development of HCC seen. These studies did not mention the number of patients that had cirrhosis or evidence of viral replication and most likely had patients that were asymptomatic carriers, which are at a lower risk for developing HCC. Therefore, the results are not generalizable to the majority of patients at risk for developing HCC.
While randomized controlled trials have been performed in China using patients with chronic hepatitis B, the results cannot be extrapolated to cirrhotic patients, who account for the majority of patients with HCC. No randomized trials have been performed in a cirrhotic population, so most of the data on surveillance in patients with cirrhosis come from cohort studies. Some studies have shown that patients undergoing surveillance with ultrasound and AFP have a better overall survival when compared to either historical controls or patients with HCC who did not undergo surveillance. Table 4 shows the details of these cohort studies including the number of HCC and early-stage HCC that developed during follow-up ( 38–46, 56–67 ). The results of these studies are also fraught with lead-time and length–time biases that limit their generalizability of improvements in survival with surveillance. Therefore, the impact of surveillance on mortality in patients with cirrhosis has only been assessed in non-randomized trials to date. As shown in Table 3, there has been a significant amount of heterogeneity among these studies pertaining to the sample size (ranging from 66 to 1,599), population studied (Child class A, Child class A or B, transplant candidates), the incidence of HCC (ranging from 3 to 28%), and number of early-stage HCC detected (ranging from 24 to 100%). Randomized or better controlled trials are needed in this area.
11.4 Novel Biomarkers
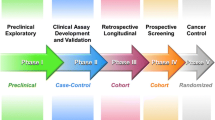
The ideal marker for HCC would be specific for HCC and not be detected in pre-malignant liver disease (i.e., cirrhosis regardless of the etiology). It should be easily accessed, easily measurable, reproducible, minimally invasive, accurate, and acceptable to patients and physicians ( 68 ). The current tests do not meet these criteria and new ones are needed. The recent developments of gene-expression microarrays, proteomics, and tumor immunology permit thousands of genes and proteins to be screened simultaneously. With the growing application of these techniques, it is anticipated that there will be an explosion of new biomarkers for cancer screening including HCC in the next decade. To establish a formal framework to guide the process of biomarker evaluation and development, a five-phase program is utilized by the Early Detection Research Network (EDRN) of the National Cancer Institute (Table 5) ( 69 ). These five phases help define criteria to determine the current status of biomarkers in the published literature, to assess how close these biomarkers are to clinical application, and to serve as a guide for future biomarker development. Table 6 shows promising biomarkers for HCC and level of evidence according to the phases of biomarker development.
11.4.1 Des-Gamma Carboxy-Prothrombin (DCP)
DCP is an abnormal prothrombin protein that is generated as a result of an acquired defect in the posttranslational carboxylation of the prothrombin precursor in malignant hepatic cells ( 70 ). A single center case-controlled study showed that DCP was more sensitive and specific than total AFP ( 71 ). Several prospective cohort studies in patients with cirrhosis without HCC have been performed to determine the performance of DCP ( 72–75 ). The sensitivities for detecting HCC ranged from 23 to 57% compared to 14 to 71% for AFP. In the largest study on DCP, 734 patients with cirrhosis were followed for a mean of 13 months (range 7–17 months) during which HCC was detected in 29 patients. The sensitivity and specificity of DCP at baseline was 41% and 90%, and 40% and 62% for AFP, respectively. Overall, AFP and DCP had equal sensitivity but DCP had better specificity. Large studies are underway to evaluate the role of DCP in the detection of early-stage HCC.
11.4.2 Lens Culinaris Agglutinin Reactive Fraction of AFP (AFP-L3)
Lens culinaris agglutinin is a plant-derived lectin that recognizes fucose residues on N-glycosylated polypeptides ( 68 ). Several variants of AFP with differences in lectin affinities have been identified. One variant, the fucosylated variant, has a high affinity of the sugar chain to lens culinaris. This variant has been shown to be more specific for HCC than total AFP ( 76 ). Prospective studies in patients with cirrhosis have shown sensitivities for AFP-L3 ranging from 55 to 75% and specificities from 68 to 90% ( 77–79 ). However, two studies included only HCC patients with elevated total AFP at baseline making it impossible to compare the accuracy of AFP-L3 with total AFP. A prospective study evaluated the clinical utility of AFP-L3 in a North American multicenter cohort ( 80 ). The authors evaluated 332 patients with HCV cirrhosis and 34 developed HCC. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for AFP (cutoff > 20 ng/mL) were 61, 71, 34, and 88%, respectively, while for AFP-L3 (cutoff 10%) were 36, 91, 51, and 85%, respectively. The main utility of AFP-L3% was that in someone with cirrhosis it increases the risk of developing HCC. At this time there is no evidence of AFP-L3’s efficacy in the surveillance of patients with cirrhosis or that these are better than AFP and ultrasound in this capacity.
11.4.3 Glypican-3
Glypican 3 is a member of the glypican family of cell-surface heparan sulfate proteoglycans, recently found to be upregulated in early-stage HCC compared to normal hepatic tissue ( 81 ). Evaluation of glypican-3 as a serum marker for HCC has been reported ( 82 ). In this study, glypican-3 expression in liver was detected using immunohistochemistry in 0/22 cirrhotics without dysplasia or HCC, 1/5 cirrhotics with high-grade dysplasia, and 21/29 HCCs. For tumors <3 cm, glypican-3 expression was detected in 11/11 and AFP in only 2/9. Using enzyme-linked immunoassay, glypican-3 was detected in the serum from 18/34 (53%) patients with HCC and only 1/20 (5%) patients with cirrhosis (p = 0.0049). More recently, it was found that glypican-3 expression was an independent histological marker for differentiating early HCC from cirrhosis ( 83 ). Further studies are needed to determine if the sensitivities can be improved in the serum in order for glypican-3 to be utilized in the surveillance for HCC.
11.4.4 Golgi Protein (GP73)
GP73 is a resident Golgi protein that is upregulated in virus-infected hepatocytes ( 84 ). Using Western blot assay, GP73 has been detected in serum with significantly higher levels among cirrhotics and patients with HCC than in normal subjects and patients with chronic hepatitis. In a phase 2 study, a total of 296 patients (152 cirrhosis controls and 144 HCC cases) were studied ( 85 ). Serum GP73 levels were significantly higher in patients with HCC compared to those with cirrhosis (p < 0.001). GP73 had a sensitivity of 69% and a specificity of 75% at the optimal cutoff point of 10 relative units, with an area under the receiver operating curve of 0.79 vs. 0.61 for AFP (p = 0.001). GP73 levels had significantly higher sensitivity (62%) than AFP (25%) for diagnosing early HCC (p < 0.0001). Moreover, GP73 levels were elevated in the serum of 57% (32/56) individuals with HCC who had serum AFP levels less than 20 ng/mL. GP73 should be tested in a larger sample set to determine the performance characteristics.
11.4.5 Glycoproteins
The fucome is the subset of polypeptides that contain the sugar fucose. Fucosylated N-glycans derived from glycoproteins in the serum of patients with HCC are greatly elevated compared to healthy individuals, and a recent study showed more than 50 fucosylated serum proteins in the woodchuck model of HCC and in human HCC ( 86 ). Fucosylated GP73 and hemopexin are examples of cases in which the measurement of these glycoproteins had sensitivities over 90% and was better than measuring the total amount. This is an interesting area of research that may lead to a significant biomarker but more validation studies are needed.
11.4.6 Human Hepatocyte Growth Factor (HHGF)
HHGF is a growth factor that has mitogenic, anti-apoptotic, and anti-fibrotic effects, and therefore, it is important in hepatocarcinogenesis. A recent study evaluated 70 patients with HCV cirrhosis and 38 patients with HCC in order to evaluate the role of HHGF in liver cancer ( 87 ). In patients with HCC, however, HGF showed little localization in cancer cells, but was noted in infiltrating mesenchymal cells in both cancerous and noncancerous regions, perhaps a measure of metastatic spread. Another study evaluated HHGF in 134 patients with HCV-related disease (62 had cirrhosis and 72 chronic hepatitis) who were followed for 4 years, 28 developing HCC ( 88 ). Human HGF had a sensitivity and specificity of 100 and 63%, respectively, at the time of HCC diagnosis. These results are preliminary and require further study but the high sensitivity is promising for HHGF being a surveillance test.
11.4.7 Insulin Growth Factor-1 (IGF-1)
Deregulation of the insulin-like growth factor (IGF) axis, including the autocrine production of IGFs, IGF-binding proteins (IGFBPs), IGFBP proteases, and the expression of the IGF receptors, has been identified in the development of hepatocellular carcinoma (HCC). IGF-1 was measured in 114 patients with HCV-related cirrhosis followed for a mean of 56±12 months; AFP and ultrasound were monitored annually ( 89 ). HCC developed in 20 patients. Among those in whom HCC developed, there was a mean annual decrease of 16 μg/L in IGF-1 levels until the diagnosis of HCC. A decrease in IGF-1 levels of 9.3 μg/L had a sensitivity of 70% for diagnosis of HCC. This is a well-done study that showed reductions in IGF-1 levels prior to the diagnosis of HCC. This marker should undergo further study as a HCC surveillance test.
11.4.8 Squamous Cellular Carcinoma Antigen (SCCA)
SSCA is a serine protease inhibitor physiologically present in the skin, which has been detected in HCC tissue ( 90 ). SCCA is strongly expressed in HCC than peritumoral tissue, and it also increases the AFP diagnostic capability up to 90% ( 91 ). A total of 961 patients, diagnosed as LC (462) and HCC (499), were enrolled to evaluate for the performance of SSCA ( 92 ). The SCCA AUC was 0.656 (95% CI 0.625–0.686), and the cutoff value was 3.8 ng/mL, showing 41.9% sensitivity and 82.6% specificity. SSCA was complementary to AFP improving the sensitivity to 80%. A large study is underway to investigate this marker in HCC.
11.4.9 Osteopontin (OPN)
OPN is a highly phosphorylated and glycosylated protein, the modification after transcription is very important to its function. In hepatocellular carcinoma (HCC), the elevated expression of OPN at mRNA levels and its relationship with metastasis and poorer prognosis of the patients have been reported. OPN in HBV-related HCC was studied recently ( 93 ). Thirty-nine of 72 (54.17%) HBV-related HCC specimens were positive for OPN with cytoplasmic staining. OPN was highly expressed in the specimens with capsular infiltration compared to those without (p < 0.05) and also was significantly related with portal vein invasion (p < 0.01) and lymph node invasion (p < 0.01). In another study of 62 HCC patients, 60 patients with chronic liver diseases, and in 60 healthy controls, OPN was measured in the plasma ( 94 ). Plasma OPN levels in the HCC patients (median 954 ng/mL, range 168–5,742) were significantly higher (p-value < 0.001) than those patients with chronic liver diseases (381 ng/mL, 29–1,688) or of a healthy control group (155 ng/mL, 10–766). Within the HCC patient group, plasma OPN level increased significantly with advancing degree of Child–Pugh class and of tumor stage. Diagnostic sensitivity and specificity of OPN for HCC was 87 and 82%, respectively (cutoff value: 617.6 ng/mL). OPN had a greater area under curve value (0.898) than AFP (0.745) or DCP (0.578), suggesting superior diagnostic accuracy of OPN. This marker has potential and should be studied further in larger trials.
11.4.10 Proteomics
Proteomics studies the complete set of proteins expressed in a given cell, tissue, or biofluid. Proteomics not only characterizes protein expression profiles but also identifies protein structures, localizations, activities, modifications, and interactions in physiological or pathological states. As proteins perform most biological functions, proteomics bridges the gap between the information coded in the genome sequence and the cellular behavior. Proteomics studies of HCC may not only elucidate the mechanisms of HCC initiation and progression but also have the potential to discover novel diagnostic and prognostic biomarkers as well as therapeutic targets. There are several techniques that can be applied to study the proteins and these include two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) and surface-enhanced laser desorption/ionization (SELDI), which combines purification of samples on a wide variety of affinity matrices and identification by time-of-flight mass spectrometry (TOF-MS) ( 95 ). However, these do not identify the proteins. Mass spectrometry is the current method of choice for the identification of proteins, as this method offers high analytical sensitivity and the capacity for high-throughput protein identification. Current studies have shown various patterns that appear to differentiate HCC from controls ( 96 ), but these studies are in their infancy and prospective studies are required.
A recent study showed the potential of the proteomic approach. A total of 10 HCC tissues from patients with HCV cirrhosis were analyzed by 2D-PAGE ( 97 ). Forty-seven protein spots that showed reproducible variation were identified by mass spectrometry, corresponding to 23 distinct genes. A positive correlation between transcript and protein level variations was observed for only 7 out of the 23 genes. Proteolytic cleavage accounted for the discrepancies between messenger RNA and protein level changes for seven genes including calreticulin, protein disulfide isomerase (PDIA3), among others. Calreticulin and PDIA3 cleavage products were detected in sera of patients with HCC. A statistically high significant difference in calreticulin and PDIA3 fragment serum levels between patients with HCC and healthy individuals was observed. Amounts of calreticulin and PDIA3 fragments were also significantly different between patients with HCC and at-risk patients (patients with cirrhosis). This showed that isoforms or cleavaged proteins may become markers for HCC.
More sensitive and specific biomarkers for HCC are urgently needed. As we have discussed in this section, there are several biomarkers that appear interesting and requires further testing because most of these have been tested in phase 1 studies. It is unlikely that one biomarker will be sufficient and more likely it will be a panel of markers. With modern advances in the study of proteins, glycoproteins, and genes, it is likely that a panel of markers may soon be identified for the early detection of HCC. For now, AFP and US are currently the best surveillance tests for HCC.
References
Cole P, Morrison AS. Basic issues in population screening for cancer. J Natl Cancer Inst 1980;64:1263–1272.
Smith RA. Screening fundamentals. J Natl Cancer Inst Monogr 1997;22:15–19.
Espey DK, Wu XC, Swan J, Wiggins C, Jim MA, Ward E, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2004. Cancer 2007;110:2119–2152.
Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis 1999;19:271–285.
Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis 2005;9:191–211.
El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology 2004;127:S27–34.
El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med 2003;139:817–823.
Tanaka Y, Hanada K, Mizokami M, Yeo AE, Shih JW, Gojobori T, Alter HJ. Inaugural Article: A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci USA 2002;99:15584–15589.
Capocaccia R, Sant M, Berrino F, Simonetti A, Santi V, Trevisani F. Hepatocellular carcinoma: trends of incidence and survival in Europe and the United States at the end of the 20th century. Am J Gastroenterol 2007;102:1661–1670; quiz 1660, 1671.
El-Serag HB, Mason AC, Key C. Trends in survival of patients with hepatocellular carcinoma between 1977 and 1996 in the United States. Hepatology 2001;33:62–65.
Collier J, Sherman M. Screening for hepatocellular carcinoma. Hepatology 1998;27:273–278.
Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35–50.
Colombo M, Kuo G, Choo QL, Donato MF, Del Ninno E, Tommasini MA, Dioguardi N, et al. Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet 1989;2:1006–1008.
Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet 1981;2: 1129–1133.
Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology 2002;36:1349–1354.
Deugnier Y, Turlin B. Iron and hepatocellular carcinoma. J Gastroenterol Hepatol 2001;16:491–494.
Farinati F, Floreani A, De Maria N, Fagiuoli S, Naccarato R, Chiaramonte M. Hepatocellular carcinoma in primary biliary cirrhosis. J Hepatol 1994;21:315–316.
Nishiyama R, Kanai T, Abe J, Hara R, Watahiki Y, Sakaguchi T, Nakamura S. Hepatocellular carcinoma associated with autoimmune hepatitis. J Hepatobiliary Pancreat Surg 2004;11:215–219.
Carbonell N, Pauwels A, Serfaty L, Fourdan O, Levy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology 2004;40:652–659.
Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208–1236.
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557–2576.
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625–1638.
Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, Decarli A, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol 2002;155:323–331.
El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004;126:460–468.
Kuper H, Tzonou A, Kaklamani E, Hsieh CC, Lagiou P, Adami HO, Trichopoulos D, et al. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer 2000;85:498–502.
Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65–73.
Velazquez RF, Rodriguez M, Navascues CA, Linares A, Perez R, Sotorrios NG, Martinez I, et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology 2003;37:520–527.
Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–699.
Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis 2005;25:181–200.
Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006; 243: 321–328.
Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med 1996;101:422–434.
Arguedas MR, Chen VK, Eloubeidi MA, Fallon MB. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: a cost-utility analysis. Am J Gastroenterol 2003;98:679–690.
Lin OS, Keeffe EB, Sanders GD, Owens DK. Cost-effectiveness of screening for hepatocellular carcinoma in patients with cirrhosis due to chronic hepatitis C. Aliment Pharmacol Ther 2004;19:1159–1172.
Meissner HI, Smith RA, Rimer BK, Wilson KM, Rakowski W, Vernon SW, Briss PA. Promoting cancer screening: Learning from experience. Cancer 2004;101:1107–1117.
Chalasani N, Said A, Ness R, Hoen H, Lumeng L. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol 1999;94:2224–2229.
Marrero JA, Hussain HK, Nghiem HV, Umar R, Fontana RJ, Lok AS. Improving the prediction of hepatocellular carcinoma in cirrhotic patients with an arterially-enhancing liver mass. Liver Transpl 2005;11:281–289.
Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47:97–104.
Cottone M, Turri M, Caltagirone M, Maringhini A, Sciarrino E, Virdone R, Fusco G, et al. Early detection of hepatocellular carcinoma associated with cirrhosis by ultrasound and alfafetoprotein: a prospective study. Hepatogastroenterology 1988;35:101–103.
Pateron D, Ganne N, Trinchet JC, Aurousseau MH, Mal F, Meicler C, Coderc E, et al. Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. J Hepatol 1994;20:65–71.
Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, Piscaglia F, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut 2001;48:251–259.
Kobayashi K, Sugimoto T, Makino H, Kumagai M, Unoura M, Tanaka N, Kato Y, et al. Screening methods for early detection of hepatocellular carcinoma. Hepatology 1985;5:1100–1105.
Sheu JC, Sung JL, Chen DS, Lai MY, Wang TH, Yu JY, Yang PM, et al. Early detection of hepatocellular carcinoma by real-time ultrasonography. A prospective study. Cancer 1985;56:660–666.
Oka H, Tamori A, Kuroki T, Kobayashi K, Yamamoto S. Prospective study of alpha-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology 1994;19:61–66.
Henrion J, Libon E, De Maeght S, Deltenre P, Schapira M, Ghilain JM, et al. Screening for hepatocarcinoma in a cohort with cirrhosis mainly of alcoholic origin. Gastroenterol Clin Biol003;27:534–539.
Zoli M, Magalotti D, Bianchi G, Gueli C, Marchesini G, Pisi E. Efficacy of a surveillance program for early detection of hepatocellular carcinoma. Cancer 1996;78: 977–985.
Santagostino E, Colombo M, Rivi M, Rumi MG, Rocino A, Linari S, Mannucci PM. A 6-month versus a 12-month surveillance for hepatocellular carcinoma in 559 hemophiliacs infected with the hepatitis C virus. Blood 2003;102:78–82.
Colli A, Fraquelli M, Conte D. Alpha-fetoprotein and hepatocellular carcinoma. Am J Gastroenterol 2006;101:1939; author reply 1940–1931.
Trevisani F, D’Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol 2001;34:570–575.
Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med 2003;139:46–50.
Di Bisceglie AM, Sterling RK, Chung RT, Everhart JE, Dienstag JL, Bonkovsky HL, Wright EC, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol 2005;43:434–441.
Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology 1995;22: 432–438.
Sangiovanni A, Colombo E, Radaelli F, Bortoli A, Bovo G, Casiraghi MA, Ceriani R, et al. Hepatocyte proliferation and risk of hepatocellular carcinoma in cirrhotic patients. Am J Gastroenterol 2001;96:1575–1580.
McMahon BJ, Bulkow L, Harpster A, Snowball M, Lanier A, Sacco F, Dunaway E, et al. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology 2000;32:842–846.
Yang B, Zhang B, Xu Y, Wang W, Shen Y, Zhang A, Xu Z. Prospective study of early detection for primary liver cancer. J Cancer Res Clin Oncol 1997;123: 357–360.
Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417–422.
Velazquez RF, Rodriguez M, Navascues CA, Linares A, Perez R, Sotorrios NG, Martinez I, et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology 2003;37:520–527.
Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol 2001;16:553–559.
Van Thiel DH, Yong S, Li SD, Kennedy M, Brems J. The development of de novo hepatocellular carcinoma in patients on a liver transplant list: frequency, size, and assessment of current screening methods. Liver Transpl 2004;10:631–637.
Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, Morabito A, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology 2004;126:1005–1014.
Tradati F, Colombo M, Mannucci PM, Rumi MG, De Fazio C, Gamba G, Ciavarella N, et al. A prospective multicenter study of hepatocellular carcinoma in Italian hemophiliacs with chronic hepatitis C. The Study Group of the Association of Italian Hemophilia Centers. Blood 1998;91:1173–1177.
Imberti D, Fornari F, Sbolli G, Buscarini E, Squassante L, Buscarini L. Hepatocellular carcinoma in liver cirrhosis. A prospective study. Scand J Gastroenterol 1993;28:540–544.
Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, Donato MF, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med 1991;325:675–680.
Cottone M, Turri M, Caltagirone M, Parisi P, Orlando A, Fiorentino G, Virdone R, et al. Screening for hepatocellular carcinoma in patients with Child’s A cirrhosis: an 8-year prospective study by ultrasound and alphafetoprotein. J Hepatol 1994;21:1029–1034.
Degos F, Christidis C, Ganne-Carrie N, Farmachidi JP, Degott C, Guettier C, Trinchet JC, et al. Hepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and death. Gut 2000;47:131–136.
Bruno S, Silini E, Crosignani A, Borzio F, Leandro G, Bono F, Asti M, et al. Hepatitis C virus genotypes and risk of hepatocellular carcinoma in cirrhosis: a prospective study. Hepatology 1997;25:754–758.
Caturelli E, Bartolucci F, Biasini E, Vigliotti ML, Andriulli A, Siena DA, Attino V, et al. Diagnosis of liver nodules observed in chronic liver disease patients during ultrasound screening for early detection of hepatocellular carcinoma. Am J Gastroenterol 2002;97:397–405.
Iavarone M, Lampertico P, Ronchi G, Del Ninno E, Zanella A, Colombo M. A prospective study of blood alpha-fetoprotein messenger RNA as a predictor of hepatocellular carcinoma in patients with cirrhosis. J Viral Hepat 2003;10:423–426.
Block TM, Marrero J, Gish RG, Sherman M, London TW, Srivastava S, et al. The degree of readiness of selected biomarkers for the early detection of Hepatocellular carcinoma: notes from a recent workshop. Cancer Biomarkers 2008;4:19–33.
Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst 2001;93:1054–1061.
Ono M, Ohta H, Ohhira M, Sekiya C, Namiki M. Measurement of immunoreactive prothrombin precursor and vitamin-K-dependent gamma-carboxylation in human hepatocellular carcinoma tissues: decreased carboxylation of prothrombin precursor as a cause of des-gamma-carboxyprothrombin synthesis. Tumour Biol 1990;11:319–326.
Marrero JA, Su GL, Wei W, et al. Des-gamma Carboxyprothrombin Can Differentiate Hepatocellular Carcinoma from Non-Malignant Chronic Liver Disease in American Patients. Hepatology 2003, 37:490.
Ikoma J, Kaito M, Ishihara T, Nakagawa N, Kamei A, Fujita N, Iwasa M, et al. Early diagnosis of hepatocellular carcinoma using a sensitive assay for serum des-gamma-carboxy prothrombin: a prospective study. Hepatogastroenterology 2002;49:235–238.
Ishii M, Gama H, Chida N, Ueno Y, Shinzawa H, Takagi T, Toyota T, et al. Simultaneous measurements of serum alpha-fetoprotein and protein induced by vitamin K absence for detecting hepatocellular carcinoma. South Tohoku District Study Group. Am J Gastroenterol 2000;95:1036–1040.
Izuno K, Fujiyama S, Yamasaki K, Sato M, Sato T. Early detection of hepatocellular carcinoma associated with cirrhosis by combined assay of des-gamma-carboxy prothrombin and alpha-fetoprotein: a prospective study. Hepatogastroenterology 1995;42: 387–393.
Shimauchi Y, Tanaka M, Kuromatsu R, Ogata R, Tateishi Y, Itano S, Ono N, et al. A simultaneous monitoring of Lens culinaris agglutinin A-reactive alpha-fetoprotein and des-gamma-carboxy prothrombin as an early diagnosis of hepatocellular carcinoma in the follow-up of cirrhotic patients. Oncol Rep 2000;7:249–256.
Taketa K, Sekiya C, Namiki M, Akamatsu K, Ohta Y, Endo Y, Kosaka K. Lectin-reactive profiles of alpha-fetoprotein characterizing hepatocellular carcinoma and related conditions. Gastroenterology 1990;99:508–518.
Sato Y, Nakata K, Kato Y, Shima M, Ishii N, Koji T, Taketa K, et al. Early recognition of hepatocellular carcinoma based on altered profiles of alpha-fetoprotein. N Engl J Med 1993;328:1802–1806.
Shiraki K, Takase K, Tameda Y, Hamada M, Kosaka Y, Nakano T. A clinical study of lectin-reactive alpha-fetoprotein as an early indicator of hepatocellular carcinoma in the follow-up of cirrhotic patients. Hepatology 1995;22:802–807.
Wang SS, Lu RH, Lee FY, Chao Y, Huang YS, Chen CC, Lee SD. Utility of lentil lectin affinity of alpha-fetoprotein in the diagnosis of hepatocellular carcinoma. J Hepatol 1996;25:166–171.
Sterling RK, Jeffers L, Gordon F, Sherman M, Venook AP, Reddy KR, et al. Clinical utility of AFP-L3% measurement in North American patients with HCV-related cirrhosis. Am J Gastroenterol 2007;102:2196–205.
Song HH, Filmus J. The role of glypicans in mammalian development. Biochimica et Biophysica Acta 2002;1573:241–246.
Capurro M, Wanless IR, Sherman M, et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterol 2003;125:89–97.
Llovet JM, Chen Y, Wurmbach E, Roayaie S, Fiel MI, Schwartz, et al. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology 2006 Dec;131(6):1758–1767.
Kladney RD, Bulla GA, Guo L, Mason AL, Tollefson AE, Simon DJ, Koutoubi Z, Fimmel CJ. GP73, a novel Golgi-localized protein upregulated by viral infection. Gene 2000;249:53–65.
Marrero JA, Romano P, Steele L, Fimmel C, Lok AS, Block T. GP73, a resident Golgi glycoprotein, is a novel serum marker for Hepatocellular Carcinoma. J Hepatology 2005;43:1007–1012.
Block TM, Comunale MA, Lowman MA, Steele LF, Romano PR, Fimmel CJ, et al. Use of targeted glycoproteomics to identify serum glycoproteins that correlate with liver cancer in woodchucks and humans. Proc Natl Acad Sci USA 2005;102:779–784.
Yamagami H, Moriyama M, Tanaka N, Arakawa Y. Detection of serum and intrahepatic human hepatocyte growth factor in patients with type C liver diseases. Intervirology 2001;44:36–42.
Yamagamim H, Moriyama M, Matsumura H, Aoki H, Shimizu T, Saito, et al. Serum concentrations of human hepatocyte growth factor is a useful indicator for predicting the occurrence of hepatocellular carcinomas in C-viral chronic liver diseases. Cancer 2002;95:824–834.
Mazzioti G, Sorvillo F, Morisco F, et al. Serum insulin-like growth factor-1 evaluation as a useful tool for predicting the risk of developing hepatocellular carcinoma in patients with hepatitis c virus-related cirrhosis. Cancer 2002;95:2539–2545.
Beneduce L, Castaldi F, Marino M, Quarta S, Ruvoletto M, Benvegnu L, et al. Squamous cell carcinoma antigen-immunoglobulin M complexes as novel biomarkers for hepatocellular carcinoma. Cancer 2005;103(12):2558–2565.
Guido M, Roskams T, Pontisso P, Fassan M, Thung SN, Giacomelli L et al. Squamous cell carcinoma antigen in human liver carcinogenesis. J Clin Pathol 2007.
Giannelli G, Fransvea E, Trerotoli P, Beaugrand M, Marinosci F, Lupo L et al. Clinical validation of combined serological biomarkers for improved hepatocellular carcinoma diagnosis in 961 patients. Clin Chim Acta 2007;383(1–2):147–152.
Xie H, Song J, Du R, Liu K, Wang J, Tang, et al. Prognostic significance of osteopontin in hepatitis B virus-related hepatocellular carcinoma. Dig Liver Dis 2007 Feb;39(2):167–172.
Kim J, Ki SS, Lee SD, Han CJ, Kim YC, Park SH, et al. Elevated plasma osteopontin levels in patients with hepatocellular carcinoma. Am J Gastroenterol 2006 Sep;101(9):2051–2059.
Feng JT, Shang S, Beretta L. Proteomics for the early detection and treatment of hepatocellular carcinoma. Oncogene 2006;25:3810–3817.
Chignard N, Beretta L. Proteomics for hepatocellular carcinoma marker discovery. Gastroenterology 2004;127:S120–S125.
Chignard N, Shang S, Wang H, Marrero J, Bréchot C, Hanash S, Beretta L. Cleavage of endoplasmic reticulum proteins in hepatocellular carcinoma: Detection of generated fragments in patient sera. Gastroenterology 2006;130:2010–2022.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Humana Press, a part of Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Marrero, J.A. (2009). Screening and Biomarkers for Hepatocellular Carcinoma. In: Carr, B. (eds) Hepatocellular Carcinoma. Current Clinical Oncology. Humana Press. https://doi.org/10.1007/978-1-60327-376-3_11
Download citation
DOI: https://doi.org/10.1007/978-1-60327-376-3_11
Published:
Publisher Name: Humana Press
Print ISBN: 978-1-60327-373-2
Online ISBN: 978-1-60327-376-3
eBook Packages: MedicineMedicine (R0)