Abstract
Stress is associated with the activation of a number of central physiological systems, which act to enhance arousal and modulate attentional, memory, and other behavioral processes. The net consequence of these actions better permits the organism to contend with a challenging situation and react promptly and effectively when similar conditions are reencountered. It has long been known that stress is associated with a robust activation of the locus coeruleus and other noradrenergic systems. Moreover, evidence indicates a prominent involvement of central noradrenergic systems in a variety of behavioral and cognitive processes associated with stress, including arousal, memory, and attention. Under normal conditions, these actions are likely beneficial to the individual. However, under conditions of extreme stress/trauma, stressor-induced sensitization of noradrenergic systems and long-term actions of norepinephrine may well prove maladaptive. Consistent with this hypothesis, available evidence indicates a prominent involvement of noradrenergic systems in the behavioral pathology associated with various stress-related disorders, particularly post-traumatic stress disorder (PTSD). In particular, there is strong evidence for an involvement of noradrenergic systems in PTSD-related hyperarousal, intrusive memories, and sleep disturbances. Consistent with this, recent studies suggest that pharmacological disruption of noradrenergic neurotransmission may well be efficacious in treating these symptoms of PTSD. Combined, available information indicates that the central noradrenergic systems likely contribute to a broad spectrum of behavioral symptoms of PTSD and that pharmacological treatments targeting noradrenergic neurotransmission will prove clinically beneficial.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
OVERVIEW
The current conceptualization of stress as a behavioral state elicited by challenging or threatening events arises from nearly a century of research, starting with the seminal work of Cannon (1) and Selye (2). These studies identified physiological systems that were similarly affected by disparate environmental events, which had in common a potential to threaten animal well-being. Initially, emphasis was placed on stressor-induced activation of peripheral systems, primarily endocrine systems. This work identified the activation of both peripheral catecholamine systems and the pituitary-adrenal axis as hallmark features of the state of stress. The activation of these systems results in enhanced ability of the animal to physically contend with a challenging situation. More recently, emphasis has been placed on the neurobiology of the affective, cognitive, and behavioral components of stress.
This raises the long-standing issue of which psychological features define the state of stress. In contrast to the well-delineated physiological indices of stress, the affective and cognitive features of stress remain less clear. The extent to which stress has an affective component that can or cannot be dissociated from anxiety is not clear. Regardless of the exact configuration of cognitive and affective responses associated with stress, it appears that a heightened level of readiness for action is paramount to a state of stress. A prominent component of this preparatory state is an elevated level of arousal, defined for the purposes of this review as a heightened sensitivity to environmental stimuli.
Sustained arousal can be a considerable drain on physiological resources, regardless of whether it is precipitated by aversive or pleasant events. Indeed, the concept of eustress was introduced to acknowledge that pleasant events that are nonetheless challenging and arousing can produce a physiological state similar to that seen in the presence of aversive conditions (distress; 3). Regardless of whether negative affect is an obligatory component to the state of stress, there is a strong relationship between arousal level and a variety of state-dependent processes affected in stress, including attention, memory, and sensory information processing. These observations suggest the working hypothesis that at least a subset of the physiological and cognitive/behavioral components of stress may be independent of affective valence (pleasant vs. unpleasant) and more closely aligned with arousal level, motivational state, or the need for action.
It has long been known that stress is associated with a robust activation of the locus coeruleus (LC) and other noradrenergic systems, resulting in increased rates of norepinephrine (NE) release widely throughout the brain. Moreover, these noradrenergic systems are known to modulate a variety of behavioral and cognitive processes associated with stress. Consistent with this, evidence demonstrates a causal role of brain noradrenergic systems in a variety of behavioral and cognitive components of stress. Under normal conditions, these actions are likely beneficial to the individual. However, under conditions of extreme stress/trauma, actions of NE may well prove maladaptive. Consistent with this hypothesis, available evidence indicates a prominent involvement of noradrenergic systems in the behavioral pathology associated with various stress-related disorders, particularly post-traumatic stress disorder (PTSD).
THE LOCUS COERULEUS-NORADRENERGIC SYSTEM
Norepinephrine is a prominent neuromodulatory transmitter within the brain. NE acts at three major receptor families, α1, α2, and β, each comprised of multiple subtypes. The α1- and β-receptors exist primarily postsynaptically, whereas α2-receptors are present both pre- and postsynaptically. The LC is the major source of brain NE (4). This nucleus is composed of a small number of neurons, approximately 1,500 per nucleus in rat, several thousand in monkey, and 10,000–15,000 in humans. Despite these relatively small numbers, LC neurons possess immensely ramified axons, permitting the nucleus to project broadly throughout the neuraxis (4), excluding the basal ganglia. Importantly, the LC is the sole provider of NE to hippocampus and neocortex, regions critical for higher cognitive and affective function. Despite the widespread distribution of noradrenergic efferent fibers within the brain, there is substantial regional specificity of noradrenergic fiber distribution across cortical and subcortical structures (5). This regional heterogeneity likely has important functional ramifications.
Discharge Activity of LC Neurons
LC neurons fire in two distinct activity modes: tonic and phasic. Tonic activity is characterized by relatively low-frequency, sustained, and highly regular discharge patterns. Tonic discharge activity is state dependent, with LC neurons displaying highest discharge rates during waking, slower rates during slow-wave sleep, and minimal activity during rapid eye movement (REM) sleep (,7). Within waking, sustained increases in tonic discharge rates are elicited by environmental stimuli that elicit sustained increases in electroencephalographic (EEG) and behavioral indices of arousal (7,8).
LC neurons also display phasic alterations in discharge rates in response to both unconditioned and conditioned salient sensory stimuli (7,9). These phasic responses are observed with a relatively short latency and are comprised of a brief burst of two or three action potentials followed by a sustained period of suppression of discharge activity (approximately 300–700 ms). Phasic responses are observed in association with overt attending to a novel stimulus within a particular environmental location (e.g., an orienting response). Phasic responses are less robust during lower levels of arousal/vigilance (10) as well as higher levels of tonic discharge activity, including in stress. For example, both hypotension stress and corticotropin-releasing hormone elevate tonic discharge activity and reduce sensory-driven phasic discharge (11). Thus, stress likely interferes with behavioral processes dependent on phasic LC discharge.
Plasticity of the LC-Noradrenergic System in Stress
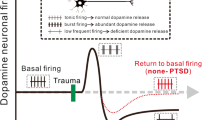
Central noradrenergic systems possess robust compensatory mechanisms that permit adjustment to long-term alterations in activity. These alterations are observed in response to damage (e.g., lesions) as well as environmental (e.g., stress) and pharmacological (e.g., antidepressant) manipulations. In the case of stress, prolonged or repeated exposure to stressors such as foot shock, cold, or restraint decrease β-receptor-driven accumulation of cyclic adenosine monophosphate (cAMP) (12)Ѐ The stressor-induced downregulation of the β-dependent cAMP response appears to result largely from a reduction in α1-receptor potentiation of the β-receptor cAMP response (12). Repeated exposure to a stressor also attenuates LC neuronal responsivity and NE release to the same (homotypic) stressor (13,14). Although repeated presentation of certain stressors results in tolerance to the LC-activating actions of those stressors, enhanced responsivity of LC neurons to repeated immobilization stress has been observed (15), indicating that tolerance to a given stressor is not obligatory.
Tolerance to stressor-induced LC activation is in contrast to the ability of both acute and chronic stressors to increase levels of the rate-limiting enzyme in NE biosynthesis, tyrosine hydroxylase (16). Thus, although chronic/repeated stressors do not tend to increase LC neuronal discharge, they do result in increased capacity of the system to release NE due to elevated rates of NE synthesis (for review, see Ref. 5). These observations raise the question of which conditions would utilize an increase in NE synthetic capacity. Insight into this issue is provided by the observation that, in contrast to homotypic stressors, repeated/chronic stress results in an increased responsiveness of the LC-noradrenergic system to presentation of a different (heterotypic) stressor. For example, chronic cold stress results in larger increases in NE efflux in response to tail shock (14) or tail pinch (17). These observations are consistent with an increase in responsivity of LC neurons to excitatory input seen in anesthetized animals that had been previously exposed to chronic stress (18).
Thus, during prolonged exposure to a stressor, the LC-noradrenergic system develops an increased capacity to respond to additional challenges. As reviewed later, stress-related sensitization of noradrenergic systems may play a critical role in PTSD.
Sensitivity of the LC-Noradrenergic System to Appetitive Stimuli
Extensive evidence indicates a robust activation of the LC-noradrenergic system by a variety of stressors (for review, see Ref. 5). The early demonstration of a sensitivity of LC neurons to stressors suggested a possibly selective role of the LC in stress and led to a number of hypotheses concerning alarm- or anxiety-specific functions of these neurons. However, subsequent studies in unanesthetized animals demonstrated a sensitivity of tonic and phasic LC discharge as well as NE release to both appetitive as well as aversive stimuli(7,19,20). Combined, these observations suggest that both tonic and phasic LC-NE neurotransmission is more closely related to the overall salience, arousing or motivating nature of a given stimulus rather than affective valence.
AROUSAL-ENHANCING ACTIONS OF THE LC-NORADRENERGIC SYSTEM
Enhanced arousal is a primary component of the state of stress. The fact that LC neurons increase firing rates in anticipation of waking and waking-associated forebrain activation suggests the hypothesis that LC neurons help induce the waking state. Substantial evidence collected since 1990 provides strong support for this hypothesis.
Noradrenergic Modulation of Cortical and Thalamic Neuronal Activity State In Vitro
Cortical and thalamic neurons display distinct activity modes during sleeping and waking. Thus, during slow-wave sleep, these neurons are hyperpolarized and display a burst-type activity mode that is associated with a relative insensitivity to incoming sensory information. In contrast, during waking these neurons display a single-spike mode associated with the efficient and accurate processing of sensory information (21,22). Consistent with the described increase in LC discharge rates during waking, in vitro, NE induces a shift in the firing pattern of cortical and thalamic neurons from a burst mode to a waking-like single spike mode (23).
Effects of LC Neuronal Discharge Activity on EEG and Behavioral Indices of Arousal
The small size of the LC, situated in close proximity to a variety of brain stem structures, presents a substantial challenge for the selective manipulation of LC neuronal discharge rates. A combined recording/infusion probe was developed that permitted a greater degree of anatomical localization of intratissue infusions (24). Using this approach, it was demonstrated that unilateral LC activation, produced by a small infusion of the cholinergic agonist bethanechol, elicits a robust bilateral activation of cortical and hippocampal EEG (25). In contrast, bilateral suppression of LC neuronal discharge activity via infusion of an α2-agonist produces a robust increase in slow-wave activity in cortical and hippocampal EEG (78). Combined, these and other observations indicate the LC is a potent modulator of forebrain EEG state, with unilateral LC neuronal discharge activity causally related to the bilateral maintenance of EEG activity patterns associated with arousal.
NE Acts Within the Basal Forebrain to Promote Arousal
A number of subcortical structures have been implicated in the regulation of cortical and hippocampal activity state, including the general region of the basal forebrain encompassing the medial septal area (MSA)/diagonal band of Broca, the general region of the anterior-medial hypothalamus, encompassing the medial preoptic area (MPOA), and the substantia innominata (SI; for review, see Ref. 5). Moreover, each of these regions receives a prominent noradrenergic innervation from the LC (26). Therefore, a series of microinfusion studies was conducted to assess the degree to which NE acts within these regions to modulate the behavioral state.
These studies demonstrated potent EEG-activating and wake-promoting actions of NE via actions at both β- and α1-receptor subtypes located within MSA and MPOA, but not SI. For example, in sleeping, unanesthetized rats, β- and α1-receptor stimulation within MSA and MPOA produced a robust and additive increase in time spent awake (for review, see Ref. 5). In contrast, when infused into SI, neither NE, a β-agonist, an α1-agonist, or the indirect noradrenergic agonist amphetamine exerted wake-promoting actions (for review, see Ref. 5). It is important to note that although the LC provides a majority of noradrenergic input to the MPOA and MSA, two areas within which NE acts to promote arousal, other noradrenergic nuclei also contribute to the noradrenergic innervation of these regions (e.g., 26). Thus, although the LC plays a critical role in the regulation of arousal, other noradrenergic systems likely also exert arousal-promoting actions.
NE Is Necessary for Alert Waking: Synergistic Sedative Actions of α1- and β-Receptor Blockade
As described, α1- and β-receptors exert additive wake-promoting actions. Consistent with this, combined β-receptor and α1-blockade blockade (intracerebroventricular timolol and intraperitoneal prazosin, respectively) exerts additive sedative actions, resulting in a profound increase in large-amplitude slow-wave activity in cortical EEG in animals exposed to an arousal-increasing and stress-inducing, brightly lit novel environment (27). This increase in slow-wave activity is in contrast to the minimal EEG effects observed with β-receptor blockade alone or the high-voltage spindles elicited by α1-receptor blockade (27).
Enhanced LC Discharge Activity Contributes to Stressor-Induced Activation of the Forebrain
The described observations suggest a potentially critical role of the LC-NE system in stressor-induced arousal. Consistent with this hypothesis, bilateral suppression of LC neuronal activity, via peri-LC infusions of an α2-agonist (clonidine), prevented EEG activation elicited by hypotension-stress in the anesthetized rat (28). These results provide direct support for a causal role of the LC-noradrenergic system in stressor-induced arousal.
Summary: LC Modulation of Arousal in Stress
A large body of information demonstrates a prominent role for the LC-noradrenergic system in the modulation of EEG and behavioral indices of arousal. Additional evidence demonstrates a critical role of the LC in stressor-induced activation of the forebrain. Combined, these observations suggest the prominent participation of this neurotransmitter system in stressor-induced increases in arousal. Stressor-induced sensitization of the LC-NE system could contribute to elevated arousal levels associated with PTSD and other stress-related disorders.
THE LC-NORADRENERGIC SYSTEM MODULATES SENSORY INFORMATION PROCESSING WITHIN CORTICAL AND THALAMIC CIRCUITS
During periods of environmental demand (e.g., stress), information collection and processing are critical for guidance of appropriate behavior. Sensory information processing is highly state-dependent (for review, see Ref. 5). Given the described state-dependent nature of the LC-NE system, this system may well contribute to state-dependent modulation of sensory information processing during stress.
A large body of work indicates complex modulatory actions of NE on discharge properties of cortical and thalamic sensory neurons (for review, see Ref. 5). These actions include increasing the “signal-to-noise” ratio of evoked responding (both excitatory and inhibitory responses) as well as “gating” of neuronal responses to previously subthreshold stimuli. Importantly, the electrophysiological actions of NE on sensory cortical neuronal activity follows a nonmonotonic, inverted U-shaped dose-response relationship, similar to that described for noradrenergic modulation of cognitive function (29). Combined, these observations indicate that, within neocortex, NE exerts a complicated array of modulatory actions. Such actions are likely of particular importance under conditions of threat/stress when a rapid and accurate behavioral response is required.
MODULATORY ACTIONS OF THE LC-NORADRENERGIC SYSTEM ON NEURONAL PLASTICITY
Long-term survival requires behavioral, and thus neural, plasticity. As described, the LC-noradrenergic system displays long-term, stressor-induced alterations in a variety of cellular processes. Additional information indicates that the LC system modulates long-term alterations in synaptic efficacy and gene transcription posited to underlie learning and memory. Combined, these actions may contribute to stress-related long-term alterations in behavior.
Long-Term Modulatory Actions of the LC-Noradrenergic System on Synaptic Efficacy Within Neuronal Ensembles
Long-lasting, experience-dependent alterations in responsiveness to afferent information are observed within large-population neuronal ensembles. For example, long-term potentiation (LTP) refers to a long-lasting increase in synaptic strength that results when excitatory synapses are rapidly and repetitively stimulated for brief periods (tetanic stimulation). Experimentally, this has been most intensively studied within the hippocampal formation and is manifested as an increase in the population spike to subsequent punctate stimulation of hippocampal afferent paths. That LTP is readily observed in a structure believed to be critical for memory function has stimulated interest in LTP as a possible mechanism underlying memory.
The LC-NE system is a potent modulator of LTP. For example, when tested in vitro, depletion of NE decreases LTP in the dentate gyrus (30), whereas NE application elicits a β-receptor-dependent enhancement of LTP in CA3 (31). NE also produces a long-lasting enhancement of synaptic efficacy in both the dentate gyrus and CA1 region of the hippocampus in the absence of tetanic stimulation (32,33). In vivo, enhancement of NE neurotransmission by LC activation, α2-antagonist administration, or direct application of NE results in an increase in the population spike recorded in the dentate gyrus (32,34). These last actions involve both β- and α1-receptors (35,36).
An additional form of NE-dependent plasticity has also been described in neocortex in which NE elicits a long-term synaptic depression of the population response recorded from layer III of visual cortex (37). Overall, these observations indicate a potentially prominent role of the LC-NE system in mediating long-lasting modifications in neurotransmission within large populations of forebrain neurons. It is particularly intriguing that these actions are observed in structures implicated in learning and memory. Such actions may be particularly critical for dealing rapidly and effectively when environmental situations that pose a threat (e.g., stress) are reencountered. Excessive activation of these systems may manifest in an excessive and potentially detrimental sensitivity to otherwise mild environmental events/stressors.
Facilitatory Actions of the LC-Noradrenergic System on Transcription Rates of Immediate-Early and Other Plasticity-Related Genes
Long-term alterations in behavior likely involve alterations in rates of gene transcription and protein production. A set of immediate-early genes (IEGs) has been identified that are activated rapidly by a variety of neuromodulators. Many of these genes act as “transcription regulators,” regulating gene transcription rates. Through these actions, IEGs may provide an intervening step through which relatively short-term alterations in neuronal activity are transduced into long-term alterations in neuronal function and behavior (38).
Evidence indicates a prominent role of the LC-NE system in the regulation of IEGs. For example, increases in NE release result in an increase in messenger ribonucleic acid (mRNA) and protein levels for a variety of IEGs in the neocortex and amygdala (for review, see Ref. 5). Interestingly, stress is associated with similar activating effects on IEG expression (39–41). Importantly, the activating effects of stressor-induced increases in NE neurotransmission on IEG expression are attenuated with pretreatment of either β- or α1-antagonists or LC lesions (42). These observations indicate that stressor-induced alterations in IEG expression are dependent on stressor-induced increases in NE release.
MODULATORY ACTIONS OF THE LC-NORADRENERGIC SYSTEM ON COGNITIVE PROCESSES
The described actions of the LC efferent system suggest a widespread influence of this neurotransmitter pathway on information processing within a variety of LC terminal fields. Indeed, substantial evidence suggests the LC-noradrenergic system plays a prominent role in a variety of behavioral/cognitive processes related to the collection, processing, retention, and utilization of sensory information. Importantly, actions of NE appear to play a prominent role in stressor-induced alterations in at least a subset of these processes.
The LC-Noradrenergic System Modulates Attention
The ability to regulate attention is an important aspect of behavior. This may be particularly true under stressful conditions that pose a threat to the animal. The actions of NE on cortical/thalamic neuronal activity reviewed indicate that NE facilitates processing of relevant sensory signals. These observations suggest that the LC-noradrenergic system might enhance cognitive function under “noisy” conditions in which irrelevant stimuli could impair performance. Results from pharmacological and lesion studies conducted in rodents, monkeys, and humans largely support these hypotheses. For example, NE depletion produces deficits in the performance on a variety of tasks when irrelevant stimuli are presented during testing (for review, see Ref. 43). Thus, the addition of distracting visual stimuli at the choice point in a T maze produces a greater disruption of performance in NE-depleted rats than in sham-treated animals (for review, see Ref. 5). Similarly, the presentation of irrelevant auditory stimuli impairs sustained attention in rats with forebrain NE depletion, although these animals perform normally under nondistracting conditions (44). Further, NE depletion increases conditioned responses to irrelevant stimuli while decreasing responses to relevant stimuli (45–47). Thus, overall, impairment of noradrenergic neurotransmission has an impact on attentional and other cognitive tasks under conditions associated with high-demand or increased arousal.
The LC-noradrenergic system may be particularly sensitive to novel environmental stimuli. For example, enhanced LC discharge rates are observed when rats encounter novel stimuli within a familiar environment (48). Further, pharmacological manipulations that enhance NE release increase physical contact/interaction with a novel stimulus located within a familiar environment (49). In contrast, when examined in a novel environment, enhanced NE neurotransmission decreases attention to an individual object, possibly reflecting enhanced scanning of the environment (50,51). Interestingly, stress produces a similar decrease in focused attention that is reversed by α1-receptor blockade (51).
Combined, these observations suggest an involvement of noradrenergic systems in the regulation of attentional processes, including sustained or focused attention. Initial electrophysiological recordings suggested the potential involvement of both phasic and tonic LC discharge and performance in a vigilance task (9). In these studies, moderate levels of tonic discharge, correlating with moderate arousal levels, were associated with high levels of performance and robust phasic LC responses. When tonic levels were too low (sedation) or too high (high arousal, scanning attention), phasic discharge was reduced, and performance was impaired. Although this was originally interpreted to suggest a role for both phasic and tonic discharge in sustained attention, subsequent work indicated that phasic LC discharge most closely correlates with behavioral responding in this task rather than attention to a sensory stimulus (52). Nonetheless, these studies indicate a sensitivity of sustained attention to fluctuations in tonic LC discharge, indicating an optimal level of tonic LC discharge is necessary for maximal levels of sustained attention.
The LC-Noradrenergic System Modulates Working Memory
The prefrontal cortex (PFC) is involved in a variety of cognitive, behavioral, and physiological processes, many of which are affected in stress. NE modulates PFC neuronal activity and PFC-dependent behavior (for review, see Ref. 53). The actions of NE on PFC-dependent behavior have been most comprehensively studied in the context of working memory. A large body of work demonstrates NE acts directly within the PFC to produce an inverted U-shaped modulation of working memory, with both low and high levels of NE neurotransmission associated with impaired working memory (for review, see Ref. 53). For example, decreased NE neurotransmission impairs working memory, an effect that is reversed by local infusion of postsynaptic-preferring α2-agonists (e.g., guanfacine; 53). Conversely, stressor-induced impairment in working memory is reversed by intra-PFC infusion of α1-antagonists (54,55). Based on these and other observations, it has been hypothesized that moderate levels of NE release associated with nonstress conditions activate high-affinity α2A-receptors, whereas release of higher levels of NE (i.e., stress) activates lower-affinity α1-receptors (53).
LC-Noradrenergic System Modulates Arousal-Related Memory
Memory strength can be enhanced by stressful and emotionally arousing conditions. Steroid (e.g., glucocorticoids) and catecholamine (e.g., epinephrine) hormones participate in this arousal-related enhancement of memory (for review, see Ref. 56). Circulating epinephrine stimulates release of central NE via stimulation of β-receptors located on vagal afferents (56). Evidence indicates that NE action at β-receptors within the amygdala plays a critical role in the memory-enhancing actions of both arousing stimuli and circulating epinephrine (56). The basolateral nucleus of the amygdala appears to be a critical site within the amygdala for the memory-modulating effects of NE. Thus, posttraining infusions of NE into the basolateral nucleus of the amygdala enhance spatial learning, while β-antagonist infusions impair performance in this task (57) as well as an inhibitory avoidance task (58,58). Further, glucocorticoid-induced enhancement of performance in an inhibitory avoidance task is prevented by the blockade of basolateral amygdala β1- or β2-receptors (60). Basolateral amygdala α1-receptors also facilitate performance in an inhibitory avoidance task (61). This facilitatory action of α1-receptors on memory appears to result from the α1-dependent enhancement of β-receptor-mediated cAMP production (61,62). In support of a role of NE in emotion-related memory in humans, Cahill, Prins, Weber, and McGaugh (63) demonstrated that β-receptor blockade in human subjects blocks the enhanced memory typically observed with emotionally activating images.
These observations indicate a prominent role of NE, via actions within the basolateral amygdala, in the consolidation of memory under high-arousal, stressful conditions. Memory involves not only the consolidation of information following an event, but also the retrieval and subsequent reconsolidation of that information (64). Additional information suggests that the modulatory actions of basolateral amygdala NE on memory consolidation are not universally observed across different types of memories. Thus, in a conditioned fear (conditioned freezing) paradigm, posttraining blockade of basolateral amygdala β-receptors had minimal effects on auditory fear conditioning (65). In contrast, intrabasolateral amygdala β-receptor blockade interfered with reconsolidation in this paradigm (65).
Combined, these observations suggest NE acts within the basolateral amygdala to strengthen consolidation or reconsolidation of aversive and emotionally arousing events.
THE LC IN STRESS-RELATED DISORDERS: PTSD
Introduction
The information reviewed indicates noradrenergic systems have an impact on widespread neural circuits involved in the regulation of arousal and the collection, processing, and responding to sensory information. Moreover, noradrenergic systems participate in a variety of behavioral, cognitive, and physiological responses associated with stress. These observations suggest a potentially prominent role of NE in stress-related disorders, including PTSD. In this discussion, it is worth noting that much of the impetus behind the initial speculation of an anxiogenic action of NE was the observation that stressors were particularly potent at activating the LC-NE system. As reviewed, subsequent work demonstrated comparable sensitivity/responsivity of LC neurons to both aversive and appetitive stimuli. These observations indicate that increased release of NE per se does not produce a negative affective state, such as anxiety. Such a conclusion apparently contradicts results from studies in humans that indicate increased anxiety following peripheral manipulations that increase NE neurotransmission (66). However, the relationship between generalized arousal, which is likely sensitive to peripheral manipulations of noradrenergic neurotransmission, and anxiety has not been fully explored in humans. Thus, the extent to which results obtained in humans indicate direct versus indirect actions of central noradrenergic systems on anxiety-related circuits remains unclear.
Despite these caveats, it is clear that noradrenergic systems are highly responsive to stressful stimuli and mediate a variety of stress-related physiological, behavioral, and cognitive processes. These observations suggest that, at the very least, the LC and other noradrenergic systems may contribute to certain affect-independent components of stress-related disorders.
Norepinephrine and PTSD
Among stress-related disorders, the strongest case for an involvement of noradrenergic systems can be made for PTSD (for review, see Ref. 67). For example, PTSD is associated with the dysregulation of a variety of processes influenced by central noradrenergic systems. This includes dysregulation of arousal and long-term memory as well as working memory, attention and other PFC-dependent processes (68–70). As reviewed, NE not only acutely modulates a variety of behavioral processes but also can induce long-term alterations in plasticity-related gene transcription, neuronal reactivity, and memory. Thus, the activation of central noradrenergic systems under intense/traumatic stressful events could contribute to certain long-lasting behavioral attributes of PTSD that result from trauma exposure.
The described work indicates that via actions at β-receptors located within the basolateral amygdala, NE strengthens memories for emotionally arousing events. It has been posited that this mechanism may contribute to certain symptoms associated with PTSD, including long-lasting and intrusive memories. Consistent with this hypothesis, clinical studies indicated that the administration of the β-antagonist propranolol within close proximity of a traumatic event, or while remembering that event, lessens behavioral and physiological symptoms of PTSD (71–73).
Beyond long-term alterations in neuronal circuitry induced by NE at the time of a traumatic event, evidence further indicates that noradrenergic systems may be dysregulated in PTSD. Specifically, noradrenergic systems appear to be hyperreactive in PTSD (for review, see Ref. 74). Hyperactivity of peripheral NE systems is observed in response to auditory reminders of trauma (75) and in response to pharmacological challenge with the α2-antagonist yohimbine (74). Based on the preclinical evidence reviewed, it is expected that excessive activity/reactivity of noradrenergic systems in PTSD would have a broad impact on arousal, memory, and attentional systems. Thus, noradrenergic hyperactivity could well contribute to a variety of symptoms associated with PTSD. Consistent with this, α2-antagonist challenge (to increase NE release) has been documented to cause both panic attacks and flashbacks in a large proportion of the PTSD patients, effects not seen with either placebo or yohimbine treatment in control subjects (74). Moreover, α1-antagonist treatment has been demonstrated to reduce trauma-related nightmares and other sleep-related disturbances seen in PTSD (76,77).
SUMMARY
A defining feature of stressful conditions is the need to confront challenging, or threatening, conditions. Associated with this is the need to acquire and process sensory information rapidly and efficiently to make an accurate response selection. Long-term survival may be dependent on behavioral plasticity to better contend with, or avoid, a threatening environmental stimulus when it is reencountered. Evidence reviewed in this chapter argues for a prominent role of the LC-noradrenergic system in a variety of physiological, cognitive, and behavioral processes associated with information processing, response selection, and behavioral plasticity. Stress is associated with elevated rates of NE release. Thus, it is not surprising that evidence indicates an involvement of the LC-noradrenergic system in stressor-induced alterations in a variety of these processes (e.g., arousal, working memory, high-arousal-related memory, IEG expression). These actions of the LC-noradrenergic system are likely independent of affective valence (e.g., appetitive vs. aversive) and are dependent only on whether a stimulus is salient (relevant) to the organism.
Under normal conditions, the long-term actions of NE, whether at the level of the gene (IEGs), neural ensembles (LTP), or behavior (memory) likely facilitate rapid and accurate response selection when a stimulus is reencountered. However, under extreme conditions associated with extreme activation of the LC-NE system, these long-term changes may result in an excessive sensitivity of arousal, memory, or other systems. In addition, these extreme conditions may result in a sensitization of the LC-NE system to otherwise innocuous stimuli. In support of these hypotheses, available evidence indicates hyperactivity/reactivity of noradrenergic systems in PTSD. Moreover, symptoms associated with PTSD are reduced by pharmacological interference with noradrenergic neurotransmission. Combined, this information indicates a prominent role for central noradrenergic systems in PTSD.
REFERENCES
Cannon, W. B. (1914) The emergency function of the adrenal medulla in pain and the major emotions. Am J Physiol 33, 356–72.
Selye, H. (1946) The general adaptation syndrome and the diseases of adaptation. J Clin Endocrinol Metab 6, 117–230.
Selye, H. (1975) Confusion and controversy in the stress field. J Human Stress 1, 37–44.
Foote, S. L., Bloom, F. E., and Aston-Jones, G. (1983) Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev 63, 844–914.
Berridge, C. W., and Waterhouse, B. D. (2003) The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42, 33–84.
Hobson, J. A., McCarley, R. W., and Wyzinski, P. W. (1975) Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science 189, 55–58.
Foote, S. L., Aston-Jones, G., and Bloom, F. E. (1980) Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci U S A 77, 3033–37.
Aston-Jones, G., and Bloom, F. E. (1981) Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci 1, 876–886.
Rajkowski, J., Kubiak, P., and Aston-Jones, G. (1994) Locus coeruleus activity in monkey: phasic and tonic changes are associated with altered vigilance. Brain Res Bull 35, 607–16.
Aston-Jones, G., and Bloom, F. E. (1981) Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci 1, 887–900.
Valentino, R. J., and Foote, S. L. (1987) Corticotropin-releasing factor disrupts sensory responses of brain noradrenergic neurons. Neuroendocrinology 45, 28–36.
Stone, E. A. (1987) Central cyclic-AMP-linked noradrenergic receptors: new findings on properties as related to the actions of stress. Neurosci Biobehav Rev 11, 391–98.
Abercrombie, E. D., and Jacobs, B. L. (1987) Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. II. Adaptation to chronically presented stressful stimuli. J Neurosci 7, 2844–48.
Nisenbaum, L. K., Zigmond, M. J., Sved, A. F., and Abercrombie, E. D. (1991) Prior exposure to chronic stress results in enhanced synthesis and release of hippocampal norepinephrine in response to a novel stressor. J Neurosci 11, 1478–84.
Pavcovich, L. A., Cancela, L. M., Volosin, M., Molina, V. A., and Ramirez, O. A. (1990) Chronic stress-induced changes in locus coeruleus neuronal activity. Brain Res Bull 24, 293–96.
Stone, E. A., Freedman, L. S., and Morgano, L. E. (1978) Brain and adrenal tyrosine hydroxylase activity after chronic footshock stress. Pharmacol Biochem Behav 9, 551–53.
Finlay, J. M., Zigmond, M. J., and Abercrombie, E. D. (1995) Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience 64, 619–28.
Simson, P. E., and Weiss, J. M. (1988) Altered activity of the locus coeruleus in an animal model of depression. Neuropsychopharmacology 1, 287–295.
Feenstra, M. G., Teske, G., Botterblom, M. H., and de Bruin, J. P. (1999) Dopamine and noradrenaline release in the prefrontal cortex of rats during classical aversive and appetitive conditioning to a contextual stimulus: interference by novelty effects. Neurosci Lett 272, 179–82.
Feenstra, M. G. (2000) Dopamine and noradrenaline release in the prefrontal cortex in relation to unconditioned and conditioned stress and reward. Prog Brain Res 126, 133–63.
Steriade, M., Domich, L., and Oakson, G. (1986) Reticularis thalami neurons revisited: activity changes during shifts in states of vigilance. J Neurosci 6, 68–81.
McCormick, D. A., and Bal, T. (1997) Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci 20, 185–215.
McCormick, D. A., Pape, H. C., Williamson, A. (1991) Actions of norepinephrine in the cerebral cortex and thalamus: implications for function of the central noradrenergic system. Prog Brain Res 88, 293–305.
Adams, L. M., and Foote, S. L. (1988) Effects of locally infused pharmacological agents on spontaneous and sensory-evoked activity of locus coeruleus neurons. Brain Res Bull 21, 395–400.
Berridge, C. W., and Foote, S. L. (1991) Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci 11, 3135–45.
España, R. A., and Berridge, C. W. (2006) Organization of noradrenergic efferents to arousal-related basal forebrain structures. J Comp Neurol 496, 668–83.
Berridge, C. W., and España, R. A. (2000) Synergistic sedative effects of noradrenergic alpha(1)- and beta- receptor blockade on forebrain electroencephalographic and behavioral indices. Neuroscience 99, 495–505.
Page, M. E., Berridge, C. W., Foote, S. L., and Valentino, R. J. (1993) Corticotropin-releasing factor in the locus coeruleus mediates EEG activation associated with hypotensive stress. Neurosci Lett 164, 81–84.
Devilbiss, D. M., and Waterhouse, B. D. (2000) Norepinephrine exhibits two distinct profiles of action on sensory cortical neuron responses to excitatory synaptic stimuli. Synapse 37, 273–82.
Stanton, P. K., and Sarvey, J. M. (1985) Depletion of norepinephrine, but not serotonin, reduces long-term potentiation in the dentate gyrus of rat hippocampal slices. J Neurosci 5, 2169–76.
Hopkins, W. F., and Johnston, D. (1984) Frequency-dependent noradrenergic modulation of long-term potentiation in the hippocampus. Science 226, 350–52.
Stanton, P. K., and Sarvey, J. M. (1987) Norepinephrine regulates long-term potentiation of both the population spike and dendritic EPSP in hippocampal dentate gyrus. Brain Res Bull 18, 115–19.
Heginbotham, L. R., and Dunwiddie, T. V. (1991) Long-term increases in the evoked population spike in the CA1 region of rat hippocampus induced by beta-adrenergic receptor activation. J Neurosci 11, 2519–27.
Harley, C. W., and Sara, S. J. (1992) Locus coeruleus bursts induced by glutamate trigger delayed perforant path spike amplitude potentiation in the dentate gyrus. Exp Brain Res 89, 581–87.
Chaulk, P. C., and Harley, C. W. (1998) Intracerebroventricular norepinephrine potentiation of the perforant path-evoked potential in dentate gyrus of anesthetized and awake rats: A role for both alpha- and beta-adrenoceptor activation. Brain Res 787, 59–70.
Kitchigina, V., Vankov, A., Harley, C., and Sara, S. J. (1997) Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur J Neurosci 9, 41–47.
Kirkwood, A., Rozas, C., Kirkwood, J., Perez, F., and Bear, M. F. (1999) Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci 19, 1599–1609.
Milbrandt, J. (1987) A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science 238, 797–99.
Gubits, R. M., Smith, T. M., Fairhurst, J. L., and Yu, H. (1989) Adrenergic receptors mediate changes in c-fos mRNA levels in brain. Brain Res Mol Brain Res 6, 39–45.
Bing, G. Y., Filer, D., Miller, J. C., and Stone, E. A. (1991) Noradrenergic activation of immediate early genes in rat cerebral cortex. Brain Res Mol Brain Res 11, 43–46.
Bing, G., Stone, E. A., Zhang, Y., and Filer, D. (1992) Immunohistochemical studies of noradrenergic-induced expression of c- fos in the rat. CNS Brain Res 592, 57–62.
Stone, E. A., Zhang, Y., John, S., Filer, D., and Bing, G. (1993) Effect of locus coeruleus lesion on c-fos expression in the cerebral cortex caused by yohimbine injection or stress. Brain Res 603, 181–85.
Mehta, M. A., Sahakian, B. J., and Robbins, T. W. (2001) Comparative psychopharmacology of methylphenidate and related drugs in human volunteers, patients with ADHD, and experimental animals. Solanto, M. V., Arnsten, A. F. T., and Castellanos, F. X., ed., Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. New York: Oxford University Press; 303–31.
Carli, M., Robbins, T. W., Evenden, J. L., and Everitt, B. J. (1983) Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res 9(3), 361–80.
Lorden, J. F., Callahan, M., and Dawson, R., Jr. (1980) Depletion of central catecholamines alters amphetamine- and fenfluramine-induced taste aversions in the rat. J Comp Physiol Psychol 94, 99–114.
Selden, N. R., Robbins, T. W., and Everitt, B. J. (1990) Enhanced behavioral conditioning to context and impaired behavioral and neuroendocrine responses to conditioned stimuli following ceruleocortical noradrenergic lesions: support for an attentional hypothesis of central noradrenergic function. J Neurosci 10, 531–39.
Selden, N. R., Everitt, B. J., and Robbins, T. W. (1991) Telencephalic but not diencephalic noradrenaline depletion enhances behavioural but not endocrine measures of fear conditioning to contextual stimuli. Behav Brain Res 43, 139–54.
Vankov, A., Herve-Minvielle, A., and Sara, S. J. (1995) Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci 7, 1180–87.
Devauges, V., and Sara, S. J. (1990) Activation of the noradrenergic system facilitates an attentional shift in the rat,. Behav Brain Res 39, 19–28.
Arnsten, A. F., Segal, D. S., Loughlin, S. E., and Roberts, D. C. (1981) Evidence for an interaction of opioid and noradrenergic locus coeruleus systems in the regulation of environmental stimulus-directed behavior. Brain Res 222, 351–63.
Berridge, C. W., and Dunn, A. J. (1989) Restraint-stress-induced changes in exploratory behavior appear to be mediated by norepinephrine-stimulated release of CRF. J Neurosci 9, 3513–21.
Clayton, E. C., Rajkowski, J., Cohen, J. D., and Aston-Jones, G. (2004) Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. J Neurosci 24, 9914–20.
Arnsten, A. F. (2007) Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex 17(suppl 1), i6–15.
Arnsten, A. F., and Goldman-Rakic, P. S. (1998) Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry 55, 362–68.
Birnbaum, S., Gobeske, K. T., Auerbach, J., Taylor, J. R., and Arnsten, A. F. (1999) A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry 46, 1266–74.
McGaugh, J. L. (2000) Memory-a century of consolidation. Science 287, 248–51.
Hatfield, T., and McGaugh, J. L. (1999) Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiol Learn Mem 71, 232–39.
Ferry, B., and McGaugh, J. L. (1999) Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiol Learn Mem 72, 8–12.
Ferry, B., Roozendaal, B., and McGaugh, J. L. (1999) Role of norepinephrine in mediating stress hormone regulation of long- term memory storage: a critical involvement of the amygdala. Biol Psychiatry 46, 1140–52.
Quirarte, G. L., Roozendaal, B., and McGaugh, J. L. (1997) Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci U S A 94, 14048–53.
Ferry, B., Roozendaal, B., and McGaugh, J. L. (1999) Involvement of alpha1-adrenoceptors in the basolateral amygdala in modulation of memory storage. Eur J Pharmacol 372, 9–16.
Ferry, B., Roozendaal, B., and McGaugh, J. L. (1999) Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between beta- and alpha1-adrenoceptors. J Neurosci 19, 5119–23.
Cahill, L., Prins, B., Weber, M., and McGaugh, J. L. (1994) Beta-adrenergic activation and memory for emotional events. Nature 371, 702–4.
Dudai, Y. (2004) The neurobiology of consolidations, or, how stable is the engram?. Annu Rev Psychol 55, 51–86.
Debiec, J., and Ledoux, J. E. (2004) Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 129, 267–72.
Charney, D. S., Heninger, G. R., and Redmond, D. E. Jr. (1983) Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine. Life Sci 33, 19–29.
Southwick, S. M., Bremner, J. D., Rasmusson, A., Morgan, C. A., III, Arnsten, A., and Charney, D. S. (1999) Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry 46, 1192–1204.
Barrett, D. H., Green, M. L., Morris, R., Giles, W. H., and Croft, J. B. (1996) Cognitive functioning and posttraumatic stress disorder. Am J Psychiatry 153, 1492–94.
Bremner, J. D., Scott, T. M., Delaney, R. C., et al. (1993) Deficits in short-term memory in posttraumatic stress disorder. Am J Psychiatry 150, 1015–19.
Clark, C. R., McFarlane, A. C., Morris, P., et al. (2003) Cerebral function in posttraumatic stress disorder during verbal working memory updating: a positron emission tomography study. Biol Psychiatry 53, 474–81.
Pitman, R. K., Sanders, K. M., Zusman, R. M. et al. (2002) Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry 51, 189–92.
Vaiva, G., Ducrocq, F., Jezequel, K. et al. (2003) Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry 54, 947–49.
Brunet, A., Orr, S. P., Tremblay, J., Robertson, K., Nader, K., and Pitman, R. K. (2008) Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res 42, 503–6.
Southwick, S. M., Krystal, J. H., Morgan, C. A., et al. (1993) Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry 50(4):266–74.
Blanchard, E. B., Kolb, L. C., Prins, A., Gates, S., and McCoy, G. C. (1991) Changes in plasma norepinephrine to combat-related stimuli among Vietnam veterans with posttraumatic stress disorder. J Nerv Ment Dis 179, 371–73.
Taylor, F., and Raskind, M. A. (2002) The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clin Psychopharmacol 22, 82–85.
Raskind, M. A., Peskind, E. R., Hoff, D. J., et al. (2007) A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry 61, 928–34.
Berridge, C. W., Page, M. E., Valentino R. J., and Foote S. L. (1993) Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus, Neuroscience 55, 381–393.
Acknowledgments:
The author gratefully acknowledge support by the University of Wisconsin Graduate School and Public Health Service grants DA10681, DA00389, and MH62359.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Humana Press, a part of Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Berridge, C. (2009). The Locus Coeruleus-Noradrenergic System and Stress: Implications for Post-Traumatic Stress Disorder. In: LeDoux, J., Keane, T., Shiromani, P. (eds) Post-Traumatic Stress Disorder. Humana Press. https://doi.org/10.1007/978-1-60327-329-9_10
Download citation
DOI: https://doi.org/10.1007/978-1-60327-329-9_10
Published:
Publisher Name: Humana Press
Print ISBN: 978-1-60327-328-2
Online ISBN: 978-1-60327-329-9
eBook Packages: MedicineMedicine (R0)