Abstract
A megaureter is in the broadest sense a purely descriptive term meant to indicate any ureter that is abnormally dilated. This label does not define a distinct pathologic entity per se, but rather encompasses a wide spectrum of both physiologic and pathophysiologic processes which culminate in a dilated ureter. Confusion arises when the terms megaureter, primary megaureter, and congenital megaureter are used interchangeably to specifically refer to a particular subset of all megaureters. In most instances, these labels are used to refer to those patients who present with either a primary non-refluxing obstructed megaureter or neonatal non-refluxing, non-obstructed megaureter. We hope to construct a framework for understanding the causes that lead to the development of a megaureter and to assist the primary care provider in formulating an appropriate and effective management strategy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
A megaureter is in the broadest sense a purely descriptive term meant to indicate any ureter that is abnormally dilated. This label does not define a distinct pathologic entity per se, but rather encompasses a wide spectrum of both physiologic and pathophysiologic processes which culminate in a dilated ureter. Confusion arises when the terms megaureter, primary megaureter, and congenital megaureter are used interchangeably to specifically refer to a particular subset of all megaureters. In most instances, these labels are used to refer to those patients who present with either a primary non-refluxing obstructed megaureter or neonatal non-refluxing, non-obstructed megaureter. We hope to construct a framework for understanding the causes that lead to the development of a megaureter and to assist the primary care provider in formulating an appropriate and effective management strategy.
Incidence and Epidemiology
Megaureter represents a relatively common anomaly of the newborn urinary tract. Overall, megaureters are thought to be the second leading cause of neonatal obstructive uropathy following only obstructions found at the ureteropelvic junction (UPJ). The vast majority of what constitute primary or congenital megaureters (meaning those found to be non-refluxing and either obstructed or unobstructed) were reportedly identified as the presumed cause for urinary tract dilation in 23 % of cases of all prenatal urinary dilation. With the increased utilization of fetal sonography, we expect megaureters to continue to be a prominent diagnosis found on neonatal evaluation.
With respect to gender differences, primary megaureters apparently occur roughly 2–4 times more often in boys than girls and are thought to occur slightly more often on the left side (1.6–4.5) than the right. Bilateral megaureters are thought to account for about 25 % of all cases. Of additional consideration is the reported association in 10–15 % of megaureters with a contralateral absent or dysplastic kidney which poses important management implications.
Definitions and Classification
By convention, any ureter that is larger in diameter than 7–8 mm is defined as a megaureter. Of crucial importance is the understanding of what processes give rise to the dilated ureter, moving beyond the simple descriptive nature of the term. The international classification for megaureters was established by Smith et al. in 1977. In this nomenclature, three major types of megaureters are emphasized based on the presence or absence of reflux and/or obstruction at the ureterovesical junction. The megaureters in this system are classified as either (1) refluxing, (2) obstructed, or (3) non-refluxing and non-obstructed. An additional category of obstructed and refluxing also deserves mention after its recognition as rare but distinct entity. Furthermore, each of these main categories is further subdivided into either an intrinsic primary ureteral etiology or a secondary non-ureteral etiology.
Refluxing Megaureters
Primary Refluxing Megaureters
Primary refluxing megaureters encompass what we more traditionally have come to think of as dilating vesicoureteral reflux. In primary refluxing megaureters, the ureterovesical junction is presumed to be incompetent allowing for cyclical retrograde flow of urine into the ureter leading to progressive ureteral and upper urinary tract dilation. More regarding this particular subset of megaureters can be found elsewhere in the chapter on vesicoureteral reflux. Typically, the use of the term primary megaureter has not come to include this category which is more often thought of as simply vesicoureteral reflux.
Secondary Refluxing Megaureter
There are two types of secondary megaureters. Both syndromes require treatment of the underlying process, of which a megaureter is only one component. First, patients with the megacystis-megaureter syndrome are found to have bilateral high-grade vesicoureteral reflux along with a large thin-walled bladder created by a constant cycling of urine from a large-volume dilating reflux. The second possible systemic cause of secondary refluxing megaureter is the prune belly syndrome, also known as either Eagle-Barrett’s syndrome or triad syndrome. Patients with prune belly syndrome may have ureterectasis as a part of a constellation of genitourinary findings. The ureteral dilation seen may be due to a variety of reasons including a secondary refluxing megaureter. These are only two specific examples of secondary refluxing megaureters, and the clinician must consider other causes as well.
Obstructed Megaureters
Primary Obstructed Megaureters
Primary obstructive megaureters (POMs), along with the non-refluxing and non-obstructed megaureters, comprise what are typically referred to as primary or congenital megaureters. Primary obstructed megaureters are thought to be a result of an adynamic distal ureteral segment which creates a functional and/or true anatomic obstruction at the ureterovesical junction. Histologic studies of this distal aperistaltic segment have confirmed the presence of an abnormal collagen ultrastructure and altered ureteral concentrations of the neurotransmitter acetylcholinesterase. What was initially that to be a process analogous to Hirschsprung’s disease was refuted by the identification of appropriate ureteral ganglia migration. Other than the presumed distal adynamic segment, other infrequent conditions that can cause a primary obstructed megaureter include congenital ureteral strictures and obstructing ureteral folds or valves.
It is at times difficult to distinguish primary obstructing megaureters from primary non-refluxing and non-obstructing megaureters as the definition of obstruction is subject to the vagaries of existing radiographic studies.
Secondary Obstructing Megaureter
The vast majority of secondary obstructed megaureters are related to functional obstructions associated with an elevated intravesical pressures and/or bladder outlet obstruction. In patients with neuropathic or non-neuropathic dysfunctional bladders, elevated intravesical pressures exceeding 40 cm H2O have been shown to generate enough resistance to impede flow of urine across a ureterovesical junction leading to ureteral dilatation and ultimately, renal deterioration. Patients with both spinal dysraphisms (tethered cord, myelomeningocele, etc.) and infravesical obstruction (posterior urethral valves, urethral atresia) are prime examples of secondary causes of ureteral obstruction. Aggressive management is imperative in these situations to avoid prolonged transmission of high pressure to the upper tracts that leads to renal deterioration.
Non-obstructed and Non-refluxing Megaureters
Primary Non-refluxing and Non-obstructed Megaureter
Primary non-refluxing and non-obstructed megaureters comprise the vast majority of neonatal megaureters encountered in practice. They are believed to be a clinically benign entity resulting from the polyuria of transitional nephrology. Fetal polyuria is marked by an immaturity of effective glomerular filtration, renovascular resistive indices, and overall concentrating ability creating a production of 4–6 times the normal amount of urine production seen later in infancy. High-volume urinary production leads to a state of transient ureteral dilation giving rise to a megaureter. Another potential contributing factor is the delayed maturation of distal ureteral architecture that transiently generates ureteral dilatation until full maturation occurs. Adding to this effect are the elevated voiding pressures of the infantile bladder resulting from a discoordinate urethrovesical unit. As previously mentioned, it becomes somewhat arbitrary as to what constitutes a non-obstructed system given the subjective nature of diuretic renal scans.
Secondary Non-refluxing, Non-obstructed Megaureter
Secondary causes for a non-refluxing and non-obstructed megaureter include conditions that induce a state of polyuria including lithium toxicity, diabetes insipidus, and sickle cell nephropathy to name a few. Additionally, a transitory paralysis of normal ureteral peristalsis can be seen with bacterial endotoxin-mediated dilation in the context of an acute urinary tract infection. Just as in the other causes of secondary megaureters, the treatment lies in the treatment of the underlying condition.
Refluxing and Obstructed Megaureter
The refluxing, obstructing megaureter represents a rare phenomenon that was not initially incorporated into the international classification system, but deserves mention as its own distinct category. Of the various categories, the refluxing and obstructing megaureter is the most difficult one to intuitively understand and conceptualize. Most of these rare cases occur in the context of ureteral ectopia, whereby a ureteral orifice aberrantly located in the bladder neck may be both incompetent causing reflux and become obstructed when the bladder neck musculature becomes contracted. Alternatively, a fixed, incompetent ureterovesical junction may lead to concomitant reflux and obstruction.
History and Physical Exam
Increasingly, the diagnosis of a megaureter is being made on the basis of a prenatal screening ultrasound. Prior to the widespread utilization of fetal ultrasound, most megaureters presented with a constellation of clinical symptoms which subsequently lead to a diagnosis. This dichotomous presentation of the primary megaureter must be taken into consideration during the formulation of a management strategy given that it appears that they may represent differing phenotypic representations of a common set of circumstances.
For the most part, the antenatally detected megaureter represents a clinically asymptomatic process that appears to resolve spontaneously as the ureterovesical complex matures over time. A relative small group of these prenatally detected patients eventually manifest clinical symptoms and likely represent the subset of patients that in the past would have gone on to be diagnosed based on clinical symptoms.
Children with clinical symptoms leading to the diagnosis of a megaureter may have urinary tract infections, abdominal pain, gross and microscopic hematuria, and in extreme cases, renal insufficiency. There are no specific findings on the physical exam that direct the differential diagnosis toward a megaureter. Aside from a nonspecific finding of an abdominal or flank mass in severe cases, megaureters rarely demonstrate overt physical findings. Diagnosis is usually dependent on radiographic imaging.
Evaluation
Laboratory Evaluation
There are no specific laboratory tests required for the diagnosis of a megaureter. In the presence of a urinary tract infection, a urinalysis and urine culture are helpful to direct antibiotic therapy. Additional studies which may be useful include measurements of serum creatinine and estimated glomerular filtration rate to provide an assessment of overall renal function.
Radiographic Evaluation
The diagnosis of a megaureter is usually based on radiographic findings. Accurate classification is paramount to the formulation of a therapeutic management plan. Radiographic investigations may provide both structural and functional information regarding the megaureter.
Ultrasonography
The first step in the evaluation process begins with the clear identification of an abnormally dilated ureter on an imaging study. Currently, a vast majority of megaureters are detected by ultrasound (US), either as part of routine fetal screening or as the first-line imaging modality of choice in the clinically symptomatic pediatric patient. Ultrasound is the preferred initial imaging modality of choice for a variety of reasons. It affords the clinician not only structural detail of the entire urinary system (renal parenchyma, ureter, and bladder) but also is readily accessible and relatively inexpensive and, most importantly, presents no significant ionizing radiation and has minimal risks. It is for these reasons that we advocate ultrasonography (US) as the first imaging modality in the evaluation of the megaureter or of pediatric urinary symptoms.
Intravenous Pyelography
Intravenous pyelography (IVP) provides structural detail of the affected ureter and distal ureterovesical junction as well as gives some idea regarding overall renal function. Limitations of IVP include the effect of renal immaturity on the ability to adequately visualize the urinary tract system and the level of ionizing radiation that is delivered to the pediatric patient. The use of IVP in the evaluation of megaureters is now largely historic but can occasionally be useful in helping to identify the location of the ureteral obstruction.
Computed Tomography
Computed tomography (CT) provides excellent structural detail of the urinary tract. We do not advocate its use in the initial investigation of megaureters given its significant exposure to ionizing radiation and failure to provide any additional level of benefit. However, it is not unheard of for a pediatric patient with nonspecific abdominal or flank pain to undergo a CT scan as a diagnostic study, yielding a diagnosis of a megaureter.
Magnetic Resonance Urography
Magnetic resonance urography (MRU) provides an excellent structural examination of the urinary tract. Additionally, the use of intravenous gadolinium enhancement affords the opportunity to assess functional information and may in the future become the study of choice in evaluating a host of urologic conditions. Currently, it remains an expensive and relatively inaccessible technology that often requires sedation. For these reasons, we do not advocate MRU as a first-line investigation at this time, but reserve it for cases involving more complex anatomical considerations (ureteral duplication, ureteral ectopia, etc.). We anticipate that as the image speed increases, sedation will become easier to manage even in the smaller babies, and MRU will become more widely utilized.
Voiding Cystourethrogram
Voiding cystourethrograms (VCUG) are essential for identifying the presence of vesicoureteral reflux in the context of a diagnosis of a megaureter. Additionally, the VCUG provides structural insight into the urethra, bladder neck, and bladder to assess the presence of secondary causes of a megaureter. Its proper performance is relatively easy to achieve and, with the utilization of spot fluoroscopy, the amount of ionizing radiation can be kept to a reasonable level. Also, in the rare instance of refluxing and obstructing megaureters, the VCUG can provide information that would suggest an obstructive component which may not have otherwise been apparent.
Diuretic Renal Scans
Diuretic renal scans (DRS) provide an important assessment of function by providing differential renal function and assessment of urinary tract obstruction. The renal scan is far from being an ideal examination and remains controversial in terms of how to interpret what constitutes urinary obstruction. Most diuretic renal scans are standardized to attempt to bring uniformity to the way the studies are performed. The radionuclide agents most often used are diethylenetriaminepentaacetic acid (99mTc-DTPA) or mercaptoacetyltriglycine (MAG3). An attempt to standardize hydration status, Lasix administration, and calculation of regions of interest is also made in an attempt to ensure reproducibility and accuracy. Despite these attempts, renal scans remain highly subjective and controversial in their ability to predict true urinary obstruction that would merit surgical interventions. Some have suggested that the drainage washout curves and t1/2 times are not accurate and that a detrimental change in overall differential renal function provides the best indication of obstruction. Another consideration in utilizing renal scans is the relative lack of tubular maturity in the neonatal kidney that may prevent an accurate assessment of function and obstruction. Some authors recommend delaying a renal scan until approximately 3 months of age when the kidneys have achieved maturity.
Whitaker Perfusion Study
Alternatively, the Whitaker perfusion study can be performed to infer an obstructive uropathy based on differential pressure studies. This study has largely fallen out of favor due to its invasive nature (the requirement of percutaneous nephrostomy tubes) and relatively high margin of error in the face of a dilated, compliant collecting system. For these reasons, we do not recommend the Whitaker perfusion study, as we do not feel it offers any advantage over the renal scans except in cases where poor renal function results in poor concentration of radionuclide. In these cases, subjective assessment of drainage at the ureterovesical junction may help determine the need for surgery.
Management
The management of primary or congenital megaureters has evolved over the past few decades as our understanding of the natural history of megaureters has grown. The principle philosophy behind therapeutic intervention in children with megaureter is preservation of renal function. Many children with primary megaureter improve the degree of dilation over time. It is reasonable to assume that a majority of these boys and girls spontaneously resolve or improve without the need for surgical intervention. With that in mind, the therapeutic strategy is therefore predicated on understanding the fundamental structural and functional etiologies that pertain to the megaureter. Additionally, the clinician must be able to recognize the potential for a secondary megaureter caused by another underlying disease process. The treatment of secondary causes of megaureter, regardless of the type of megaureter, is the aggressive treatment of the underlying etiology. Examples previously given of causes of secondary megaureters include neuropathic bladders, prune belly syndrome, and diabetes insipidus.
In the case of primary refluxing megaureters, the treatment recommendations consist largely of medical management with antibiotic prophylaxis and careful observation. Surgical interventions are reserved for those patients who persist with breakthrough urinary tract infections, pyelonephritis, and/or have documented renal scarring or deterioration. This topic is also somewhat controversial and is covered in more detail in the chapter addressing vesicoureteral reflux.
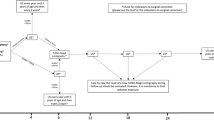
The primary non-refluxing, obstructed and non-refluxing, non-obstructed megaureters comprise two distinct categories of megaureters that likely lie on a continuous spectrum and present a therapeutic challenge in identifying which patients require intervention. In the clear case of an unambiguously obstructed ureter, few would disagree that surgical correction is warranted in order to prevent further renal deterioration. Unfortunately, the lack of an accurate and precise measurement of what truly constitutes an obstructed system leaves us unable to definitively answer which patients are clearly at risk, which patients are clearly obstructed, and which patients only require further observation. What was once uniformly treated with surgical reconstruction, the current knowledge that 70–87 % of these patients will either spontaneously resolve or improve has tempered our approach to the patient with the primary megaureter. Most pediatric urologists would now advocate a conservative course of expectant management consisting of antibiotic prophylaxis and serial radiographic surveillance. The development of recurrent febrile urinary tract infections or significant renal deterioration would prompt surgical intervention to mitigate further renal sequelae. Once again, the area of what constitutes significant renal deterioration is somewhat controversial and is subjective and largely based on clinical experience.
When initially confronted with a patient who is a diagnosed with a megaureter, one must take into consideration the circumstances of the diagnosis.
Was the patient diagnosed based on clinical symptoms or detected incidentally on fetal imaging? Presumably, a patient who presents with a clinical manifestation has transgressed into a clinically significant obstruction requiring a more timely evaluation. More frequently, the latter scenario is now what most clinicians encounter. Although it is reassuring to know that a vast majority of prenatally detected patients resolve spontaneously, one must be careful not to summarily discount the potential for these patients to later manifest significant disease. It is important to recognize that the clinical symptoms are preceded by a potentially lengthy preclinical period of ureteral dilation.
Which patients with a megaureter should be referred to a pediatric urologist? Given that it can be difficult for even pediatric urologist to discriminate which patients will require intervention on initial evaluation, we recommend that all patients who are diagnosed with a megaureter should be thoroughly evaluated by a pediatric urologist.
Should patients be on antibiotic prophylaxis? The protective effect of antibiotic prophylaxis remains one of the most controversial issues in pediatric urology. There is yet to be any conclusive evidence that antibiotic therapy confers a true benefit in the setting, but it is still a widely held belief that an obstructed and infected urinary system poses a serious threat to the safety of the child. This is an area that will require well-designed prospective randomized clinical trials to lend clarity to the issue. In the meantime, we still advocate the initial institution of daily low-dose prophylaxis (amoxicillin 25–50 mg/kg, trimethoprim 2 mg/kg) until a full risk assessment can be performed, including the exclusion of a refluxing megaureter.
Conclusions
In conclusion, the term megaureter is a descriptive label that incorporates a wide spectrum of both physiologic and pathophysiologic causes. As we see a greater number of children diagnosed on ultrasound with a megaureter, it is important that the primary care provider be familiar with larger context of what processes give rise to a congenitally dilated ureter and have a working knowledge of the general categories of megaureter.
Suggested Reading
Atala A, Keating MA. Vesicoureteral reflux and megaureter. In: Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Campbell’s urology. 7th ed. Philadelphia: WB Saunders Co; 1998. p. 1859.
Shokeir AA, Nijman RJ. Primary megaureter: current trends in diagnosis and treatment. BJU Int. 2000;86(7):861–8.
Shukla AR, Cooper J, Patel RP, et al. Prenatally detected primary megaureter: a role for extended followup. J Urol. 2005;173(4):1353–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Kim, S.S., Austin, J.C., Canning, D.A. (2014). Megaureter. In: Rabinowitz, R., Hulbert, W., Mevorach, R. (eds) Pediatric Urology for the Primary Care Physician. Current Clinical Urology. Humana Press, New York, NY. https://doi.org/10.1007/978-1-60327-243-8_10
Download citation
DOI: https://doi.org/10.1007/978-1-60327-243-8_10
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-60327-242-1
Online ISBN: 978-1-60327-243-8
eBook Packages: MedicineMedicine (R0)