Abstract
Enhanced biological phosphorus removal (EBPR) processes developed for wastewater treatment are mainly based on the enrichment of activated sludge with phosphorus-accumulating organisms under alternative anaerobic–aerobic conditions. According to the literature information of the EBPR processes, this chapter attempts to review the biochemical models, microbiology of the EBPR processes, and the main operating parameters that may influence the performance of the EBPR processes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Activate Sludge
- Terminal Restriction Fragment Length Polymorphism
- Sequencing Batch Reactor
- Phosphorus Removal
- Aerobic Granule
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
15.1 Introduction
It is well known that excess nutrients such as nitrogen (N) and phosphorus (P) in water bodies result in serious eutrophication, which is currently a global problem. Eutrophication may affect the general aspects of water bodies, decreasing its aesthetic appeal and making necessary treatment for drinking water more difficult and expensive. Aquatic life is also adversely affected by this excess vegetable matter due to its depletion of oxygen, slowing down the currents and sometimes producing toxic matters. In response to the harmful effects of nutrients, more and more stringent regulations for controlling nutrient discharge in receiving waters have been implemented in many countries, e.g., typical effluent standards that require nitrogen and phosphorus concentrations in effluent must be less than 3 mg total nitrogen/l and 1 mg P/l, respectively. However, it should be noted that the phosphorus levels vary by region and water body. In some regions, more stringent phosphorus discharge limit was set. The Spokane River in Washington, U.S., is a prime example. The regulators there set a phosphorus discharge limit for treatment plants of 50 μg ∕ L (1).
N and P are two necessary elements for the growth of algae, while P input is considered more critical since many of the cyanobacteria are diazotrophic, capable of satisfying their N requirements from the fixation of atmospheric nitrogen (2, 3). Chemical precipitation with alum, ferric chloride, and lime has been widely used as a proven technology for phosphorus removal (4). However, chemical treatment for P removal has the disadvantages of high chemical costs, chemical handling and storage requirements, increased chemical slurry production, and subsequent slurry handling and disposal costs. Biological processes are cost-effective and environmentally sound alternatives to the chemical treatment of nutrient-containing wastewater (5). Biological phosphorus removal (BPR) from wastewaters is based on the enrichment of activated sludge with phosphate accumulating organisms, namely PAOs (6, 7). These PAOs are able to accumulate P in bacterial cells in the form of polyphosphate (polyP) granules in excess levels normally required to satisfy the metabolic demands of growth, such a storage process is commonly referred to as enhanced biological phosphorus removal or EBPR in short (8, 9). In past decades, many treatment plants had been designed and built to deliberately reduce not only organic carbon and nitrogen but also phosphorus by EBPR process. The advantages of EBPR over the chemical precipitation include reduced sludge production, obviation of effluent salinity problems experienced with the chemical process, easier management, and significantly higher reuse potential of produced sludge.
15.2 Biochemical Models for Enhanced Biological Phosphorus Removal
In 1955, Greenburg et al. (10) proposed that activated sludge could take up phosphorus at a level beyond its normal microbial growth requirements. Subsequently, Srinath et al. (11) reported in 1959 that soluble phosphorus in mixed liquor decreased rapidly to below 1 mg/L under varying conditions of aeration. In 1965, Levin and Shapiro (12) further proposed the concept of excess biological phosphorus removal, i.e., “luxury uptake” with alternating anaerobic/aerobic sequence of biological treatment systems, which is the accepted mechanism of P removal. The EBPR process operates on the basis of alternating anaerobic and aerobic conditions with substrates being supplied in the anaerobic stage, while a specific group of bacteria capable of accumulating extra phosphates beyond the anabolic needs, namely P-accumulating organisms (PAOs), can be selected ecologically by this way. Under anaerobic conditions, PAOs tend to release phosphorus and uptake simple organic carbon. In the subsequent aerobic phase, PAOs prefer to generate energy by metabolizing the previously stored organic carbon. As a result, the cell polyphosphate pools are replenished. In such an anaerobic–aerobic alternative process, both phosphorus and organic carbon present in the wastewater stream are considerably reduced.
The EBPR is mainly based on a series of biochemical reactions involved in anaerobic and aerobic phases. So far, two main biochemical models for EBPR have been commonly accepted, i.e., the Comeau/Wentzel model and the Mino model for convenience. The common assumption of these two models is that alternative anaerobic/aerobic phases are essential for the growth of bacteria that can accumulate phosphate in the form of intracellular polyP granules and perform EBPR; short chain fatty acids are taken up and stored in the form of polyhydroxyalkanoates (PHA) as typical internal carbon source in the anaerobic phase, and production of polyP and glycogen at the expense of the stored PHA during the subsequent aerobic phase without extracellular and easily biodegradable carbon left over.
15.2.1 The Comeau/Wentzel Model
The Comeau/Wentzel model was initially developed in 1985 (9, 13). The salient points of this model are that (1) the model accepts the genus Acinetobacter as typical of the PAO group, and the carbon and phosphorus biochemical pathways specific to Acinetobacter spp. are recognized in this model; (2) the ATP/ADP and the NADH/NAD ratios are identified as the key parameters that may regulate these pathways.
15.2.1.1 Under Anaerobic Conditions
The high extracellular acetate concentration allows passive diffusion of acetate into the cell. In the Comeau/Wentzel model, the intracellular acetate is activated to acetyl-CoA by coupled ATP hydrolysis, while the ATP hydrolysis releases cations (e.g., K+ or Mg2 +) and the anion H2PO4 −. The cations are released to the bulk solution via a proton mediated antiport protein carrier, and the phosphorus is released via a hydroxyl mediated antiport protein carrier. Two acetyl-CoA molecules condense to form acetoacetyl-CoA, which is further reduced by NAD(P)H2 to form hydroxybutyryl-CoA, which then is polymerized to form poly-β-hydroxybutyrate (PHB). Conversion of intracellular acetate to PHB maintains a favorable concentration gradient for further diffusion of acetate into the cell. Organisms with stored PHB are able to use these as carbon and energy sources to grow and to assimilate phosphate to synthesize polyP under aerobic conditions.
To supply the reducing power (NAD(P)H2) needed to convert acetoacetyl-CoA to hydroxybutyryl-CoA, part of acetate is metabolized via the tricarboxylic acid (TCA) cycle. As a result, partial acetate is oxidized to carbon dioxide by TCA cycle for providing reducing power; meanwhile partial acetate is used for formation of PHB, which was proposed by Matsuo (14), Comeau et al. (13), and Wentzel et al. (9). The ATP required in the process is regenerated from ADP by transfer of an energy-rich phosphoryl group from polyphosphate (polyP) to the ADP. Originally, this transfer was proposed to be direct, catalyzed by the enzyme ATP, e.g., polyphosphate phosphotransferase according to the following reaction:
However, evidence shows that there is an intermediate step in the ATP generation mediated by the combined action of the enzymes and AMP, i.e., polyphosphate phosphotransferase and adenylate kinase according to the following reactions (15):
Whichever pathway is operative, the net result is a decrease in the stored polyP concentration and a generation of ATP. Conversion of acetate (Ac) to PHB (C4H6O2) n can be summarized as follows:
Metabolism of acetate via the TCA cycle for production of reducing power can be written as:
The net result of these processes can be expressed as:
It appears from Eq. (15.6) that for every acetate utilized, one ATP is required, and one ADP and one Pi are generated. This gives a theoretical molar ratio of acetate uptake to P release of 1:1.
15.2.1.2 Under Aerobic Conditions
In the Comeau/Wentzel model, PHB is broken down and used for either anabolic or catabolic metabolism. In anabolism, carbon skeletons generated from PHB are incorporated into cell mass. In catabolism, the PHB is broken down to acetyl-CoA, which enters the TCA and associated glyoxylate cycles. Reducing equivalents (NADH2) generated in these cycles are subsequently oxidized via the electron transfer pathway, and simultaneous oxidative phosphorylation generates ATP. The ATP generated is further used for cell energy requirements (e.g., biosynthesis) and synthesis of polyP. Phosphate uptake for polyP synthesis occurs via the hydroxyl mediated antiport, and cation uptake via the proton mediated antiport. However, it should be pointed out that the model does not explain the increase in intracellular carbohydrate (16) and increase in extracellular carbohydrate (17).
15.2.2 The Mino Model
The Mino model was developed to explain observations on a laboratory-scale anaerobic/aerobic system receiving an artificial substrate of acetate, propionate, glucose, and peptone and observations on batch tests conducted using sludge from the laboratory-scale system. In the laboratory-scale anaerobic/aerobic system, Mino et al. (16) measured the changes in soluble P, polyP, PHB, acetate, and intracellular carbohydrate. They observed a decrease of intracellular carbohydrates in the anaerobic phase and an increase in the subsequent aerobic phase. Evaluation of these results is hampered by uncertainty as to whether the analytical methodology used to determine carbohydrate adequately differentiated between extracellular and intracellular carbohydrates, while in some methods, extracellular carbohydrates are not separated from intracellular carbohydrates. For this reason, in describing the Mino model, no differentiation is made between extracellular and intracellular carbohydrates. Another point that requires clarification is whether the changes in PHB and carbohydrates are mediated by the same organism type, or by different organism types that may present in the mixed culture systems. To explain their results, Mino et al. (16) assumed that a single organism type would cause the observed changes in both carbohydrates and PHB. Obviously, this point requires further experimental clarification. Below is a brief description of the Mino model.
15.2.2.1 Under Anaerobic Conditions
Acetate is first taken up by the organism, and intracellular acetate is activated to acetyl-CoA by coupled hydrolysis of ATP (P released to the bulk solution). The ATP required in Eq. (15.2) is supplied by the accumulated polyP. PHB is synthesized from acetyl-CoA (AcCoA) according to the following reaction:
Up to this stage the Mino model is in agreement with the Comeau/Wentzel model. The main difference between the Comeau/Wentzel and the Mino model is the production of reducing equivalents required for the conversion of acetyl-CoA to PHB. The Mino model suggests that reducing equivalents is produced by the conversion of glycogen to acetyl-CoA via pyruvate, and not by oxidation for acetyl-CoA via TCA cycle. Under anaerobic conditions, intracellularly stored glycogen (C6H10O5) n is converted to pyruvic acid via the Embden-Meyerhof-Panas (EMP) pathway with the production of reducing equivalents (NADH2). The pyruvic acid is further converted to acetyl-CoA with the production of carbon dioxide. The overall reaction for the breakdown of carbohydrate to acetyl-CoA can be expressed as follows:
Thus, the reducing equivalents (NADH2) required in the reduction of acetate to PHB under the anaerobic conditions are supplied by the consumption of carbohydrate via the EMP pathway. By combining the reaction for the consumption of glycogen with that of the activation and conversion of acetate to PHB, the following net reaction for changes in intracellular carbon is obtained:
This reaction is in agreement with the observation by Bordacs and Chiesa (18), i.e., almost no 14CO2 is produced from [14C]-acetate during the anaerobic period, which indicates that the acetate taken up anaerobically is not oxidized to CO2, and thus not metabolized through the TCA cycle. Other experimental evidence shows that glycogen is involved in the anaerobic metabolisms of EBPR sludges (19, 20). With the increase of evidence favoring a key role of glycogen in EBPR, the Mino model is now widely accepted. However, the possibility of partial functioning of the TCA cycle cannot be totally excluded. The experimental results by using 13C labeled acetate as substrate showed that a small fraction of acetate was metabolized through the TCA cycle under anaerobic conditions supplying 30% of the reducing power for PHA formation (21). Thus, it seems that the oxidation of acetyl-CoA via TCA cycle can meet the demands of reducing power of PHA synthesis, and the oxidation of glycogen to acetyl-CoA will provide the remainder (21, 22).
For the bioenergetics of anaerobic substrate assimilation and PHA synthesis by PAOs, glycogen catabolism is thought to provide ATP for PHA production besides ATP from polyP degradation, and the amount of energy produced by glycogen depends on the pathway for glycogen catabolism (20, 22, 23). In the study by Mino et al. (16), nitrate concentrations in the laboratory-scale systems and batch tests were not reported. In this case, if nitrate was discharged to the anaerobic phase or was present at the beginning of the batch tests, an uptake of acetate for denitrification would take place without concomitant P release. This would partially explain the high ratio of acetate uptake to P release, i.e., 3.4:1 as reported by Mino et al. (16).
15.2.2.2 Under Aerobic Conditions
The anaerobically stored PHA is further utilized as the energy and carbon source to recover the glycogen and polyP levels. As a result, the stored PHA decreases and soluble orthophosphate is taken up by the sludge with the increase in intracellular glycogen and polyP.
15.2.3 The Adapted Mino Model
15.2.3.1 Under Anaerobic Conditions
Compared to the Mino model, the reducing equivalents in the adapted Mino model that convert acetate to PHB are supplied by consuming carbohydrates through the Entner–Doudoroff (ED) pathway. In fact, this has a significant influence on the stoichiometry of P release and acetate uptake because consumption of carbohydrates through the ED pathway produces markedly less energy than that produced through the EMP pathway, thus more energy production via polyP breakdown will be necessary to convert acetate to acetyl-CoA. Consumption of carbohydrates via the ED pathway can be written as follows:
Equation (15.10) shows that only 2 ATPs are produced, while Eq. (15.8) indicates that in the EMP pathway, 3 ATPs are generated per carbohydrate consumed. Acetyl-CoA produced by the consumption of carbohydrates is further converted to PHB according to Eq. (15.7). Combining Eqs. (15.10) and (15.7) gives the overall equation for the consumption of carbohydrates:
Assume that Eq. (15.4) is acceptable for the production of PHB from acetate; the overall process can be summarized as:
Note that NAD and NADP are used interchangeably, i.e., either form can be used in PHB synthesis. Comparison of Eq. (15.12) with Eq. (15.9) for the EMP pathway shows that in the ED pathway 4Ps are released for every 6Ac taken up, i.e., molar ratio of Ac taken up to P released is about 1.5:1; however, in the EMP pathway 6 moles of Ac are taken up for every 3 moles of P released, i.e., molar ratio of Ac taken up to P released is 2:1.
15.2.3.2 Under Aerobic Conditions
The adapted model follows the Mino model for PHB utilization, P uptake and polyP formation, cell synthesis, and carbohydrate regeneration from the PHB. The regeneration of carbohydrates from PHB assumes that the organisms possess the required biochemical pathways as discussed above. Necessarily, the regeneration must involve the formation of glucose from acetyl-CoA. In organisms where this conversion occurs, acetate undergoes an anabolic sequence known as the glyoxylate cycle. A mechanism for carbohydrate regeneration has been proposed as follows: PHB is broken down to acetyl-CoA via normally accepted biochemical pathways (9, 24). Acetyl-CoA is further converted to phosphoenolpyruvate via malate and oxaloacetate. In fact, phosphoenolpyruvate is an intermediate in the ED pathway and can be converted to carbohydrate by a reversal of the ED pathway. In both the Mino and the adapted Mino models, the formation of carbohydrates under aerobic conditions is essential for PHB formation under the subsequent anaerobic condition. Conceptually, the formation of a carbon storage sink under the carbon limiting conditions may present difficulties, and merits further study.
There is apparent consensus regarding many key features of the organism behaviors mediating EBPR and the biochemical pathways involved. However, little experimental biochemical data are yet available to validate any of these empirical models, and even NMR data are unable to fully explain the behaviors of the different communities since the structure/function relationships of the populations involved are completely unknown (25). In addition, many aspects of the biochemical models are still not fully understood, e.g., (1) the source of reducing equivalents (NADH required in the reduction step for converting acetate to PHB) remains to be unclearly defined; (2) experiments carried out to verify biochemical models in terms of the key features (PHB/P and acetate/P ratios) could not offer quantitative consistency with predictions, etc (26). Therefore, in order to successfully design and manage the EBPR process, a sound understanding of the energetic metabolism and biochemical pathways of this process is essential.
15.3 Microbiology of the Ebpr Processes
The biochemical models developed for EBPR are mainly based on the assumption that there is a typical group of microorganisms dominating the EBPR process. So far, phosphorus accumulating organisms (PAOs) and their competitors, the non-polyphosphate glycogen accumulating organisms (GAOs) have been identified. Evidence shows that the EBPR communities are very diverse phylogenetically, as are the non-EBPR activated sludge communities.
15.3.1 Phosphorus Accumulating Organisms
Acinetobacter spp. was the first group of phosphorus accumulating organisms (PAOs) isolated and identified for EBPR (8). The PAOs are clusters of coccobacillus-shaped microorganisms containing polyphosphate, and are dominant in the EBPR process (27–32). A number of the organisms associated with phosphorus removal have been isolated from the EBPR processes, including Lampropedia (33), Microlunatus phosphovorus (34), Micropruina glycogenica (35) and Tetrasphaera spp. (36). However, none of these isolates exhibits all the characteristics that EBPR sludge should possess, e.g., some isolates lack the anaerobic acetate metabolisms (acetate uptake and its conversion to PHA for storage coupled with hydrolysis of stored polyP and consequent release of orthophosphate under anaerobic conditions) (37).
The disadvantages associated with analyzing natural microbial communities using culture-dependent methods have been discussed extensively in the literature. Thus, culture-independent approaches, including chemotaxonomic methods such as quinone profiling (38) and molecular methods, e.g., the fluorescence in situ hybridization (FISH) (39), the clone library approach, denaturing gradient gel electrophoresis (DGGE) (40), and terminal restriction fragment length polymorphisms (T-RFLP) (41), have been used for studying the microbiology of EBPR.
Chemotaxonomic markers have been used for the analysis of microbial community composition, in which the presence of certain cell components may indicate the presence and relative abundances of particular bacterial populations (38). For example, diaminopropane was used as a marker for members of the genus Acinetobacter present in full-scale plants with high P removal (42). The type of respiratory quinone in biological samples can be quantitatively determined, and the quinone patterns should explicitly reflect the chemotaxonomic composition of the examined samples, e.g., ubiquinone Q-8, diagnostic of the β-Proteobacteria was the most abundant quinone in both EBPR and non-EBPR biomass samples, and not Q-9 associated with the γ-Proteobacteria, including Acinetobacter spp (43). Menaquinone profiles may also change during the EBPR, and had been suggested as possible useful chemical indicators for monitoring P removal (44).
A fluorescent antibody staining technique developed for the identification of Acinetobacter revealed that the number of Acinetobacter in the EBPR processes studied was less than 10% of total bacteria and could not account for the EBPR observed (45). So far, more and more evidence shows that Acinetobacter spp. would not be real PAOs (46, 47). The FISH with the group-specific oligonucleotide probes targeting rRNA further revealed that there was an underestimation of bacteria belonging to the β-subclass of Proteobacteria and an overestimation of bacteria in the γ-subclass of Proteobacteria with culture-dependent method (47). Therefore, it appears that the culture-dependent enumerations of the γ-subclass bacteria of the genus Acinetobacter in the EBPR plants would result in significant overestimation. FISH using a probe specific for Acinetobacter also showed that the number of Acinetobacter was very small, i.e., the role of Acinetobacter in the biological P removal process might be insignificant (48). In addition, the number of DNA sequences found for the Actinobacteria were much lower than the number of these bacteria detected by the other methods (e.g., FISH); these may suggest that their DNA is not as readily obtained as that from other bacteria (49, 50).
Bacterial community structures of P-removing and non-P-removing sludges have been studied and further compared by 16S rDNA clone library analysis (49). It was found that in both sludges, the predominant bacterial group represented in the clones was the β-Proteobacteria at a level of 28%, while the Rhodocyclus group within the β-Proteobacteria was represented more in the reactor with greater P removal (49). However, it should be realized that determination of the microbial community structure using this method may not be representative because of a relatively low number of clones examined, which would not represent the full species abundance in the sludges.
So far, β-Proteobacteria has been reported to be the most abundant bacteria in different activated sludge processes when FISH was used as the method of analysis (47, 51, 52). Using FISH technique, Bond et al. (53) found that two subgroups of the β-Proteobacteria comprised 55% of all bacteria in an efficiently operating laboratory-scale EBPR reactor. Olsen et al. (54) described the lab-scale PAOs-enriched, high-performing EBPR cultures by analyzing the full-cycle rRNA, while Hesselmann et al. (55) firstly reported the definitive phylogenetic placement of the β-Proteobacteria-2 subgroup PAO as a close relative of Rhodocyclus spp. and named the organism “Candidatus Accumulibacter phosphates” or Accumulibacter for short. Using FISH and post-FISH chemical staining techniques, Crocetti et al. (56) further demonstrated that the Accumulibacter cells were able to cycle polyP according to EBPR. This in turn provides support to the finding by Hesselmann et al. (55).
Single strand conformational polymorphism (SSCP) and 16S rDNA clone library analysis have been used to study the microbial ecology of efficient and deteriorated EBPR (57, 58). SSCP showed an abundance of Accumulibacter in the microbial ecosystem as well as the prominent appearance of other bacteria, notably some γ-Proteobacteria and microorganisms closely related to Haliscomenobacter in the Bacteroidetesphylum (58). Increasing evidence shows that Accumulibacter is a PAO in both laboratory- and full-scale EBPR processes (59, 60). Thus, Accumulibacter has been presumed to be the first of many confirmed PAOs. Additional PAO candidates may include Actinobacteria (47), α-Proteobacteria (61), and γ-Proteobacteria (62).
15.3.2 Non-polyphosphate Glycogen Accumulating Organisms
Tetrad-arranged cocci had been found in glucose-fed EBPR process with a poor phosphorus removal, and they were called glycogen-accumulating organisms (GAOs), but these GAOs could grow well when acetate was the carbon source in anaerobic–aerobic reactors (63, 64). GAOs are often described as large oval cells (2 − 3 μm in diameter) that form compact aggregates, and apparently attach together with extracellular slime (64). So far it is known that the GAOs may out-compete the PAOs in anaerobic–aerobic EBPR systems under some conditions. It has been hypothesized that the GAOs could assimilate glucose anaerobically better than the PAOs, and eventually used it for the production of PHA, which could be further metabolized under subsequent aerobic conditions for glycogen formation. These seem to imply that the GAOs are selectively favored and become predominant populations under certain conditions. Since the GAOs are unable to synthesize polyP under aerobic condition, this leads to the failure of the EBPR in the GAOs-dominant process (23). As noted by Mino et al. (23), a deeper insight into the biodiversity of PAOs and GAOs is strongly needed for optimizing the EBPR under different operating conditions, which may in turn select and enrich different PAOs and GAOs.
15.4 Biological Phosphorus Removal Processes
To date, all biological phosphorus removal processes developed are based on alternative aerobic and anaerobic cycle operation.
15.4.1 Process Description
15.4.1.1 PhoStrip Process
The PhoStrip process was first reported in 1965 (12, 65), which is a combination of both biological and chemical phosphorus removal processes. The PhoStrip process has been referred to as a side-stream process since a portion of the return activated sludge flow is diverted for phosphorus stripping and subsequent precipitation with lime. Because a percentage of the return sludge that is subjected to anaerobic conditions for different detention times in the stripper tank is adjustable, a wide range of phosphorus removal can be achieved. Control of the side-stream permits phosphorus removal to be divided between supernatant from the stripper and waste activated sludge, i.e., phosphorus removal is carried out by chemical precipitation or in the waste biological sludge. In this process, an effluent concentration less than 1 mg/L total phosphorus can be achieved with less dependence on the BOD strength of the influent wastewater. A large percentage of the phosphorus removal is tied up as lime sludge, which causes less concern than handling a phosphorus-rich waste biological sludge. Compared with the direct chemical addition to an activated sludge aeration basin for phosphorus precipitation, the PhoStrip process may require a lower chemical dosage, and is cost-effective because the lime dosage is a function of the alkalinity and not the amount of phosphorus to be removed, as is the case for alum and iron salts. This potential advantage is dependent on wastewater alkalinity, phosphorus concentration, and relative chemical costs.
15.4.1.2 The Bardenpho Process
The Bardenpho process is an activated sludge process specially designed to accomplish biological phosphorus and nitrogen removal. The original process developed by Barnard (66) is a single-sludge, four-stage (anoxic–aeration–anoxic–aeration) system intended for nitrogen removal through nitrification and denitrification. For the purpose of P removal, the Bardenpho process has been modified by adding an anaerobic stage ahead of the original four-stage Bardenpho nitrogen removal system. Such a modification allows for the creation of an anaerobic–aerobic contacting condition necessary for biological phosphorus uptake. In the modified Bardenpho process (67), the recycled activated sludge separated from the clarifier is mixed with the influent wastewater prior to the anaerobic contactor. Such a mixing strategy can initiate luxury phosphorus uptake by releasing phosphates first. The mixed liquor from the anaerobic contactor then flows into the first anoxic denitrification stage in which it is further mixed with the internally recycled mixed liquor from the aerobic nitrification zone. In the first anoxic stage, nitrate is denitrified to nitrogen gas using the influent BOD as carbon source. About 70% of the nitrate–nitrogen produced in the system can be removed in the first anoxic stage. Then, the mixed liquor flows into the aerobic nitrification zone in which luxury phosphorus uptake, ammonium oxidation, and additional BOD removal occur. Following the aerobic nitrification stage, a second anoxic stage can further provide the possibility to enhance additional denitrification, which is designed to remove additional nitrate in order to minimize nitrate fed back to the anaerobic contactor. The final aerobic stage provides a short time period of mixed liquor aeration prior to clarification to minimize anaerobic conditions and phosphorus release in the second clarifier.
15.4.1.3 Anaerobic/Oxic Process
The Anaerobic/Oxic process, namely A/O process, was initially developed for the removal of phosphorus and/or nitrogen from wastewater (68, 69). The A/O is a single-sludge suspended growth system that combines anaerobic, anoxic, and aerobic zones in sequence. The anaerobic and the aerobic stages are divided into a number of equal size complete-mixed compartments. Partition of several compartments makes the hydraulic flow approaching plug-flow and prevents backmixing. For the removal of phosphorus, three compartments are commonly used for the anaerobic stage and three or more for the aerobic stage. Recycled sludge from the clarifier is mixed with influent wastewater in the anaerobic section so that there is sorption of BOD by biomass, with accompanying phosphorus release necessary for biological phosphorus removal. The anaerobic section is covered and equipped with mechanical mixers for mixing but not aeration. The oxic stage required for the oxidation of BOD and uptake of the phosphorus released in the anaerobic stage, is aerated with air or pure oxygen. Phosphorus is removed by the discharge of the waste sludge from the system, which may contain 4–6% P by dry weight. The achievable phosphorus concentration in effluent is dependent on the rate of sludge wasting, which is controlled by the operating solids residence time (SRT). Relatively short SRTs and high organic loading rates are the key features of the A/O process (67). Compared to the Bardenpho process, this results in greater sludge production and more phosphorus removal per unit of BOD removal in the system. However, the choice of further sludge stabilization methods (anaerobic or aerobic digestion) must account for the amount of phosphorus released during stabilization as well as the effect of recycle streams from the stabilization units on facility performance.
When necessary, nitrification can be accomplished in the oxic section operated at a properly selected SRT and organic loading suitable for the growth of nitrifying bacteria. When denitrification is required, the anoxic section is included between the anaerobic and oxic sections, namely A2 ∕ O process. The anoxic section is deficient in dissolved oxygen, but chemically bound oxygen in the form of nitrate or nitrite is introduced by recycling nitrified mixed liquor from the oxic section back to the anoxic section. Internal recycle flow of 100–300% has been used, and consequently 40–70% of nitrate–nitrogen removal can be achieved by this way.
15.4.1.4 The UCT Process
The UCT process for biological phosphorus removal is the modification of the Bardenpho process. In the UCT process, the recycled activated sludge is directed to the anoxic stage instead of the anaerobic stage as in the Bardenpho process (67, 70). In fact, the UCT process is based on the finding that initial phosphorus removal efficiency could be negatively affected by nitrate–nitrogen entering the anaerobic stage. Nitrate may serve as an electron acceptor during the biological oxidation of BOD entering the anaerobic stage. This in turn results in competition for the soluble, readily biodegradable BOD that would normally be converted to fermentation products for subsequent use by the biological phosphorus-removing bacteria in the anaerobic zone in the absence of nitrate–nitrogen. In the Bardenpho or A/O process, the ratio of the nitrate–nitrogen in the sludge recycled to the anaerobic stage and the soluble BOD available in the influent to that zone determines if sufficient BOD will remain after denitrification to produce a necessary concentration of the fermentation products for biological phosphorus removal. For wastewaters with a relatively high ratio of TKN to BOD, the effect of nitrate–nitrogen in the recycled sludge on anaerobic zone fermentation may be significant for these two processes.
15.4.1.5 The Modified Activated Sludge Process
In practice, the existing activated sludge systems can be changed operationally to create an anaerobic fermentation zone ahead of the aeration zone for biological phosphorus removal. This modified activated sludge process typically involves turning off air flow or aerators in the front of the activated sludge basin.
15.4.1.6 Combined Process for Biological Phosphorus Removal
A stripper is added in a combined biological system for phosphorous removal. The stripper consists of a complete mix tank for anaerobic contact of a side-stream of return activated sludge followed by a clarifier for separation of the stripped sludge. This combination allows for more than 97% total phosphorus removal compared to 40–50% removal for the anaerobic–aerobic sequence without the stripper (67). This process is operated with a relatively low organic loading, and nitrification can also be realized. High nitrate production may have effect on the phosphorus removal efficiency in the anaerobic–aerobic system without the stripper.
15.4.1.7 SBR Process
Sequencing batch reactor (SBR) has been widely applied for biological phosphorus removal. The SBR is a fill-and-draw activated sludge system. The operation steps of a SBR basically consist of a fill period in which flow is diverted to one of the SBR tanks while the other tank(s) operates in the reaction, settle, effluent withdrawal, or idle operation sequences (70). After the fill period, the reactor contents are mixed, but not aerated, to provide the anaerobic period for phosphorus release and uptake of soluble fermentation products. The next step is the aeration period followed by a settling period without aeration and mixing. The effluent is then withdrawn at the end of the settling period. After the effluent withdrawal, a variable length of idle time may occur, which depends on the influent flow rate (70). Combined with the step-feed strategy, a high degree removal of total phosphorus ( > 98%), total nitrogen ( > 97%), and total COD ( > 95%) was consistently and reliably achieved after a 3-month start-up period in SBR (71).
15.4.1.8 Granular Sludge Process
During the past few years, aerobic granules for organic carbon removal and for simultaneous carbon and nitrogen removal have been developed in aerobic sequencing batch reactors (SBR) (72–74). Compared to conventional activated sludge flocs, aerobic granular sludge has a regular, dense and strong physical structure, good settling ability, high biomass retention, and the ability to withstand shock-loading rate. So far, almost all biological phosphorus removal is carried out by activated sludge. With the development of granular sludge technology, Lin et al. (75) first produced phosphorus-accumulating microbial granules in alternative anaerobic and aerobic sequencing batch reactors at different substrate P/COD ratios for biological phosphorus removal. The structure of the granules became more compact and dense as the substrate P/COD ratio increased and the P uptake by granules fell within the range of 1.9–9.3% by weight, which is comparable with the uptake obtained in conventional enhanced biological phosphorus removal processes.
A recent study showed that the chemical precipitation-related P in granules only accounted for less than 10% of the total P accumulation, i.e., biological storage was mainly responsible for the observed P accumulation in microbial granules (76). According to the elemental analyses (76), the empirical formulas of P-accumulating granules developed at different substrate P/COD ratios were generated, and significant difference in the elemental compositions between P-accumulating and non P-accumulating granules was observed, indicating a shift in microbial association. The substantial accumulation of calcium and magnesium ions was also found in the P-accumulating granules, and was closely related to the polyphosphate accumulated in granules. The granular sludge technology for biological phosphorus removal is expected to overcome problems encountered in the suspended growth P removal process, such as sludge bulking, large treatment plant space, secondary P release in a clarifier, higher production of waste sludge.
The application of this granular technology to an abattoir wastewater in a sequencing batch reactor showed that P removal was over 98% when influent total P was 217 mg/L (77). Meanwhile, the results showed that single granular SBR could realize the simultaneous COD, N, and P removal in abattoir wastewater (77). In addition, P-removal efficiency by steady-state aerobic granules at temperatures of 20, 15, and 8∘C were over 95% (78). All these results showed that aerobic granular sludge technology offers a possibility to design compact wastewater treatment plants based on simultaneous COD, N and P removal in one sequencing batch reactor (78–80). In addition, it has been proven that selection for slow-growing organisms PAO improved the granule stability, particularly at low oxygen concentrations (80). Therefore, the simultaneous removal of COD, N, and P is favorable to the long-term operation stability of aerobic granular sludge.
15.4.2 Process Applications and Limitations
The PhoStrip, Bardenpho, and A/O processes are capable of removing total phosphorus from 4 to 12 mg/L normally found in municipal wastewaters to 1–2 mg/L (67). Industrial practice shows that the PhoStrip process can consistently produce effluent with a soluble phosphorus concentration less than 1.0 mg/L as PO4 − P. However, due to variability in flow and wastewater characteristics as well as other operation reasons, excursions above 1 mg total phosphorous/l in treated effluent are often encountered (70). The PhoStrip process is particularly applicable to cases where only phosphorus removal is required, i.e., with no requirement on nitrification. Basically, the PhoStrip process is not applicable when hydraulic retention time in the aeration unit exceeds 10 h, or when significant nitrification occurs in the system. If nitrification is necessary, PhoStrip can be used in conjunction with the first stage of a two-stage activated sludge process, or a single-stage activated sludge system, while modifications, e.g., increased hydraulic retention time in the anaerobic unit, would be required in order to compensate for the effect of nitrate.
The A/O process is often applied for phosphorus removal with or without nitrification. Concentration of total phosphorus in the treated effluent from the A/O process is usually in the range of 1.5–3.0 mg/L. Since significant amounts of effluent phosphorus are associated with the suspended solids, filtration of the effluent from the A/O process would be necessary in order to produce water with total phosphorus less than 1 mg/L. It is also possible to design the A/O process for denitrification. However, the capability of the A/O process for the phosphorus removal and complete nitrification–denitrification remains undemonstrated. It should be noted that phosphorus-rich waste sludge is generated from the A/O process. Aerobic or anaerobic digestion has been commonly employed to stabilize this sludge through removing degradable portions of volatile solids. In particular, when anaerobic digestion is chosen, the digested liquor contains high concentration of phosphorus and should be treated by chemical precipitation of phosphorus before returning to the front end of the A/O system (67).
The Bardenpho process is an effective process designed for the removal of both the phosphorus and total nitrogen, while it is rarely used when only phosphorus removal is desired. It seems hard for the Bardenpho process to produce an effluent with total phosphorus less than 2 mg/L or soluble phosphate less than 1 mg P/l without supplemental mineral addition. If the total phosphorus in the effluent below 1 mg/L is required, filters are needed for further polishing the effluent from the Bardenpho process. Since SRT in the Bardenpho process is typically maintained at about 20 days and can be as high as 40 days, the excess sludge generated from the system is well stabilized. Regarding the UCT process, it is generally suitable for the treatment of wastewaters with influent TKN to COD ratios greater than 0.08 or influent COD to TKN ratios less than 12.0 (81).
15.5 Factors Affecting EBPR
In the operation of full-scale EBPR, difficulties in assuring stable and reliable system performance have been recognized. Failure of EBPR process has occurred in the laboratory as well as in full-scale wastewater treatment plants without a clear cause (82–84). It has been believed that a possible reason may be due to the competition between PAOs and GAOs induced by the operational conditions, such as substrate, sludge age, anaerobic/oxic time ratio, and so on.
15.5.1 Type of Substrate
The success of activated sludge plants for EBPR depends on the composition of wastewater to a certain extent (13, 85). Tam et al. (86) showed that the addition of readily biodegradable carbon significantly enhanced the nutrient removal while fructose or starch supplementation was detrimental to P-removal. Glucose, propionate, and amino acid rich synthetic wastewater are also extremely detrimental to P-removal (87). Glucose, which may induce accumulation of the GAOs, is the least effective carbon source and is not recommended for biological phosphorous removal (88). In the study of the influence of wastewater biodegradability on EBPR in batch tests with different kinds of carbohydrates, e.g., saccharose, cellobiose, starch, and cellulose, Martinez et al. (89) reported that soluble carbohydrates allowed an EBPR mechanism, but particulate carbohydrates seemed to cause non-biological P-removal. Among all kinds of substrates tested, volatile fatty acids are the most effective substrate for EBPR since those non volatile fatty acid substrate need to be converted to acetic acid first for further take-up by bacteria (84, 90). It has been proved in batch tests with prefermentation of glucose that the addition of VFA of two to five carbon chain lengths with the exception of propionate would result in greater P-removal (91, 92). As carbon source, acetate, and butyrate were equally good for P-removal whereas propionate was the least efficient VFA for EBPR (93). In addition, branched VFA have been shown to be superior to their linear counterparts for more efficient P-removal. Basically, 1 mg of phosphorus removal requires about 7–9 mg of VFAs (94). Besides direct addition of VFAs as substrate for P-removal, VFAs can also be produced on-site by other wastewater (95).
15.5.2 Organic Loading
Influent organic loading is an important parameter which determines the extent of excess phosphorus removal in a biological process since low COD loading results in poor P-removal, and excessive COD loading can lead to deterioration of the BPR process. High influent acetate concentrations would negatively affect BPR (96), e.g., increasing the influent acetate concentration to 400 mg/L led to efficient anaerobic P-removal in a biofilm system; however, further increasing the influent acetate concentration to above 600 mg/L resulted in cessation of anaerobic P-release and subsequently a deterioration of the P-removal capability (97). At higher COD loading rates, the sludge appears to convert the influent organics first to a storage product, namely 3-hydroxyvalerate, which is mainly utilized by the GAOs. This would result in a failure of phosphorus removal process (98). The ratio of influent acetate, or BOD, or COD to P also exerts an influence on the BPR removal capability. The influent to the anaerobic zone of the BPR system should have a ratio of BOD5 to the total P higher than 20:1 or COD:P ratio greater than 40:1 for achieving an effluent P concentration of less than 1.0 mg/L (99).
15.5.3 Magnesium and Potassium
In the EBPR process, evidence shows that Mg2 + and K+ concentrations in bulk solution tend to increase in anaerobic phase and decrease in subsequent aerobic phase. Furthermore, the charge ratio of cation decrease to phosphate decrease in the bulk solution is one mole positive charge decrease per mole phosphate decrease (13). This is mainly due to the fact that one positive charge is required to stabilize one phosphate group in any polyP chain and the expulsion of each phosphate molecule from the cell needs one cationic charge from K+ or Mg2 + (100). The cation limitation would have negative effect on anaerobic P-release and acetate uptake, leading to P-removal decrease. It is likely that the magnesium and potassium concentrations would play an important role in maintaining the stability of the EBPR process. So far, there is no evidence to show that calcium is involved in EBPR. In general, there are excessive K+ and Mg2 + in municipal wastewater, i.e., no cation limitation could be assumed in the EBPR process (99). However, full-scale sewage treatment plants designed for EBPR may periodically experience short- or long-term shortage of potassium in the influent, while excess potassium strongly influences the properties of activated sludge, and results in the poor dewatering property and effluent quality (101).
15.5.4 Nitrate Content in the Influent
Evidence shows that the presence of NO x -N can disturb the release of phosphorus in the anaerobic zone and further reduce the uptake of phosphorus in the aerobic zone (67, 102, 103). The possible reason behind is that in the presence of nitrate–nitrogen in the anaerobic zone, denitrifier will consume the organic carbon readily available for PAOs. Generally, 5 g COD is consumed in denitrifying 1 g of nitrate–nitrogen in the anaerobic zone. These imply that the presence of nitrate would reduce the net influent BOD/P ratio to the system. For wastewater with a low BOD concentration, nitrate entering the anaerobic zone can significantly deplete the BOD available for conversion to anaerobic fermentation products. Depending on the amount of nitrate received, this will lead to a lowered phosphorus removal efficiency, or even prevent biological phosphorus removal. For wastewater with high soluble organic concentration, the effect of nitrate may not be significant. If soluble organic concentration is high enough, VFA reduction and phosphorus release can occur simultaneously (104). In addition, the recirculation ratio of the return activated sludge also plays an important role as it affects the amount of inflow nitrate–nitrogen to the anaerobic zone. The redox potential is another key factor determining the rate of anaerobic P-release. In general, a lower redox potential would favor the phosphate release in the anaerobic phase (105). The presence of low-concentration oxygen or other oxidizing agents, e.g., nitrate, may alter the redox potential, and thus may negatively impact the rate of phosphate release.
15.5.5 Phosphorus Loading
So far, research attention has been given to EBPR of low-phosphate wastewaters, while there are few attempts to apply biological systems to treat influents with phosphorus concentration higher than 20 mg P/l. The ratio of phosphorus to total organic carbon (P/TOC) in a system is crucial in effectively selecting PAOs as well as in giving them a competitive advantage (106). Kinetically, low phosphate loadings may suppress the growth of PAOs. This would eventually lead to the establishment of GAOs over PAOs, i.e., GAOs dominate at low P loadings (107, 108).
15.5.6 Temperature
Although EBPR processes have been applied successfully for both cold and warm wastewater, it is clear that low temperatures would pose a negative effect on biological P-removal. An incomplete P-uptake was observed in the aerobic phase at 5 and 10∘C, while at 20 and 37∘C, a complete P-uptake was achievable (109, 110). In contrast, good or even comparatively better P-removal efficiency at lower temperatures of \(5-1{5}^{\circ }\mathrm{C}\) was reported by Barnard et al. (82). These inconsistent results may be due to a poor understanding of PAOs. Panswad et al. (111) reported that the PAOs would belong to lower-range mesophiles, or perhaps psychrophiles and predominated only at 20∘C or possibly lower, while the GAOs would be classified, somewhat, as mid-range mesophilic organisms with optimum temperature between 25.0 and 32. 5∘C.
15.5.7 pH
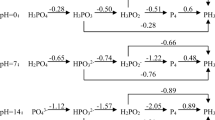
More efficient biological phosphorus removal normally occurs at pH values of 7.5–8.0. The maximum specific growth rate of Acinetobactor at a pH 8.5 was 42% higher than that at a pH of 7.0 (112). The pH between 6.5 and 7.0 has an insignificant effect on the specific phosphorus uptake rate in the aerobic zone; however, once the pH drops below 6.5, the PAO activity steadily declines, and 100% of the activity would be lost at a pH 5.2 (113). A negative effect of acidic pH on both acetate uptake and P-release in the anaerobic stage had been observed, whereas a more alkaline pH inhibited the uptake of acetate and stimulated more P-release than at acidic pH (98). The pH of a combined EBPR and BNR system requires careful monitoring since the various processes, such as nitrification, denitrification, P-release and P-uptake, all have specific pH ranges within which they can be optimized. Nitrification, in particular, appears to be sensitive to the changes in pH, e.g., the optimum pH is in the range of 7.9–8.2 for Nitrosomonas, whereas it ranges from 7.2 to 7.6 for Nitrobacter (114). The optimum pH for denitrification appears to be in between 7.0 and 8.0 (70). As discussed above, maintenance of a stable, neutral pH is essential for the stability of the EBPR process. When the influent pH was reduced from 7.2 to a weakly acidic value of 6.3, P-removal efficiency was adversely affected and 15 days were required to reestablish steady-state conditions (109).
15.5.8 Dissolved Oxygen
A combined EBPR and BNR process must satisfy many different oxygen demands from the bacterial populations present in the system. Activated sludge systems designed for carbon oxidation and nitrification typically require DO levels greater than 2 mg/L (70, 115). If the DO is too low, phosphorus removal may be reduced, incomplete nitrification will result, and a poor settling sludge may be developed. In the EBPR process, the anaerobic zone must be kept nearly free of oxygen (0.0–0.2 mg/L DO) as the presence of oxidizing substances will interfere with the EBPR process, while an oxygen concentration of 3.0–4.0 mg/L in the oxic zone has been recommended with BNR (116). In practice, the maintenance of oxygen concentrations above 4 mg/L will cause a waste of energy for aeration. Meanwhile, excessive aeration may negatively affect the EBPR process as cessation of P-uptake occurs due to the depletion of poly-hydroxy-butyrate (PHB) in an over-aerated process (6). In addition, denitrification performance could be limited due to the increase in DO recycled to the first anoxic zone.
15.5.9 Lengths of Anaerobic and Aerobic Phases
EBPR is realized through an alternative anaerobic–aerobic cycle operation; thus, the relative lengths of anaerobic and aerobic phases would have a profound effect on the performance of the EBPR process. A long anaerobic phase would aid the polyP accumulators in the competition for food against other heterotrophs capable of anaerobic substrate uptake (117, 118). The necessity and the success of longer anaerobic contact times depend on the strength and nature of the wastewater. It should be noted that unnecessary PHA oxidation in the absence of extracellular P would occur if the aerobic reaction time is longer than that required for P-uptake (119). In practice, by adjusting the aeration time to that required for P-uptake, residual PHA is sustained in the SBR and excess phosphate-uptake reaction potential (PRP) is generated during transient influent excursions in P. Consequently, a shorter anaerobic contact time would result in insufficient phosphorus release. However, at a longer anaerobic contact time, polyP microbial bacteria would be inactivated. It appears that anaerobic contact time must be optimized together with aerobic contact time in the EBPR process. An/Ox contact time ratio of 1/2 has been recommended for efficient EBPR (120).
15.5.10 Solid Retention Time
Phosphorus removal is strongly dependent on solid retention time (STR). The different populations involved in the combined nutrient removal processes have different requirements in relation to SRT (67, 70). In general, slow-growing organisms, such as nitrifying bacteria, require longer SRTs, e.g., the Bardenpho process is often operated at longer STRs in order to accomplish nitrification and denitrification. However, lower sludge yields associated with the longer SRTs would hinder the phosphorus removal capacity because a decrease in the polyP-microbial fraction in the mixed culture would occur with the increase in SRT (121). Another problem associated with a shorter SRT is poor sludge settleability. Evidence shows that the optimum SRT for EBPR should be around 10 days, leading to the maximum nutrient removal efficiencies and minimum SVI (67, 103, 122–125). It is clear that biological system should not be operated at SRT in excess of that required for overall COD, N, and P removal.
References
Christen K (2007) Phosphorus removal: how low can we go? Environ Sci Technol 41:674–674
Kortstee GJJ, Appeldorn KJ, Bonting CFC, van Niel EWJ, van Veen HW (1994) Biology of phosphate-accumulating bacteria involved in enhanced biological phosphorus removal. FEMS Microbiol Rev 15:137–153
Nixon SW, Ammerman JW, Atkinson LP, Berounsky VM, Billen G, Boicourt WC, Boynton WR, Church TM, Ditoro DM, Elmgren R, Garber JH, Giblin AE, Jahnke RA, Owens NJP, Pilson MEQ, Seitzinger SP (1996) The fate of nitrogen and phosphorus at the land sea margin of the north Atlantic ocean. Biogeochemistry 35:141–180
Morse GK, Brett SW, Lester JN (1998) Review: phosphorus removal and recovery technologies. Sci Total Environ 212:69–81
Osee Muyima NY, Momba MNB, Cloete TE (1997) Biological methods for the treatment of wastewaters. In: Cloete TE, Moyima NYO (eds) Microbial community analysis: the key to the design of biological wastewater treatment systems. IAWQ Publishers, London, pp 1–24
Brdjanovic D, Slamet A, van Loosdrecht MCM, Hooijmans CM, Alaerts GJ, Heijnen JJ (1998) Impact of excessive aeration on biological phosphorus removal from wastewater. Water Res 32:200–208
Wagner M, Loy A (2002) Bacterial community composition and function in sewage treatment systems. Curr Opin Biotechnol 13:218–227
Fuhs GW, Chen M (1975) Microbiological basis of phosphate removal in the activated sludge process for the treatment of wastewater. Microb Ecol 2:119–138
Wentzel MC, Lotter LH, Loewenthal RE, Marais GvR (1986) Metabolic behaviour of Acinetobacter spp. in enhanced biological phosphorus removal – a biochemical model. Water SA 12:209–224
Greenburg AE, Levin G, Kauffman WJ (1955) Effect of phosphorus removal on the activated sludge process. Sewage Ind Waste 27:227
Srinath EG, Sastry CA, Pillai SC (1959) Rapid removal of phosphorus from sewage by activated sludge. Experientia 15:339–345
Levin GV, Shapiro J (1965) Metabolic uptake of phosphorus by wastewater organisms. J Water Pollut Control Fed 37:800–821
Comeau Y, Hall KJ, Hancock REW, Oldham WK (1986) Biochemical model for enhanced biological phosphorus removal. Water Res 20:1511–1521
Matsuo Y (1985) Functioning of the TCA cycle under anaerobic conditions in the anaerobic aerobic acclimated activated sludge. Proc 40th Annu Conf Jpn Soc Civil Eng 40:989–990
van Groenestijn JW, Deinema MH, Zehnder AJB (1987) ATP production from polyphosphate in Acinetobacter strain 210A. Arch Microbiol 148:14–19
Mino T, Arun V, Tsuzuki Y, Matsuo T (1987) Effect of phosphorus accumulation on acetate metabolism in the biological phosphorus removal process. In: Ramadori R (ed) Biological phosphorus removal from wastewaters. Advances in water pollution control. Pergamon, Oxford, pp 27–38
Osborn DW, Lotter LH, Pitman AR, Nicholls HA (1989) Two-year study on the enhancement of biological phosphate removal by altering process feed composition (Plant and Laboratory Studies). WRC Report No. 137/2/89
Bordacs K, Chiesa SC (1989) Carbon flow patterns in enhanced biological phosphorus accumulating activated sludge cultures. Water Sci Technol 21:387–396
Satoh H, Mino T, Matsuo T (1992) Uptake of organic substrates and accumulation of polyhydroxyalkanoates linked with glycolysis of intracellular carbohydrates under anaerobic conditions in the biological excess phosphate removal process. Water Sci Technol 26:933–942
Maurer M (1997) Intracelluar carbon flow in phosphorus accumulating organisms from activated sludge systems. Water Res 31:907–917
Pereira H, Lemos PC, Reis MAM, Crespo JPSG, Carrondo MJT, Santos H (1996) Model for carbon metabolism in biological phosphorus removal processes based on in vivo 13C-NMR labelling experiments. Water Res 30:2128–2138
Hesselmann RPX, von Rummel R, Resnick SM, Hany R, Zehnder AJB (2000) Anaerobic metabolism of bacteria performing enhanced biological phosphate removal. Water Res 34:3487–3494
Mino T, van Loosdrecht MCM, Heijnen JJ (1998) Microbiology and biochemistry of the enhanced biological phosphate removal process. Water Res 32:3193–3207
Dawes EA, Senior PJ (1973) The role and regulation of energy reserve polymers in microorganisms. Adv Microb Physiol 10:135–266
Seviour RJ, Mino T, Onuki M (2003) The microbiology of biological phosphorus removal in activated sludge systems. FEMS Microbiol Rev 27:99–127
Wentzel MC, Lotter LH, Ekama GA, Loewenthal TE, Marais GvR (1991) Evaluation of biochemical models for biological excess phosphorus removal. Water Sci Technol 23:567–576
Buchan L (1983) The possible biological mechanism of phosphorus removal. Water Sci Technol 15:87–103
Lotter LH, Murphy M (1985) Identification of heterotrophic bacteria in an activated sludge plant with particular reference to polyphosphate accumulation. Water SA 11:179–184
Wentzel MC, Loewenthal RE, Ekama GA, Marais GvR (1988) Enhanced polyphosphate organism cultures in activated sludge systems-part 1: enhanced culture development. Water SA 14:81–92
Deinema MH, van Loosdrecht M, Scholten A (1985) Some physiological characteristics of Acinetobacter spp. accumulating large amounts of phosphate. Water Sci Technol 17:119–125
Streichan M, Golecki JR, Schoen G (1990) Polyphosphate accumulating bacteria from sewage plants with different processes for biological phosphorus removal. FEMS Microbiol Ecol 73: 113–124
Beacham AM, Seviour RJ, Lindrea KC (1992) Polyphosphate accumulating abilities of Acinetobacter isolates from a biological nutrient removal pilot plant. Water Res 26:121–122
Stante L, Cellamare CM, Malaspina F, Bortone G, Tilche A (1997) Biological phosphorus removal by pure culture of Lampropedia spp. Water Res 31:1317–1324
Nakamura K, Hiraishi A, Yoshimi Y, Kawaharasaki M, Masuda K, Kamagata Y (1995) Microlunatus phosphovorus gen-nov, sp-nov, a new gram-positive polyphosphate-accumulating bacterium isolating from activated sludge. Int J Syst Bacteriol 45:17–22
Shintani T, Liu WT, Hanada S, Kamagata Y, Miyaoka S, Suzuki T, Nakamura K (2000) Micropruina glycogenica gen. nov., sp, nov., a new Gram-positive glycogen-accumulating bacterium isolated from activated sludge. Int J Syst Evol Microbiol 50:201–207
Maszenan AM, Seviour RJ, Patel BKC, Schumann P, Burghardt J, Tokiwa Y, Stratton HM (2000) Three isolates of novel polyphosphate-accumulating Gram-positive cocci, obtained from activated sludge, belong to a new genus, Tetrasphaera gen. nov., and description of two new species, Tetrasphaera japonica sp, nov and Tetrasphaera australiensis sp nov. Int J Syst Evol Microbiol 50:593–603
Jenkins D, Tandoi V (1991) The applied microbiology of enhanced biological phosphate removal – accomplishments and needs. Water Res 25:1471–1478
Hiraishi A, Masamune K, Kitamura H (1989) Characterization of the bacterial population structure in an anaerobic-aerobic activated sludge system on the basis of respiratory quinine profiles. Appl Environ Microbiol 30:197–210
Amann RI, Krumholz L, Stahl DA (1990) Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol 172: 762–770
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Liu WT, Marsh TL, Cheng H, Forney LJ (1997) Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl Environ Microbiol 63:4516–4522
Auling G, Pilz F, Busse HJ, Karrasch M, Streichan M, Schon G (1991) Analysis of the polyphosphate-accumulating microflora in phosphorus-eliminating, anaerobic-aerobic activated sludge systems by using diaminopropane as a biomarker for rapid estimation of Acinetobacter spp. Appl Environ Microbiol 57:3685–3692
Hiraishi A, Ueda Y, Ishihara J (1998) Quinone profling of bacterial communities in natural and synthetic sewage activated sludge for enhanced phosphate removal. Appl Environ Microbiol 64:992–998
Fujita M, Chen H, Furamai H (1999) An investigation on microbial population dynamics in enhanced biological phosphorus removal SBR using quinone profile and PCR-DGGE techniques In: Proceedings of 7th IAWQ Asian Pacific Conference, Taipai
Cloete TE, Steyn PL (1987) A combined fluorescent antibody-membrane filter technique for enumerating Acinetobacter in activated sludge. In: Ramadori R (ed) Proc. IAWPRC Int. Conf. in Rome on method “Biological Phosphate Removal from Wastewaters”, Advances in water pollution control. Pergamon Press, Rome, pp 335–338
Hiraishi A, Morishita Y (1990) Capacity for polyphosphate accumulation of predominant bacteria in activated sludge showing enhanced phosphate removal. J Ferment Bioeng 69:368–371
Wagner M, Amann R, Lemmer H, Manz W, Schleifer KH (1994) Probing activated sludge with fluorescently labeled rRNA targeted oligonucleotides. Water Sci Technol 29:15–23
Bond PL, Keller J, Blackall LL (1998) Characterisation of enhanced biological phosphorus removal activated sludges with dissimilar phosphorus removal performances. Water Sci Technol 37:567–571
Bond PL, Hugenholtz P, Keller J, Blackall LL (1995) Bacterial community structures of phosphate-removing and non-phosphate-removing activated sludge from sequencing batch reactors. Appl Environ Microbiol 61:1910–1916
Snaidr J, Amann R, Huber I, Ludwig W (1997) Phylogenetic analysis and in situ identification of bacteria in activated sludge. Appl Environ Microbiol 63:2884–2896
Wagner M, Amann R, Lemmer H, Schleifer KH (1993) Probing activated sludge with oligonucleotides specific for proteobacteria: inadequacy of culture-dependent methods for describing microbial community structure. Appl Environ Microbiol 59:1520–1525
Sudiana IM, Mino T, Satoh H, Matsuo T (1998) Morphology, in-situ characterization with rRNA targeted probes and respiratory quinone profiles of enhanced biological phosphorus removal sludge. Water Sci Technol 38:69–76
Bond PL, Erhart R, Wagner M, Keller J, Blackall LL (1999) Identification of some of the major groups of bacteria in efficient and nonefficient biological phosphorus removal activated sludge systems. Appl Environ Microbiol 65:4077–4084
Olsen GJ, Lane DJ, Giovannoni SJ, Pace NR, Stahl DA (1986) Microbial ecology and evolution: a ribosomal RNA approach. Annu Rev Microbiol 40:337–365
Hesselmann RPX, Werlen C, Hahn D, van der Meer JR, Zehnder AJB (1999) Enrichment, phylogenetic analysis and detection of a bacterium that performs enhanced biological phosphate removal in activated sludge. Syst Appl Microbiol 22:454–465
Crocetti GR, Hugenholtz P, Bond PL, Schuler A, Keller J, Jenkins D, Blackall LL (2000) Identification of polyphosphate-accumulating organisms and design of 16S rRNA-directed probes for their detection and quantitation. Appl Environ Microbiol 66:1175–1182
Dabert P, Fleura-Lessard A, Mounier E, Delgenes JP, Moletta R, Godon JJ (2001) Monitoring of the microbial community of a sequencing batch reactor bioaugmented to improve its phosphorus removal capabilities. Water Sci Technol 43:1–8
Dabert P, Sialve B, Delgenes JP, Moletta R, Godon JJ (2001) Characterisation of the microbial 16S rDNA diversity of an aerobic phosphorus-removal ecosystem and monitoring of its transition to nitrate respiration. Appl Microbiol Biotech 55:500–509
Onuki M, Satoh H, Mino T (2001) Analysis of microbial community that performs enhanced biological phosphorus removal in activaded sludge fed with acetate. In: Tandoi V, Passino R, Blundo CM (eds) Microorganisms in activated sludge and biofilm processes. Proceedings of the 3rd International Water Association Conference. International Water Association, London, England, pp 98–105
Zilles JL, Hung C-H, Noguera DR (2001) Presence of Rhodocyclus in a full-scale wastewater treatment plant and their participation in enhanced biological phosphorous removal. In: Tandoi V, Passino R, Blundo CM (eds) Microorganisms in activated sludge and biofilm processes. Proceedings of the 3rd International Water Association Conference. International Water Association, London, England, pp 75–81
Kawaharasaki M, Tanaka H, Kanagawa T, Nakamura K (1999) In situ identification of polyphosphate-accumulating bacteria in activated sludge by dual staining with rRNA-targeted oligonucleotide probes and 4’, 6-diamidino-2-phenylindol (DAPI) at a polyphosphate-probing concentration. Water Res 33:257–265
Liu WT, Nielsen AT, Wu JH, Tsai CS, Matsuo Y, Molin S (2001) In situ identification of polyphosphate- and polyhydroxyalkanoate-accumulating traits for microbial populations in a biological phosphorus removal process. Environ Microbiol 3:110–122
Cech JB, Hartman P (1990) Glucose induced breakdown of enhanced biological phosphate removal. Environ Technol 11:651–656
Cech JB, Hartman P (1993) Competition between polyphosphate and polysaccharide accumulating bacteria in enhanced biological phosphate removal systems. Water Res 27:1219–1225
Shapiro J, Levin GV, Humberto ZG (1967) Anoxically induced release of phosphate in sewage treatment. J Water Pollut Control Fed 39:1810–1818
Barnard JL (1974) Cut P and N without chemicals. Water Waste Eng 11:33–36
Sedlak RI (1991) Phosphorus and nitrogen removal from municipal wastewater. Lewis, New York
Krichten DJ, Nicholas DM, Galdiers JV (1978) Phosphorus and BOD removal in an activated sludge system without chemical addition. Presented at the 176th national meeting, American chemical society, Miami Beach, FL
Hong SN, Krichten DJ, Kisenbauer KS, Sell RL (1982) A biological wastewater treatment system for nutrient removal. Presented at the workshop on biological phosphorus removal in municipal wastewater treatment, Annapolis, MD
Metcalf & Eddy (2003) Wastewater engineering, treatment, disposal and reuse. New York: McGraw-Hill
Lemaire R, Yuan ZG, Bernet N, Marcos M, Yilmaz G, Keller J (2009) A sequencing batch reactor system for high-level biological nitrogen and phosphorus removal from abattoir wastewater. Biodegradation 20:339–350
Beun JJ, Hendriks A, Loosdrecht MCM, van Morgenroth E, Wilderer PA, Heijnen JJ (1999) Aerobic granulation in a sequencing batch reactor. Water Res 33:2283–2290
Shi XY, Yu HQ, Sun YJ, Huang X (2009) Characteristics of aerobic granules rich in autotrophic ammonium-oxidizing bacteria in a sequencing batch reactor. Chem Eng J 147:102–109
Adav SS, Lee DJ, Show KY, Tay JH (2008) Aerobic granular sludge: Recent advances. Biotechnol Adv 26:411–423
Lin YM, Liu Y, Tay JH (2003) Development and characteristics of phosphorus-accumulating microbial granules in sequencing batch reactors. Appl Microbiol Biotechnol 62:430–435
Liu Y, Lin YM, Tay JH (2005) The elemental compositions of P-accumulating microbial granules developed in sequencing batch reactors. Process Biochem 40:3258–3262
Cassidy DP, Belia E (2005) Nitrogen and phosphorus removal from an abattoir wastewater in a SBR with aerobic granular sludge. Water Res 19:4817–4823
Kishida N, Kim J, Tsuneda S, Sudo R (2006) Anaerobic/oxic/anoxic granular sludge process as an effective nutrient removal process utilizing denitrifying polyphosphate-accumulating organisms. Water Res 40:2303–2310
de Kreuk MK, Pronk M, van Loosdrecht MCM (2005) Formation of aerobic granules and conversion processes in an aerobic granular sludge reactor at moderate and low temperatures. Water Res 39:4476–4484
de Kreuk M, Heijnen JJ, van Loosdrecht MCM (2005) Simultaneous COD, nitrogen, and phosphate removal by aerobic granular sludge. Biotechnol Bioeng 90:761–769
Gerber A, Simpkins MJ, Winter CT, Scheepers JA (1982) Biological nutrient removal from wastewater effluents: Performance Evaluation of the Full PHOREDOX and UCT processes. Contract report 52, council for scitific and industrial research, Pretoria, South Africa
Barnard JL, Stevens GM, Leslie PJ (1985) Design strategies for nutrient removal plants. Water Sci Technol 17:233–242
Fukase T, Shibata M, Miyaji Y (1985) The role of an anaerobic stage on the biological phosphorus removal. Water Sci Technol 17:69–80
Pitman AR, Trim BC, van Dalsen L (1988) Operating experience with biological nutrient removal at the Hohannesburg Bushkoppie works. Water Sci Technol 20:51–61
Wentzel MC, Ekama GA, Marais GvR (1992) Processes and modeling of nitrification denitrification biological excess phosphorus removal systems – a review. Water Sci Technol 25:59–82
Tam NFY, Wong YS, Leung G (1992) Significance of external carbon sources on simultaneous removal of nutrients from wastewater. Water Sci Technol 26:1047–1055
Randall AA, Benefield LD, Hill WE (1994) Effect of fermentation products on enhanced biological phosphorus removal, polyphosphate storage, and microbial population dynamics. Water Sci Technol 30:213–219
Satoh H, Mino T, Matsuo T (1994) Deterioration of enhanced biological phosphorus removal by the domination of microorganisms without poly-phosphate accumulation. Water Sci Technol 30:203–211
Martinez AD, Canizares PC, Mayor LR, Camacho JV (2001) Short-term effects of wastewater biodegradability on biological phosphorus removal. J Environ Eng 127:259–265
Ruel SM, Comeau Y, Heduit A, Deronzier G, Ginestet P, Audic JM (2002) Operating conditions for the determination of the biochemical acidogenic potential of wastewater. Water Res 36:2337–2341
Randall AA, Benefield LD, Hill WE, Nicol JP, Boman GK, Jing SR (1997) The effect of volatile fatty acids on enhanced biological phosphorus removal and population structure in anaerobic/aerobic sequencing batch reactors. Water Sci Technol 35:153–160
Hood C, Randall AA (2001) A biochemical hypothesis explaining the response of enhanced biological phosphorus removal biomass to organic substrates. Water Res 35:2758–2766
Rustrian E, Delgenes JP, Moletta R (1996) Effect of the volatile fatty acids on phosphate uptake parameters by pure cultures of Acinetobacter spp. Lett Appl Microbiol 23:245–248
Barnard JL (1993) Prefermentation in biological nutrient removal plants. Proceedings of the Joint CSCE-ASCE National Conference on Environmental Engineering, Montreal, Quebec, Canada, 12–14 July 1993, pp 1767–1774
Maharaj I, Elefsiniotis P (2001) The role of HRT and low temperature on the acidphase anaerobic digestion of municipal and industrial wastewaters. Bioresour Technol 76:191–197
Randall CW, Chapin RW (1997) Acetic acid inhibition of biological phosphorus removal. Water Environ Res 69:955–960
Morgenroth E, Wilderer PA (1998) Modeling of enhanced biological phosphorus removal in a sequencing batch biofilm reactor. Water Sci Technol 37:583–587
Liu WT, Mino T, Matsuo T, Nakamura K (1996) Biological phosphorus removal process – effect of pH on anaerobic substrate metabolism. Water Sci Technol 34:25–32
Randall CW, Barnard JL, Stensel HD (1992) Design and retrofit of wastewater treatment plants for biological nutrient removal. Technomic Publishing, Lancaster, pp 25–78
Romanski J, Heider M, Wiesmann U (1997) Kinetics of anaerobic orthophosphate release and substrate uptake in enhanced biological phosphorus removal from synthetic wastewater. Water Res 31:3137–3145
Murthy SN, Novak JT (1998) Effects of potassium ion on sludge settling, dewatering and effluent properties. Water Sci Technol 37:317–324
Barnard JL (1976) A review of biological phosphorus removal in the activated sludge process. Water SA 2:136–144
Choi YS, Shin EB, Lee YD (1996) Biological phosphorus removal from wastewater in a single reactor combining anaerobic and aerobic conditions. Water Sci Technol 34:179–186
Rabinowitz B (1985) The role of specific substrates in excess biological phosphorus removal. Ph.D. Thesis, the University British Columbia, Vancouver, British Columbia, Canada
Rensink JH, van der Ven J, van Pamelen G, Fedder F, Majoor E (1997) The modified Renphosystem: a high biological nutrient removal system. Water Sci Technol 35:137–146
Liu WT, Marsh TL, Forney LJ (1998) Determination of the microbial diversity of anaerobic – aerobic activated sludge by a novel molecular biological technique. Water Sci Technol 37: 417–422
Randall AA, Benefield LD, Hill WE (1997). Induction of phosphorus removal in an enhanced biological phosphorus removal bacterial population. Water Res 31:2869–2877
Sudiana IM, Mino T, Satoh H, Nakamura K, Matsuo T (1999) Metabolism of enhanced biological phosphorus removal and non-enhanced phosphorus removal sludge with acetate and glucose as carbon source. Water Sci Technol 39:29–35
Converti A, Rovatti M, Del Borghi M (1995) Biological removal of phosphorus from wastewaters by alternating aerobic and anaerobic conditions. Water Res 29:263–269
Brdjanovic D, van Loosdrecht MCM, Hooijmans CM, Alaerts GJ, Heijnen JJ (1997) Temperature effects on physiology of biological phosphorus removal. J Environ Eng 123:144–153
Panswad T, Doungchai A, Anotai J (2003) Temperature effect on microbial community of enhanced biological phosphorus removal system. Water Res 37:409–415
Groenestijn JW, Deinema MH (1985) Effects of cultural conditions on phosphate accumulation and release by Acinetobactor strain 210A. Proceeding of the international conference, management strategies for phosphorus in the environment, Lisbon, Portugal, July 1–4, 1985
Tracy KD, Flammino A (1985) Kinetics of biological phosphorus removal. Presented at the 58th annual water pollution control federation conference, Kansas city, Missouri, October 1985
Alleman JE (1984) Elevated nitrite occurrence in biological wastewater treatment systems. Water Sci Technol 17:409–419
Louzeiro NR, Mavinic DS, Oldham WK, Meisen A, Gardner IS (2002) Methanolinduced biological nutrient removal kinetics in a full-scale sequencing batch reactor. Water Res 36:2721–2732
Shehab O, Deininger R, Porta F, Wojewski T (1996) Optimising phosphorus removal at the Ann Arbor wastewater treatment plant. Water Sci Technol 34:493–499
Matsuo Y (1994) Effect of the anaerobic solids retention time on enhanced biological phosphorus removal. Water Sci Technol 30:193–202
Goncalves RF, Rogalla F (2000) Optimising the A/O cycle for phosphorus removal in a submerged biofilter under continuous feed. Water Sci Technol 41:503–508
Dassanayake CY, Irvine RL (2001) An enhanced biological phosphorus removal (EBPR) control strategy for sequencing batch reactors (SBRs). Water Sci Technol 43:183–189
Chiou RJ, Ouyang CF, Lin KH, Chuang SH (2001) The characteristics of phosphorus removal in an anaerobic/aerobic sequential batch biofilter reactor. Water Sci Technol 44:57–65
Rodrigo MA, Seco A, Penyaroja JM, Ferrer J (1996) Influence of sludge age on enhanced phosphorus removal in biological systems. Water Sci Technol 34:41–48
Chang WC, Chiou RJ, Ouyang CF (1996) The effect of residual substrate utilization on sludge settling in an enhanced biological phosphorus removal process. Water Sci Technol 34:425–430
Chang CH, Hao OJ (1996) Sequencing batch reactor system for nutrient removal: ORP and pH profiles. J Chem Technol Biotechnol 67:27–38
Kargi F, Uygur A (2002) Nutrient removal performance of a sequencing batch reactor as a function of the sludge age. Enzyme Microb Technol 31:842–847
Wang LK, Ivanov V, Tay JH, Hung YT (2010) Environmental Biotechnology. Humana Press, Totowa, NJ, pp 783–814
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Liu, YQ., Liu, Y., Tay, JH., Hung, YT. (2010). Biological Phosphorus Removal Processes. In: Wang, L., Tay, JH., Tay, S., Hung, YT. (eds) Environmental Bioengineering. Handbook of Environmental Engineering, vol 11. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-60327-031-1_15
Download citation
DOI: https://doi.org/10.1007/978-1-60327-031-1_15
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-58829-493-7
Online ISBN: 978-1-60327-031-1
eBook Packages: EngineeringEngineering (R0)