Abstract
Ovarian cancers are malignancies for which improved therapeutic approaches are urgently needed. The development of chemoresistance in ovarian high-grade serous carcinoma is almost inevitable, and researchers are constantly seeking new pathways to target in order to improve the dismal survival rates of women diagnosed with this disease. The Notch pathway in ovarian cancer represents a promising subject for research into new ovarian cancer treatment modalities. Over the last 12 years, the major Notch proteins (Notch1 and NOTCH3), prominent Notch ligands (JAG1 and DLL4), and downstream proteins (Hes1 and DLGAP5) have begun to be studied in ovarian cancers. The roles of Notch in conferring chemoresistance and acting in angiogenesis have also been demonstrated. Additionally, GSI and DLL4 inhibitors as well as Notch antibodies continue to be explored in both clinical and nonclinical settings. It is clear that future studies are needed in order to translate the results from these preclinical studies into practice. Most importantly, it is crucial to demonstrate the safety and efficacy of Notch-based therapy in ovarian cancer patients. There is still much work to be done in examining the pathways and proteins with which Notch may be associated as well as in developing more specific and more effective means of inhibiting Notch pathway components.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Ovarian Cancer Background
Ovarian cancer is the most lethal gynecologic malignancy; fewer than 50% of women with advanced ovarian cancers will survive beyond 5 years. In the case of high-grade serous carcinoma (HGSC), which is almost always diagnosed at advanced stages (stage III or IV), only 30% of patients will survive beyond 5 years. In fact, the overall mortality of ovarian cancer has only slightly improved in the last three decades. In addition to the lack of effective detection for the disease at its early stages, one of the main reasons for the sluggish improvement in treatment outcomes is the frequent development of chemoresistance in advanced ovarian cancers. Standard chemotherapy for ovarian cancer patients is composed of a combination of platinum- and taxane-based drugs administrated by cycles of treatment. Most ovarian cancer patients initially respond to chemotherapy; however, at least 80% of those initial responders will experience recurrence, most within 18 months of initial response to chemotherapy [1]. Recurrent disease will be resistant to, or will likely become resistant to, platinum therapy. This phenomenon is the primary cause of the bleak prognosis of this deadly disease.
Although ovarian cancer is often referred to as a single disease, it is more accurately a heterogeneous group of diseases. Based on differences in morphological and clinical features, pathogenesis pathways, and unique molecular genetic alterations, epithelial ovarian cancer can broadly be divided into two groups [2]: type I, which includes low-grade serous carcinoma, clear cell carcinoma, endometrioid carcinoma, mucinous carcinoma, and malignant Brenner tumor, and type II, which mainly consists of high-grade serous carcinoma, as well as carcinosarcoma and undifferentiated carcinoma. The prevalence of the five most common subtypes is presented in Fig. 6.1, with other subtypes constituting less than 1% of the total diagnosed epithelial ovarian cancers.
The prevalence of the five most common ovarian cancer subtypes. High-grade serous carcinoma (including peritoneal primary, carcinosarcoma, and tubal carcinoma) is indicated in light blue, low-grade serous carcinoma in orange, clear cell carcinoma in gray, endometrioid carcinoma in yellow, and mucinous carcinoma in dark blue
Type I tumors are typically genetically stable, relatively indolent, and confined to the ovary when they present [3]. Each subtype of type I ovarian cancer presents with a distinct molecular genetic profile and morphology. Common and defining genetic alterations in type I cancers include mutations in KRAS, BRAF, and ERBB2 (low-grade serous carcinoma) [4]; PIK3CA (clear cell carcinoma) [5]; CTNNB1, PTEN, and PIK3CA (low-grade endometrioid carcinoma) [6]; KRAS (mucinous carcinoma) [7, 8]; and CDKN2A (Brenner tumors) [9]. One genetic feature that unites type I ovarian cancers is a lack of TP53 mutations [6]. Type II tumors, on the other hand, account for around 75% of epithelial ovarian cancers and are more aggressive than their type I counterparts [3]. They possess more homogenous morphologies than do type I tumors; exhibit solid, glandular, and papillary patterns; and are diagnosed based on the dominant pattern they exhibit. Type II tumors exhibit TP53 mutations in over 95% of cases as well as frequent CCNE1 amplification but rarely exhibit mutations characteristic of type I tumors [6, 10]. They are highly unstable chromosomally and present as advanced stage disease in over 75% of cases. High-grade serous carcinoma, which accounts for the vast majority of type II ovarian cancer and is highly aggressive, also exhibits BRCA inactivation (either by mutation or promoter methylation) in 40–50% of cases [11].
As a result of the major genetic and morphologic differences between type I and type II ovarian cancers, clinical presentation also differs between these two groups. Type I cancers are slow-growing and are usually confined to the ovaries when diagnosed. Patients may present with early- or late-stage disease, and the frequency of stage at diagnosis depends largely on the subtype [6]. In contrast, the vast majority of type II tumors are at advanced stages when they are diagnosed [12], and at the time of diagnosis, they have almost always spread outside of the ovaries [6]. In addition, type II tumors recur more frequently than do type I tumors [13].
The differences between type I and type II tumors also have implications for approaches to early detection and treatment. Because type I tumors are generally slower growing and are more confined to the ovary, it is typically easier to recognize these malignancies at earlier stages. Since it is so rare to observe type II tumors at stages I or II, there have been significant efforts to improve early detection strategies. Approaches have included examining fallopian tubes removed from women at risk for ovarian cancer (due to BRCA1/BRCA2 mutations) to look for evidence of ovarian cancer precursor lesions [14] and refocusing on detecting low-volume disease (as this may be the best predictor of prognosis) [15, 16]. Recently, applying a liquid Pap smear has been proposed for detection of malignant cells or DNA at early or low-volume stage in ovarian cancers [17], as a significant fraction of ovarian cancer may be derived from fallopian tube epithelial cells, which are easily dislodged and flow through the uterine cavity and cervix.
As of now, type I and type II ovarian cancers are largely treated via platinum-based chemotherapy, i.e., alternating cycles of taxane and a platinum-based agent. Identifying pathways and mutations important to specific subtypes of ovarian cancer will be important to the development of novel therapeutic agents. We must carefully examine pathways that contribute to chemoresistance and tumorigenesis and evaluate the utility of drugs that can be used to target these pathways either alone or in combination with extant therapeutic agents.
It should be clear that “ovarian cancer” is not a single, unified disease, but a collection of various subtypes with distinct genetic profiles, morphologies, and cell origins [2]. In discussing the presence and role of a specific gene or pathway in ovarian cancer, it is important to refrain from generalizing findings to all ovarian cancer subtypes. The majority of literature concentrates on high-grade serous carcinomas, the deadliest and the most common form of ovarian cancer. We will attempt to address the role of the Notch pathway in high-grade ovarian serous carcinoma and examine how targeting the Notch pathway could lead to new treatment modalities in this deadly disease.
6.2 Notch Signaling Pathway: Background
The Notch signaling pathway is evolutionally conserved and regulates a broad spectrum of functions that include cell-fate determination, cell communication, tissue patterning, and cell differentiation, proliferation, and apoptosis. While there is only one Notch receptor and two ligands in insects, four different Notch receptors, named Notch1, Notch2, Notch3, and Notch4, are present in mammals; this was likely an adaptation to deal with the complex and pleiotropic needs of mammals. Additionally, there are five different Notch ligands in mammals, including members of the Jagged family (JAG1 and JAG2), as well as members of the Delta-like family (DLL1, DLL3, and DLL4). This leads to the possibility of different ligand and receptor combinations in mammalian systems. However, when examining cancer tissues, we observed predominant forms of ligand-receptor pairs in specific types of tumor tissues. For example, of the four different Notch receptors, Notch3 is the most frequently amplified and overexpressed in ovarian cancer [18, 19]. Of the ligands, JAG1 and DLL4 have been shown in the literature to be overexpressed in ovarian cancer [20,21,22]. However, based on gene and transcriptome analysis of TCGA ovarian HGSC dataset, DLL3 appears to be the predominate form in this tumor type. This will be detailed in the Sects. 6.4 and 6.7.
The Notch receptors are large transmembrane proteins, each consisting of an extracellular fragment that contains many epidermal growth factor (EGF)-like repeats (e.g., 36 in Notch1), a transmembrane domain, and an intracellular cytoplasmic domain (NICD) . The Notch heterodimer is auto-inhibited by a negative regulatory region (NRR), which contains a heterodimerization domain and three Lin12/Notch repeats [23]. Notch3 differs slightly from the other Notch receptors. Unlike Notch1 and Notch2, Notch3 does not have a complete transactivation domain (TAD). This may explain why the NOTCH intracellular domain (NICD) of Notch3 has weaker transactivation activity than that of Notch1 and Notch2 [24, 25]. There are further differences in the amino acid identity between Notch1 and Notch3 in several intracellular domains, such as the ankyrin repeat region, the RBP-jκ-associated molecule (RAM) domain, and the C-terminal region, as well as in the EGF repeats of the extracellular region [24, 25]. Both the JAG and DLL ligands also contain EGF-like repeats; the JAG proteins typically have 15–16, while the DLL proteins typically have 6–8 [26]. Repeats 11 and 12 are required for Notch binding [27,28,29]. Also required is a degenerate EGF-like repeated entitled the DSL domain shared by both the JAG and DLL ligands [30,31,32]. Other minor structural differences between the two include two additional DOS motifs (tandem EGF repeats) adjacent to the DSL domain and a C-rich domain adjacent to the single transmembrane domain (TMD) residue in the JAG proteins [33]. The binding of the Notch ligands to EGF-repeat region of the Notch extracellular domain is necessary to induce subsequent cleavage steps on the transmembrane domain of NOTCH [34, 35].
The basic signal transmission steps of Notch signaling are generally conserved across different Notch isoforms. The Notch receptor located at the plasma membrane binds to one of its ligands located at the plasma membrane of an adjacent juxtaposed cell. This triggers serial cleavage events on the Notch receptor. First, it is cleaved by the metalloprotease ADAM or TACE , releasing an extracellular fragment that remains bound to the ligand; the remaining cytoplasmic component is then cleaved by gamma secretase to generate the Notch intracellular domain (NICD). NICD then migrates to the nucleus, where it repels a corepressor and binds to the CBF1/Su(H)/Lag-1 (CSL) complex [36]. With the recruitment of additional coactivators (such as MAML1), the CSL complex is converted from a repressor to an activator of transcription. This in turn activates transcription of Notch target proteins [33].
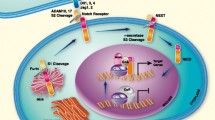
The Notch pathway in ovarian cancer is unique in terms of its (1) dominant Notch receptor, (2) dominant Notch ligand(s), (3) regulatory proteins, and (4) transcriptionally activated genes. As stated above, NOTCH3 was found to be amplified and overexpressed in serous carcinomas and can thus be considered the dominant receptor [18, 19]. It is constitutively activated during tumor development. Notch1 has also been found to be active in ovarian cancer [37, 38]. There is some discussion, however, as to which Notch ligands are most dominant. One study suggests that DLL4 is overexpressed in up to 72% of carcinomas [22]. Yet, the general finding in other reports seems to be that JAG1 is the most dominant Notch ligand (although both JAG1 and DLL4, as well as JAG2, are observed ligands of Notch in ovarian cancer) [20, 21, 39]. Third, as shown in Fig. 6.2, WWP2 has been discovered to be a negative regulator of Notch3 signaling in ovarian cancer [40]. It directly interacts with and mono-ubiquinates post-secretase-cleaved Notch3 protein fragments, promoting their sorting to and degradation in lysosomes. This is thought to be one of the mechanisms by which NOTCH receptor signals are downregulated in human cancers [40].
The Notch pathway in ovarian cancer. The Notch receptor at the plasma membrane binds a ligand located on an adjacent plasma membrane. ADAM or TACE cleaves Notch3 (S2 cleavage), resulting in the separation of the extracellular Notch component. The remaining protein is then cleaved by the gamma secretase complex, resulting in the formation of the Notch3 intracellular domain (NICD3). The NICD3 fragment can then enter the nucleus, where it binds to the CSL complex, converting it to an activator of transcription with the recruitment of coactivators such as MAML1. Alternatively, the repressor WWP2 can ubiquinate Notch3, leading to its degradation in the endosome/lysosome compartments
Finally, different target genes may be transcriptionally activated by different NICD. Since the transcription cofactors are likely to be unique in different cell and tissue types, these Notch transcriptional modifiers are likely to facilitate the tissue-level complexity in the Notch transcription regulation. Based on recent genome-wide ChIP-seq studies using antibody specific to NICD1 or NICD3, Hes and Hey proteins are conserved NOTCH target genes across different cancer cell types, from T-cell lymphoblastic leukemia (T-ALL) to ovarian and breast cancers [41,42,43,44,45]. Hes1 and Hey are both members of the helix-loop-helix (bHLH) family of transcription factors. Members of this family repress the transcription of tissue-specific transcription factors; Notch may thereby maintain stem cells through inhibiting differentiation [26, 46,47,48]. A variety of potential genes transcriptionally activated by Notch3 in ovarian cancer have been revealed by genome-wide chromatin immunoprecipitation (ChIP) and integrated transcriptome analyses [43]. However, the analyses are currently restricted to cancer cell lines. It will be critical to perform similar kind of experiments on tissues or at least on primary cell cultures.
6.3 NOTCH3 Signaling in Ovarian Cancer
In ovarian cancer tissues, gene amplification of chromosome 19p13.12, a locus containing the NOTCH3 gene, was first discovered using digital karyotyping and FISH techniques, and its associated overexpression was identified in ovarian high-grade serous carcinomas [18]. The discovery of NOTCH3 gene amplification in ovarian cancers was later confirmed via SNP array technique, including in experiments performed by The Cancer Genome Atlas (TCGA) [18, 19, 49]. Gene amplification of the NOTCH3 locus was found to be present in approximately 12% of serous carcinomas [18]. NOTCH3 inhibition and silencing resulted in decreased proliferation and induction of apoptosis in Notch3-expressing cell lines [18]. One of the potential Notch3 signal-initiating ligands in ovarian serous carcinoma is JAG1, which would form a juxtacrine circuit with Notch and promote proliferation of ovarian cancer cells in the intraperitoneal cavity [20]. Putative targets of NOTCH3 include Hes1 and Hes4, canonical downstream targets of the Notch1 signaling pathway, and newly identified target genes using genome-wide ChIP approaches [43]. These include DLGAP5, a mitotic apparatus organizing protein [43], ZNF155, and NRARP (unpublished data). Expression of NICD3 was shown to result in the upregulation of embryonic stem cell markers as well as ABCB1, an ATP-binding cassette family member responsible for drug efflux and multidrug resistance [50]. Various studies demonstrated that Notch3 is upregulated in chemoresistant tumors, may confer platinum resistance, and may correlate with worse disease outcome when the signaling is reactivated [50,51,52]. Thus, targeting Notch3 may represent a vital treatment option to overcoming chemoresistance in ovarian tumors.
Early attempts to isolate cancer stem cell-like cells (CSCs) in ovarian cancer , a special self-renewing cell population, identified Notch1 upregulation in isolated CSC spheroid cells, which also showed increased levels of CD44 and CD117 [53]. Later study demonstrated that Notch3 is expressed in ovarian CSC populations isolated from ascites [54] and that inhibition of NOTCH signaling may sensitize cells to platinum treatment [50]. Since ovarian CSCs are tightly linked to treatment failure, CSCs will be more explicitly defined, and this topic will be discussed further in the Sect. 6.5.
The role of Notch signaling in angiogenesis and vascular development has also become increasingly clear. Global knockout of NOTCH1 [55] and endothelium-specific knockout of JAG1 [56] both result in embryonic death with severe vascular defects in mice. NOTCH1 was also shown to be crucial for vascular endothelial growth factor (VEGF)-induced postnatal angiogenesis [57]. While NOTCH3 knockout does not cause the same lethality, the knockout mice show abnormalities in arterial structure and myogenic response, as well as a defect in postnatal maturation of vascular smooth muscle cells [58]. The effect of Notch3 on regulating smooth muscle is also evident when examining CADASIL, an autosomal dominant disease caused by a mutation in the NOTCH3 gene on chromosome 19 [59]. Disulfide bond formation between mutated Notch3 and other proteins is thought to lead the Notch3 extracellular domain to accumulate near vascular smooth muscle cells, leading to smooth muscle cell degeneration [60].
The role of Notch in ovarian tumor angiogenesis was first explored a decade later, when Hu et al. showed a relationship between angiogenesis regulator VEGF and Notch ligand DLL4 in ovarian tumors, reporting a link between DLL4 overexpression and poor overall survival and response to anti-VEGF therapy [22]. Thanapprapasr et al. built on this finding to propose DLL4/Notch signaling as a new approach to anti-angiogenesis therapy in ovarian cancer [61]. Over the past several years, research into anti-DLL4 and anti-JAG1 as possible anti-angiogenic Notch therapeutic strategies has also expanded. The roles of DLL4 and JAG1 in angiogenesis may explain why a number of studies have found a link between inhibition of Notch3 in ovarian cancer and reduced angiogenesis [62, 63]. The Sect. 6.6 will discuss these findings in more detail.
6.4 Genetic Alterations of Members of the Notch Signaling Pathway in Ovarian Cancer
Somatic genetic alteration is a hallmark of cancer, as it often leads to aberrant signaling pathways and disruption of cellular function, which together propel tumorigenesis. Comprehensive molecular genetic analysis of major tumor types, including ovarian HGSC, has been completed by TCGA, a US government-funded research initiative. With a publically available dataset from TCGA [19], we analyzed genetic alterations including mutation, amplification, and deletion of NOTCH1, NOTCH2, NOTCH3, and NOTCH4 and WWP2 in a number of different cancer types. NOTCH1, NOTCH2, and NOTCH4 are altered in ovarian cancer but at rates significantly lower than NOTCH3, which is altered in 12% of ovarian HGSCs, most often via gene amplifications (Fig. 6.3a). This data largely agrees with previous reports concerning NOTCH3 gene amplification in ovarian carcinomas published by our group [18, 49]. Minimal amplicon mapping has pinpointed NOTCH3 located at the core of the amplified region [18, 49]. Other co-amplified genes within this amplicon include BRD4, a BET (bromodomain and extra terminal domain) family protein that could potentially cooperate with Notch3 to promote cell dedifferentiation, repopulation, and other key steps in the tumorigenesis [18, 19].
Notch proteins, signal modifiers, and ligands are altered at different frequencies in ovarian cancer, and Notch ligand DLL3 alterations are associated with worse overall survival. (a) The alteration frequencies of Notch proteins Notch1, Notch2, Notch3, Notch-negative regulator WPP2, and Notch ligands JAG1, JAG2, DLL1, DLL3, and DLL4 are shown for ovarian high-grade serous carcinoma (TCGA, Nature 2011; retrieved from cBioPortal) [19]. Cases are represented with rectangles. Red rectangles indicate cases with gene amplification, blue indicate cases with gene deletion, and green indicate cases with gene mutation. Gray rectangles are cases in which the gene is unaffected. (b) Survival curves for high-grade serous carcinoma cases with and without alterations in DLL3 mRNA expression (left) and with and without alterations in DLL3 DNA copy number (right). Data is retrieved from cBioPortal (TCGA, Nature 2011) [19]. The y-axes denote the percentage of surviving patients, while the x-axes denote the time in months. Red curves represent cases with alterations in DLL3, and blue curves represent cases without alterations. The p-value is indicated in both plots
Among the regulatory players that could fine-tune Notch3 signaling, we chose to analyze WWP2, a NEDD4-like E3 ubiquitin-protein ligase, which was found to mono-ubiquitinate NOTCH3 and target it to the lysosomal degradation pathway [40]. In ovarian cancer, WWP2, which localizes at chromosome 16q22.1, is deleted in approximately 2% of cases and downregulated in approximately 17% of tumors. Therefore, downregulation or deletion of WWP2 could, in theory, unleash the pre-programmed ubiquitination/degradation route of Notch3 and enhance its signaling activity [40].
Notch ligands, including JAG1, JAG2, DLL1, DLL3, and DLL4, can also be altered in ovarian cancer. Based on the analysis of TCGA ovarian HGSC dataset, rare amplification events were found in the JAG1 (1.6%) and JAG2 (3%) gene loci, while rare deletion events (~2%) were observed in the DLL1 or DLL4 locus. Contrarily, DLL3 is heavily amplified in ovarian cancers, with a 9% alteration rate (Fig. 6.3a). Furthermore, DLL3 amplification or overexpression in ovarian HGSC is significantly correlated with dismal overall survival (Fig. 6.3b). This finding is significant, as DLL4 is, by far, the best-studied DLL ligand in ovarian cancer. It will be important to delineate functional receptor-ligand pair of Notch in ovarian tumorigenesis and, based on this finding, develop rationale approaches targeting the Notch pathway.
6.5 Chemoresistance
Perhaps one of the most significant obstacles in improving the dismal survival of patients with ovarian cancer is to overcome the development of resistance to platinum-based therapy. The response to platinum agents can be classified according to three categories. The first group of patients is platinum sensitive. These individuals, who account for approximately 80% of the total patients, show a complete response to the first-line platinum therapy. The second group is platinum refractory, in which the patients fail to respond to initial platinum therapy, with either stable or progressive disease during treatment [64]. Although platinum-sensitive and platinum-resistant patients begin as distinct groups, approximately 50% of platinum-sensitive patients develop resistance during the course of chemotherapy treatment. If the tumor recurs within 6 months after the final treatment, it is considered platinum resistant , and those patients will be treated with non-platinum agents [64].
The Notch pathway has been implicated in the development of chemoresistance and may represent a promising target for overcoming this major barrier to effective treatment. The first research into the role of Notch in platinum resistance in ovarian cancer emerged in 2010, when it was found that Notch confers stem cell-like properties in ovarian HGSC [50]. Subsequent study noted NOTCH3 overexpression in platinum-resistant cells, as well as correlation of NOTCH3 overexpression with worse progression-free and overall survival in patients with advanced ovarian cancer [51]. Inhibiting Notch signaling with GSI in combination with cisplatin was found to prolong survival compared to cisplatin alone in xenograft mouse model of ovarian cancer [52].
It has been argued that one force behind the development of chemoresistance is the existence of cancer stem cell-like cells (CSCs) in the tumor microenvironment. The research on CSCs is still largely in its early stages, and thus there is still work to be done in elucidating the defining markers of CSCs and the pathways that regulate them over a variety of cancer types. These cells, which constitute only a small percentage of the bulk population of tumor cells, are refractory to primary chemotherapy. Unlike cells of the bulk population, CSCs are capable of subsequently regenerating tumor cells and repopulating the tumor microenvironment, leading to recurrence. In general, CSCs are defined according to several criteria, including self-renewal, occupation of a small percentage of the tumor population, ability to reproduce tumors in vivo, differentiation into non-tumorigenic cells, and expression of distinct cell surface markers [52]. CSCs share a number of characteristics with adult stem cells, including those that confer increased chemoresistance, such as enhanced DNA repair and increased levels of membrane efflux transporters.
The Notch signaling pathway has been shown to control survival, proliferation, maintenance, and cell fate in somatic stem cells [65], as well as to participate in regulating CSC functions over many types of cancers [66, 67]. Inhibition of Notch in ovarian cancer decreases the population of ovarian CSCs, suggesting that Notch may play a role in stem cell self-renewal and maintenance in ovarian cancer [52]. Notch1 [53] and Notch3 [52] upregulation have both been observed in the chemoresistant CSC populations. Additionally, CD44, Nanog, Oct4, drug transporters (MDR1, ABCG2, ABCB5), DNA repair genes (ATM, BRCA2), and platinum resistance-associated genes such as Connexi43/Gja1 and Cyp1a1 have been reported in ovarian CSCs [52]. Upregulation of ATP-binding cassette transporters may be a major mechanism contributing to multidrug chemoresistance [52]. In fact, overexpression of the Notch3 intracellular domain (NICD3) has been shown toconfer resistance to platinum in ovarian cancer [68] and to upregulate Nanog, Oct4, ABCB1, and other embryonic stem cell-associated genes such as Klf4, Rex1, Rif1, and Sall4 [50]. Upregulation of ABCB1, an ATP-binding cassette, may indicate increased drug efflux and thus a decrease in the accumulation of carboplatin in ovarian cancer cells, which is likely a factor in chemoresistance. The modulation of the other stem cell markers further supports the putative role of Notch signaling in CSC repopulation in ovarian cancer.
6.6 Angiogenesis
Notch also plays a key role in angiogenesis and vascular development in certain biological contexts. It was shown that both global knockout of NOTCH1 [55] and endothelium-specific knockout of JAG1 [56] result in severe vascular defects and embryonic cell death in mice, while knockout of NOTCH3 results in defects in arterial structure and myogenic response [58]. When taken together, these results indicate that the Notch signaling pathway plays an essential role in regulating embryonic vascular morphogenesis and remodeling. Interestingly, NOTCH3 knockout does not produce lethality as NOTCH1 and JAG1 knockouts do. Instead, NOTCH3 knockout mice present with deficient postnatal maturation of vascular smooth muscle cells, a phenomenon similar to that resulting from cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) , a disease caused by a mutation in the NOTCH3 gene on chromosome 19 [59]. In patients with CADASIL, disulfide bonds form between mutated Notch3 and neighboring proteins, causing the Notch3 extracellular domain to accumulate near vascular smooth muscle. This, in turn, leads to smooth muscle degeneration [60].
In cancer, the Notch signaling pathway has also been implicated in angiogenesis and the development of blood vessels in ovarian tumors, including vessel maturation, pericyte recruitment, branching, and cell differentiation, proliferation, survival, and apoptosis [22]. Angiogenesis is a process that could be targeted for controlling the growth of ovarian tumors, including proteins involved in the Notch signaling pathway. As angiogenesis is necessary for tumor growth and metastasis, targeting tumor vasculature via the Notch signaling pathway holds particular therapeutic promise due to the presumed genetic stability of tumor endothelial cells [22]. Recent work has been in characterizing the effects of inhibiting Notch ligands, regulators, and modulators on angiogenesis in ovarian tumors.
The clinical promise of targeting ovarian tumor angiogenesis was initially reported using VEGF inhibitors such as bevacizumab in patients with ovarian tumors [69]. In ovarian cancers, an increased level of VEGF, which is known to play a key role in neovascularization [22, 70, 71], is inversely correlated with patient survival [72, 73]. Treatment of ovarian tumors with VEGF inhibitors in combination with paclitaxel resulted in decreased tumor burden in preclinical models [74]. While there is a demonstrable clinical benefit in using VEGF inhibitors, there are some limitations. Such therapies are not universally effective for ovarian cancers, and initially sensitive tumors often develop resistance following treatment [22]. However, the VEGF pathway is also known to participate in crosstalk with the Notch signaling pathway [75], which has led to the Notch signaling pathway being regarded as an alternate clinical target to VEGF inhibitor in ovarian cancer therapeutics. It has been shown that Notch1 is downstream of VEGF signaling and is critical for VEGF-induced postnatal angiogenesis [57]. A recent study has also demonstrated that VEGF participates in the ovarian cytokine TNF network, an autocrine malignant cell network that also includes IL6, CXCL12, and CXCR4. The Notch signaling pathway was highly enriched in association with this TNF network. Protein kinase CK2 was posited as a key signaling node of this pathway; inhibition of CK2 resulted in decreased Notch signaling and reduced angiogenesis [76].
In order to interfere with angiogenesis in tumor formation, the Notch signaling pathway can be targeted by blocking expression of Notch ligands DLL4 and JAG1, both of which are shown to be overexpressed in ovarian cancers [20,21,22] and both of which play a role in angiogenesis. DLL4 is induced by VEGF, and downregulates angiogenesis by decreasing VEGF receptor expression, allowing DLL4 and VEGF to form a sort of regulatory loop [22, 77, 78]. DLL4 is overexpressed in up to 72% of ovarian cancers [79], is correlated with worse patient outcome, and is a predictor of a poor response to anti-VEGF treatment [22]. Blockade of DLL4 in tumor endothelial cells with a human monoclonal antibody, REGN421, in xenograft mouse models engineered to express human DLL4 results in reduced Notch signaling in solid tumors and surrounding blood vessels, as well as the dysregulation of angiogenic processes via VEGF. This subsequently causes the formation of hyper-sprouted vessels possessing increased vascular structure but decreased vascular perfusion and leads to dose-dependent inhibition of ovarian tumor growth [80]. Chronic inhibition of DLL4 alone has been thought to foster a reversible pathological activation of endothelial cells and vascular tumorigenesis; however, the antitumor effect of DLL4 inhibition can be maintained without the consequences of chronic inhibition if used in concert with VEGF inhibition [80]. The concomitant use of a VEGF inhibitor such as bevacizumab and REGN421 results in a strong antitumor effect in xenograft ovarian tumor mouse models and demonstrates that the critical regulatory interaction between VEGF and DLL4 can be disrupted to a clinical benefit [80].
In ovarian cancer, JAG1 has been found to be upregulated in tumor and tumor-associated endothelial cells; silencing JAG1 has also been found to decrease angiogenesis [20, 81,82,83]. The ligand JAG1, which is predominantly upregulated in ovarian tumors, has been shown to promote angiogenesis by inhibiting the expression of anti-angiogenic VEGFR1/sFLT2. Through the use of Notch decoys that selectively block the signaling activities of DLL4 and JAG1, it was shown that the ligands have opposing effects on ovarian tumor vessel density, but inhibition of either ligand results in decreased vascular perfusion and tumor growth in vitro [32]. In the presence of the JAG1 decoy, increased levels of VEGFR1/sFLT2 and disrupted pericyte coverage reduce angiogenic sprouting and vessel perfusion, quelling tumor growth [32].
6.7 Notch-Based Antitumor Therapy
6.7.1 Gamma Secretase Inhibitors
Gamma secretase inhibitors (GSIs) have been studied in a variety of solid tumors and were at one point regarded as the most promising approach to Notch-based therapy. After ligand binding, the Notch receptor is cleaved by two sets of enzymes, an ADAM metalloprotease and gamma secretase, yielding the intracellular cytoplasmic domain fragment (NICD), which migrates to the nucleus to initiate transcription. GSI could, in principle, block the release of NICD from the plasma membrane and thus suppresses NOTCH signaling (Fig. 6.4). Single agent GSI has been found to induce cell growth inhibition, G2-M cell-cycle arrest, and apoptosis associated with Notch1 downregulation and its downstream effectors in cell line and animal models of ovarian cancer [84]. However, Phase I studies of single agent GSIs have revealed limited to no antitumor activities [85, 86]. Unfortunately, long-term tolerability of GSIs may also be an impediment to therapy, as the vast majority of patients experience some level of adverse effects [86]. One of the most serious adverse effects is GI toxicity and diarrhea due to goblet cell metaplasia of the small intestine [87].
Mechanisms of several Notch therapeutic strategies in ovarian cancer. Therapeutic approaches to treating ovarian cancer via Notch pathway inhibition include anti-Notch3 antibody, anti-DLL4 antibody, and gamma secretase inhibitor (GSI). Anti-Notch3 antibody binds to a negative regulatory (NRR) region, stabilizing it in an “off” state and thereby preventing cleavage by ADAM/TACE. DLL4 antibody binds to DLL4, preventing Notch binding and thus Notch pathway activation. GSIs bind to the gamma secretase protein, preventing the final cleavage step: generation of the Notch intracellular domain (NICD). Notch therefore fails to enter the nucleus
In ovarian cancer, a recent Phase II study of single agent gamma secretase inhibitor RO4929097 in patients with recurrent platinum-resistant ovarian cancer was largely unsuccessful, with no objective responses to the drug [88]. Combined therapy with a platinum agent, however, may prove more efficacious. Studies from several research groups have indicated that cisplatin and GSI co-therapy has a synergistic cytotoxic effect compared with monotherapies in both platinum-resistant and platinum-sensitive patients [50, 52]. This combination therapy has been shown to eliminate both CSCs and bulk tumor cells and to increase the effects of DNA damage, G2-M cell-cycle arrest, and apoptosis more than monotherapy. This may be because GSI sensitizes cells to cisplatin-induced DNA damage and enhances the rate of tumor cell death. Therefore, to bring GSI into clinics for cancer treatment, future research effort should focus on fine-tuning the dosages of GSI perhaps in combination therapy setting, organ site-specific drug delivery, or nanoparticle-based slow-releasing strategy to limit unwanted toxicity by monotherapy and to enhance cancer-specific targeting efficacy [86].
6.7.2 DLL Antibodies
Delta-like 4, or DLL4 , is a dominant Notch ligand in tumor as well as tumor-associated blood vessels. Notch signaling mediated via DLL4 is critical for tumor angiogenesis. Therefore, DLL4 represents a valid target for tumor inhibition (Fig. 6.4). A Phase I trial of DLL4 monoclonal antibody enoticumab developed by Regeneron Pharmaceuticals was launched in year 2016 for patients with advanced solid tumors. Enoticumab treatment has led to response and stable disease in patients with ovarian cancer, not only in those with serous carcinomas but also in those with endometrioid carcinomas [89]. Anti-DLL4 therapy could also be used in combination with VEGF inhibitor, due to interactions detailed in Sect. 6.6 [80]. Progress along this research front remains to be seen. It is worth restating that anti-DLL4 antibodies result in the nonproductive proliferation of poorly differentiated blood vessels [90], which may affect our ability to effectively deliver chemotherapeutic agents through the vasculature. Anti-DLL4 antibodies raise some of the same questions as do GSIs, though there have yet to be serious adverse effects recorded.
Since DLL3 was found to be a dominant ligand in ovarian cancer (Fig. 6.3a), future research should be invested in developing therapeutic grade anti-DLL3 antibody for use in ovarian cancer. Recently, a DLL3-targeted antibody-drug conjugate (ADC) has been developed and evaluated for use in high-grade pulmonary neuroendocrine tumors, such as small cell lung cancer (SCLC) and large cell neuroendocrine carcinoma (LCNEC) [91]. A single course of the ADC SC16LD6.5 (also known as rovalpituzumab tesirine, Rova-T™ ) was shown to rapidly debulk tumors and lead to progression-free responses in the majority of mice bearing SCLC and LCNEC PDX tumors [91, 92]. The efficacy of this drug was highly correlated with DLL3 expression [91]. DLL3 is thought to differ from other members of the Notch family in that it is localized to the Golgi apparatus [92]. Interestingly, DLL3 is thought to interact with Notch1 and DLL1 during development in the Golgi apparatus, inhibiting them from activating Notch signaling through causing their retention in the Golgi or degradation [91, 93, 94]. Thus, future work is needed to elucidate the function of DLL3 before rationale therapeutics could be designed.
6.7.3 Notch Antibodies
Given the lack of specificity of GSIs and anti-DLL inhibitors and potential serious side effects associated with these pan-Notch inhibitors, the focus in Notch-based therapy has recently shifted to the generation of antibodies that are specific to individual Notch paralogs. A study by Wu et al. developed separate anti-Notch1 and anti-Notch2 antibodies and examined their effects both tumor cell growth and angiogenesis in preclinical T-cell acute lymphoblastic leukemia (T-ALL) models [95]. Analysis of co-crystal structure revealed that the antibodies function through stabilizing the negative regulatory regions (NRRs) of the Notch receptors; without a conformational change in receptor, ADAM protease cleavage cannot take place [95] (see Figs. 6.2 and 6.4.). Although GSI-related inhibition of both Notch1 and Notch2 receptors produces serious intestinal toxicity, targeting either Notch1 or Notch2 through its respective NRR does not produce this unwanted effect [95]. Most importantly, targeting Notch1 potently inhibited tumor growth as well as deregulation of angiogenesis associated with anti-Notch1 antibody. These results suggest that targeting NOTCH3 in a similar fashion could increase the specificity as well as limit the toxicity of Notch therapy in ovarian cancer [35].
Like Notch1 and Notch2, Notch3 also has an NRR region that locks the Notch3 receptor in an “off” state, resulting in resistance to ADAM protease cleavage [95]. A recent study of anti-Notch3 antibodies has found that the extant inhibitory anti-Notch3 antibody antagonizes Notch3 signaling through stabilizing the NRR but that it could not regress tumor xenografts in mice with NOTCH3 signaling [96]. They subsequently found that constructing an antibody-drug conjugate (ADC) by conjugating non-inhibitory or inhibitory anti-Notch3 antibody to an auristatin-based microtubule inhibitor (a type of ADC) resulted in dramatic antitumor activity and tumor regressions in breast, lung, and ovarian preclinical models. They were also able to regress OVCAR3 ovarian high-grade serous xenografts with Notch overexpression and were refractory to platinum drug or to anti-VEGF therapy. An ongoing phase I clinical trial will determine the safety and efficacy of a non-inhibitory anti-NOTCH3 ADC [96].
Recently, an anti-Notch2/anti-Notch3 antibody, OMP-59R5 (tarextumab), has yielded promising antitumorigenic effects in a xenograft study of a variety of solid tumors, including pancreatic, triple-negative breast, small cell lung , and serous ovarian tumors. In the pancreatic and ovarian models, OMP-59R5 significantly downregulated Hes1, Notch2, and Notch3 in the tumors and Hes1, Rgs5, and Notch3 in the stroma. In the pancreatic tumor model, OMP-59R5 significantly reduced CSC frequency. In the breast tumor model, it led to improved vascular stability – which, in contrast to the angiogenic effects of anti-DLL4 antibodies, would improve chemotherapeutic delivery – through downregulation of Rgs5, which regulates tumor pericytes [97]. Overall, OMP-59R5 represents a promising new treatment modality, and future clinical trials will further reveal its efficacy.
6.8 Summary
The Notch pathway is just one facet of the complex molecular landscape of ovarian cancer; however, it is ripe with potential to deepen our understanding of this deadly disease. The link between major receptors, ligands, and downstream proteins has spurred questions as to the Notch pathway’s role in the development of chemoresistance as well as angiogenesis, furthering our insight into how carcinomas become viable and lethal. As for clinical applications, progress has mostly remained in its infancy, though recent advances in preclinical and phase I studies have been promising. Overall, Notch signaling constitutes an exciting avenue into the molecular landscape of ovarian cancer, and further offers a potential bevy of novel therapies.
References
Ledermann, J. A., Raja, F. A., Fotopoulou, C., et al. (2013). Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 24(Suppl 6), vi24–vi32.
Kurman, R. J., & Shih, I. (2016). Seromucinous tumors of the ovary. What’s in a name? International Journal of Gynecological Pathology, 35(1), 78–81.
Kurman, R. J., & Shih, I. M. (2010). The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. The American Journal of Surgical Pathology, 34, 433–443.
Kuo, K. T., Guan, B., Feng, Y., et al. (2009). Analysis of DNA copy number alterations in ovarian serous tumors identifies new molecular genetic changes in low-Grade and high-grade carcinomas. Cancer Research, 69, 4036–4042.
Cho, K. R. (2009). Ovarian cancer update: Lessons from morphology, molecules, and mice. Archives of Pathology & Laboratory Medicine, 133(11), 1775–1781.
Cho, K. R., & Shih, I. M. (2009). Ovarian cancer. Annual Review of Pathology: Mechanisms of Disease, 4, 287–313.
Mok, S. C., Bell, D. A., Knapp, R. C., et al. (1993). Mutation of K-ras protooncogene in human ovarian epithelial tumors of borderline malignancy. Cancer Research, 53(7), 1489–1492.
Auner, V., Kriegshauser, G., Tong, D., et al. (2009). KRAS mutation analysis in ovarian samples using a high sensitivity biochip assay. BMC Cancer, 9, 111.
Kuhn, E., Ayhan, A., Shih, I., et al. (2014). The pathogenesis of atypical proliferative Brenner tumor: An immunohistochemical and molecular genetic analysis. Modern Pathology, 27(2), 231–237.
Ahmed, A. A., Etemadmoghadam, D., Temple, J., et al. (2010). Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. The Journal of Pathology, 221(1), 49–56.
Senturk, E., Cohen, S., Dottino, P. R., et al. (2010). A critical re-appraisal of BRCA1 methylation studies in ovarian cancer. Gynecologic Oncology, 119(2), 376–383.
Seidman, J. D., & Khedmati, F. (2008). Exploring the histogenesis of ovarian mucinous and transitional cell (Brenner) neoplasms and their relationship with Walthard cell nests: A study of 120 tumors. Archives of Pathology & Laboratory Medicine, 132(11), 1753–1760.
Skirnisdottir, I., Seidal, T., & Akerud, H. (2015). Differences in clinical and biological features between Type I and Type II tumors in FIGO stages I-II epithelial ovarian carcinoma. International Journal of Gynecological Cancer, 25(7), 1239–1247.
Rabban, J. T., Garg, K., Crawford, B., et al. (2014). Early detection of high-grade tubal serous carcinoma in women at low risk for hereditary breast and ovarian cancer syndrome by systematic examination of fallopian tubes incidentally removed during benign surgery. The American Journal of Surgical Pathology, 38(6), 729–742.
Bristow, R. E., Gossett, D. R., Shook, D. R., et al. (2002). Micropapillary serous ovarian carcinoma: Surgical management and clinical outcome. Gynecologic Oncology, 86(2), 163–170.
Jacobs, I. J., Skates, S. J., MacDonald, N., et al. (1999). Screening for ovarian cancer: A pilot randomised controlled trial. Lancet, 353(9160), 1207–1210.
Tie, J., Wang, Y., Tomasetti, C., et al. (2016). Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Science Translational Medicine, 8(346), 346ra92.
Park, J. T., Li, M., Nakayama, K., et al. (2006). Notch3 gene amplification in ovarian cancer. Cancer Research, 66(12), 6312–6318.
Cancer Genome Atlas Research Network. (2011). Integrated genomic analyses of ovarian carcinoma. Nature, 474(7353), 609–615.
Choi, J. H., Park, J. T., Davidson, B., et al. (2008). Jagged-1 and Notch3 juxtacrine loop regulates ovarian tumor growth and adhesion. Cancer Research, 68(14), 5716–5723.
Chen, X., Stoeck, A., Lee, S. J., et al. (2010). Jagged1 expression regulated by Notch3 and Wnt/β-catenin signaling pathways in ovarian cancer. Oncotarget, 1(3), 210–218.
Hu, W., Lu, C., Dong, H. H., et al. (2011). Biological roles of the Delta family Notch ligand Dll4 in tumor and endothelial cells in ovarian cancer. Cancer Research, 71(18), 6030–6039.
Sanchez-Irizarry, C., Carpenter, A. C., Weng, A. P., et al. (2004). Notch subunit heterodimerization and prevention of ligand-independent proteolytic activation depend, respectively, on a novel domain and the LNR repeats. Molecular and Cellular Biology, 24(21), 9265–9273.
Beatus, P., Lundkvist, J., Oberg, C., et al. (2001). The origin of the ankyrin repeat region in Notch intracellular domains is critical for regulation of HES promoter activity. Mechanisms of Development, 104(1–2), 3–20.
Bellavia, D., Checquolo, S., Campese, A. F., et al. (2008). Notch3: From subtle structural differences to functional diversity. Oncogene, 27(38), 5092–5098.
Artavanis-Tsakonas, S., Rand, M. D., & Lake, R. J. (1999). Notch signaling: Cell fate control and signal integration in development. Science, 284(5415), 770–776.
Rebay, I., Fleming, R. J., Fehon, R. G., et al. (1991). Specific EGF repeats of Notch mediate interactions with Delta and Serrate: Implications for Notch as a multifunctional receptor. Cell, 67(4), 687–699.
Hambleton, S., Valeyev, N. V., Muranyi, A., et al. (2004). Structural and functional properties of the human notch-1 ligand binding region. Structure, 12(12), 1273–1283.
Cordle, J., Johnson, S., Tay, Z., et al. (2008). A conserved face of the Jagged/Serrate DSL domain is involved in Notch trans-activation and cis-inhibition. Nature Structural & Molecular Biology, 15(8), 849–857.
Henderson, S. T., Gao, D., Christensen, S., et al. (1997). Functional domains of LAG-2, a putative signaling ligand for LIN-12 and GLP-1 receptors in Caenorhabditis elegans. Molecular Biology of the Cell, 8(9), 1751–1762.
Glittenberg, M., Pitsouli, C., Garvey, C., et al. (2006). Role of conserved intracellular motifs in Serrate signalling, cis-inhibition and endocytosis. The EMBO Journal, 25(20), 4697–4706.
Kangsamaksin, T., Murtomaki, A., Kofler, N. M., et al. (2015). NOTCH decoys that selectively block DLL/NOTCH or JAG/NOTCH disrupt angiogenesis by unique mechanisms to inhibit tumor growth. Cancer Discovery, 5(2), 182–197.
Kopan, R., & Ilagan, M. X. (2009). The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell, 137(2), 216–233.
Mumm, J. S., Schroeter, E. H., Saxena, M. T., et al. (2000). A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Molecular Cell, 5(2), 197–206.
Li, K., Li, Y., Wu, W., et al. (2008). Modulation of Notch signaling by antibodies specific for the extracellular negative regulatory region of NOTCH3. The Journal of Biological Chemistry, 283(12), 8046–8054.
Andersson, E. R., & Lendahl, U. (2014). Therapeutic modulation of Notch signalling–are we there yet? Nature Reviews. Drug Discovery, 13(5), 357–378.
Rose, S. L., Kunnimalaiyaan, M., Drenzek, J., et al. (2010). Notch 1 signaling is active in ovarian cancer. Gynecologic Oncology, 117(1), 130–133.
Hopfer, O., Zwahlen, D., Fey, M. F., et al. (2005). The Notch pathway in ovarian carcinomas and adenomas. British Journal of Cancer, 93(6), 709–718.
Euer, N. I., Kaul, S., Deissler, H., et al. (2005). Identification of L1CAM, Jagged2 and Neuromedin U as ovarian cancer-associated antigens. Oncology Reports, 13(3), 375–387.
Jung, J. G., Stoeck, A., Guan, B., et al. (2014). Notch3 interactome analysis identified WWP2 as a negative regulator of Notch3 signaling in ovarian cancer. PLoS Genetics, 10(10), e1004751.
Castel, D., Mourikis, P., Bartels, S. J., et al. (2013). Dynamic binding of RBPJ is determined by Notch signaling status. Genes & Development, 27(9), 1059–1071.
Stoeck, A., Lejnine, S., Truong, A., et al. (2014). Discovery of biomarkers predictive of GSI response in triple-negative breast cancer and adenoid cystic carcinoma. Cancer Discovery, 4(10), 1154–1167.
Chen, X., Thiaville, M. M., Chen, L., et al. (2012). Defining NOTCH3 target genes in ovarian cancer. Cancer Research, 72(9), 2294–2303.
Palomero, T., Lim, W. K., Odom, D. T., et al. (2006). NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proceedings of the National Academy of Sciences of the United States of America, 103(48), 18261–18266.
Wang, H., Zou, J., Zhao, B., et al. (2011). Genome-wide analysis reveals conserved and divergent features of Notch1/RBPJ binding in human and murine T-lymphoblastic leukemia cells. Proceedings of the National Academy of Sciences of the United States of America, 108(36), 14908–14913.
Radtke, F., & Raj, K. (2003). The role of Notch in tumorigenesis: Oncogene or tumour suppressor? Nature Reviews. Cancer, 3(10), 756–767.
Leong, K. G., & Karsan, A. (2006). Recent insights into the role of Notch signaling in tumorigenesis. Blood, 107(6), 2223–2233.
Katoh, M., & Katoh, M. (2007). Integrative genomic analyses on HES/HEY family: Notch-independent HES1, HES3 transcription in undifferentiated ES cells, and Notch-dependent HES1, HES5, HEY1, HEY2, HEYL transcription in fetal tissues, adult tissues, or cancer. International Journal of Oncology, 31(2), 461–466.
Nakayama, K., Nakayama, N., Jinawath, N., et al. (2007). Amplicon profiles in ovarian serous carcinomas. International Journal of Cancer, 120(12), 2613–2617.
Park, J. T., Chen, X., Trope, C. G., et al. (2010). Notch3 overexpression is related to the recurrence of ovarian cancer and confers resistance to Carboplatin. The American Journal of Pathology, 177(3), 1087–1094.
Rahman, R. T., Nakayama, K., Rahman, M., et al. (2012). Notch3 overexpression as potential therapeutic target in advanced stage chemoresistant ovarian cancer. American Journal of Clinical Pathology, 138(4), 535–544.
McAuliffe, S. M., Morgan, S. L., Wyant, G. A., et al. (2012). Targeting Notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proceedings of the National Academy of Sciences of the United States of America, 109(43), E2939–E2948.
Zhang, S., Balch, C., Chan, M. W., et al. (2008). Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Research, 68(11), 4311–4320.
Vathipadiekal, V., Saxena, D., Mok, S. C., et al. (2012). Identification of a potential ovarian cancer stem cell gene expression profile from advanced stage papillary serous ovarian cancer. PLoS One, 7(1), e29079.
Krebs, L. T., Xue, Y., Norton, C. R., et al. (2000). Notch signaling is essential for vascular morphogenesis in mice. Genes & Development, 14(11), 1343–1352.
Xue, Y., Gao, X., Lindsell, C. E., et al. (1999). Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Human Molecular Genetics, 8(5), 723–730.
Takeshita, K., Satoh, M., Ii, M., et al. (2007). Critical role of endothelial Notch1 signaling in postnatal angiogenesis. Circulation Research, 100(1), 70–78.
Domenga, V., Fardoux, P., Lacombe, P., et al. (2004). Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes & Development, 18(22), 2730–2735.
Joutel, A., Corpechot, C., Ducros, A., et al. (1996). Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature, 383(6602), 707–710.
Opherk, C., Duering, M., Peters, N., et al. (2009). CADASIL mutations enhance spontaneous multimerization of NOTCH3. Human Molecular Genetics, 18(15), 2761–2767.
Thanapprapasr, D., Hu, W., Sood, A. K., et al. (2012). Moving beyond VEGF for anti-angiogenesis strategies in gynecologic cancer. Current Pharmaceutical Design, 18(19), 2713–2719.
Kang, H., Jeong, J. Y., Song, J. Y., et al. (2016). Notch3-specific inhibition using siRNA knockdown or GSI sensitizes paclitaxel-resistant ovarian cancer cells. Molecular Carcinogenesis, 55(7), 1196–1209.
Hu, W., Liu, T., Ivan, C., et al. (2014). Notch3 pathway alterations in ovarian cancer. Cancer Research, 74(12), 3282–3293.
Markman, M., & Bookman, M. A. (2000). Second-line treatment of ovarian cancer. The Oncologist, 5(1), 26–35.
Koch, U., Lehal, R., & Radtke, F. (2013). Stem cells living with a Notch. Development, 140(4), 689–704.
Takebe, N., Harris, P. J., Warren, R. Q., et al. (2011). Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nature Reviews. Clinical Oncology, 8(2), 97–106.
Singh, A., & Settleman, J. (2010). EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene, 29(34), 4741–4751.
Tzeng, T. J., Cao, L., Fu, Y., et al. (2014). Methylseleninic acid sensitizes Notch3-activated OVCA429 ovarian cancer cells to carboplatin. PLoS One, 9(7), e101664.
Burger, R. A., Sill, M. W., Monk, B. J., et al. (2007). Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: A Gynecologic Oncology Group Study. Journal of Clinical Oncology, 25(33), 5165–5171.
Senger, D. R., Galli, S. J., Dvorak, A. M., et al. (1983). Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science, 219(4587), 983–985.
Paley, P. J., Staskus, K. A., Gebhard, K., et al. (1997). Vascular endothelial growth factor expression in early stage ovarian carcinoma. Cancer, 80(1), 98–106.
Yamamato, S., Konishi, I., Mandai, M., et al. (1997). Expression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: Correlation with clinicopathology and patient survival, and analysis of serum VEGF levels. British Journal of Cancer, 76(9), 1221–1227.
Hartenbach, E. M., Olson, T. A., Goswitz, J. J., et al. (1997). Vascular endothelial growth factor (VEGF) expression and survival in human epithelial ovarian carcinomas. Cancer Letters, 121(2), 169–175.
Hu, L., Hofmann, J., Zaloudek, C., et al. (2002). Vascular endothelial growth factor immunoneutralization plus Paclitaxel markedly reduces tumor burden and ascites in athymic mouse model of ovarian cancer. The American Journal of Pathology, 161(5), 1917–1924.
Holderfield, M. T., & Hughes, C. C. (2008). Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circulation Research, 102(6), 637–652.
Kulbe, H., Iorio, F., Chakravarty, P., et al. (2016). Integrated transcriptomic and proteomic analysis identifies protein kinase CK2 as a key signaling node in an inflammatory cytokine network in ovarian cancer cells. Oncotarget, 7(13), 15648–15661.
Li, J. L., & Harris, A. L. (2009). Crosstalk of VEGF and Notch pathways in tumour angiogenesis: Therapeutic implications. Frontiers in Bioscience (Landmark Ed), 14, 3094–3110.
Thurston, G., & Kitajewski, J. (2008). VEGF and Delta-Notch: Interacting signalling pathways in tumour angiogenesis. British Journal of Cancer, 99(8), 1204–1209.
Groeneweg, J. W., Foster, R., Growdon, W. B., et al. (2014). Notch signaling in serous ovarian cancer. Journal of Ovarian Research, 7, 95.
Kuhnert, F., Chen, G., Coetzee, S., et al. (2015). Dll4 blockade in stromal cells mediates antitumor effects in preclinical models of ovarian cancer. Cancer Research, 75(19), 4086–4096.
Lu, C., Bonome, T., Li, Y., et al. (2007). Gene alterations identified by expression profiling in tumor-associated endothelial cells from invasive ovarian carcinoma. Cancer Research, 67(4), 1757–1768.
Jung, S. G., Kwon, Y. D., Song, J. A., et al. (2010). Prognostic significance of Notch 3 gene expression in ovarian serous carcinoma. Cancer Science, 101(9), 1977–1983.
Shah, M. M., Zerlin, M., Li, B. Y., et al. (2013). The role of Notch and gamma-secretase inhibition in an ovarian cancer model. Anticancer Research, 33(3), 801–808.
Chen, X., Gong, L., Ou, R., et al. (2016). Sequential combination therapy of ovarian cancer with cisplatin and γ-secretase inhibitor MK-0752. Gynecologic Oncology, 140(3), 537–544.
Krop, I., Demuth, T., Guthrie, T., et al. (2012). Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. Journal of Clinical Oncology, 30(19), 2307–2313.
Pant, S., Jones, S. F., Kurkjian, C. D., et al. (2016). A first-in-human phase I study of the oral Notch inhibitor, LY900009, in patients with advanced cancer. European Journal of Cancer, 56, 1–9.
Milano, J., McKay, J., Dagenais, C., et al. (2004). Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicological Sciences, 82(1), 341–358.
Diaz-Padilla, I., Wilson, M. K., Clarke, B. A., et al. (2015). A phase II study of single-agent RO4929097, a gamma-secretase inhibitor of Notch signaling, in patients with recurrent platinum-resistant epithelial ovarian cancer: A study of the Princess Margaret, Chicago and California phase II consortia. Gynecologic Oncology, 137(2), 216–222.
Chiorean, E. G., LoRusso, P., Strother, R. M., et al. (2015). A phase I first-in-human study of enoticumab (REGN421), a fully human delta-like ligand 4 (Dll4) monoclonal antibody in patients with advanced solid tumors. Clinical Cancer Research, 21(12), 2695–2703.
Noguera-Troise, I., Daly, C., Papadopolous, N. J., et al. (2006). Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature, 444(7122), 1032–1037.
Saunders, L. R., Bankovich, A. J., Anderson, W. C., et al. (2015). A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Science Translational Medicine, 7(302), 302ra136.
Dylla, S. J. (2016). Toppling high-grade pulmonary neuroendocrine tumors with a DLL3-targeted trojan horse. Molecular and Cellular Oncology, 3(2), e1101515.
Chapman, G., Sparrow, D. B., Kremmer, E., et al. (2011). Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Human Molecular Genetics, 20(5), 905–916.
Serth, K., Schuster-Gossler, K., Kremmer, E., et al. (2015). O-fucosylation of DLL3 is required for its function during somitogenesis. PLoS One, 10(4), e0123776.
Wu, Y., Cain-Hom, C., Choy, L., et al. (2010). Therapeutic antibody targeting of individual Notch receptors. Nature, 464(7291), 1052–1057.
Geles, K. G., Gao, Y., Sridharan, L., et al. (2015). Abstract 1697: Therapeutic targeting the NOTCH3 receptor with antibody drug conjugates. Cancer Research, 75(15), 1697.
Yen, W. C., Fischer, M. M., Axelrod, F., et al. (2015). Targeting Notch signaling with a Notch2/Notch3 antagonist (tarextumab) inhibits tumor growth and decreases tumor-initiating cell frequency. Clinical Cancer Research, 21(9), 2084–2095.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media, LLC, part of Springer Nature
About this chapter
Cite this chapter
Gerry, E., Singh, V., Wang, TL. (2018). Notch in Ovarian Cancer. In: Miele, L., Artavanis-Tsakonas, S. (eds) Targeting Notch in Cancer. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-8859-4_6
Download citation
DOI: https://doi.org/10.1007/978-1-4939-8859-4_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-8857-0
Online ISBN: 978-1-4939-8859-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)