Abstract
Spinal cord injury (SCI) can lead to moderate to severe muscle paralysis, loss of lower-limb functionality and often results in a reduced physical activity. As a consequence, people with SCI demonstrate numerous metabolic and contractile transformations such as leg muscles atrophy, a transformation from slow, fatigue-resistant fibers to fast, fatigable fibers, a decreased vascularization. Appropriate exercise and most especially exercise using functional electrical stimulation (FES) is now well-known to have beneficial effects on muscle characteristics, force output, exercise capacity, but also bone mineral density and cardiovascular parameters. For example, increases in muscle mass and strength, oxidative capacity and vascularization have been reported after several weeks of FES exercise (FES-strengthening, FES-cycling or FES-rowing) in people with SCI.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Spinal Cord Injury
- Functional Electrical Stimulation
- Paralyzed Muscle
- Peak Blood Flow
- Spinal Cord Injury Individual
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Spinal cord injury (SCI) induces substantial changes in paralyzed muscles. Although variable, most muscles below the neurologic level of injury rapidly become atrophied and convert toward predominantly fast type IIx fibers due to the removal of chronic efferent neural activity. As a result, muscles become weak, highly fatigable, and have a fast contractile speed. With that said, significant evidence now exists to support the beneficial effects of physical activity, and more precisely electrically induced activity on the paralyzed muscles for people with SCI. The aim of this chapter is to examine the role of activity in defining metabolic and contractile adaptations after SCI. We will first present the main metabolic and contractile alterations observed after SCI and then describe the impact of physical activity (i.e., electrically induced activity) on these alterations. More precisely, we will focus on muscle atrophy, changes in myotypology, muscle metabolism, vascularization and contractile properties.
3.1 Metabolic and Contractile Alterations After SCI
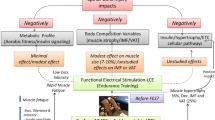
Figure 3.1 presents the main alteration observed after spinal cord injury, with a particular focus on muscle changes.
Main alterations observed in people with spinal cord injury. MHC myosin heavy chain, ATP Adenosine Tri-Phosphate, SDH Succinate DeHydrogenase, right up arrow increases with spinal cord injury, right down arrow decreases with spinal cord injury. Adapted from Deley et al. (2015)
3.1.1 Muscle Atrophy
Significant muscle fiber atrophy, a reduction in the size and/or number of muscle fibers, occurs after SCI. For example, mean area of the Vastus Lateralis muscles of 3.535 ± 1.795 μm2 have been reported in people with complete SCI whereas values averaged 5.392 ± 1.327 μm2 in able-bodied control subjects (Ditor et al. 2004). This atrophy occurs rapidly with significant changes in cross-sectional area and muscle volume within 6 weeks of injury (Gorgey and Dudley 2007). Atrophy occurs in type II fibers before type I fibers (Lotta et al. 1991). Muscle atrophy may be secondary to denervation atrophy or disuse atrophy. Denervation atrophy after SCI results from injury to motoneurons in the spinal cord or to the motor nerves in the ventral roots through which they exit (Gordon and Mao 1994). Disuse atrophy occurs as a result of loss of muscle activation due to disruption to the central and segmental synaptic drive to intact spinal motoneurons (Peckham et al. 1976; Gordon and Pattullo 1993). Muscles are usually referred to as paralyzed in both cases.
Denervation Atrophy
Muscles that lose all of their innervation undergo drastic and rapid wasting. Generally, the proportion of muscles that suffer complete denervation after SCI is small (Peckham et al. 1976). However, many muscle fibers sustain partial denervation as a result of the damage to motoneurons in an affected spinal cord segment. For example, in patients with C5 complete tetraplegia, the paralyzed thenar muscles lose as much as 50 %–90 % of their normal complement of motor innervation. Prevention or reversal of denervation atrophy in these cases will depend on the capacity of the nerves of surviving motoneurons nerves to grow and reinnervate as many denervated muscle fibers as possible. As it will be detailed in the second part of this chapter, muscle fibers may survive and contract in response to electrical stimulation to develop enough force to perform functional movements.
Disuse Atrophy
Muscle wasting after spinal cord injury is generally attributed to the muscle inactivity induced by the loss of the synaptic inputs from spinal cord segments to spinal motoneurons. However, several studies suggest that much of the disuse atrophy of the paralyzed muscles occurs secondary to changes in muscle length or loading conditions, rather than to the decline in neuromuscular activity (Gordon and Pattullo 1993; Roy et al. 1991). The magnitude of disuse atrophy is variable after spinal cord lesions but does not necessarily correlate with the decline in neuromuscular activity (Gordon and Pattullo 1993). Although neural activity is generally reduced after spinal cord lesions it can also vary considerably depending on the type of lesion and the level of spasticity (Gordon and Pattullo 1993; Roy et al. 1991; Alaimo et al. 1984).
Disuse atrophy is more pronounced in muscles that normally bear weight, especially those crossing single joints since they contain a large proportion of slow fatigue-resistant muscle fibers, which are largely responsible for maintaining posture (Gordon and Pattullo 1993). For example, in patients with C5 complete tetraplegia, the paralyzed tibialis anterior muscles develop isometric forces very similar to those in nondisabled individuals whereas the quadriceps femoris muscle, which normally lifts the lower limb by extending the knee, shows significant atrophy after SCI (Peckham et al. 1976). A similar atrophy occurs after space flight, hind-limb suspension, and limb immobilization, wherein muscles undergo shortening contractions that are not resisted by a normal 1oad (Gordon and Pattullo 1993). These findings also suggest that changes in loading or length of paralyzed muscles after spinal cord lesions are responsible, at least in part, for the atrophy that occurs.
3.1.2 Myotypology
In addition to muscle atrophy, people with SCI also demonstrate a fiber-type transformation with a shift toward a homogeneous muscle composed of predominantly type II fibers (Dudley-Javoroski and Shields 2008). According to most studies, in the quadriceps muscle, the fiber-type transformation, with down-regulation of type I fibers and upregulation of type IIA and IIx fibers, begins between 4 and 7 months post SCI (Biering-Sorensen et al. 2009). Similar results were found for the soleus and gastrocnemius muscles. It has also been suggested that early post SCI (<1 month) myosin heavy chain (MCH) isoform composition remains relatively stable (Burnham et al. 1997). A transitional period is seen between 1 and 20 months post SCI with a progressive drop in the proportion of slow MHC isoform fibers and a rise in the proportion that co-express both the fast and slow MHC isoform. By approximately 70 months post SCI, a new steady state has been reached characterized by almost exclusively fast MHC isoform expression.
However, some studies have reported that type I fiber decrease is not always correlated with the length of time post-injury. For example, Ditor et al. (2004) found that the vastus lateralis muscles of those with SCI had a higher proportion of their area represented by type I fibers compared with values in the literature for healthy able-bodied (52.6 ± 25.3 % vs. 36 ± 11.3 %, respectively) (Gorgey and Dudley 2007). These authors suggested that muscle spasticity as a result of upper motor neuron injuries explain these results. Thus, although it is clear that SCI induces a fiber-type transformation toward type II fibers, it also appears that there might be important, unexplained interindividual differences.
3.1.3 Muscle Metabolism
As mentioned above, there appears to be a general reduction in absolute activity of metabolic enzymes after SCI, with a shift in the metabolic profile of muscle fibers toward the fast glycolytic type. Oxidative enzymatic activity starts to decline a few months post-SCI and may reflect the transformation from slow to faster muscle fibers. For example, low values of citrate synthase activity and mitochondrial DNA in the vastus lateralis of individuals with paraplegia have been reported as compared to controls (Wang et al. 1999). In addition, these authors found that the significant relationships between citrate synthase activity, mitochondrial DNA content and aerobic exercise capacity were sustained in individuals with paraplegia with no intrinsic muscle disease. More recently, magnetic resonance spectroscopy (31P-MRS) has reported ∼50 % reduction in the rate of phosphocreatine recovery after exercise in skeletal muscle of persons with SCI (McCully et al. 2011).
Gerrits et al. (2003) reported a 67 % decrease in succinate deshydrogenase (SDH) activity in type II fibers and a 48 % decrease in type I fibers in the tibialis anterior muscle in those >2 years post injury (Gerrits et al. 2003). In addition, Castro et al. (1999) suggested that there is a relative independence of metabolic enzyme levels and inactivation within the first months after SCI; long-term but not short-term inactivation (and the consequent unloading of human skeletal muscle) reduces aerobic-oxidative enzyme levels (Castro et al. 1999). Na+, K+-ATPase concentration has also been demonstrated to be decreased in the paralyzed vastus lateralis as compared with control subjects (141.6 ± 50.0 vs. 339 ± 16 μmol/g wet weight) and a significant negative correlation to years since injury (Ditor et al. 2004). Sarcolemmal lactate/H+ transport capacity is also lower in SCI individuals than in normally physically active subjects suggesting that prolonged muscle inactivity reduces the lactate/H+ transport capacity of human muscle (Pilegaard et al. 1998).
Proteins associated with the Ca2+-ATPase of the sarcoplasmic reticulum, responsible for re-sequestering Ca2+ into the sarcoplasmic reticulum from the myoplasm, also undergo transitions following SCI (Scott et al. 2006). Talmadge et al. (2002) demonstrated that the fast isoform of sarcoplasmic reticulum Ca2+-ATPase is upregulated soon after SCI in paralyzed human muscle and that the proportion of fibers with the slow isoform of sarcoplasmic reticulum Ca2+-ATPase alone was decreased by 30 % at 6 weeks and 65 % at 24 weeks (Talmadge et al. 2002). At the same time, the hybrid sarcoplasmic reticulum Ca2+-ATPase fibers, containing both slow and fast sarcoplasmic reticulum Ca2+-ATPase, was increased nearly fivefold by 24 weeks. However, no significant difference was found in the proportion of fibers containing only MHC I between SCI and control individuals at either time-point (6 weeks and 24 weeks).
3.1.4 Muscle Contractile Properties
It is well known that the ability of muscles to produce and sustain force depends on their fiber type composition and metabolic profile (as well as the general nutritional and cardiovascular state of the organism). These fibers vary in their oxidative and glycolytic enzyme profiles and their corresponding susceptibility to fatigue (Enoka 1988). Thus, it can be easily understood that the histochemical changes associated with changes in contractile properties prevent paralyzed muscles from performing repeated, high-intensity contractions (Dudley-Javoroski and Shields 2008).
For example, Scott et al. (2006) found that the paralyzed muscles of subjects with SCI contract faster (+14 %), relax faster (+38 %), are weaker (−62 % of peak twitch force) and less resistant to fatigue (fatigue ratio −35 %) than non-paralyzed individuals (Scott et al. 2006). These authors also reported that compared to control subjects, those with SCI had twitch-to-tetanus ratios that were 84 % and 127 % greater in nonfatigued and fatigued conditions, respectively. They suggested that possible explanations for the increased twitch-to-tetanus ratio of paralyzed muscle are shifts in fiber type toward the fast-twitch phenotype, changes in muscle stiffness, and changes in muscle length.
Several studies have shown markers of oxidative capacity to be correlated with increased muscle fatigue in those with SCI (Gerrits et al. 2003; Rochester et al. 1995a). As mentioned above, other possibilities include the fiber-type transformation with a shift to predominantly type II fatigable fibers (Gerrits et al. 2003; Talmadge et al. 2002; Rochester et al. 1995a) and ensuing impairments in Ca2+ handling (Castro et al. 2000). However, characteristics of muscle fatigue in people with SCI seem to vary with fatiguing protocols, stimulation intensity, and the muscle being investigated. For example, during 2 min of intermittent tetanic stimulation, a decrease in muscle excitability does not appear to be a significant contributing factor to the greater fatigue after SCI and the mechanisms more likely relate to changes in excitation–contraction coupling and/or muscle oxidative capacity. Another factor inducing rapid fatigue in paralyzed muscles may be impaired muscle blood flow, which would limit oxygen and energy supply to the exercising muscle and allow accumulation of potassium and metabolic products such as lactate and inorganic phosphate.
Gerrits et al. (2000) showed that fatigue resistance was negatively correlated with time since injury. The lesser fatigue resistance at some point stabilizes, and subsequently muscle will show properties characteristic of fast fatigable muscles. At the same time, speed-related contractile properties change toward faster contractile properties in muscles. More precisely, fatigue resistance has been shown to decrease rapidly for the first 1.7 years post-injury, with smaller decreases occurring in subsequent years (Shields and Dudley-Javoroski 2006). As many rehabilitation strategies (for example, functional electrical stimulation) are dependent on the muscle being able to perform repeated contractions, a greater understanding of the processes of muscle fatigue in paralyzed muscles might allow an optimization of rehabilitation efforts.
3.1.5 Vascularization
Olive et al. (2003) found that femoral artery diameter (0.48 ± 0.06 vs 0.76 ± 0.14, SCI vs. able-bodied) and femoral artery maximal blood flow (1220 ± 240 vs 2050 ± 520 mL min−1) were lower individuals with SCI than in able-bodied individuals (Olive et al. 2003). However, these reductions are only evident for absolute values since femoral artery diameter and maximal blood flow per unit muscle volume did not differ between individuals with SCI and able-bodied individuals. These authors also found a fivefold greater half-time to peak blood flow at the beginning of exercise and a threefold greater recovery of blood flow at the end of exercise in individuals with SCI vs. able-bodied. However, they concluded that increased muscle fatigue in those with SCI was not associated with the magnitude of the muscle blood flow response to electrical stimulation. The prolonged time to peak blood flow may contribute.
3.2 Effects of Activity on Muscle Adaptations After SCI
Exercise recommendations for individuals with SCI include the use of Functional Electrical Stimulation (FES) to facilitate exercise and allow the achievement of greater exercise intensities (Jacobs and Nash 2004). Indeed, FES artificially activates paralyzed muscles and it may offset the rapid process of skeletal muscle atrophy, regional adiposity and impaired metabolic profile. Recent research has noted the utility loading the paralyzed skeletal muscles to improve musculoskeletal, metabolic and cardiovascular health in persons with SCI (Gorgey et al. 2012). Figure 3.2 presents the main adaptations to exercise in people with SCI with FES exercise.
Main effects of physical activity in people with spinal cord injury. MHC myosin heavy chain, ATP Adenosine Tri-Phosphate, SDH Succinate DeHydrogenase, right up arrow increases after training, right down arrow decreases after training, “equal to” unchanged after training. Adapted from Deley et al. (2015)
3.2.1 Electrical Stimulation (ES)
Generally speaking, ES involves application of a series of intermittent stimuli to skeletal muscles, with the main objective to trigger visible muscle contractions by activating the intramuscular nerve branches (Hultman and Sjöholm 1983). Functional electrical stimulation (FES) applies this electric current to activate the damaged or disabled neuromuscular system in a coordinated manner to generate a functionally useful movement such as leg flexion/extension for cycling or rowing (Liberson et al. 1961). It serves to generate a train of impulses that grossly imitates the neural triggers that would have normally passed through the spinal cord to the appropriate peripheral nerves below spinal cord lesion. These stimuli thus initiate action potentials in the peripheral nerves, which in turn activate muscle contractions in the associated muscles fibres (Rattay et al. 2003). Pulses, delivered via adhesive electrodes positioned on selected muscles, are defined by several characteristics such as pulse width, duration, frequency, waveform and duty cycle (the total time to complete one on/off cycle). These characteristics directly influence torque production but also the rate of muscle fatigue. Indeed, muscle fatigue may be an important factor for FES programs, limiting practical utilization (Isakov et al. 1986). The ideal stimulation pattern for activation during FES, according to the purpose (strengthening, cycling or rowing), would therefore be one that produces sufficiently high forces while minimizing fatigue [for review see Deley et al. (2015)].
3.2.2 Effects on Muscle Atrophy
Several studies have found that ES and FES exercise may reverse or limit muscle atrophy, and muscle fibers may shift their morphological characteristics more toward that similar to sedentary, able-bodied individuals. For example, Dudley et al. (1999) found that a program of electrically-induced knee extensions, performed twice weekly over an 8-week period is effective to reverse muscular atrophy of the quadriceps muscles (Dudley et al. 1999). Further, Baldi et al. (1998) found that FES cycling was more effective than unloaded ES-induced isometric contractions in preventing muscle atrophy in those with SCI less than 3 months post injury (Baldi et al. 1998). In addition, several studies have shown that FES-cycling training leads to a hypertrophy of the thigh and calf muscles (Chilibeck et al. 1999; Kjaer et al. 2001). It has also been reported that the mean cross sectional area (CSA) of muscle fibers is higher in those trained with FES-cycling or FES-rowing as compared with untrained able-bodied individuals and is similar to trained able-bodied individuals after 8 weeks of cycle training (Andersen and Henriksson 1977).
Studies have evaluated the effects of FES-cycling training on changes in spastic muscle tone with equivocal results. One study found that FES-cycling training effectively reduces spasticity (Krause et al. 2008) whereas another study reported lesser duration and frequency of spasticity, but greater spasticity intensity (Arnold et al. 1992). This could result from greater muscle strength after FES-cycling training.
Lastly, in denervated and degenerated muscle biopsies, more than 50 % of myofibers have a diameter smaller than 10 μm, whereas FES-trained subjects demonstrate more than 50 % of myofibers with a diameter larger than 30 μm. This seems to result from both an increase in size of the surviving fibers and a regeneration of new myofibers (Kern et al. 2004). In addition, myofibrils that were completely disarranged without training appear structurally normal after training. The excitation-contraction coupling apparatus is reorganized and its association with the myofibrils appears to be normal. This coordinated reorganization may provide the structural basis for the improved capacity of FES-trained muscle to respond to electrical impulses. Figure 3.3 presents the effects of 10 weeks of FES-cycling on both the overall CSA and the fiber distributions in people with SCI.
Effect of 10 weeks of FES-cycling in people with Spinal Cord Injury. The diameter of the circles is representative of the overall cross sectional area (Vastus Lateralis muscle) before (PRE) and after (POST) training (+129 %). The repartition of fiber types is also presented before and after training. Adapted from Crameri et al. (2002)
3.2.3 Effects on Myotypology
FES training appears to modify the histochemical properties of the muscle through conversion of type IIb fibers to type IIa (Mohr et al. 1997). Several studies reported that FES-cycling training converted the skeletal muscle fiber-type toward more oxidative muscle fibers (Andersen et al. 1996; Rochester et al. 1995b), with concomitant increases in concentration of oxidative enzymes and mitochondria in the paralyzed muscle groups. Moreover, Crameri et al. (2002) found an overall 129 % increase in cross-sectional area of all muscle fibers with a threefold increase in type IIx fibers and a 36 % increase in type IIa fibers after 10 weeks of FES-cycling (Crameri et al. 2002). However, MHC IIx significantly decreased (from 67.9 % to 44.1 %) over the 10 weeks, while a corresponding significant increase (from 30.4 % to 49.6 %) in MHC IIa was detected. In addition, Andersen et al. (1996) reported that 12 months of FES-cycling training induced a marked switch in MHC expression from about equal amounts of MHC IIa and MHC IIb to an almost dominance of MHC IIa: after 12 months almost all fibers (91.2 %, P < 0.05) contained only MHC IIa. The number of fibers containing only MHC IIb was 2.3 % and the fibers co-expressing MHC IIa and IIb had decreased to 4.6 %. The amount of fibers containing only MHC I never exceeded 0.5 %. On the other hand, Harridge et al. (2002) found that 4–9 weeks of chronic stimulation (2–6 h per day) does not evoke any significant change in relative MHC content, but found evidence of upregulation of the mRNA for the MHC I isoform and downregulation of the MHC IIx isoform (Harridge et al. 2002). Hence, a longer-duration training program may be necessary to cause changes at the protein level.
3.2.4 Effects on Metabolism
Several experiments have found important effects of ES training on muscle metabolism in those with SCI. For example, Gerrits et al. (2003) reported that 12 weeks of daily electrical stimulation training resulted in a significant increase of 76 ± 26 % (P < 0.05) in total SDH activity in the trained vastus lateralis muscle suggesting increased oxidative capacity (Gerrits et al. 2003). Moreover, Crameri et al. (2002) found increased hexokinase (+100 %) and citrate synthase activity (+132 %) after 10 weeks of FES-cycling (Crameri et al. 2002), and Chilibeck et al. (1999) reported increased glucose transporter (GLUT-1 and GLUT-4) protein levels in paralyzed skeletal muscles of individuals with SCI after 8 weeks of FES-cycling (Chilibeck et al. 1999). Hence, it is not surprising that 12 months of FES-cycling three times a week induced increases in glycolytic enzymes (hexokinase (HK): +150 %, lactate dehydrogenase (LDH): +40 %, citrate synthase (CS): +100 % and 3-hydroxyacyl-CoA dehydrogenase (HAD): +70 %) activities (Kjaer et al. 2001). After reducing the amount of training to once per week, HK, LDH and CS activities remained elevated above basal levels, whereas HAD returned to pretraining levels. Hence, most improvements in glycolytic and mitochondrial oxidative enzyme activity induced by long-term training can be maintained in SCI individuals even with a marked reduction in training frequency.
3.2.5 Effects on Contractile Properties
Muscle strength increases after FES-strengthening protocols (Dudley et al. 1999). Increases in the maximal workload or total power output at the end of exercise tests, as well as decreases in muscle fatigue have also been reported (Shields and Dudley-Javoroski 2006; Dudley et al. 1999; Ingjer 1979). For example, Shields and Dudley-Javoroski (2006) reported greater torque, torque-time integral and fatigue-index of up to 50 % in trained versus untrained limbs (2 years on plantar flexors muscles) (Shields and Dudley-Javoroski 2006). Moreover, Wheeler et al. (2002) found a 25 % enhancement in the distance rowed during an incremental test after 12 weeks of FES-rowing (Wheeler et al. 2002) and Crameri et al. (2002) found that the total work performed during the ergometer testing increased from 51.8 ± 39.3 to 112.9 ± 33.5 kJ after FES-cycling training (Crameri et al. 2002). Contractile properties also increase towards values for able-bodied. Muscles contract slower as shown by a 45 % increase in torque rise time after 2 years of ES training (Shields and Dudley-Javoroski 2006). It has also been suggested that half-relaxation time return to able-bodied values after 4 weeks of ES training (Rochester et al. 1995a).
3.2.6 Effects on Vascularization
Crameri et al. (2002) reported an increase in capillarization after after 10 weeks of FES-cycling. Moreover, the capillary/fiber ratio in those with SCI trained with FES-cycling or FES-rowing is only than in trained able-bodied individuals (Ingjer 1979). Interestingly, FES-rowing may result in a ratio higher than both the trained SCI cyclists and able-bodied subjects. These changes in capillarity are reflected in blood flow. Taylor et al. (1993) found that training with the Odstock functional electrical standing system during 3 months produced an average increase of 115 % in thigh blood flow so that these values were similar to those of the control group (Taylor et al. 1993). This increased leg blood flow in SCI may also be due, in part to an endothelin-1 pathway (Thijssen et al. 2007). The same authors also demonstrated that 4 weeks of arm combined with ES-induced leg exercise leads to vascular adaptations in the exercised tissues (thigh) but not in non-stimulated passive tissue (calf) (Thijssen et al. 2005). Similarly, Gerrits et al. (2001) reported a 29 % increase in resting femoral artery blood flow after 4 weeks of FES-cycling (Gerrits et al. 2001). These improvements seem to be directly related to the training program being performed. Indeed, Sabatier et al. (2006), who did not find any modification in femoral arterial diameter and in blood flow after 18 weeks of low volume resistance training, concluded that the volume of training was probably insufficient to evoke any changes (Sabatier et al. 2006).
References
Alaimo MA, Smith JL, Roy RR, Edgerton VR (1984) EMG activity of slow and fast ankle extensors following spinal cord transection. J Appl Physiol 56:1608–1613
Andersen P, Henriksson J (1977) Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol 270:677–690
Andersen JL, Mohr T, Biering-Sørensen F, Galbo H, Kjær M (1996) Myosin heavy chain isoform transformation in single fibres from m. vastus lateralis in spinal cord injured individuals: effects of long-term functional electrical stimulation (FES). Pflügers Arch Eur J Physiol 431:513–518
Arnold PB, McVey PP, Farrell WJ, Deurloo TM, Grasso AR (1992) Functional electric stimulation: its efficacy and safety in improving pulmonary function and musculoskeletal fitness. Arch Phys Med Rehabil 73:665–668
Baldi JC, Jackson RD, Moraille R, Mysiw WJ (1998) Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord 36:463–469
Biering-Sorensen B, Kristensen IB, Kjaer M, Biering-Sorensen F (2009) Muscle after spinal cord injury. Muscle Nerve 40:499–519
Burnham R, Martin T, Stein R, Bell G, MacLean I, Steadward R (1997) Skeletal muscle fibre type transformation following spinal cord injury. Spinal Cord 35:86–91
Castro MJ, Apple DF, Hillegass EA, Dudley GA (1999) Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol 80:373–378
Castro MJ, Apple DF, Melton-Rogers S, Dudley GA (2000) Muscle fiber type-specific myofibrillar Ca(2+) ATPase activity after spinal cord injury. Muscle Nerve 23:119–121
Chilibeck PD, Bell G, Jeon J, Weiss CB, Murdoch G, MacLean I, Ryan E, Burnham R (1999) Functional electrical stimulation exercise increases GLUT-1 and GLUT-4 in paralyzed skeletal muscle. Metabolism 48:1409–1413
Crameri RM, Weston A, Climstein M, Davis GM, Sutton JR (2002) Effects of electrical stimulation-induced leg training on skeletal muscle adaptability in spinal cord injury. Scand J Med Sci Sport 12:316–322
Deley G, Denuziller J, Babault N (2015) Functional electrical stimulation: cardiorespiratory adaptations and applications for training in paraplegia. Sports Med 45:71–82
Ditor DS, Hamilton S, Tarnopolsky MA, Green HJ, Craven BC, Parise G, Hicks AL (2004) Na+,K+-ATPase concentration and fiber type distribution after spinal cord injury. Muscle Nerve 29:38–45
Dudley GA, Castro MJ, Rogers S, Apple DF (1999) A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol 80:394–396
Dudley-Javoroski S, Shields RK (2008) Muscle and bone plasticity after spinal cord injury: review of adaptations to disuse and to electrical muscle stimulation. J Rehabil Res Dev 45:283–296
Enoka RM (1988) Muscle strength and its development. New perspectives. Sport Med 6:146–168
Gerrits HL, De Haan A, Sargeant AJ, Dallmeijer A, Hopman MTE (2000) Altered contractile properties of the quadriceps muscle in people with spinal cord injury following functional electrical stimulated cycle training. Spinal Cord 38(4):214–224
Gerrits HL, de Haan A, Sargeant AJ, van Langen H, Hopman MT (2001) Peripheral vascular changes after electrically stimulated cycle training in people with spinal cord injury. Arch Phys Med Rehabil 82:832–839
Gerrits HL, Hopman MTE, Offringa C, Engelen BGM, Sargeant AJ, Jones DA, Haan A (2003) Variability in fibre properties in paralysed human quadriceps muscles and effects of training. Pflügers Arch Eur J Physiol 445:734–740
Gordon T, Mao J (1994) Muscle atrophy and procedures for training after spinal cord injury. Phys Ther 74:50–60
Gordon T, Pattullo MC (1993) Plasticity of muscle fiber and motor unit types. Exerc Sport Sci Rev 21:331–362
Gorgey AS, Dudley GA (2007) Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 45:304–309
Gorgey AS, Mather KJ, Cupp HR, Gater DR (2012) Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc 44:165–174
Harridge SDR, Andersen JL, Hartkopp A, Zhou S, Biering-Sørensen F, Sandri C, Kjaer M (2002) Training by low-frequency stimulation of tibialis anterior in spinal cord-injured men. Muscle Nerve 25:685–694
Hultman E, Sjöholm H (1983) Energy metabolism and contraction force of human skeletal muscle in situ during electrical stimulation. J Physiol 345:525–532
Ingjer F (1979) Effects of endurance training on muscle fibre ATP-ase activity, capillary supply and mitochondrial content in man. J Physiol 294:419–32
Isakov E, Mizrahi J, Najenson T (1986) Biomechanical and physiological evaluation of FES-activated paraplegic patients. J Rehabil Res Dev 23:9–19
Jacobs PL, Nash MS (2004) Exercise recommendations for individuals with spinal cord injury. Sports Med 34:727–751
Kern H, Boncompagni S, Rossini K, Mayr W, Fanò G, Zanin ME, Podhorska-Okolow M, Protasi F, Carraro U (2004) Long-term denervation in humans causes degeneration of both contractile and excitation-contraction coupling apparatus, which is reversible by functional electrical stimulation (FES): a role for myofiber regeneration?. J Neuropathol Exp Neurol 63:919–931
Kjaer M, Mohr T, Biering-Sørensen F, Bangsbo J (2001) Muscle enzyme adaptation to training and tapering-off in spinal-cord-injured humans. Eur J Appl Physiol 84:482–486
Krause P, Szecsi J, Straube A (2008) Changes in spastic muscle tone increase in patients with spinal cord injury using functional electrical stimulation and passive leg movements. Clin Rehabil 22:627–634
Liberson WT, Holmquest HJ, Scot D, Dow M (1961) Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch Phys Med Rehabil 42:101–105
Lotta S, Scelsi R, Alfonsi E, Saitta A, Nicolotti D, Epifani P, Carraro U (1991) Morphometric and neurophysiological analysis of skeletal muscle in paraplegic patients with traumatic cord lesion. Paraplegia 29:247–252
McCully KK, Mulcahy TK, Ryan TE, Zhao Q (2011) Skeletal muscle metabolism in individuals with spinal cord injury. J Appl Physiol 111:143–148
Mohr T, Andersen JL, Biering-Sørensen F, Galbo H, Bangsbo J, Wagner A, Kjaer M (1997) Long-term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord 35(1)
Olive JL, Dudley GA, McCully KK (2003) Vascular remodeling after spinal cord injury. Med Sci Sports Exerc 35:901–907
Peckham PH, Mortimer JT, Marsolais EB (1976) Upper and lower motor neuron lesions in the upper extremity muscles of tetraplegics. Paraplegia 14:115–121
Pilegaard H, Mohr T, Kjaer M, Juel C (1998) Lactate/H+ transport in skeletal muscle from spinal-cord-injured patients. Scand J Med Sci Sports 8:98–101
Rattay F, Resatz S, Lutter P, Minassian K, Jilge B, Dimitrijevic MR (2003) Mechanisms of electrical stimulation with neural prostheses. Neuromodulation 6:42–56
Rochester L, Barron MJ, Chandler CS, Sutton RA, Miller S, Johnson MA (1995a) Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects. 2. Morphological and histochemical properties. Paraplegia 33:514–522
Rochester L, Chandler CS, Johnson MA, Sutton RA, Miller S (1995b) Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects. 1. Contractile properties. Paraplegia 33:437–449
Roy RR, Baldwin KM, Edgerton VR (1991) The plasticity of skeletal muscle: effects of neuromuscular activity. Exerc Sport Sci Rev 19:269–312
Sabatier MJ, Stoner L, Mahoney ET, Black C, Elder C, Dudley GA, McCully K (2006) Electrically stimulated resistance training in SCI individuals increases muscle fatigue resistance but not femoral artery size or blood flow. Spinal Cord 44:227–233
Scott WB, Lee SCK, Johnston TE, Binkley J, Binder-Macleod SA (2006) Contractile properties and the force-frequency relationship of the paralyzed human quadriceps femoris muscle. Phys Ther 86:788–799
Shields RK, Dudley-Javoroski S (2006) Musculoskeletal plasticity after acute spinal cord injury: effects of long-term neuromuscular electrical stimulation training. J. Neurophysiol 95:2380–2390
Talmadge RJ, Castro MJ, Apple DF, Dudley GA (2002) Phenotypic adaptations in human muscle fibers 6 and 24 wk after spinal cord injury. J Appl Physiol 92:147–154
Taylor PN, Ewins DJ, Fox B, Grundy D, Swain ID (1993) Limb blood flow, cardiac output and quadriceps muscle bulk following spinal cord injury and the effect of training for the Odstock functional electrical stimulation standing system. Paraplegia 31:303–310
Thijssen DHJ, Heesterbeek P, Van Kuppevelt DJM, Duysens J, Hopman MTE (2005) Local vascular adaptations after hybrid training in spinal cord-injured subjects. Med Sci Sports Exerc 37:1112–1118
Thijssen DHJ, Ellenkamp R, Kooijman M, Pickkers P, Rongen GA, Hopman MTE, Smits P (2007) A causal role for endothelin-1 in the vascular adaptation to skeletal muscle deconditioning in spinal cord injury. Arterioscler Thromb Vasc Biol 27:325–331
Wang H, Hiatt WR, Barstow TJ, Brass EP (1999) Relationships between muscle mitochondrial DNA content, mitochondrial enzyme activity and oxidative capacity in man: alterations with disease. Eur J Appl Physiol Occup Physiol 80:22–27
Wheeler GD, Andrews B, Lederer R, Davoodi R, Natho K, Weiss C, Jeon J, Bhambhani Y, Steadward RD (2002) Functional electric stimulation–assisted rowing: increasing cardiovascular fitness through functional electric stimulation rowing training in persons with spinal cord injury. Arch Phys Med Rehabil 83:1093–1099
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 The American Physiological Society
About this chapter
Cite this chapter
Deley, G. (2016). Role of Activity in Defining Metabolic and Contractile Adaptations After SCI. In: Taylor, J. (eds) The Physiology of Exercise in Spinal Cord Injury. Physiology in Health and Disease. Springer, Boston, MA. https://doi.org/10.1007/978-1-4939-6664-6_3
Download citation
DOI: https://doi.org/10.1007/978-1-4939-6664-6_3
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4939-6662-2
Online ISBN: 978-1-4939-6664-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)