Abstract
Hypertension is common, difficult to diagnose, and poorly controlled in dialysis patients. There remains controversy on the diagnosis, treatment, and prognosis of hypertension in dialysis. This chapter describes the latest evidence on epidemiology, diagnosis, management, and prognosis of hypertension in dialysis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Dialysis
- Hypertension
- Interdialytic hypertension
- Intradialytic hypertension
- Ambulatory blood pressure
- Home blood pressure
- Dry weight
Introduction
Hypertension is both a cause and a consequence of the spectrum of chronic kidney disease (CKD) to end stage renal disease (ESRD ). Despite steady improvement, mortality remains high in the dialysis population with a 5-year survival rate only at 40 % [1], which compares poorly to some advanced cancers [2]. Cardiovascular events remain the leading cause of death in dialysis [3], with hypertension an important contributor. Thus the diagnosis and management of hypertension in dialysis is a vital topic to all dialysis patients and providers. This chapter will review the epidemiology, diagnosis, treatment, and prognosis of hypertension in the dialysis population.
Epidemiology
Blood pressure in the hemodialysis (HD) patient is a moving target that is influenced by when and where it is measured, complicating diagnosis. The epidemiology of hypertension in HD therefore can vary depending not only on the BP cutoffs employed but also on when and where BP is measured: in the HD clinic before or after dialysis (called peridialytic BP) versus outside the dialysis unit using ambulatory BP monitoring (ABPM) or home BP monitoring during the interdialytic period . Table 7.1 summarizes the various methods of assessing BP in HD.
Epidemiology Using Peridialytic BP Measurements
Multiple observational studies using peridialytic BP have found a high prevalence of hypertension in HD ranging from 62 to 86 %; notably, these studies used different threshold BP values varying from 140/90 to 160/90 mmHg and variously included antihypertensive drug use in their definitions of hypertension [4–7]. More recent analyses of randomized trials have found similar rates of hypertension.
The Hemodialysis (HEMO) Study was a landmark large multicenter trial of HD dose and dialyzer flux on survival [8] that recruited clinically stable HD patients [9]. An analysis of the baseline characteristics of the first 1238 subjects randomized into the HEMO Study between March 1995 and April 1998 found 72 % of the cohort to be hypertensive, defined as peridialytic BP ≥140/90 mmHg during the baseline HD session [10]. This rate of hypertension was despite 74.2 % of the cohort receiving antihypertensive medications, with a median of 1.0 drug per subject.
The BP data from an even larger randomized double blind and placebo controlled trial of sodium ferric gluconate was examined in detail with similar results [11]. The original trial enrolled 2535 clinically stable HD patients between August 1999 and October 2000 [12]. Using a definition of hypertension as pre-HD BP >150/85 averaged over 1 week or the use of antihypertensive medications, an analysis of baseline data found a prevalence of 86 % for hypertension in this cohort [11]. Within the hypertensive subjects only 30 % were controlled, while 12 % weren’t treated pharmacologically and 58 % were treated but still uncontrolled [11], which is similar to previous reports [6].
Epidemiology Using Ambulatory BP Measurements
A recent single center study of 369 prevalent and clinically stable HD patients employed 44 h interdialytic ABPM and found a prevalence for hypertension at 86 % using a definition of hypertension as average ambulatory BP of ≥135/85 mmHg or antihypertensive drug use; [13] this prevalence is similar to prior small studies of ABPM in HD [14]. In the cohort of 369 patients, hypertension was treated with medications in 89 % of patients but was controlled adequately in only 38 % of patients [13].
Epidemiology in Peritoneal Dialysis Patients
It has been suggested that peritoneal dialysis (PD ) controls hypertension better than HD, with a single center study of 56 prevalent and clinically stable PD patients finding that only 9 % of the cohort was hypertensive with BP >140/90 mmHg by standardized auscultated BP as compared to a hypertension prevalence of 56 % in the same center’s HD unit [15]. Similarly, control of BP was compared in the retrospective Peritoneal Dialysis Core Indicators Study in the mid-1990s that found among the 926 PD patients with BP data only 35 % were hypertensive with BP >150/90 mmHg with the cohort having an average BP of 139/80 as compared to a contemporaneous cohort of HD patients whose average pre-HD BP was 151/79 and post-HD BP 137/74 mmHg [16]. A larger study using United States Renal Data System (USRDS) data from the Dialysis Morbidity and Mortality Wave 2 study in the late 1990s found that from 1034 PD patients 54 % had SBP >140 mmHg while on a mean of 1.6 antihypertensive medications [17].
While the above reported prevalence values for hypertension in PD patients are less than that generally reported for HD, this is not a universal finding. A prospective study of 504 prevalent and clinically stable PD patients found a hypertension prevalence of 88 % defined as BP >140/90 mmHg or use of antihypertensive medications, and of the hypertensive patients only 16 % were adequately controlled [18]. Additionally 24 h ABPM was performed, and of the 414 adequate examinations hypertension was present in 69 % based on BP load >30 %, with load defined as the percent of ambulatory BP readings >140/90 mmHg during the day or >120/80 mmHg at night. Also utilizing ABPM, a study of 22 HD and 24 PD patients that were well matched for major clinical characteristics found no significant difference in either daytime or nighttime BP between the two groups [19]. Thus while there is a suggestion that PD may control hypertension better than HD there is no conclusive evidence that this is the case .
Diagnosis
ABPM is the accepted gold standard for diagnosing hypertension in the general population and in the ESRD population on dialysis [20–23]. ABPM not only permits the diagnosis of nocturnal hypertension, it is also superior to peridialytic BP measurements in correlating end organ damage manifest as left ventricular hypertrophy (LVH ) [24] and predicting the outcome of mortality [25]. The proper ABPM technique includes employing a validated monitor [26] to measure BP every 20 min during the day from 6 AM to 10 PM and then every 30 min at night from 10 PM to 6 AM [27]. This prolonged interval of measurement permits observation of the full change in BP during the interdialytic period, where SBP increases an average of 2.5 mmHg every 10 h [28, 29]. Unfortunately, ABPM is also cumbersome to use, especially over 44 continuous hours, so it remains a research technique in ESRD.
As ABPM is not used routinely, more convenient methods of measuring BP and diagnosing hypertension must be employed. Since BP changes both during the HD session and during the interdialytic interval there remains uncertainty about how best to diagnose and manage hypertension in the HD population, which may contribute to both undertreatment [14, 30] and overtreatment of hypertension [31]. Further complicating matters, HD patients have significant seasonal variability in BP with lower BP during the summer and higher during the winter [32], possibly due to temperature mediated vasodilation or sweat induced volume losses.
Classically, the peridialytic BP measures taken before and after an HD session have been used to diagnose and manage hypertension, and while there are no randomized clinical trials to guide goal BP recommendations in ESRD, longstanding professional guidelines have employed peridialytic BP values . The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines recommend to target pre-HD BP <140/90 mmHg and post-HD BP <130/80 mmHg [33]. Unfortunately, making treatment decisions based on peridialytic BP can be associated with adverse outcomes, as shown by a study of 11 HD units in London, England that found those HD units with more patients reaching the post-HD BP goal had significantly more episodes of symptomatic hypotension requiring saline infusion [34]. Peridialytic BP is highly variable such that the variability within a given patient over time is similar to the variability between patients [35], possibly due in part to these measurements often being made without attention to technique [36]. While peridialytic BP does have a statistically significant relationship to the gold standard interdialytic ambulatory BP [37], it is because of the inherent variability in peridialytic measurements that a meta-analysis of 18 studies comparing peridialytic BP and interdialytic ABPM found very wide limits of agreement between the techniques such that peridialytic BP provides a very imprecise estimate of interdialytic BP [38]. In the meta-analysis the limits of agreement between pre-HD SBP and interdialytic ambulatory SBP ranged from +41.7 mmHg to −25.2 mmHg while the limits of agreement for post-HD SBP were similarly broad, which illustrates the reduced clinical utility of a diagnosis of hypertension or normotension based on peridialytic BP [38].
A readily available alternative to peridialytic BP is to use all the BP measurements made during a single mid-week HD session to calculate a median BP, which is easier to calculate at the bedside compared to mean BP. A study of 150 chronic HD patients found that median intradialytic BP had the best reproducibility and was superior to either pre- or post-HD BP or their average for predicting 44 h interdialytic ambulatory BP [39]. In this study median intradialytic SBP >140 mmHg during a mid-week HD session had an 80 % sensitivity and specificity for diagnosing hypertension by gold standard ABPM [39]. Additionally, median intradialytic BP has been shown to change in response to interventional reduction in dry weight to reduce BP [40], further supporting the clinical usefulness of this measure.
Home BP monitoring is the third alternative to clinically inconvenient gold standard ABPM, as home BP monitoring is the recommended method to routinely diagnose and manage BP in general hypertension populations by both the American Heart Association [41] and the European Society of Hypertension [42] and its use is feasible and practical both in patients with CKD and ESRD [43, 44]. Home BP monitoring is superior to peridialytic BP measurements by every methodological and clinical standard. This includes superior correlation with gold standard ABPM [45], week to week reproducibility [46], the ability to reflect BP changes from interventional probing of dry weight [46], correlation to the end organ damage of LVH [24, 47], and predicting outcomes including cardiovascular events [48] and mortality [48, 49]. Table 7.2 summarizes the advantages of home BP monitoring .
Two small randomized trials have found home BP monitoring to be beneficial versus usual care for management of BP in HD patients. The first randomized 34 patients to home BP monitoring plus usual care versus usual care only over 12 weeks and found that the home BP group had significantly lower BP at the end of the study [50]. A subsequent trial of 65 HD patients randomized participants to usual hypertension care based on pre-HD BP versus open label monthly home BP monitoring for 6 months [51]. At the end of the trial the home BP monitoring group had a significant reduction in ambulatory SBP both from baseline and versus the usual care group, which had no change in ambulatory SBP from baseline. However the primary endpoint of reduction in echocardiographic LVH was no different between groups, possibly due to lack of power and variability in the timing of the echocardiograms relative to the HD schedule [51].
As with ABPM , home BP increases between HD sessions, at an average rate of 4 mmHg every 10 h [52], so it is important to adequately sample home BP at spaced intervals between HD sessions. Randomized trials have used protocols of home BP monitoring performed twice daily over 4–7 days once per month [51, 53], which is a reasonable regimen for routine clinical use and decision making. Measurements performed more than once per month may be needed in more unstable patients including those recently hospitalized. Table 7.3 summarizes the suggested method of home BP monitoring.
There are no randomized trials comparing goal BP levels in the dialysis population using any of the available BP methods including peridialytic BP, intradialytic BP, home BP, or interdialytic ambulatory BP. However, the American Heart Association defines hypertension as home BP >135/85 mmHg on average for the general population [41], so a goal interdialytic home BP target of ≤140/90 mmHg is reasonable [54], as was used in a recent large randomized trial of BP control in HD [53].
Intradialytic Hypertension
While the focus of diagnosis and treatment of hypertension in chronic HD is on interdialytic BP between HD sessions, the case of intradialytic hypertension merits special mention. BP normally declines during the HD, but approximately 5–15 % of chronic HD patients have a paradoxical rise in BP during the HD session [55]. Intradialytic hypertension has been described variously and there is currently no uniformly recognized definition. Definitions have included (1) a change in SBP or mean arterial pressure from pre-HD to post-HD over various thresholds from >0 mmHg to ≥10 mmHg change [56, 57], (2) a positive slope after regression of all intradialytic SBP values [58], or (3) BP increase during or immediately following HD resulting in post-HD BP >130/80 mmHg [55].
Recently it has been recognized that intradialytic hypertension is associated with worse outcomes. Inrig and colleagues performed a secondary analysis of a randomized trial in 443 prevalent HD subjects and found intradialytic hypertension to be significantly associated with greater mortality at 6 months [56]. Similarly, in a subsequent observational study of a cohort of 1748 incident HD patients Inrig and colleagues found 2-year survival to be significantly decreased for each 10 mmHg increase in SBP from pre-HD to post-HD BP, however this relationship was limited to those whose pre-HD SBP was <120 mmHg [59]. Most recently, a prospective cohort study of 115 prevalent HD patients found an average pre-HD to post-HD rise in SBP of >5 mmHg to significantly predict all-cause and cardiovascular mortality [60].
Intradialytic hypertension has been associated with interdialytic hypertension as measured by 44 h ABPM [57, 58], so it is not surprising that the same mechanisms implicated in causing interdialytic hypertension between HD sessions have also been implicated in causing intradialytic hypertension during the HD session [55], but the preponderance of evidence currently points to volume overload and endothelial dysfunction. Markers of volume overload such as increased cardiothoracic ratio have been associated with intradialytic hypertension [60], but most importantly interventional trials have shown volume removal though dry weight reduction improves intradialytic hypertension. An early study from the mid-1990s included seven patients with intradialytic hypertension and found them all to have marked cardiac dilation and to be very hypertensive with mean pre-HD BP 172/99 mmHg despite medications [61]. All subjects had subsequent reduction in dry weight with an average weight loss of 6.7 kg that was associated with an improvement in pre-HD BP by 46/21 mmHg despite discontinuation of all BP meds. More recently a secondary analysis of the Dry Weight Reduction in Hypertensive Hemodialysis Patients (DRIP) trial [62] regressed the intradialytic BP values for the 150 trial subjects and found that the quintile of subjects with the greatest reduction in dry weight, more than 0.94 kg reduction after the first 4 weeks of the trial, also had the most positive BP slope at baseline as they were the only quintile with intradialytic hypertension by this definition [58]. Importantly, after dry weight reduction this same quintile had reduction in BP slopes to finish the trial, meaning their intradialytic hypertension had resolved such that their BP slopes were similar to the other subjects. Thus intradialytic hypertension appears to be a marker of volume overload that is amenable to dry weight reduction .
Additionally, endothelial dysfunction has been identified as an important mediator as there is evidence for both a rise in endothelin-1 levels [63] and a decrease in nitric oxide during HD [64] in patients with intradialytic hypertension. The contribution of endothelial function was investigated in 25 HD patients recruited in an 8-week pilot study with a before–after design using carvedilol [65], which has been shown to block endothelin-1 release in vitro [66]. Subjects were administered carvedilol up to a dose of 50 mg twice a day, and while endothelin-1 levels were unchanged on carvedilol, flow mediated dilation significantly improved [65]. Of clinical importance, the frequency of intradialytic hypertension was significantly reduced from 77 % of HD sessions down to 28 % of sessions and average 44 h interdialytic ambulatory SBP was also reduced from 155 to 148 mmHg with carvedilol treatment.
Thus based on the available evidence, a renewed focus on addressing volume overload should be a priority for those patients with a paradoxical rise in BP on HD, and specifically targeting endothelial dysfunction with agents such as carvedilol can also be considered. The features of intradialytic hypertension are summarized in Table 7.4.
Treatment
As an introduction to the specifics of treating hypertension on dialysis, it’s instructive to briefly review the major modifiable causes of hypertension in this population with a focus on those etiologies that can currently be addressed. As both a cause and a consequence of kidney disease, the pathophysiology of hypertension in CKD not only shares commonalities with the general hypertensive population but also has causes that are unique to kidney disease and its treatment. Table 7.5 summarizes the major modifiable causes of hypertension in ESRD.
Risk Factors in Common with the General Population
Patients with CKD often carry a burden of pre-existing primary hypertension prior to the recognition of their kidney disease. Additionally, risk factors in the general hypertensive population are similarly present in the CKD population including obesity, excessive salt intake, alcohol consumption, and physical inactivity. Obstructive sleep apnea (OSA ) is an additional comorbidity that is important to consider in more detail.
In the general population OSA frequently coexists with hypertension [67–69], with hypopnea leading to hypoxemia and ultimately to sympathetic activation. OSA is strongly linked with resistant hypertension as the presence of OSA is a risk factor for resistant hypertension and the severity of OSA correlates with the severity of hypertension [70–72]. Given this link it is important to note that OSA is a very common in the setting of CKD with the prevalence increasing with declining renal function [73], culminating in a prevalence over 50 % for those patients on dialysis [73, 74]. The association between OSA and resistant hypertension is similarly strong in ESRD as a recent cohort study of subjects with advanced CKD including 75 subjects on HD and 20 on PD found a sevenfold increased risk of resistant hypertension in those dialysis patients with severe OSA [75]. Interestingly, there is a growing recognition that OSA itself is caused or exacerbated by volume overload that leads to parapharyngeal edema, which worsens at bedtime in the recumbent position, both in patients without CKD [69] and in those with ESRD [76, 77].
While most studies of continuous positive airway pressure for OSA in the non-CKD population find significant improvement in BP, this improvement is typically modest compared to medication [78], with CPAP use yielding a 1.7 mmHg improvement in mean 24 h ambulatory BP [79].
Causes of Hypertension Unique to End Stage Renal Disease
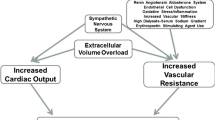
The two classic mechanisms felt to be responsible for hypertension in the “renoprival” state of ESRD are volume overload and an inappropriately activated renin–angiotensin–aldosterone system (RAAS ) [80]. Sodium loading has long been clinically recognized as a major and essential contributor to hypertension both in those with normal renal function [81] and in the setting of kidney disease. As the glomerular filtration rate (GFR) declines less sodium is filtered leading to sodium retention and to an expanded extracellular fluid volume. The increased plasma volume leads to increased cardiac output and then to increased total peripheral resistance, whereby normal renal autoregulation would lead to a pressure natriuresis and normalization of BP [82], however this natriuresis is incomplete or absent in advanced CKD and ESRD and the increased total peripheral resistance persists. Randomized trial evidence confirms that ultrafiltration for volume removal improves BP on HD [62].
The other classically recognized contributor is an inappropriately activated RAAS [83], possibly provoked by renal ischemia in patients with renovascular disease or by regional ischemia due to renal fibrosis. Unsurprisingly, angiotensin converting enzyme inhibitor (ACEi ) therapy has been shown to be effective at reducing BP in dialysis patients [84, 85].
Among the novel mechanisms of hypertension in advanced kidney disease, sympathetic overactivity is now widely accepted as a contributor. Increased catecholamine levels [86] and increased catecholamine sensitivity [87] in CKD were both demonstrated in the 1980s, and increased catecholamine levels have been shown to predict cardiovascular events and mortality in chronic HD patients [88]. Unidentified uremic toxins were originally thought to provoke this sympathetic overactivity, however Converse and colleagues implicated the diseased kidneys themselves via experiments wherein they measured muscle sympathetic nerve activity in three groups of subjects: those on chronic HD with their native kidneys, those on chronic HD status post bilateral nephrectomy, and normal controls [89]. They found increased sympathetic activity and higher BP in those chronic HD patients still with their native kidneys but those subjects who were surgically anephric have sympathetic nerve activity and BP similar to the normal controls. Uremic toxins don’t appear to be the cause of the increased renal afferent nerve signals that increase sympathetic activity, as demonstrated by studies of patients who have normally functioning renal transplants and still retain their native kidneys; [90] but renal ischemia of the native kidneys is a likely contributor [91].
Lastly, medications contribute to hypertension in ESRD. Conventional medications such as over-the-counter nonsteroidal anti-inflammatory drugs and decongestants can exacerbate hypertension, however erythropoiesis stimulating agents (ESA ) are commonly prescribed for the anemia of CKD and resultant hypertension has been recognized since the early days of ESA use in ESRD [92]. The incidence of hypertension provoked by ESA administration is associated with the ESA dose but is independent of red blood cell mass or viscosity [93, 94]. While the exact mechanism of how ESA use causes hypertension is unknown, the current evidence suggests that it is mediated via vasoconstrictor effects, likely through increased levels of endothelin-1 or increased vasoconstrictive response to that peptide [95–97].
Up to 30 % of dialysis patients develop hypertension or require an adjustment in antihypertensive medications with ESA use [98, 99], while the rise in BP with ESA use typically ranges from 5 to 8 mmHg in SBP and 4–6 mmHg in DBP [100]. The rise in BP with ESA administration is more likely in those with baseline hypertension [101] or a family history of hypertension [102]. There is unfortunately a paucity of evidence to guide the prevention of ESA induced hypertension but recommended strategies include changing to subcutaneous administration, reducing the goal hemoglobin level in those who are unresponsive to ESA therapy, minimizing the ESA dose by starting low and increasing slowly, and avoiding ESA use entirely [54].
Volume Control
The focus of nonpharmacologic treatment of hypertension on dialysis is to treat volume overload through complementary strategies both to reduce sodium intake by dietary sodium restriction and individualization of the dialysate sodium while also augmenting sodium removal by dry weight reduction, providing adequate time on dialysis, and considering frequent dialysis. Table 7.6 summarizes the nonpharmacologic treatment of hypertension in dialysis. The archetype for this management of hypertension on dialysis is reported by Charra and colleagues from Tassin, France where patients are dialyzed for extended hours on a low sodium dialysate and low sodium diet is emphasized to the point where low sodium bread is provided to the patients [103]. They report excellent control of BP despite antihypertensive medication use at only 1–2 % [104], as well as low mortality with a 5-year survival rate reported at 87 % [105], which is more than twice the current reported 5-year survival rate in the USA [1]. More recently a trial of low sodium diet and dry weight reduction in 19 hypertensive HD patients with a before–after design found this combined strategy reduced echocardiographic left ventricular hypertrophy [106]. Similar results have been reported in PD from a single center where all 47 of the center’s hypertensive patients had their antihypertensive medications withdrawn and BP was subsequently successfully controlled in 37 patients with a combination of strict low sodium diet and added ultrafiltration [107].
Dry Weight Reduction
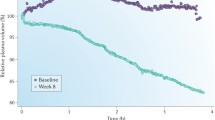
Malignant hypertension was common in ESRD prior to the advent of chronic HD and since those earliest days ultrafiltration has been recognized as an effective means of BP control in ESRD [108], including for the very first chronic HD patient in the USA, Clyde Shields, under the care of Dr. Belding Scribner [109]. Only recently has the randomized controlled DRIP trial of dry weight reduction definitively confirmed those original observations [62]. The DRIP trial recruited 150 chronic and stable HD patients with hypertension confirmed by 44 h interdialytic ABPM despite being on an average of 2.6 antihypertensive medications who were then randomized in a two to one ratio to intervention versus usual care for the 8-week trial. All subjects had their antihypertensive medications and their prescribed time on HD kept stable, and all were visited by a study physician on each HD session during the trial. The intervention group received progressive reduction in dry weight by at least 0.2 kg each HD session until they had symptoms of hypovolemia. Compared to the control group at 8 weeks, the intervention group had 1 kg of weight reduction and average 44 h interdialytic ambulatory BP improved by 6.6/3.3 mmHg [62]. Notably, by design those in the intervention group necessarily had to have symptoms of hypovolemia before dry weight reduction was stopped, but despite this requirement there was no change in any domain of the Kidney Disease Quality of Life questionnaire during the trial .
Management of Dry Weight
Unfortunately there is no single universally accepted definition of dry weight, but a reasonable standard is that used by Sinha and Agarwal who define the dry weight as the lowest tolerated post-HD weight achieved via gradual change in post-HD weight at which there are minimal signs or symptoms of either hypovolemia or hypervolemia [110]. Thus, achieving and maintaining an adequately low dry weight is a hands-on and iterative process that requires attention to details beyond only the prescribed dry weight [111], including adherence to a low sodium diet, minimization of dialysate sodium content, providing adequate time on HD, and consideration of more frequent dialysis.
When deciding whether to adjust the dry weight prescription, the first step is the assessment for volume overload. Unfortunately, while the routine clinical exam performs well at detecting acute or massive volume overload, it performs poorly at detecting subtle and chronic volume overload [110]. This is exemplified by a cross sectional study of 150 chronic HD patients that found the presence of pedal edema to have no correlation with putative objective markers of volume overload including brain natriuretic peptide, echocardiographic inferior vena cava diameter, or relative plasma volume slope [112]. As another example, it is important to consider that all the hypertensive subjects of the DRIP trial were at their clinical dry weight as determined by their primary nephrologist to start the trial, yet the subjects of the intervention group had their dry weight successfully reduced, which resulted in a clinically significant improvement in 44 h ambulatory BP [62]. This further illustrates the difficulty in detecting subtle volume overload that if removed by means of dry weight reduction will improve BP.
A number of experimental objective measures of volume status have been studied including natriuretic peptides , inferior vena cava diameter, relative plasma volume monitoring, and bioelectrical impedance analysis [113]. The latter two have the most supporting evidence with a secondary analysis of the DRIP trial showing that baseline relative plasma volume monitoring identified the most volume overloaded subjects, who subsequently had the largest average reduction in weight at 1.5 kg and the largest improvement in ambulatory SBP at 12.6 mmHg [114]. Most recently, a randomized trial of bioelectrical impedance analysis to guide dry weight management in a cohort of largely normotensive HD subjects found a significant improvement in LVH as well as improvement in peridialytic BP despite reductions in antihypertensive drug use for the intervention group [115].
However, these objective measures of volume status remain investigational and remain to be adequately validated. Therefore the onus is on the treating nephrologist to have a high index of suspicion for occult volume overload. Signs that should prompt consideration for reduction in dry weight include uncontrolled hypertension, especially in those patients who are on multiple medications such as in the DRIP trial where subjects were on an average of 2.6 antihypertensive drugs at baseline [62]. Numerous studies have shown that greater antihypertensive drug use is associated with worse control of hypertension [13, 116], which is plausibly due to inadequately addressed volume overload which could be improved with reduction in dry weight. Table 7.7 summarizes the clinical signs of volume overload.
Another sign to consider reduction in dry weight is a low interdialytic weight gain . This comes from the observation that interdialytic weight gain tends to rise when dry weight is reduced and vice versa [117]. Additionally the secondary analysis of the DRIP trial that employed relative plasma volume monitoring found the flattest relative plasma volume slopes, corresponding to the most volume overload, in the group with the lowest ultrafiltration volume [114]. This is not surprising considering the mechanism of relative plasma volume monitoring, however the ultrafiltration volume generally equals the interdialytic weight gain, and, as noted above, subsequent reduction in dry weight per the trial protocol in the group with the lowest interdialytic weight gain resulted in the greatest weight loss at 1.5 kg and in the greatest reduction in 44 h ambulatory SBP at 12.6 mmHg compared to any of the other groups with higher interdialytic weight gains. Thus low interdialytic weight gain may be a sign of occult volume overload and low interdialytic weight gain should not be considered to be synonymous with euvolemia [118]. As a purely practical matter, a low interdialytic weight gain also makes it easier to challenge the dry weight.
It is important to note that while volume overload is a major contributor to hypertension in ESRD and volume removal is the foundation of hypertension control in ESRD , that hypertension and volume overload are not equivalent. The presence or absence of hypertension doesn’t definitively rule volume overload either in or out. This is illustrated by a study of 500 HD patients using bioelectric impedance that found 33 % of the cohort to be euvolemic and normotensive based on peridialytic BP, while 10 % were hypervolemic yet still normotensive, and 13 % were euvolemic but still hypertensive [119]. The distinction is even more important when outcomes are considered. Volume overload has been shown to be independently associated with mortality both when assessed by bioelectrical impedance [120] and by relative plasma volume monitoring [121], even after adjusting for BP in both studies. So while the presence or absence of hypertension is an important finding to guide the clinical assessment of volume status in dialysis, the treating nephrologist must keep an open mind and look for other confirming signs.
The recommended method to reduce dry weight is in decrements as small as 0.2–0.3 kg per HD session based on the recognition that even small changes in dry weight can improve BP, as the DRIP trial had only 1 kg reduction in the dry weight of the intervention group yet found a large change in ambulatory SBP, and other trials of dry weight management have similarly found significant BP reduction with similar changes in dry weight of only 1 kg or less [122, 123]. An added benefit of making small and gradual changes in dry weight is that it builds trust in patients who are often reluctant to permit their dry weight to be lowered for fear of provoking symptoms such as cramping.
There are risks to challenging dry weight including increased risk of clotted vascular access [49], accelerated loss of residual renal function [107], and increased frequency of intradialytic hypotension, which has been associated with myocardial stunning [124] and increased mortality [125]. As the DRIP trial lasted only 8 weeks long-term randomized trials are needed to examine the balance between benefits and risks of dry weight reduction .
Dietary Sodium Restriction
Despite recent observational evidence questioning the benefits of a low sodium diet for the general population [126], there is ample randomized trial evidence supporting the efficacy of low sodium diet to treat resistant hypertension in those without kidney disease [127], to treat hypertension in stage 3–4 CKD [128], and for reduction of proteinuria and albuminuria in diabetic nephropathy [129]. In the HD patient sodium intake provokes increased interdialytic weight gain [130] which also leads to increased ultrafiltration rates, both of which are associated with cardiovascular mortality [131, 132]. Restricting sodium intake reduces interdialytic weight gain, which will also practically improve the ability to achieve an adequately low dry weight with dialysis [133, 134]. However, in the dialysis patient, reducing dietary sodium intake should be followed by probing dry weight to manage hypertension better. In the absence of probing dry weight, the full benefit of restricting dietary sodium intake may not be realized. The American Heart Association recommends <1500 mg (equivalent to 65 mmol) daily intake [135], which is a reasonable prescription for dialysis patients [54]. Notably, except for hyponatremia treatment, there is no rational role for fluid restriction in dialysis patients [130, 136].
Dialysate Sodium Reduction
In the earliest days of chronic HD low dialysate sodium concentrations were used and sodium removal on HD was thus in part due to diffusion in addition to convective removal with ultrafiltration. As the efficiency of dialyzers improved and dialysis times were reduced, higher dialysate sodium concentrations became the norm to reduce hemodynamic instability, cramping, and symptoms of disequilibrium [137] and initial studies suggested that hypertension wasn’t a complication [138]. However, more recently it has been recognized that higher dialysate sodium concentrations will reduce or reverse the diffusive removal of sodium on HD, which undermines the effective management of volume control [139]. As an example of the impact of dialysate sodium concentration, in a pilot study that reduced dialysate sodium from 137.8 to 135.6 mmol/L stepwise over 7 weeks, net sodium removal was significantly increased from 383 to 480 mmol per HD session [140].
Numerous studies have shown interdialytic weight gain to be directly related to dialysate sodium concentration with higher dialysate sodium leading to higher intradialytic weight gain [140–142]. Increased interdialytic weight gain is also seen with sodium profiles, also called sodium ramping, where the dialysate sodium concentration generally starts high and then is gradually reduced during the HD session [141, 143, 144]. While higher dialysate sodium concentrations are prescribed to promote hemodynamic stability, the resulting higher interdialytic weight gain can lead to higher ultrafiltration rates which lead to the very hemodynamic instability originally to be avoided. Additionally, more recent studies of higher dialysate sodium concentrations, whether constant or with a profile, have been associated with higher BP in some [143, 144], but not all investigations [142].
An alternative to avoid the vicious cycle above is to individualize the dialysate sodium prescription to the patient’s pre-HD serum sodium. The importance of individualization is illustrated by a cross sectional study of 1084 HD patients that examined the difference between the individual dialysate sodium concentration and the patient’s pre-HD serum sodium and found that this difference is directly related to interdialytic weight gain, with a higher dialysate sodium concentration relative to the pre-HD serum sodium being associated with greater weight gain [145]. A single-blind crossover study of 27 HD patients illustrated one method of individualizing the dialysate sodium concentration by first dialyzing all patients with a standard 138 mmol/L sodium dialysate for 3 weeks and then on a dialysate sodium concentration set to 0.95 multiplied by the pre-HD serum sodium for 3 weeks [146]. On the low sodium dialysate prescription significant reductions were seen in interdialytic weight gain by 0.6 kg, in the frequency of intradialytic hypotension, and in the pre-HD SBP in the hypertensive subjects. Based on these findings it is reasonable to recommend that dialysate sodium be individualized to avoid being higher than the individual patient’s pre-HD serum sodium and possibly as low as 0.95 multiplied by the serum sodium in hypertensive individuals or those with high interdialytic weight gain precluding the achievement of an adequately low dry weight.
A trial in 25 PD patients employed a before–after design to investigate the use of low sodium dialysate over 2 months [147]. All subjects had one exchange per day changed to a low sodium solution, but 10 subjects had the dextrose concentration increased to compensate for reduced osmolality while 15 subjects had no change to their dextrose concentration. The first group had significantly improved BP along with markers of improved volume overload, suggesting that reducing dialysate sodium is only useful if it is accompanied by adequate ultrafiltration .
Adequate Time on Dialysis
Despite reducing dietary sodium intake and the dialysate sodium concentration to reduce interdialytic weight gain, many attempts to reduce dry weight to improve BP will be precluded in many patients due to intradialytic hypotension or symptoms on HD including cramping. In these patients increasing the HD time can make ultrafiltration easier to tolerate thus facilitating the achievement of an adequate dry weight. Shorter HD times have recently been shown in a secondary analysis of the DRIP trial to be associated both with higher BP and slower improvement in BP when dry weight is reduced [148]. A randomized crossover trial of 38 HD patients evaluated time on HD by assigning subjects to 2 weeks of 4 h versus 5 h HD sessions and found significantly less intradialytic hypotension and post-HD orthostatic hypotension during the longer HD runs [149]. An added salutary effect of longer HD time is that for a given amount of interdialytic weight gain, an increased HD time will lead to a lower ultrafiltration rate, which has been associated with mortality [132].
It is for all these reasons that the European Best Practice Guidelines recommend that HD should be delivered at least three times weekly for a total duration of at least 12 h, unless substantial residual renal function remains [150]. However, in the USA a recent cohort study among 32,000 HD patients found that the average single HD session was only 217 min and that one quarter of patients dialyzed less than 3 h and 15 min per session [151]. The lower average treatment times in the USA are likely due to the practice of reducing the prescribed time to achieve a minimum goal Kt/V, however this practice should be avoided on account of the potential deleterious effects that shorter treatment time can have on volume status and hypertension [152].
Frequent Dialysis
An additional strategy to treat patients who cannot achieve an adequately low dry weight on a conventional three times weekly HD schedule is to consider a change in modality to more frequent dialysis. Observational studies have shown frequent HD to be associated with reductions in BP despite lower antihypertensive drug use [153, 154], as well as with improvements in LVH [154]. More recently randomized trials have confirmed some of these findings with trial of 52 patients that assigned subjects to either conventional 3 times weekly HD versus 6 nights weekly nocturnal HD for 6 months and found significantly improved BP, lower antihypertensive drug use, and improvement left ventricular hypertrophy in the nocturnal HD group [155]. The subsequent Frequent Hemodialysis Network (FHN ) Nocturnal trial recruited 87 HD patients and randomized them to 3 times weekly conventional HD versus 6 nights weekly nocturnal HD, and weekly average pre-HD BP was significantly improved in the nocturnal HD group despite a reduction in antihypertensive medication use; however, the trends toward improvement in the primary endpoints including LVH were nonsignificant, possibly due to lack of power from difficultly with subject recruitment [156]. The companion FHN trial of daily HD recruited 245 patients who were randomized to 3 times weekly conventional HD versus 6 days weekly HD for 12 months and this trial did find significantly reduced hazard for both coprimary composite endpoints of death or increase in left ventricular mass and death or decrease in the physical-health composite score [157]. Both weekly average pre-HD SBP and the number of antihypertensive medications for the intervention group were reduced significantly, as well. These improvements in BP and LVH are plausibly due to better control of volume [158], especially when it is recognized that the daily HD group had significantly more ultrafiltration per week at 10.58 L on average compared to 8.99 L for the control group [157].
Pharmacologic Treatment
Patients with ESRD are routinely excluded from drug trials, limiting the evidence base from which to make recommendations for antihypertensive drug therapy. Two meta-analyses of randomized trials employing antihypertensive drugs in dialysis have found significant improvements in the cardiovascular event rates associated with treatment [159, 160], which was particularly pronounced in subjects with hypertension [160]. However, the trials included in these meta-analyses were highly heterogeneous, most trials weren’t limited to hypertensive patients, and only two trials targeted a specific BP goal.
Despite the benefits from the use of antihypertensive medication in ESRD, it must be emphasized that greater use of antihypertensive medications is associated with worse control of hypertension [13, 116], which is plausibly due to inadequately treated volume overload in those cases. Thus the first step in treating hypertension in ESRD should be to address volume overload as able. All classes of antihypertensive medications have roles in the treatment of hypertension in ESRD [161], as detailed below.
Diuretics
While published evidence is lacking, diuretics are often used to address hypertension and volume overload in patients with significant residual renal function, which includes those new to HD and nonoliguric patients on PD. In the setting of advanced renal failure higher doses of diuretics will be necessary to be effective [162]. However, in anuric patients even doses of furosemide as high as 250 mg intravenously are ineffective [163]. While some investigators have suggested that thiazide diuretics exert an antihypertensive vasodilator effect [164, 165], the placebo controlled administration of thiazides to anuric dialysis patients has been shown to have no effect on BP [166]. Thus, the role for diuretics in the treatment of hypertension in dialysis is at best limited to the subset of patients with significant residual renal function .
Beta-Blockers
Beta-adrenergic blocking agents have well-established benefits in the non-dialysis population including in the setting of heart failure [167] and coronary artery disease [168]. As cardiovascular events are the leading cause of death in ESRD and increased sympathetic nervous system overactivity is common [89], beta-blockers are an attractive therapy in this population. A retrospective cohort study of PD patients found beta-blocker use to be associated with a significantly reduced risk of new onset heart failure or the composite endpoint of new onset heart failure and cardiac mortality [169]. Cice and colleagues recruited 114 HD patients with reduced left ventricular ejection fraction <35 % and randomized them to carvedilol versus placebo and reported significantly improved 2-year survival in the carvedilol group [170].
Despite these encouraging findings, enthusiasm for beta-blockers as first line pharmacological treatment for hypertension in dialysis is tempered by the non-dialysis experience where beta-blockers are not recommended for initial monotherapy of hypertension [171, 172], consequently ACEi medications are often instead recommended for initial therapy of hypertension [173]. While head to head studies of antihypertensives are few, a recent randomized controlled trial in HD comparing lisinopril to atenolol begins to address the question of which medication to prescribe first for hypertension in HD.
The Hypertension in Hemodialysis Patients Treated with Atenolol or Lisinopril (HDPAL) trial recruited 200 chronic HD patients with hypertension confirmed by 44 h interdialytic ABPM and echocardiographic LVH and randomized them to lisinopril or atenolol based therapy for 12 months to determine which drug is superior for reduction of LVH [53]. All patients were treated to target goal home BP ≤140/90 mmHg checked monthly, first by maximizing the study drug, then by addition of other drugs, sodium restriction, and reduction in dry weight. The trial was terminated early by an independent data safety monitoring board for cardiovascular safety because of significantly more serious adverse cardiovascular events in the lisinopril group, which had 43 events in 28 subjects compared to only 20 events in 16 subjects in the atenolol group (incidence rate ratio 2.36, P = 0.001). Similarly, the combined serious adverse events of myocardial infarction, stroke, hospitalization for heart failure, or cardiovascular death occurred 23 times in 17 subjects in the lisinopril group compared to only 11 events in 10 subjects in the atenolol group (incidence rate ratio 2.29, P = 0.002). LVH improved in both groups but no differences between drug groups were found.
While 44 h ambulatory BP improved similarly in both groups measured at baseline, 3 months, 6 months, and 12 months, the monthly home BP was consistently lower in the atenolol group despite significantly more antihypertensive medications and greater dry weight reduction in the lisinopril group [53]. Thus atenolol appears to be superior to lisinopril in terms of cardiovascular event rates and BP reduction. Based on the findings of this head to head comparison of atenolol and lisinopril in HD patients it is recommended that beta-blockers be the first line therapy for hypertension. Table 7.8 summarizes the recommendations for pharmacologic therapy for hypertension in dialysis. Atenolol in particular may be practically useful as it can be dosed just 3 times per week after HD, as was the protocol in the HDPAL trial, and this schedule has been previously shown to significantly reduce 44 h interdialytic ambulatory BP [174]. Three times weekly dosing permits the possibility of directly observed administration of atenolol in the HD unit to improve compliance with the antihypertensive regimen .
Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers
It cannot be concluded from the HDPAL trial that ACEi medications are harmful because there was no placebo controlled group in the study [53]. Indeed, ACEi and angiotensin receptor blocker (ARB) drugs are mainstays of therapy in pre-dialysis CKD [175] and in cardiovascular disease [176, 177]. Both ACEi [84, 85, 178] and ARB medications [179] have been shown to improve BP in ESRD. As with atenolol, both lisinopril [84] and trandolapril [178] have been shown to be effective at lowering BP when dosed only three times weekly after HD.
Three randomized clinical trials have examined ACEi or ARB therapy in HD patients with cardiovascular events as the primary endpoint, and they warrant more detailed mention. The only randomized clinical trial investigating an ACEi and cardiovascular events in HD patients is the Fosinopril in Dialysis Study (FOSIDIAL) [85], which recruited 397 chronic HD patients with LVH who were followed for a 2-week run-in period on fosinopril, and those who tolerated the therapy were randomized to fosinopril or placebo and treated to goal peridialytic <160/90 mmHg for 24 months [180]. No benefit was found for fosinopril in decreasing cardiovascular events [85].
Two randomized controlled trials have investigated ARB use in HD and found a benefit for cardiovascular events. The first enrolled 80 HD patients who were randomized to candesartan or open label usual care for a planned 3 years [181]. The trial was stopped early on account of an interim analysis that found significant and substantial benefit for candesartan for the primary endpoint of cardiovascular events as well as for mortality with zero deaths in the candesartan group and 18.9 % mortality in the control group [181]. The second trial included 360 HD patients randomized to open label ARB therapy with losartan, valsartan, or candesartan versus usual care with goal peridialytic SBP <150 mmHg over 3 years [182]. ARB treatment significantly reduced the primary endpoint of cardiovascular events with a 49 % reduction in risk of cardiovascular event (HR = 0.51, P = 0.002).
In the setting of the divergent outcomes for the three randomized clinical trials using ACEi or ARB drugs above, a recent meta-analysis examined the pooled results for cardiovascular events in the 837 total subjects in these trials and found a trend toward benefit with a relative risk for cardiovascular events at 0.66, but this did not reach statistical significance (95 % confidence interval 0.35 to 1.25, P = 0.20) [183]. Each trial had different definitions for cardiovascular events, and not surprisingly significant heterogeneity was found. Similarly, a systematic review of ACEi and ARB therapy in PD patients included three randomized clinical trials and found no improvement in cardiovascular events for the intervention groups [184].
As ACEi and ARB therapy has been shown to delay progression of pre-dialysis chronic kidney disease [185], these agents may be effective at preserving residual renal function in dialysis patients, which is emerging as an important goal for both PD and HD patients [186]. Two randomized controlled open label trials in PD, one investigating ramipril in 60 subjects [187] and the other studying valsartan in 34 subjects [188], both found active treatments reduced the rate of decline in GFR, while a meta-analysis pooled the difference between the intervention groups and the control groups at 12 months and found a clinically and statistically significant benefit of 0.9 mL/min/1.73 m2 in favor of the intervention groups [184]. In HD however, a recent randomized placebo controlled trial of irbesartan over 1 year in 82 nonoliguric HD patients found no benefit for irbesartan in decline of GFR or development of anuria [189].
The risk of hyperkalemia with ACEi or ARB use in dialysis appears low based on the evidence from the aforementioned randomized controlled trials in both HD [85, 181, 182] and PD [187]. ACEi or ARB agents are a reasonable second choice after beta-blockers for an antihypertensive medication in dialysis based on their tolerability, the evidence of benefit in the non-dialysis population, and the randomized trial evidence of benefit on intermediate end points such as reduction in LVH [183].
Calcium Channel Blockers
Amlodipine has been shown to be effective versus placebo in improving BP in a randomized trial of 251 hypertensive HD patients where the primary endpoint of cardiovascular events showed no improvement for active treatment [190]. Calcium channel blockers have the practical benefits of being well tolerated and requiring only once a day dosing, however ACEi or ARB therapy is preferred before calcium blockers based on head to head trials showing calcium channel blockers to be significantly inferior for regression of LVH [191, 192].
Centrally Acting Alpha Agonists
These medications are typically reserved only for those patients whose BP is uncontrolled on the combination of beta-blocker, ACEi or ARB, plus calcium channel blocker. To minimize pill burden and dosing schedule, it is recommended to avoid oral clonidine and to instead use the long acting clonidine patch, which can be administered once a week at the dialysis unit as directly observed therapy. As the clonidine patch can be expensive, a cheaper alternative is oral guanfacine, dosed only once daily at bedtime to minimize the impact of dose related drowsiness.
Vasodilators
Direct vasodilator agents are usually reserved as last line therapy for hypertension in ESRD. However, hydralazine use is becoming more common based on trial evidence of benefit for heart failure in the African-American population in combination with isosorbide dinitrate [193], but it is important to recognize that this combination of medications has not been studied in ESRD. Furthermore the pill burden and requirement of three times daily dosing of hydralazine makes it less attractive for use in ESRD. It is for that reason that minoxidil is usually preferable to hydralazine on account of its antihypertensive effectiveness with only once daily dosing in the setting of CKD .
Mineralocorticoid Receptor Antagonists
Mineralocorticoid receptor antagonists have well-established roles in the non-dialysis population for treatment of resistant hypertension [194] and heart failure [195, 196]. In the dialysis population spironolactone has been shown to significantly reduce 24 h ambulatory BP by 10.9/5.8 mmHg in a randomized controlled double blind trial of 76 hypertensive dialysis patients on HD or PD treated with spironolactone 25–50 mg daily versus placebo over 12 weeks [197]. Recently the Dialysis Outcomes Heart Failure Aldosterone Study (DOHAS) clinical trial randomized 309 HD patients to spironolactone 25 mg daily versus open label usual care for 3 years and found both cardiovascular death and hospitalizations and all-cause mortality were significantly improved in the spironolactone group [198]. Importantly, spironolactone therapy was discontinued for hyperkalemia in only three patients during the trial. While the results of the DOHAS trial are very promising, they require confirmation in future blinded randomized trials to balance the risk of hyperkalemia with the potential benefits before the routine use of spironolactone for hypertension in HD can be recommended .
Invasive Treatment
The history of invasive treatments for hypertension dates back at least to the 1930s when surgical sympathectomy was employed for essential hypertension [199]. As efficacious oral antihypertensive agents with tolerable side effect profiles were discovered, surgery fell out of favor as a treatment for simple hypertension. However, in the early era of chronic HD in the 1960s and 1970s it was recognized that a subset of patients with ESRD didn’t achieve adequate control of hypertension despite ultrafiltration on HD and the use of the antihypertensive medications of the day [80]. As high renin levels were common in these cases, bilateral nephrectomy was advocated as an effective means to reduce renin levels and BP, though it was recognized even then that other mechanisms likely were responsible for the improved BP after nephrectomy [80]. With the introduction of ACEi drugs bilateral nephrectomy too became much less common.
However, Converse and colleagues demonstrated that sympathetic overactivity from renal afferent nerves are a major source of hypertension in ESRD [89], and with the invention of a radiofrequency catheter based approach to target the renal nerves there has been renewed interest in renal sympathectomy via the endovascular approach [91]. A pilot study of renal denervation by radiofrequency ablation was performed in 12 chronic HD patients with uncontrolled hypertension and office BP was reduced in the 9 patients who had the procedure versus unchanged BP in those 3 patients whose atrophic renal arteries precluded the endovascular denervation procedure [200]. However, enthusiasm for renal denervation must be tempered by the experience with renal denervation in the resistant hypertension population without CKD where initial trials showed promise [201] but when a randomized, sham placebo controlled, and blinded trial was performed there was no BP reduction for the intervention [202]. While ESRD patients are an ideal group who may benefit from this therapy, it remains to be seen whether the disappointing result from the randomized controlled trial of endovascular renal denervation will preclude further development of this technique .
Prognosis
Despite a strong and direct relationship between hypertension and cardiovascular and all-cause mortality [203] and copious evidence of benefit for treatment of hypertension in the non-dialysis population [175], the relationship between BP and outcomes in dialysis patients remains a topic of controversial [204, 205]. Various studies have found an association between peridialytic hypertension and strokes [206], heart failure [207], arrhythmias [208], cardiovascular events [209], and all-cause mortality [210]. However, other studies suggest that peridialytic hypertension is protective and lower BP is associated with worse mortality [4, 211–213], and the risk of normotensive BP is magnified when BP is considered as a time dependent co-variate [211, 213]. This paradoxical relationship between BP and mortality has been termed the “reverse epidemiology” of hypertension [205] and has raised concern that treatment of hypertension may be harmful [214].
When examining the prognostic value of hypertension in dialysis it is important to additionally consider severity of illness and dialysis vintage as well. This is illustrated by a retrospective cohort study of 2770 prevalent PD patients where a fully adjusted analysis found higher SBP, DBP, mean arterial pressure, and pulse pressure to be associated with decreased mortality during the first year on dialysis [215]. However, higher SBP and pulse pressure were associated with increased mortality for those patients on dialysis ≥6 years. Similar findings have been shown in a cohort of 16,959 HD patients where SBP <120 mmHg was associated with increased mortality within the first 2 years of starting dialysis, but SBP >150 mmHg was associated with increased mortality among those that survived at least 3 years [216]. These findings suggest lower BP may be an indicator of more severe illness in those patients new to dialysis who are likely to have advanced chronic but unstable systemic comorbidities that recently culminated in ESRD , whereas in the survivors that have been on dialysis for at least 6 years have a more normal relationship between hypertension and outcomes because they are less acutely ill. This explanation is further bolstered by the subgroup in the PD cohort who were listed for transplant within 6 months of starting dialysis, as in this healthier subgroup higher SBP, DBP, mean arterial pressure, and pulse pressure were not associated with improved mortality during the first year of dialysis [215].
The technique of BP measurement also contributes to the controversial relationship between BP and outcomes as the reverse epidemiology of hypertension is primarily a phenomenon of peridialytic BP values. However, ambulatory BP has a strong relationship with mortality on HD, first demonstrated by Amar and colleagues in a study of 57 HD patients [217]. Agarwal and colleagues have confirmed the relationship between ambulatory BP and mortality in a cohort of 150 HD patients, and in the same cohort they also demonstrated home BP to similarly have a strong relationship with mortality [49]. In an expanded cohort of 326 HD patients followed for a mean of 32 months Agarwal subsequently has shown increased mortality at the extremes of ambulatory and home BP, and that mortality was best at a home SBP 120–130 mmHg and ambulatory SBP 110–120 mmHg, while peridialytic BP had no relationship with mortality in this cohort [25]. Most recently an analysis of the Chronic Renal Insufficiency Cohort (CRIC) study compared pre-HD SBP and out of HD unit SBP for prediction of mortality in the 403 subjects who started HD since the start of the study [218]. There were 98 deaths over a mean follow-up of 2.7 years and pre-HD SBP showed a U-shaped relationship to mortality consistent with reverse epidemiology of hypertension. However, in the 326 subjects who had BP checked out of the HD unit in a standardized manner during a research visit, there was a significant and direct linear relationship between BP and mortality with hazard ratio 1.26 for every 10 mmHg rise in SBP, which further emphasizes the importance of BP measurement technique when considering prognosis [218].
Thus while there is concern for reverse epidemiology of hypertension when analyzing peridialytic BP, which would suggest that lowering BP would be harmful in HD patients, the evidence from ambulatory and home BP studies doesn’t support those conclusions, nor does the evidence from two meta-analyses of randomized clinical trials of antihypertensive medication use in HD which find cardiovascular benefit rather than harm with active treatment [159, 160].
References
Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2015;65(6 Suppl 1):A7.
O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96(19):1420–5.
U.S. Renal Data System, USRDS 2013 Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013.
Salem MM. Hypertension in the hemodialysis population: a survey of 649 patients. Am J Kidney Dis. 1995;26(3):461–8.
Mittal SK, Kowalski E, Trenkle J, McDonough B, Halinski D, Devlin K, et al. Prevalence of hypertension in a hemodialysis population. Clin Nephrol. 1999;51(2):77–82.
Rahman M, Dixit A, Donley V, Gupta S, Hanslik T, Lacson E, et al. Factors associated with inadequate blood pressure control in hypertensive hemodialysis patients. Am J Kidney Dis. 1999;33(3):498–506.
Rahman M, Fu P, Sehgal AR, Smith MC. Interdialytic weight gain, compliance with dialysis regimen, and age are independent predictors of blood pressure in hemodialysis patients. Am J Kidney Dis. 2000;35(2):257–65.
Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–9.
Greene T, Beck GJ, Gassman JJ, Gotch FA, Kusek JW, Levey AS, et al. Design and statistical issues of the hemodialysis (HEMO) study. Control Clin Trials. 2000;21(5):502–25.
Rocco MV, Yan G, Heyka RJ, Benz R, Cheung AK. Risk factors for hypertension in chronic hemodialysis patients: baseline data from the HEMO study. Am J Nephrol. 2001;21(4):280–8.
Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med. 2003;115(4):291–7.
Michael B, Coyne DW, Fishbane S, Folkert V, Lynn R, Nissenson AR, et al. Sodium ferric gluconate complex in hemodialysis patients: adverse reactions compared to placebo and iron dextran. Kidney Int. 2002;61(5):1830–9.
Agarwal R. Epidemiology of interdialytic ambulatory hypertension and the role of volume excess. Am J Nephrol. 2011;34(4):381–90.
Cheigh JS, Milite C, Sullivan JF, Rubin AL, Stenzel KH. Hypertension is not adequately controlled in hemodialysis patients. Am J Kidney Dis. 1992;19(5):453–9.
Boudville NC, Cordy P, Millman K, Fairbairn L, Sharma A, Lindsay R, et al. Blood pressure, volume, and sodium control in an automated peritoneal dialysis population. Perit Dial Int. 2007;27(5):537–43.
Rocco MV, Flanigan MJ, Beaver S, Frederick P, Gentile DE, McClellan WM, et al. Report from the 1995 Core Indicators for Peritoneal Dialysis Study Group. Am J Kidney Dis. 1997;30(2):165–73.
Goldfarb-Rumyantzev AS, Baird BC, Leypoldt JK, Cheung AK. The association between BP and mortality in patients on chronic peritoneal dialysis. Nephrol Dial Transplant. 2005;20(8):1693–701.
Cocchi R, Degli EE, Fabbri A, Lucatello A, Sturani A, Quarello F, et al. Prevalence of hypertension in patients on peritoneal dialysis: results of an Italian multicentre study. Nephrol Dial Transplant. 1999;14(6):1536–40.
Tonbul Z, Altintepe L, Sozlu C, Yeksan M, Yildiz A, Turk S. Ambulatory blood pressure monitoring in haemodialysis and continuous ambulatory peritoneal dialysis (CAPD) patients. J Hum Hypertens. 2002;16(8):585–9.
Townsend RR, Ford V. Ambulatory blood pressure monitoring: coming of age in nephrology. J Am Soc Nephrol. 1996;7(11):2279–87.
Mansoor GA, White WB. Ambulatory blood pressure monitoring is a useful clinical tool in nephrology. Am J Kidney Dis. 1997;30(5):591–605.
Peixoto AJ, Santos SF, Mendes RB, Crowley ST, Maldonado R, Orias M, et al. Reproducibility of ambulatory blood pressure monitoring in hemodialysis patients. Am J Kidney Dis. 2000;36(5):983–90.
Thompson AM, Pickering TG. The role of ambulatory blood pressure monitoring in chronic and end-stage renal disease. Kidney Int. 2006;70(6):1000–7.
Agarwal R, Brim NJ, Mahenthiran J, Andersen MJ, Saha C. Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension. 2006;47(1):62–8.
Agarwal R. Blood pressure and mortality among hemodialysis patients. Hypertension. 2010;55(3):762–8.
Peixoto AJ, Gray TA, Crowley ST. Validation of the SpaceLabs 90207 ambulatory blood pressure device for hemodialysis patients. Blood Press Monit. 1999;4(5):217–21.
Agarwal R, Lewis RR. Prediction of hypertension in chronic hemodialysis patients. Kidney Int. 2001;60(5):1982–9.
Agarwal R, Light RP. Arterial stiffness and interdialytic weight gain influence ambulatory blood pressure patterns in hemodialysis patients. Am J Physiol Renal Physiol. 2008;294(2):F303–8.
Kelley K, Light RP, Agarwal R. Trended cosinor change model for analyzing hemodynamic rhythm patterns in hemodialysis patients. Hypertension. 2007;50(1):143–50.
Cannella G, Paoletti E, Ravera G, Cassottana P, Araghi P, Mulas D, et al. Inadequate diagnosis and therapy of arterial hypertension as causes of left ventricular hypertrophy in uremic dialysis patients. Kidney Int. 2000;58(1):260–8.
Bishu K, Gricz KM, Chewaka S, Agarwal R. Appropriateness of antihypertensive drug therapy in hemodialysis patients. Clin J Am Soc Nephrol. 2006;1(4):820–4.
Argiles A, Mourad G, Mion C. Seasonal changes in blood pressure in patients with end-stage renal disease treated with hemodialysis. N Engl J Med. 1998;339(19):1364–70.
K/DOQI Workgroup: K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1–153.
Davenport A, Cox C, Thuraisingham R. Achieving blood pressure targets during dialysis improves control but increases intradialytic hypotension. Kidney Int. 2008;73(6):759–64.
Rohrscheib MR, Myers OB, Servilla KS, Adams CD, Miskulin D, Bedrick EJ, et al. Age-related blood pressure patterns and blood pressure variability among hemodialysis patients. Clin J Am Soc Nephrol. 2008;3(5):1407–14.
Rahman M, Griffin V, Kumar A, Manzoor F, Wright Jr JT, Smith MC. A comparison of standardized versus “usual” blood pressure measurements in hemodialysis patients. Am J Kidney Dis. 2002;39(6):1226–30.
Conion PJ, Walshe JJ, Heinle SK, Minda S, Krucoff M, Schwab SJ. Predialysis systolic blood pressure correlates strongly with mean 24-hour systolic blood pressure and left ventricular mass in stable hemodialysis patients. J Am Soc Nephrol. 1996;7(12):2658–63.
Agarwal R, Peixoto AJ, Santos SF, Zoccali C. Pre- and postdialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol. 2006;1(3):389–98.
Agarwal R, Metiku T, Tegegne GG, Light RP, Bunaye Z, Bekele DM, et al. Diagnosing hypertension by intradialytic blood pressure recordings. Clin J Am Soc Nephrol. 2008;3(5):1364–72.
Agarwal R, Light RP. Median intradialytic blood pressure can track changes evoked by probing dry-weight. Clin J Am Soc Nephrol. 2010;5(5):897–904.
Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52(1):10–29.
Parati G, Stergiou GS, Asmar R, de Leeuw P, Imai Y, Imai Y, et al. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens. 2008;26(8):1505–26.
Agarwal R. Role of home blood pressure monitoring in hemodialysis patients. Am J Kidney Dis. 1999;33(4):682–7.
Agarwal R, Peixoto AJ, Santos SF, Zoccali C. Out-of-office blood pressure monitoring in chronic kidney disease. Blood Press Monit. 2009;14(1):2–11.
Agarwal R, Andersen MJ, Bishu K, Saha C. Home blood pressure monitoring improves the diagnosis of hypertension in hemodialysis patients. Kidney Int. 2006;69(5):900–6.
Agarwal R, Satyan S, Alborzi P, Light RP, Tegegne GG, Mazengia HS, et al. Home blood pressure measurements for managing hypertension in hemodialysis patients. Am J Nephrol. 2009;30(2):126–34.
Moriya H, Ohtake T, Kobayashi S. Aortic stiffness, left ventricular hypertrophy and weekly averaged blood pressure (WAB) in patients on haemodialysis. Nephrol Dial Transplant. 2007;22(4):1198–204.
Moriya H, Oka M, Maesato K, Mano T, Ikee R, Ohtake T, et al. Weekly averaged blood pressure is more important than a single-point blood pressure measurement in the risk stratification of dialysis patients. Clin J Am Soc Nephrol. 2008;3(2):416–22.
Alborzi P, Patel N, Agarwal R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol. 2007;2(6):1228–34.
Kauric-Klein Z, Artinian N. Improving blood pressure control in hypertensive hemodialysis patients. CANNT J. 2007;17(4):24–6.
da Silva GV, de Barros S, Abensur H, Ortega KC, Mion Jr D. Home blood pressure monitoring in blood pressure control among haemodialysis patients: an open randomized clinical trial. Nephrol Dial Transplant. 2009;24(12):3805–11.
Agarwal R, Light RP. Chronobiology of arterial hypertension in hemodialysis patients: implications for home blood pressure monitoring. Am J Kidney Dis. 2009;54(4):693–701.
Agarwal R, Sinha AD, Pappas MK, Abraham TN, Tegegne GG. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol Dial Transplant. 2014;29(3):672–81.
Agarwal R, Flynn J, Pogue V, Rahman M, Reisin E, Weir MR. Assessment and management of hypertension in patients on dialysis. J Am Soc Nephrol. 2014;25(8):1630–46.
Inrig JK. Intradialytic hypertension: a less-recognized cardiovascular complication of hemodialysis. Am J Kidney Dis. 2010;55(3):580–9.
Inrig JK, Oddone EZ, Hasselblad V, Gillespie B, Patel UD, Reddan D, et al. Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int. 2007;71(5):454–61.
Van Buren PN, Kim C, Toto R, Inrig JK. Intradialytic hypertension and the association with interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol. 2011;6(7):1684–91.
Agarwal R, Light RP. Intradialytic hypertension is a marker of volume excess. Nephrol Dial Transplant. 2010;25(10):3355–61.
Inrig JK, Patel UD, Toto RD, Szczech LA. Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: a secondary analysis of the Dialysis Morbidity and Mortality Wave 2 Study. Am J Kidney Dis. 2009;54(5):881–90.
Yang CY, Yang WC, Lin YP. Postdialysis blood pressure rise predicts long-term outcomes in chronic hemodialysis patients: a four-year prospective observational cohort study. BMC Nephrol. 2012;13:12.
Cirit M, Akcicek F, Terzioglu E, Soydas C, Ok E, Ozbasli CF, et al. ‘Paradoxical’ rise in blood pressure during ultrafiltration in dialysis patients. Nephrol Dial Transplant. 1995;10(8):1417–20.
Agarwal R, Alborzi P, Satyan S, Light RP. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension. 2009;53(3):500–7.
El-Shafey EM, El-Nagar GF, Selim MF, El-Sorogy HA, Sabry AA. Is there a role for endothelin-1 in the hemodynamic changes during hemodialysis? Clin Exp Nephrol. 2008;12(5):370–5.
Chou KJ, Lee PT, Chen CL, Chiou CW, Hsu CY, Chung HM, et al. Physiological changes during hemodialysis in patients with intradialysis hypertension. Kidney Int. 2006;69(10):1833–8.
Inrig JK, Van BP, Kim C, Vongpatanasin W, Povsic TJ, Toto R. Probing the mechanisms of intradialytic hypertension: a pilot study targeting endothelial cell dysfunction. Clin J Am Soc Nephrol. 2012;7(8):1300–9.
Saijonmaa O, Metsarinne K, Fyhrquist F. Carvedilol and its metabolites suppress endothelin-1 production in human endothelial cell culture. Blood Press. 1997;6(1):24–8.
Goodfriend TL, Calhoun DA. Resistant hypertension, obesity, sleep apnea, and aldosterone: theory and therapy. Hypertension. 2004;43(3):518–24.
Drager LF, Diegues-Silva L, Diniz PM, Bortolotto LA, Pedrosa RP, Couto RB, et al. Obstructive sleep apnea, masked hypertension, and arterial stiffness in men. Am J Hypertens. 2010;23(3):249–54.
Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens. 2012;26(5):281–7.
Goncalves SC, Martinez D, Gus M, de Abreu-Silva EO, Bertoluci C, Dutra I, et al. Obstructive sleep apnea and resistant hypertension: a case–control study. Chest. 2007;132(6):1858–62.
Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58(5):811–7.
Ruttanaumpawan P, Nopmaneejumruslers C, Logan AG, Lazarescu A, Qian I, Bradley TD. Association between refractory hypertension and obstructive sleep apnea. J Hypertens. 2009;27(7):1439–45.
Nicholl DD, Ahmed SB, Loewen AH, Hemmelgarn BR, Sola DY, Beecroft JM, et al. Declining kidney function increases the prevalence of sleep apnea and nocturnal hypoxia. Chest. 2012;141(6):1422–30.
Forni OV, Ogna A, Pruijm M, Bassi I, Zuercher E, Halabi G, et al. Prevalence and diagnostic approach to sleep apnea in hemodialysis patients: a population study. Biomed Res Int. 2015;2015:103686.
Abdel-Kader K, Dohar S, Shah N, Jhamb M, Reis SE, Strollo P, et al. Resistant hypertension and obstructive sleep apnea in the setting of kidney disease. J Hypertens. 2012;30(5):960–6.
Tada T, Kusano KF, Ogawa A, Iwasaki J, Sakuragi S, Kusano I, et al. The predictors of central and obstructive sleep apnoea in haemodialysis patients. Nephrol Dial Transplant. 2007;22(4):1190–7.
Park J, Campese VM. Resistant hypertension and obstructive sleep apnea in end-stage renal disease. J Hypertens. 2012;30(5):880–1.
Pepin JL, Tamisier R, Barone-Rochette G, Launois SH, Levy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;182(7):954–60.
Haentjens P, Van MA, Moscariello A, De WS, Poppe K, Dupont A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167(8):757–64.
Lazarus JM, Hampers C, Merrill JP. Hypertension in chronic renal failure. Treatment with hemodialysis and nephrectomy. Arch Intern Med. 1974;133(6):1059–66.
Murphy RJ. The effect of “rice diet” on plasma volume and extracellular fluid space in hypertensive subjects. J Clin Invest. 1950;29(7):912–7.
Guyton AC, Coleman TG, Cowley Jr AV, Scheel KW, Manning Jr RD, Norman Jr RA. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med. 1972;52(5):584–94.
Schalekamp MA, Beevers DG, Briggs JD, Brown JJ, Davies DL, Fraser R, et al. Hypertension in chronic renal failure. An abnormal relation between sodium and the renin-angiotensin system. Am J Med. 1973;55(3):379–90.
Agarwal R, Lewis R, Davis JL, Becker B. Lisinopril therapy for hemodialysis hypertension: hemodynamic and endocrine responses. Am J Kidney Dis. 2001;38(6):1245–50.
Zannad F, Kessler M, Lehert P, Grunfeld JP, Thuilliez C, Leizorovicz A, et al. Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 2006;70(7):1318–24.
Ishii M, Ikeda T, Takagi M, Sugimoto T, Atarashi K, Igari T, et al. Elevated plasma catecholamines in hypertensives with primary glomerular diseases. Hypertension. 1983;5(4):545–51.
Beretta-Piccoli C, Weidmann P, Schiffl H, Cottier C, Reubi FC. Enhanced cardiovascular pressor reactivity to norepinephrine in mild renal parenchymal disease. Kidney Int. 1982;22(3):297–303.
Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105(11):1354–9.
Converse Jr RL, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327(27):1912–8.
Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, et al. Sympathetic nerve activity in end-stage renal disease. Circulation. 2002;106(15):1974–9.
Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, et al. Sympathetic activation in chronic renal failure. J Am Soc Nephrol. 2009;20(5):933–9.
Buckner FS, Eschbach JW, Haley NR, Davidson RC, Adamson JW. Hypertension following erythropoietin therapy in anemic hemodialysis patients. Am J Hypertens. 1990;3(12 Pt 1):947–55.
Abraham PA, Macres MG. Blood pressure in hemodialysis patients during amelioration of anemia with erythropoietin. J Am Soc Nephrol. 1991;2(4):927–36.
Kaupke CJ, Kim S, Vaziri ND. Effect of erythrocyte mass on arterial blood pressure in dialysis patients receiving maintenance erythropoietin therapy. J Am Soc Nephrol. 1994;4(11):1874–8.
Carlini R, Obialo CI, Rothstein M. Intravenous erythropoietin (rHuEPO) administration increases plasma endothelin and blood pressure in hemodialysis patients. Am J Hypertens. 1993;6(2):103–7.
Carlini RG, Dusso AS, Obialo CI, Alvarez UM, Rothstein M. Recombinant human erythropoietin (rHuEPO) increases endothelin-1 release by endothelial cells. Kidney Int. 1993;43(5):1010–4.
Bode-Boger SM, Boger RH, Kuhn M, Radermacher J, Frolich JC. Recombinant human erythropoietin enhances vasoconstrictor tone via endothelin-1 and constrictor prostanoids. Kidney Int. 1996;50(4):1255–61.
Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med. 1989;111(12):992–1000.
Eschbach JW, Kelly MR, Haley NR, Abels RI, Adamson JW. Treatment of the anemia of progressive renal failure with recombinant human erythropoietin. N Engl J Med. 1989;321(3):158–63.
Krapf R, Hulter HN. Arterial hypertension induced by erythropoietin and erythropoiesis-stimulating agents (ESA). Clin J Am Soc Nephrol. 2009;4(2):470–80.
Lebel M, Kingma I, Grose JH, Langlois S. Effect of recombinant human erythropoietin therapy on ambulatory blood pressure in normotensive and in untreated borderline hypertensive hemodialysis patients. Am J Hypertens. 1995;8(6):545–51.
Ishimitsu T, Tsukada H, Ogawa Y, Numabe A, Yagi S. Genetic predisposition to hypertension facilitates blood pressure elevation in hemodialysis patients treated with erythropoietin. Am J Med. 1993;94(4):401–6.
Charra B. Control of blood pressure in long slow hemodialysis. Blood Purif. 1994;12(4–5):252–8.
Chazot C, Charra B, Laurent G, Didier C, Vo VC, Terrat JC, et al. Interdialysis blood pressure control by long haemodialysis sessions. Nephrol Dial Transplant. 1995;10(6):831–7.
Charra B, Calemard E, Ruffet M, Chazot C, Terrat JC, Vanel T, et al. Survival as an index of adequacy of dialysis. Kidney Int. 1992;41(5):1286–91.
Ozkahya M, Toz H, Qzerkan F, Duman S, Ok E, Basci A, et al. Impact of volume control on left ventricular hypertrophy in dialysis patients. J Nephrol. 2002;15(6):655–60.
Gunal AI, Duman S, Ozkahya M, Toz H, Asci G, Akcicek F, et al. Strict volume control normalizes hypertension in peritoneal dialysis patients. Am J Kidney Dis. 2001;37(3):588–93.
Vertes V, Cangiano JL, Berman LB, Gould A. Hypertension in end-stage renal disease. N Engl J Med. 1969;280(18):978–81.
Scribner BH. A personalized history of chronic hemodialysis. Am J Kidney Dis. 1990;16(6):511–9.
Sinha AD, Agarwal R. Can chronic volume overload be recognized and prevented in hemodialysis patients? Semin Dial. 2009;22:480.
Agarwal R, Weir MR. Dry-weight: a concept revisited in an effort to avoid medication-directed approaches for blood pressure control in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(7):1255–60.
Agarwal R, Andersen MJ, Pratt JH. On the importance of pedal edema in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3(1):153–8.
Jaeger JQ, Mehta RL. Assessment of dry weight in hemodialysis: an overview. J Am Soc Nephrol. 1999;10(2):392–403.
Sinha AD, Light RP, Agarwal R. Relative plasma volume monitoring during hemodialysis aids the assessment of dry weight. Hypertension. 2010;55(2):305–11.
Hur E, Usta M, Toz H, Asci G, Wabel P, Kahvecioglu S, et al. Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: a randomized controlled trial. Am J Kidney Dis. 2013;61(6):957–65.
Grekas D, Bamichas G, Bacharaki D, Goutzaridis N, Kasimatis E, Tourkantonis A. Hypertension in chronic hemodialysis patients: current view on pathophysiology and treatment. Clin Nephrol. 2000;53(3):164–8.
Sinha AD, Agarwal R. What are the causes of the ill effects of chronic hemodialysis? The fallacy of low interdialytic weight gain and low ultrafiltration rate: lower is not always better. Semin Dial. 2014;27(1):11–3.
Hecking M, Karaboyas A, Antlanger M, Saran R, Wizemann V, Chazot C, et al. Significance of interdialytic weight gain versus chronic volume overload: consensus opinion. Am J Nephrol. 2013;38(1):78–90.
Wabel P, Moissl U, Chamney P, Jirka T, Machek P, Ponce P, et al. Towards improved cardiovascular management: the necessity of combining blood pressure and fluid overload. Nephrol Dial Transplant. 2008;23(9):2965–71.
Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, et al. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant. 2009;24(5):1574–9.
Agarwal R. Hypervolemia is associated with increased mortality among hemodialysis patients. Hypertension. 2010;56(3):512–7.
Zhu F, Kuhlmann MK, Sarkar S, Kaitwatcharachai C, Khilnani R, Leonard EF, et al. Adjustment of dry weight in hemodialysis patients using intradialytic continuous multifrequency bioimpedance of the calf. Int J Artif Organs. 2004;27(2):104–9.
Zhou YL, Liu J, Sun F, Ma LJ, Han B, Shen Y, et al. Calf bioimpedance ratio improves dry weight assessment and blood pressure control in hemodialysis patients. Am J Nephrol. 2010;32(2):109–16.
Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4(5):914–20.
Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66(3):1212–20.
O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371(7):612–23.
Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell’Italia LJ, et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009;54(3):475–81.
McMahon EJ, Bauer JD, Hawley CM, Isbel NM, Stowasser M, Johnson DW, et al. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol. 2013;24(12):2096–103.
Suckling RJ, He FJ, Macgregor GA. Altered dietary salt intake for preventing and treating diabetic kidney disease. Cochrane Database Syst Rev. 2010;12, CD006763.
Ramdeen G, Tzamaloukas AH, Malhotra D, Leger A, Murata GH. Estimates of interdialytic sodium and water intake based on the balance principle: differences between nondiabetic and diabetic subjects on hemodialysis. ASAIO J. 1998;44(6):812–7.
Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van WD, Bunnapradist S, Horwich TB, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. 2009;119(5):671–9.
Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 2011;79(2):250–7.
Krautzig S, Janssen U, Koch KM, Granolleras C, Shaldon S. Dietary salt restriction and reduction of dialysate sodium to control hypertension in maintenance haemodialysis patients. Nephrol Dial Transplant. 1998;13(3):552–3.
Charra B. Fluid balance, dry weight, and blood pressure in dialysis. Hemodial Int. 2007;11(1):21–31.
Appel LJ, Frohlich ED, Hall JE, Pearson TA, Sacco RL, Seals DR, et al. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the American Heart Association. Circulation. 2011;123(10):1138–43.
Tomson CR. Advising dialysis patients to restrict fluid intake without restricting sodium intake is not based on evidence and is a waste of time. Nephrol Dial Transplant. 2001;16(8):1538–42.
Ogden DA. A double blind crossover comparison of high and low sodium dialysis. Proc Clin Dial Transplant Forum. 1978;8:157–65.
Cybulsky AV, Matni A, Hollomby DJ. Effects of high sodium dialysate during maintenance hemodialysis. Nephron. 1985;41(1):57–61.
Santos SF, Peixoto AJ. Revisiting the dialysate sodium prescription as a tool for better blood pressure and interdialytic weight gain management in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3(2):522–30.
Manlucu J, Gallo K, Heidenheim PA, Lindsay RM. Lowering postdialysis plasma sodium (conductivity) to increase sodium removal in volume-expanded hemodialysis patients: a pilot study using a biofeedback software system. Am J Kidney Dis. 2010;56(1):69–76.
Daugirdas JT, Al-Kudsi RR, Ing TS, Norusis MJ. A double-blind evaluation of sodium gradient hemodialysis. Am J Nephrol. 1985;5(3):163–8.
Barre PE, Brunelle G, Gascon-Barre M. A randomized double blind trial of dialysate sodiums of 145 mEq/L, 150 mEq/L, and 155 mEq/L. ASAIO Trans. 1988;34(3):338–41.
Sang GL, Kovithavongs C, Ulan R, Kjellstrand CM. Sodium ramping in hemodialysis: a study of beneficial and adverse effects. Am J Kidney Dis. 1997;29(5):669–77.
Song JH, Lee SW, Suh CK, Kim MJ. Time-averaged concentration of dialysate sodium relates with sodium load and interdialytic weight gain during sodium-profiling hemodialysis. Am J Kidney Dis. 2002;40(2):291–301.
Munoz MJ, Sun S, Chertow GM, Moran J, Doss S, Schiller B. Dialysate sodium and sodium gradient in maintenance hemodialysis: a neglected sodium restriction approach? Nephrol Dial Transplant. 2011;26(4):1281–7.
de Paula FM, Peixoto AJ, Pinto LV, Dorigo D, Patricio PJ, Santos SF. Clinical consequences of an individualized dialysate sodium prescription in hemodialysis patients. Kidney Int. 2004;66(3):1232–8.
Davies S, Carlsson O, Simonsen O, Johansson AC, Venturoli D, Ledebo I, et al. The effects of low-sodium peritoneal dialysis fluids on blood pressure, thirst and volume status. Nephrol Dial Transplant. 2009;24(5):1609–17.
Tandon T, Sinha AD, Agarwal R. Shorter delivered dialysis times associate with a higher and more difficult to treat blood pressure. Nephrol Dial Transplant. 2013;28(6):1562–8.
Brunet P, Saingra Y, Leonetti F, Vacher-Coponat H, Ramananarivo P, Berland Y. Tolerance of haemodialysis: a randomized cross-over trial of 5-h versus 4-h treatment time. Nephrol Dial Transplant. 1996;11 Suppl 8:46–51.
Tattersall J, Martin-Malo A, Pedrini L, Basci A, Canaud B, Fouque D, et al. EBPG guideline on dialysis strategies. Nephrol Dial Transplant 2007;22 Suppl 2:ii5–21.
Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med. 2011;365(12):1099–107.
Twardowski ZJ. Treatment time and ultrafiltration rate are more important in dialysis prescription than small molecule clearance. Blood Purif. 2007;25(1):90–8.
Woods JD, Port FK, Orzol S, Buoncristiani U, Young E, Wolfe RA, et al. Clinical and biochemical correlates of starting “daily” hemodialysis. Kidney Int. 1999;55(6):2467–76.
Chan CT, Floras JS, Miller JA, Richardson RM, Pierratos A. Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int. 2002;61(6):2235–9.
Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA. 2007;298(11):1291–9.
Rocco MV, Lockridge Jr RS, Beck GJ, Eggers PW, Gassman JJ, Greene T, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80(10):1080–91.
Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363(24):2287–300.
Agarwal R. Frequent versus standard hemodialysis. N Engl J Med. 2011;364(10):975–6.
Heerspink HJ, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, Jardine MJ, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2009;373(9668):1009–15.
Agarwal R, Sinha AD. Cardiovascular protection with antihypertensive drugs in dialysis patients: systematic review and meta-analysis. Hypertension. 2009;53(5):860–6.
Levin NW, Kotanko P, Eckardt KU, Kasiske BL, Chazot C, Cheung AK, et al. Blood pressure in chronic kidney disease stage 5D-report from a kidney disease: improving global outcomes controversies conference. Kidney Int. 2010;77(4):273–84.
Brater DC. Diuretic therapy. N Engl J Med. 1998;339(6):387–95.
Hayashi SY, Seeberger A, Lind B, Gunnes S, Alvestrand A, do Nascimento MM, et al. Acute effects of low and high intravenous doses of furosemide on myocardial function in anuric haemodialysis patients: a tissue Doppler study. Nephrol Dial Transplant. 2008;23(4):1355–61.
Pickkers P, Hughes AD, Russel FG, Thien T, Smits P. Thiazide-induced vasodilation in humans is mediated by potassium channel activation. Hypertension. 1998;32(6):1071–6.
Eladari D, Chambrey R. Identification of a novel target of thiazide diuretics. J Nephrol. 2011;24(4):391–4.
Bennett WM, McDonald WJ, Kuehnel E, Hartnett MN, Porter GA. Do diuretics have antihypertensive properties independent of natriuresis? Clin Pharmacol Ther. 1977;22(5 Pt 1):499–504.
Foody JM, Farrell MH, Krumholz HM. beta-Blocker therapy in heart failure: scientific review. JAMA. 2002;287(7):883–9.
Teo KK, Yusuf S, Furberg CD. Effects of prophylactic antiarrhythmic drug therapy in acute myocardial infarction. An overview of results from randomized controlled trials. JAMA. 1993;270(13):1589–95.
Abbott KC, Trespalacios FC, Agodoa LY, Taylor AJ, Bakris GL. beta-Blocker use in long-term dialysis patients: association with hospitalized heart failure and mortality. Arch Intern Med. 2004;164(22):2465–71.
Cice G, Ferrara L, D’Andrea A, D’Isa S, Di BA, Cittadini A, et al. Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41(9):1438–44.
Mancia G, De BG, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25(6):1105–87.
Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Mbewu A, Opie LH. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2012;11, CD002003.
Denker MG, Cohen DL. Antihypertensive medications in end-stage renal disease. Semin Dial. 2015;28(4):330–6.
Agarwal R. Supervised atenolol therapy in the management of hemodialysis hypertension. Kidney Int. 1999;55(4):1528–35.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20.
Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown Jr EJ, Cuddy TE, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327(10):669–77.
Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349(20):1893–906.
Zheng S, Nath V, Coyne DW. ACE inhibitor-based, directly observed therapy for hypertension in hemodialysis patients. Am J Nephrol. 2007;27(5):522–9.
Saracho R, Martin-Malo A, Martinez I, Aljama P, Montenegro J. Evaluation of the Losartan in Hemodialysis (ELHE) Study. Kidney Int Suppl. 1998;68:S125–9.
Zannad F, Kessler M, Grunfeld JP, Thuilliez C. FOSIDIAL: a randomised placebo controlled trial of the effects of fosinopril on cardiovascular morbidity and mortality in haemodialysis patients. Study design and patients’ baseline characteristics. Fundam Clin Pharmacol. 2002;16(5):353–60.
Takahashi A, Takase H, Toriyama T, Sugiura T, Kurita Y, Ueda R, et al. Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis--a randomized study. Nephrol Dial Transplant. 2006;21(9):2507–12.
Suzuki H, Kanno Y, Sugahara S, Ikeda N, Shoda J, Takenaka T, et al. Effect of angiotensin receptor blockers on cardiovascular events in patients undergoing hemodialysis: an open-label randomized controlled trial. Am J Kidney Dis. 2008;52(3):501–6.
Tai DJ, Lim TW, James MT, Manns BJ, Tonelli M, Hemmelgarn BR. Cardiovascular effects of Angiotensin converting enzyme inhibition or Angiotensin receptor blockade in hemodialysis: a meta-analysis. Clin J Am Soc Nephrol. 2010;5(4):623–30.
Akbari A, Knoll G, Ferguson D, McCormick B, Davis A, Biyani M. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in peritoneal dialysis: systematic review and meta-analysis of randomized controlled trials. Perit Dial Int. 2009;29(5):554–61.
Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139(4):244–52.
Krediet RT. How to preserve residual renal function in patients with chronic kidney disease and on dialysis? Nephrol Dial Transplant. 2006;21 Suppl 2:ii42–ii6.
Li PK, Chow KM, Wong TY, Leung CB, Szeto CC. Effects of an angiotensin-converting enzyme inhibitor on residual renal function in patients receiving peritoneal dialysis. A randomized, controlled study. Ann Intern Med. 2003;139(2):105–12.
Suzuki H, Kanno Y, Sugahara S, Okada H, Nakamoto H. Effects of an angiotensin II receptor blocker, valsartan, on residual renal function in patients on CAPD. Am J Kidney Dis. 2004;43(6):1056–64.
Kjaergaard KD, Peters CD, Jespersen B, Tietze IN, Madsen JK, Pedersen BB, et al. Angiotensin blockade and progressive loss of kidney function in hemodialysis patients: a randomized controlled trial. Am J Kidney Dis. 2014;64(6):892–901.
Tepel M, Hopfenmueller W, Scholze A, Maier A, Zidek W. Effect of amlodipine on cardiovascular events in hypertensive haemodialysis patients. Nephrol Dial Transplant. 2008;23(11):3605–12.
London GM, Pannier B, Guerin AP, Marchais SJ, Safar ME, Cuche JL. Cardiac hypertrophy, aortic compliance, peripheral resistance, and wave reflection in end-stage renal disease. Comparative effects of ACE inhibition and calcium channel blockade. Circulation. 1994;90(6):2786–96.
Shibasaki Y, Masaki H, Nishiue T, Nishikawa M, Matsubara H, Iwasaka T. Angiotensin II type 1 receptor antagonist, losartan, causes regression of left ventricular hypertrophy in end-stage renal disease. Nephron. 2002;90(3):256–61.
Taylor AL, Ziesche S, Yancy C, Carson P, D’Agostino Jr R, Ferdinand K, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351(20):2049–57.
Chapman N, Dobson J, Wilson S, Dahlof B, Sever PS, Wedel H, et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49(4):839–45.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–17.
Pitt B, Williams G, Remme W, Martinez F, Lopez-Sendon J, Zannad F, et al. The EPHESUS trial: eplerenone in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction. Eplerenone Post-AMI Heart Failure Efficacy and Survival Study. Cardiovasc Drugs Ther. 2001;15(1):79–87.
Ni X, Zhang J, Zhang P, Wu F, Xia M, Ying G, et al. Effects of spironolactone on dialysis patients with refractory hypertension: a randomized controlled study. J Clin Hypertens (Greenwich). 2014;16(9):658–63.
Matsumoto Y, Mori Y, Kageyama S, Arihara K, Sugiyama T, Ohmura H, et al. Spironolactone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol. 2014;63(6):528–36.
Allen EV. Sympathectomy for essential hypertension. Circulation. 1952;6(1):131–40.
Schlaich MP, Bart B, Hering D, Walton A, Marusic P, Mahfoud F, et al. Feasibility of catheter-based renal nerve ablation and effects on sympathetic nerve activity and blood pressure in patients with end-stage renal disease. Int J Cardiol. 2013;168(3):2214–20.
Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373(9671):1275–81.
Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–401.
Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13.
Dorhout Mees EJ. Hypertension in haemodialysis patients: who cares? Nephrol Dial Transplant. 1999;14(1):28–30.
Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th annual fall conference and scientific sessions. Hypertension. 2005;45(4):811–7.
Kawamura M, Fijimoto S, Hisanaga S, Yamamoto Y, Eto T. Incidence, outcome, and risk factors of cerebrovascular events in patients undergoing maintenance hemodialysis. Am J Kidney Dis. 1998;31(6):991–6.
Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int. 1996;49(5):1379–85.
De Lima JJ, Lopes HF, Grupi CJ, Abensur H, Giorgi MC, Krieger EM, et al. Blood pressure influences the occurrence of complex ventricular arrhythmia in hemodialysis patients. Hypertension. 1995;26(6 Pt 2):1200–3.
Takeda A, Toda T, Fujii T, Shinohara S, Sasaki S, Matsui N. Discordance of influence of hypertension on mortality and cardiovascular risk in hemodialysis patients. Am J Kidney Dis. 2005;45(1):112–8.
Tomita J, Kimura G, Inoue T, Inenaga T, Sanai T, Kawano Y, et al. Role of systolic blood pressure in determining prognosis of hemodialyzed patients. Am J Kidney Dis. 1995;25(3):405–12.
Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, et al. “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int. 1998;54(2):561–9.
Port FK, Hulbert-Shearon TE, Wolfe RA, Bloembergen WE, Golper TA, Agodoa LY, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3):507–17.
Li Z, Lacson Jr E, Lowrie EG, Ofsthun NJ, Kuhlmann MK, Lazarus JM, et al. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis. 2006;48(4):606–15.
Lacson Jr E, Lazarus JM. The association between blood pressure and mortality in ESRD-not different from the general population? Semin Dial. 2007;20(6):510–7.
Udayaraj UP, Steenkamp R, Caskey FJ, Rogers C, Nitsch D, Ansell D, et al. Blood pressure and mortality risk on peritoneal dialysis. Am J Kidney Dis. 2009;53(1):70–8.
Stidley CA, Hunt WC, Tentori F, Schmidt D, Rohrscheib M, Paine S, et al. Changing relationship of blood pressure with mortality over time among hemodialysis patients. J Am Soc Nephrol. 2006;17(2):513–20.
Amar J, Vernier I, Rossignol E, Bongard V, Arnaud C, Conte JJ, et al. Nocturnal blood pressure and 24-hour pulse pressure are potent indicators of mortality in hemodialysis patients. Kidney Int. 2000;57(6):2485–91.
Bansal N, McCulloch CE, Rahman M, Kusek JW, Anderson AH, Xie D, et al. Blood pressure and risk of all-cause mortality in advanced chronic kidney disease and hemodialysis: the chronic renal insufficiency cohort study. Hypertension. 2015;65(1):93–100.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Sinha, A.D. (2016). Hypertension in the Dialysis Patient. In: Singh, A., Agarwal, R. (eds) Core Concepts in Hypertension in Kidney Disease. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-6436-9_7
Download citation
DOI: https://doi.org/10.1007/978-1-4939-6436-9_7
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-6434-5
Online ISBN: 978-1-4939-6436-9
eBook Packages: MedicineMedicine (R0)