Abstract
Accurate blood pressure (BP) measurement is essential in the evaluation and management of hypertensive patients. Office BP measurement must follow basic precepts to generate accurate readings. Home and ambulatory BP monitoring provide greater reliability and reproducibility than office readings and are associated with greater ability to predict hypertension-related outcomes in patients with hypertension, including those with chronic kidney disease, thus representing the preferred method for the diagnosis of hypertension. Although clinical trial evidence to support the use of home and ambulatory BP to guide treatment among patients with chronic kidney disease is sparse, observational data suggest that these techniques add value to the management of hypertensive patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Blood pressure measurement
- Blood pressure monitoring
- Home blood pressure
- Ambulatory blood pressure
- White coat hypertension
- Masked hypertension
- Chronic kidney disease
- Kidney transplantation
Hypertension is highly prevalent in chronic kidney disease (CKD). It is estimated that up to 80 % of patients have high blood pressure (BP) by the time they reach advanced stages of kidney disease (glomerular filtration rate <15 ml/min), and remains highly prevalent among dialysis [1] and kidney transplant [2] patients. Because of the importance of BP control to decrease cardiovascular risk and limit the progression of CKD, adequate assessment of hypertension is essential to the care of CKD patients. In this chapter, we review relevant aspects of the assessment of BP in the office and out-of-office environments in patients with CKD (not on dialysis) and with kidney transplants. Issues related to dialysis patients are discussed in Chap. 7.
General Elements of the Assessment of Hypertension Burden
Besides the careful measurement of BP, the evaluation of hypertension in patients with CKD should include the same general approach as used in any patient with hypertension. Other critical components of the clinical evaluation include the consideration of features that suggest secondary causes of hypertension other than CKD itself, the identification of comorbid conditions that may impact on treatment decisions, the discussion of lifestyle practices and preferences that may affect management, and the systematic evaluation of extra-renal target organ involvement, such as cerebrovascular disease, cognitive impairment, left ventricular hypertrophy, heart failure, coronary disease, and peripheral arterial disease.
Mounting evidence indicates that the objective assessment of extracellular fluid volume expansion and systemic hemodynamics can improve BP management. Such measurements can be obtained with different non-invasive technologies. Although it is cumbersome to directly measure extracellular fluid volume, various methods exist to estimate it indirectly and include bioimpedance, ultrasonographic measurement of inferior vena cava diameter and collapsibility with inspiration, estimation of right atrial pressure with hepatic vein flow patterns, or through thoracic ultrasound to estimate the amount of extravascular lung water. Systemic hemodynamics, on the other hand, can be easily determined non-invasively through echocardiography, impedance cardiography, bioreactance, and several oscillometric methods. Impedance cardiography can simultaneously measure volume (thoracic fluid content) and hemodynamic variables (cardiac output, systemic vascular resistance). In patients with resistant hypertension, use of this technology to guide treatment resulted in better BP control in two randomized trials [3, 4]. The experience in non-dialysis CKD is small, but some have called for more extensive use of formal volume assessment in the treatment of hypertension [5], particularly in the setting of kidney disease, where extracellular fluid volume expansion is common, and often covert.
Most relevant to the present discussion is the careful measurement of BP both in the office and in the home and ambulatory setting. The following sections will cover each of these elements in detail.
Principles of Blood Pressure Measurement
Adequate management decisions demand accurate BP measurement. Cuff-based brachial BP is the most commonly used method to measure BP, typically in the office setting. However, current evidence progressively points to the value of out-of-office BP methods, such as 24-hour BP monitoring (ABPM) and home BP monitoring, as superior methods to evaluate BP burden and BP-related risk in hypertensive patients [6–8].
Conventional Office Blood Pressure Measurement
BP measurement is traditionally made in the office using either the auscultatory technique or oscillometric method (manual or automated cuff following specific proprietary algorithms to impute systolic and diastolic BP). In some countries such as in the USA, mercury sphygmomanometers are now seldom available in clinical practice because of environmental concerns [9], so most measurements are made either with aneroid devices or electronic oscillometric manometers. Both types of manometers are accurate but should have periodic maintenance to ensure that they are properly calibrated. This is typically done every 12 months or anytime poor function is suspected or anticipated (such as following drop from height of the instrument).
Most patients should have their BP measured in the arm in the seated position [10]. In selected situations, such as malformations, injuries or vascular disease of the upper extremities, or when comparisons of BP levels in the upper and lower extremities is warranted, it may be necessary to use thigh measurements with an appropriately sized thigh cuff, which should be obtained in the supine position to allow the cuff to be at the level of the heart. Thigh cuffs are most easily used with an oscillometric automated device, but can also be used with the auscultatory method (Korotkoff sounds are auscultated in the popliteal fossa). Table 2.1 provides the essential elements of proper BP measurement in the office.
Inter-Arm Blood Pressure Differences
As noted in Table 2.1, patients should have their BP measured in both arms at the time of their initial evaluation and periodically thereafter. Differences >10 mmHg between arms can occur in a variable proportion of hypertensive patients (average ~14 %) [11]. Inter-arm BP differences had been thought to be due to occlusive arterial disease of the upper extremities, but this has not been confirmed by prospective imaging studies [11], and the underlying mechanisms remain uncertain, possibly related to vascular calcification and arterial stiffness. Regardless of this uncertainty there is general agreement that clinical decisions should be made based on BP levels from the arm with higher BP.
A recent meta-analysis indicates that the presence of an inter-arm SBP difference >10 mmHg is associated with a 2.7-fold increase in risk of fatal and non-fatal cardiovascular events in populations with increased vascular risk, including one with CKD [11]. Reproducibility of the difference, however, is limited. In one study of 443 patients with simultaneous bilateral measurements on two separate occasions, the reproducibility of an inter-arm difference >10 mmHg or >20 mmHg was only 27 % and 41 %, respectively [12]. Therefore, while recognizing inter-arm differences should be noted to optimize decisions on which BP level to use on a given visit, the limited reproducibility makes the prognostic implications of that difference still uncertain.
Pseudohypertension
Pseudohypertension is the detection of spuriously elevated BP due to poor compressibility of the brachial artery and its branches. In the past, the Osler maneuver, or palpation of the radial artery during cuff inflation above the systolic BP level, was purported to effectively diagnose pseudohypertension. However, several studies have repudiated this, and it is no longer considered to be a useful test [10]. Therefore, if pseudohypertension is being considered in a patient, the only definitive means of confirming it is through arterial cannulation and direct measurement of intra-arterial pressure.
The Auscultatory Gap
A common source of error when using the auscultatory method is the auscultatory gap, which consists of a prolonged period of disappearance of Korotkoff sounds after their initial appearance. Therefore, if the cuff is not inflated high enough, the observer may record an incorrectly low systolic BP. The auscultatory gap is most common in older patients with underlying vascular disease and systolic hypertension with wide pulse pressure [10]. It can be easily avoided by identification of the systolic BP through palpation of the radial or brachial artery so as to guarantee that the cuff is being inflated to a pressure that is above the systolic BP. The auscultatory gap does not occur with oscillometric BP mea surements.
Orthostatic Blood Pressure Measurement s
Orthostatic hypotension is common in patients treated for hypertension, especially in older patients (8–34 %) [13, 14]. It is recommended that standing BP be obtained as a screen for orthostatic hypotension in elderly patients with hypertension, as well as in patients at increased risk of autonomic dysfunction, such as those with diabetes and kidney disease [7, 15]. Orthostatic vital signs (heart rate and BP) should be obtained after at least 5 min in the supine position followed by immediate assumption of the standing position for up to 3 min [14]. The practical difficulties of following this method in a busy office cannot be ignored, so it is acceptable to compare values in the seated position with those after standing for 1 min. This method is less sensitive for the detection of orthostatic hypotension but is better than no measurement at all [14]. The definition of orthostatic hypotension is a drop in BP >20/10 mmHg after 3 min of standing [16]. Among patients with supine hypertension, the required systolic fall in BP for the diagnosis is >30 mmHg because the level of baseline supine BP is directly proportional to the orthostatic BP drop [14, 16]. Integration of the heart rate response to changes in BP with standing is important to guide the differential diagnosis and further evaluation of orthostatic hypotension. In the absence of medications that slow heart rate, the lack of a rise in heart rate by at least 20 bpm in response to hypotension suggests baroreflex or sympathetic autonomic dysfunction. Conversely, patients with an appropriate heart rate response likely have volume depletion or excessive vasodilatation.
Office BP Measurement During Exercise
BP measurement is necessary during exercise stress testing, which is commonly performed in the office setting. There are issues related to both the measurement and the interpretation of BP levels during exercise. BP measurement may be difficult during exercise; auscultatory measurements can be plagued by difficulties hearing Korotkoff sounds due to equipment noise, and many of the available automatic devices are inaccurate during exercise testing or have not been appropriately validated in this setting. As a general rule, the auscultatory method should be used preferentially, as it is less susceptible to systematic or random error during exercise. Oscillometric measurements are not recommended to assess BP response to exercise. Some automated devices are available that concurrently record Korotkoff sounds with EKG which enable better separation of signal from noise during exercise.
On average, systolic BP increases by ~10 mmHg per metabolic equivalent (MET) of exercise (~30 mmHg during the early stages of aerobic exercise and by 50–60 mmHg above baseline at peak exercise), with average increases higher in men than women [17]. Diastolic BP response is less adequately characterized; typically it stays the same or is slightly lower but may increase during exercise. Despite lack of formal guidelines, the generally accepted upper limit of BP during peak exercise is 210/110 mmHg for men and 190/110 mmHg for women [17].
Several studies suggest that the delayed rate of recovery of systolic BP after exercise has been associated with the presence of coronary artery disease.
Automated Office Blood Pressure Measurement
Multiple office BP measurements can now be performed automatically while the patient is alone in the room. The devices are programmed to perform several sequential readings (typically 6), discard the first reading, and provide an average that is used as the value for the visit. Using this method, the white coat effect is largely eliminated [18, 19]. In addition, this automated approach results in better correlations with ambulatory BP averages and left ventricular mass than routine office BP [19, 20]. Obviously, this may lead to significant slowing of patient flow in physician offices, but if planned appropriately, can be performed as the patient waits while the clinician see other patients. Using this technology is particularly relevant for patients who are being treated for hypertension and those who cannot or do not want to perform self-measured BP monitoring in the home environment (see below). This idea was initially launched by the BpTRU company (and the method is often referred to as “the BpTRU”), but others are now available on the market such as the Omron HEM-907 and the Welch-Allyn ProBP 2400.
Out-of-Office Blood Pressure Monitoring
Even though office BP has been the most commonly used measure to guide hypertension diagnosis and treatment, growing evidence indicates that out-of-office techniques (home BP and ABPM) are better markers of hypertension-related risk and as such, have been increasingly used in research and clinical practice. Indications for home BP and ABPM are listed in Table 2.2 and a summary of the advantages and shortcomings of these monitoring methods is presented in Table 2.3.
Home Blood Pressure Monitoring
Home BP monitoring is performed by the patient in the home and/or work environment and is increasingly used in practice; as many as 65 % of patients with hypertension own a home monitor [21], although accessibility to low-income patients is still a problem despite the availability of low cost devices ($40–50) and coverage by many healthcare insurance plans.
Just as with office BP, it is important that the equipment works properly and fits the patient well. Home BP measurements should be obtained using the same attention to technique as described for office BP (see Table 2.1). In general, it is preferred that the automatic oscillometric method be used for self-measured BP. Unfortunately, many of the marketed devices have not been appropriately validated and may not provide accurate readings. A list of independently validated devices can be found at www.dableducational.org. The preferred devices use arm cuffs. Finger cuffs are inaccurate and wrist cuffs often provide incorrect readings because of inappropriate technique. As a result, only arm devices are recommended by current guidelines [21, 22].
The reliability of reporting of home BP values by patients has been questioned in the past. This problem has been circumvented by the universal availability of a memory function in automated BP devices. Therefore, if the clinician has concerns about the values being reported, he can ask the patient to bring the machine and personally review the values recorded in the device memory.
For most patients, a BP log obtained over 7 days before each office visit provides reproducible information that allows good prognostication and treatment decisions [22]. We instruct our patients to obtain readings in duplicate (about 1 min apart), twice daily (in the morning before taking medications and in the evening before dinner) for a 7-day period. In some clinical situations, more frequent or more prolonged monitoring may be needed. For example, patients with symptoms suggestive of intermittent hypotension may benefit from BP measurements during peak action of medications, such as in the mid-to-late morning or late evening, depending on the time when medications are taken. Patients with wide fluctuations in BP can be monitored more often to better quantify the overall BP variability, though we prefer to use 24-hour ABPM in such patients. Detailed guidelines on the use of home BP are available from the European Society of Hypertension [22] and the American Heart Association [21].
Normative values for home BP based on observed outcomes are now available [23]. These threshold levels were established using the observed cardiovascular event rates equivalent to those observed for office BP of 120/80 mmHg (“optimal”) and 140/90 mmHg (“hypertension”) in a large multinational cohort of patients [23]. Using this approach, the currently accepted level of “optimal” home BP is 121/78 mmHg, and the level defining “hypertension” is 133/82 mmHg (see Table 2.4).
Ambulatory Blood Pressure Monitoring
ABPM combines the ability to evaluate BP in the ambulatory setting with the unique feature of allowing the measurement of BP during sleep, which, as will be discussed below, provides additive prognostic information. ABPM is performed with a validated automated device (for a list, refer to www.dableducational.org) that is fitted on the patient using an appropriately sized cuff. ABP is usually performed over a 24-hour period, although most devices can run for longer periods of time as allowed by battery life and number of readings stored in the memory. In some clinical situations, 48-hour monitoring is quite useful, such as in patients undergoing hemodialysis, so that the entire interdialytic period can be evaluated. The device is programmed to inflate periodically; a typical measurement interval is every 20 min during the daytime (7 AM–11 PM) and every 30 min at night (11 PM–7 AM), though these schedules can be adjusted based on individual needs. The patient keeps a log of activities during the monitoring period including the time of going to bed and waking up and time of taking antihypertensive medications (if any). It is preferred that the periods designated as “night and day” reflect the actual periods of sleep and wakefulness obtained from the patient’s diary. Most patients accept ABPM well, although sometimes sleep is affected (<10 % of cases) and rarely, patients have bruising or pain from the frequent cuff inflations. Up-to-date guidelines that include practical information on ABPM are available from the European Society of Hypertension [24, 25].
The generally accepted indications for ABPM are listed in Table 2.2. The most commonly used indication is to rule out white coat hypertension . In fact, it is this property that has made ABPM recommended for definitive initial diagnosis of hypertension by the British Hypertension Society [6] and the United States Preventive Services Task Force [8]. Another important clinical use is in the evaluation of patients with resistant hypertension. Mounting evidence indicates that almost 40 % of patients with “office resistance” have controlled BP levels on ABPM, i.e., “office resistance” [26]. Identification of patients with true resistance is important to identify those with increased risk of adverse cardiovascular and renal outcomes [27, 28].
Similar to home BP, outcomes-based normative values are available for ABPM (Table 2.4) [29]. When interpreting an ABPM recording, the clinician needs to take into account the total number of successful measurements; a generally accepted minimum of valid readings is 20 during wakefulness and 7 during sleep [25]. The key elements of the 24-hour BP profile are the awake, asleep, and overall 24-hour BP levels, as these are the prognostic determinants in hypertension.
The blood pressure decline during sleep (“dipping”) is also calculated as the ratio between the asleep and awake BP. Normally, BP declines by ~15 % during sleep (i.e., a night/day ratio of 0.85). When evaluating the circadian BP profile based on the behavior of BP during sleep, four patterns are described:
-
1.
Dipper: normal BP decline during sleep, arbitrarily defined as between 10 and 20 %.
-
2.
Non-dipper: smaller than normal BP decline during sleep (between 0 and 10 %). This pattern is observed in 20–25 % of patients with essential hypertension, and with increasing frequency in patients with cardiovascular disease, kidney disease, and other causes of secondary hypertension.
-
3.
Reverse dipper (also called “Riser”): BP increases during sleep. This pattern is often observed in patients with advanced kidney disease, sleep apnea, or autonomic dysfunction.
-
4.
Extreme dipper: greater than normal BP fall during sleep (>20 %).
In large observational studies, extreme dippers have lower fatal and non-fatal cardiovascular event rates than those whose BP decreases by less <20 %. Reverse dippers, on the other hand, have significantly worse cardiovascular outcomes than all other patients [30].
Most software packages provide information on the 24-hour BP variability (defined as the standard deviation of systolic and diastolic BP for each of the monitoring periods) and the BP load (percentage of readings above a certain threshold). Although some data have linked high BP variability and high BP load to adverse outcomes, they do not appear to provide additional information beyond what is obtained from average BP values [31], so we give limited relevance to these values.
Integrating Home BP and ABPM into Clinical Decision Making
In deciding between home BP and ABPM , the clinician must take into account availability, costs, and patient preferences. For the initial evaluation of the patient, home BP is an adequate method, particularly in the primary care setting. In subspecialty practices, however, ABPM is more easily available and is particularly useful for patients with borderline home BP values. A systematic review of 20 studies compared the agreement between office, home and ABPM according to different BP thresholds [32]. Using a 24-hour BP average of 135/85 mmHg as the definition of HTN, an office BP of 140/90 mmHg has a sensitivity of 75 % (95 % CI, 61–85 %) and specificity of 75 % (95 % CI, 48–90 %) for the diagnosis of HTN. Likewise, a home BP average of 135/85 mmHg has a sensitivity of 86 % (95 % CI, 78–91 %) and a specificity of 62 % (95 % CI, 48–75 %) for the diagnosis. Therefore, neither office nor home BP has sufficient sensitivity or specificity for the diagnosis of HTN based on this analysis [32]. However, the use of different thresholds can produce adequate predictive values (positive and negative) that allow home BP to be integrated into the decision to obtain ABPM or not with greater precision [21]. A structured approach to this decision-making process is summarized below [21]:
-
If office BP >140/90 mmHg, perform home BP monitoring.
-
If home BP <125/76, continue to monitor (or continue same treatment).
-
If home BP >135/85 mmHg, start treatment (or escalate therapy).
-
If home BP between 125/76 and 135/85 mmHg, obtain ABPM.
-
If 24-hour ABPM average <130/80 mmHg, continue same strategy. If higher, start or increase treatment.
Prognostic Relevance of Out-of-Office BP
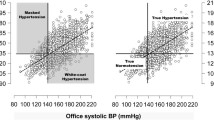
Compared to isolated office BP measurements, home BP and ABPM generally demonstrate stronger associations with target organ damage (especially left ventricular hypertrophy and proteinuria) and cardiovascular and renal endpoints in hypertension [6, 33, 34]. There are several possible reasons for the better prognostic performance of home BP and ABPM. For example, home BP and ABPM include more readings, thus leading have lower variability and higher reproducibility of results, thus leading to a more precise determination of BP levels. Moreover, both techniques allow the detection of the white coat (high BP in the office, normal at home) and masked effects (normal BP in the office, high at home), which better reflect overall BP burden to the patient. White coat hypertension affects 20–30 % of patients with a diagnosis of office hypertension [35] and has generally been associated with similar cardiovascular outcomes as normotensive individuals [36], although recent data from the International Database of Home Blood Pressure in Relation to Cardiovascular Outcome (IDHOCO ) indicated a 42 % increase in risk of CV events compared with those with normal BP in the office and at home, especially among untreated patients [37]. Interestingly, treated hypertensive patients who retained a “white coat effect” had the same overall risk as treated patients whose BP was controlled both at home and in the office. Masked hypertension , on the other hand, has a prevalence of 10–15 % in population studies and has been consistently associated with increased risk for adverse cardiovascular endpoints and mortality to a level that is identical to that of sustained hypertension [36]. Lastly, ABPM allows assessment of BP during sleep. Nighttime BP is a generally a slightly better marker of cardiovascular risk than daytime or 24-hour average BP [38–40].
In a meta-analysis of studies that took into account both office and ABPM in the assessment of cardiovascular events and mortality, only ABPM values, not office, were significant predictors of outcomes [39]. Along the same lines, a large cohort study that included simultaneous use of office and home BP to predict cardiovascular events and mortality, only home BP values were significant markers of risk [41]. The superiority of out-of-office methods over office BP has also been demonstrated in patients with resistant hypertension [28, 42], chronic kidney disease [27, 43–46], hemodialysis [47], and in the general population [48–50].
Clinical trials testing the use of office and out-of-office BP during hypertension treatment, however, have been unable to show any differences with respect to BP control or changes in left ventricular mass [51–53]. However, these studies were all relatively small and limited to 6–12 months in duration. A detailed analysis for the United States Agency for Healthcare Research and Quality demonstrated that very large sample sizes would be required for definitive clinical trials comparing office and home BP (between 6500 and 59,000 subjects followed for 10 years depending on the assumptions of baseline risk and differences between the two groups) [54], making it doubtful that such a randomized trial will be performed.
In summary, prospective cohort studies convincingly show the superiority of home BP and ABPM over office BP measurements for the diagnosis of hypertension and to predict hypertension-related outcomes. This evidence is already being incorporated into clinical practice guidelines for diagnosis of hypertension. Because it is unlikely that a definitive clinical trial will ever be performed in the treatment realm, decisions to use out-of-office BP for hypertension management are now made primarily on the basis of circumstantial evidence. We feel strongly that their use is warranted and our practice is to routinely use out-of-office BP as a guide to hypertension treatment. However, we acknowledge our practice is based on observational and not on clinical trial evidence.
Out-of-Office BP in Chronic Kidney Disease
Patients with CKD have a high prevalence of abnormal diurnal BP profiles, with decreased nocturnal BP dip [55]. Attention to this was raised by a landmark study in 1991 showing uniformly blunted circadian BP profiles in patients with CKD not on dialysis, patients on hemodialysis, and patients with a kidney transplant compared with controls matched for age, sex, office systolic BP and presence/absence of antihypertensive drug therapy [56]. Average dipping during sleep (SBP%/DBP%) was 7 %/11 % in CKD patients (vs. 18 %/24 % in controls), 4 %/8 % in hemodialysis patients (vs. 14 %/24 % in controls), and 5 %/9 % in transplantation patients (vs. 13 %/18 % in controls) [56]. Since then, many studies have confirmed these observations. In an important analysis of the African American Study of Kidney Disease (AASK ) in patients with hypertensive nephrosclerosis with an average GFR of 44 ml/min/1.73 m2, there was an 80 % combined prevalence of non-dipping (41 %) or reverse dipping (39 %) [57]. Another large cohort study of CKD patients with less severe loss of renal function (average eGFR 59 ml/min/1.73 m2) showed a 61 % prevalence of non-dipping [58]. The prevalence of non-dipping, and more importantly, reverse dipping, which is associated with the highest cardiorenal risk, increases as eGFR falls [59] (see Fig. 2.1).
Relative distribution of circadian BP profiles in patients with CKD. Prevalence of dipping classifications in terms of the sleep-time relative SBP decline—≥20 % (extreme-dipper), 10–20 % (dipper), 0–10 % (non-dipper), <0 % (riser)—of hypertensive patients with CKD in relation to stage (disease severity)—Stage 1: GFR ≥90 ml/min/1.73 m2; Stage 2: GFR 60–89 ml/min/1.73 m2; Stage 3A: GFR 45–59 ml/min/1.73 m2; Stage 3B: GFR 30–44 ml/min/1.73 m2; Stage 4: GFR 15–29 ml/min/1.73 m2; Stage 5: GFR <15 ml/min/1.73 m2. Reproduced with permission from Hermida R et al., Nephrol Dial Transplant. 2014;29:1160–7
As in essential hypertension, home BP and ABPM have been tested in their predictive ability for adverse clinical outcomes in CKD. Several small studies suggested that the rate of loss of renal function and/or increase in proteinuria was greater in non-dipping than dipping patients with different causes of CKD [60], diabetic nephropathy [61], and IgA nephropathy [62]. However, more recent, larger studies have not confirmed the relevance of non-dipping to CKD progression after adjustments for average BP and other factors [43, 45].
The evaluation of risk of progression to dialysis or death was performed in a study of 217 male patients with CKD due to multiple etiologies, mostly diabetes and hypertension (baseline eGFR 45 ml/min/1.73 m2) [44]. After a median follow-up of 3.5 years, one standard deviation of home systolic BP (21 mmHg) was associated with 84 % increased risk (95 % CI = 1.46–2.32) of death or progression to ESRD after multiple relevant adjustments including office BP [44]. In a companion paper published shortly thereafter, the same authors presented ABPM data for the same cohort showing that one standard deviation increase in 24-hour systolic BP (17 mmHg) resulted in a 62 % (95 % CI—1.21–2.18) increase in risk of dialysis or death after adjustment for clinic BP [43]. However, this prognostic advantage did not remain significant after adjustment for other clinical factors. Of the individual components of ABPM, only nighttime systolic BP was a significant predictor of death and dialysis risk on adjusted analyses (hazard ratio 1.79 for ESRD or death, 1.90 for death) [43].
In a multicenter study of 436 patients with CKD of varying etiologies, mostly hypertension, diabetes, and tubulointerstitial diseases (baseline eGFR 43 ml/min/1.73 m2), only ABPM, not office BP, was associated with cardiovascular events, progression to dialysis or death during 4.2 years of follow-up [46]. The same group of investigators recently published further data on outcomes based on the degree of BP control in the office, on ABPM, both or neither [63]. They considered office BP as being at goal if less than 140/90 mmHg, whereas ABPM was considered at goal if daytime BP was <135/85 mmHg and nighttime BP was <120/70 mmHg. Among the 489 study subjects, 17 % were controlled both at home and office, 22 % were controlled only on ABPM (i.e., a white coat effect), 15 % only in the office (i.e., a masked effect), and 47 % on neither (“uncontrolled”). The group with a “white coat effect” had similar risk of cardiovascular events, dialysis, and death as the referent group (controlled BP in both settings). Conversely, the “masked effect” and uncontrolled groups had 2.3 to 3.9-fold greater risk of negative outcomes than patients with controlled BP [63].
In a 5-year longitudinal analysis of 617 African-American patients with hypertensive nephrosclerosis found ABPM to be better than office BP for prediction of loss of renal function and cardiovascular events [45]. Both daytime and nighttime BPs were associated with increased risk of cardiovascular events despite adjustments for office BP and other variables. On the other hand, ABPM values were only associated with the composite renal endpoint (doubling of serum creatinine, dialysis, or death) in patients with controlled office BP (systolic BP <130 mmHg) [45].
In summary, similar to the general population, out-of-office BP measurements are better predictors of renal and cardiovascular outcomes in patients with CKD. However, the available data are not as strong as the studies are not as well powered as studies in other hypertensive populations, and several inconsistencies remain with respect to the prognostic role of individual ambulatory BP variables (i.e., daytime vs. nighttime vs. 24-hour average vs. dipping status).
Out-of-Office BP in Kidney Transplantation
Hypertension is present in the majority of kidney transplant recipients [64]. A recent study found only 16 % of recipients to be normotensive without the need for antihypertensive therapy [65]. This study also showed poor BP control in 44 %, while 10 % had white coat hypertension and 18 % had masked hypertension . Additionally, only 16 % of the recipients had a normal nocturnal dipping blood pressure pattern. This increased incidence of hypertension is in part a consequence of the immunosuppression regimen. In particular, corticosteroids and the calcineurin inhibitors (cyclosporine more so than tacrolimus) are associated with hypertension [66]. Furthermore, and consistent with native kidney disease, hypertension can be both a cause and a consequence of allograft renal insufficiency. A study addressing BP control by ABPM and office BP in 868 kidney transplant recipients found that only 34 % of participants had controlled ambulatory BP [64]. Circadian BP patterns showed a high proportion of reverse dippers (48 %) in addition to 34 % non-dippers, and only a small proportion (14 %) of normal dippers [64]. Another study compared office BP and ABPM in patients with CKD and patients with a kidney transplant [67]. The investigators hypothesized that the immunosuppressants would lead to a greater degree of hypertension compared to patients with CKD and similar levels of renal insufficiency. While the office-based BP levels were similar, ABPM identified a significant difference in both awake and asleep BP between the two groups (higher in transplant), and transplant recipients less often had a normal diurnal BP rhythm (21 % were dippers compared with 34 % of the CKD patients) [67]. In summary, nocturnal hypertension and non-dipping are common in transplant recipients, and likely occur to a greater extent than in patients with similar degrees of kidney dysfunction without a transplant.
Home BP monitoring has also been evaluated in kidney transplantation. Consistent with data from the general population, home BP in kidney transplant recipients better approximated ABPM than office BP readings (72 % concordance versus 54 %) [68]. Moreover, compared with ABPM reference data, HBPM was both more sensitive and specific at detecting hypertension than office-based BP measurements for the recipients studied.
Limited data are available to compare the prognostic relevance of out-of-office versus office BP in renal transplant recipients. ABPM correlates better with left ventricular mass than office BP in renal transplant patients [69, 70]. Small prospective studies have shown stronger associations between ABPM-derived BP values and serum creatinine levels [71, 72] and vascular injury in the allograft [72], although not all studies have corroborated this [73]. The only long-term study evaluating graft failure and cardiovascular events in renal transplant patients included 126 patients followed for 46 months [74]. In this study, the presence of a reverse dipper pattern on ABPM was associated with a 3.6-fold increase in risk of loss of allograft or cardiovascular event during follow-up (P = 0.02). Neither office BP nor other measures derived from ABPM were associated with the outcomes in question [74]. In summary, the strength of the association between ABPM levels and clinical outcomes in renal transplantation is weak.
One relevant point related to renal transplantation is the increasing use of ABPM to evaluate potential kidney donors, as several studies indicate that donor candidates with hypertension are at risk for worsened BP control following kidney donation [75–77] and the prevalence of white coat hypertension may be as high as 62 % in this patient group [78, 79]. Therefore, the use of ABPM allows for better risk stratification prior to donation.
References
Peixoto AJ, Santos SF. Blood pressure management in hemodialysis: what have we learned? Curr Opin Nephrol Hypertens. 2010;19(6):561–6.
Weir MR, Burgess ED, Cooper JE, Fenves AZ, Goldsmith D, McKay D, et al. Assessment and management of hypertension in transplant patients. J Am Soc Nephrol. 2015;26:1248–60.
Smith RD, Levy P, Ferrario CM, Consideration of Noninvasive Hemodynamic Monitoring to Target Reduction of Blood Pressure Levels Study Group. Value of noninvasive hemodynamics to achieve blood pressure control in hypertensive subjects. Hypertension. 2006;47(4):771–7.
Taler SJ, Textor SC, Augustine JE. Resistant hypertension: comparing hemodynamic management to specialist care. Hypertension. 2002;39(5):982–8.
Covic A, Voroneanu L, Goldsmith D. Routine bioimpedance-derived volume assessment for all hypertensives: a new paradigm. Am J Nephrol. 2014;40(5):434–40.
National Institute for Health and Clinical Excellence. The clinical management of primary hypertension in adults: clinical guideline 127. NICE, 2011.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281–357.
Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162(3):192–204.
United States Environmental Protection Agency. Eliminating mercury in hospitals. 2002 November 2002. Report No.
Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45(1):142–61.
Clark CE, Aboyans V. Interarm blood pressure difference: more than an epiphenomenon. Nephrol Dial Transplant. 2015;30(5):695–7.
Mehlsen J, Wiinberg N. Interarm difference in blood pressure: reproducibility and association with peripheral vascular disease. Int J Vas Med. 2014;2014:841542.
Judd E, Calhoun DA. Hypertension and orthostatic hypotension in older patients. J Hypertens. 2012;30(1):38–9.
Shibao C, Lipsitz LA, Biaggioni I, American Society of Hypertension Writing Group. Evaluation and treatment of orthostatic hypotension. J Am Soc Hypertens. 2013;7(4):317–24.
Wheeler DC, Becker GJ. Summary of KDIGO guideline. What do we really know about management of blood pressure in patients with chronic kidney disease? Kidney Int. 2013;83(3):377–83.
Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69–72.
Sharman JE, LaGerche A. Exercise blood pressure: clinical relevance and correct measurement. J Hum Hypertens. 2015;29(6):351–8.
Myers MG. Automated blood pressure measurement in routine clinical practice. Blood Press Monit. 2006;11(2):59–62.
Myers MG. The great myth of office blood pressure measurement. J Hypertens. 2012;30(10):1894–8.
Andreadis EA, Agaliotis GD, Angelopoulos ET, Tsakanikas AP, Chaveles IA, Mousoulis GP. Automated office blood pressure and 24-h ambulatory measurements are equally associated with left ventricular mass index. Am J Hypertens. 2011;24(6):661–6.
Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52(1):1–9.
Parati G, Stergiou GS, Asmar R, Bilo G, de Leeuw P, Imai Y, et al. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24(12):779–85.
Niiranen TJ, Asayama K, Thijs L, Johansson JK, Ohkubo T, Kikuya M, et al. Outcome-driven thresholds for home blood pressure measurement: international database of home blood pressure in relation to cardiovascular outcome. Hypertension. 2013;61(1):27–34.
O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31(9):1731–68.
Parati G, Stergiou G, O’Brien E, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32(7):1359–66.
de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57(5):898–902.
De Nicola L, Gabbai FB, Agarwal R, Chiodini P, Borrelli S, Bellizzi V, et al. Prevalence and prognostic role of resistant hypertension in chronic kidney disease patients. J Am Coll Cardiol. 2013;61(24):2461–7.
Salles GF, Cardoso CR, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168(21):2340–6.
Kikuya M, Hansen TW, Thijs L, Bjorklund-Bodegard K, Kuznetsova T, Ohkubo T, et al. Diagnostic thresholds for ambulatory blood pressure monitoring based on 10-year cardiovascular risk. Circulation. 2007;115(16):2145–52.
Fagard RH, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Night-day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Hum Hypertens. 2009;23(10):645–53.
Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Bjorklund-Bodegard K, et al. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55(4):1049–57.
Hodgkinson J, Mant J, Martin U, Guo B, Hobbs FD, Deeks JJ, et al. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ. 2011;342:d3621.
Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30(7):1289–99.
Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep. 2013;15(11):413.
Kollias A, Ntineri A, Stergiou GS. Is white-coat hypertension a harbinger of increased risk? Hypertens Res. 2014;37(9):791–5.
Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta analysis. Am J Hypertens. 2011;24(1):52–8.
Stergiou GS, Asayama K, Thijs L, Kollias A, Niiranen TJ, Hozawa A, et al. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63(4):675–82.
Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51(1):55–61.
Conen D, Bamberg F. Noninvasive 24-h ambulatory blood pressure and cardiovascular disease: a systematic review and meta-analysis. J Hypertens. 2008;26(7):1290–9.
Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Bjorklund-Bodegard K, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370(9594):1219–29.
Niiranen TJ, Hanninen MR, Johansson J, Reunanen A, Jula AM. Home-measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn-Home study. Hypertension. 2010;55(6):1346–51.
Redon J, Campos C, Narciso ML, Rodicio JL, Pascual JM, Ruilope LM. Prognostic value of ambulatory blood pressure monitoring in refractory hypertension: a prospective study. Hypertension. 1998;31(2):712–8.
Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69(7):1175–80.
Agarwal R, Andersen MJ. Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69(2):406–11.
Gabbai FB, Rahman M, Hu B, Appel LJ, Charleston J, Contreras G, et al. Relationship between ambulatory BP and clinical outcomes in patients with hypertensive CKD. Clin J Am Soc Nephrol. 2012;7(11):1770–6.
Minutolo R, Agarwal R, Borrelli S, Chiodini P, Bellizzi V, Nappi F, et al. Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch Intern Med. 2011;171(12):1090–8.
Agarwal R, Peixoto AJ, Santos SF, Zoccali C. Out-of-office blood pressure monitoring in chronic kidney disease. Blood Press Monit. 2009;14(1):2–11.
Kikuya M, Ohkubo T, Asayama K, Metoki H, Obara T, Saito S, et al. Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension. 2005;45(2):240–5.
Ohkubo T, Imai Y, Tsuji I, Nagai K, Kato J, Kikuchi N, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama. Jpn J Hypertens. 1998;16(7):971–5.
Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111(14):1777–83.
Staessen JA, Byttebier G, Buntinx F, Celis H, O’Brien ET, Fagard R. Antihypertensive treatment based on conventional or ambulatory blood pressure measurement. A randomized controlled trial. Ambulatory Blood Pressure Monitoring and Treatment of Hypertension Investigators. JAMA. 1997;278(13):1065–72.
Staessen JA, Den Hond E, Celis H, Fagard R, Keary L, Vandenhoven G, et al. Antihypertensive treatment based on blood pressure measurement at home or in the physician’s office: a randomized controlled trial. JAMA. 2004;291(8):955–64.
Verberk WJ, Kroon AA, Lenders JW, Kessels AG, van Montfrans GA, Smit AJ, et al. Self-measurement of blood pressure at home reduces the need for antihypertensive drugs: a randomized, controlled trial. Hypertension. 2007;50(6):1019–25.
Uhlig K, Patel K, Concannon TW, Balk EM, Ratichek SJ, Chang LKW, et al. Self-measured blood pressure: future research needs: identification of future research needs from comparative effectiveness review No 45. AHRQ Future Research Needs Papers. Rockville; 2012.
Peixoto AJ, White WB. Ambulatory blood pressure monitoring in chronic renal disease: technical aspects and clinical relevance. Curr Opin Nephrol Hypertens. 2002;11(5):507–16.
Baumgart P, Walger P, Gemen S, von Eiff M, Raidt H, Rahn KH. Blood pressure elevation during the night in chronic renal failure, hemodialysis and after renal transplantation. Nephron. 1991;57(3):293–8.
Pogue V, Rahman M, Lipkowitz M, Toto R, Miller E, Faulkner M, et al. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53(1):20–7.
Mojon A, Ayala DE, Pineiro L, Otero A, Crespo JJ, Moya A, et al. Comparison of ambulatory blood pressure parameters of hypertensive patients with and without chronic kidney disease. Chronobiol Int. 2013;30(1-2):145–58.
Hermida RC, Smolensky MH, Ayala DE, Fernandez JR, Moya A, Crespo JJ, et al. Abnormalities in chronic kidney disease of ambulatory blood pressure 24 h patterning and normalization by bedtime hypertension chronotherapy. Nephrol Dial Transplant. 2014;29(6):1160–7.
Timio M, Venanzi S, Lolli S, Lippi G, Verdura C, Monarca C, et al. “Non-dipper” hypertensive patients and progressive renal insufficiency: a 3-year longitudinal study. Clin Nephrol. 1995;43(6):382–7.
Farmer CK, Goldsmith DJ, Quin JD, Dallyn P, Cox J, Kingswood JC, et al. Progression of diabetic nephropathy--is diurnal blood pressure rhythm as important as absolute blood pressure level? Nephrol Dial Transplant. 1998;13(3):635–9.
Csiky B, Kovacs T, Wagner L, Vass T, Nagy J. Ambulatory blood pressure monitoring and progression in patients with IgA nephropathy. Nephrol Dial Transplant. 1999;14(1):86–90.
Minutolo R, Gabbai FB, Agarwal R, Chiodini P, Borrelli S, Bellizzi V, et al. Assessment of achieved clinic and ambulatory blood pressure recordings and outcomes during treatment in hypertensive patients with CKD: a multicenter prospective cohort study. Am J Kidney Dis. 2014;64(5):744–52.
Fernandez Fresnedo G, Franco Esteve A, Gómez Huertas E, Cabello Chaves V, Díz Gómez JM, Osorio Moratalla JM, et al. Ambulatory blood pressure monitoring in kidney transplant patients: RETENAL study. Transplant Proc. 2012;44(9):2601–2.
Czyżewski Ł, Wyzgał J, Kołek A. Evaluation of selected risk factors of cardiovascular diseases among patients after kidney transplantation, with particular focus on the role of 24-hour automatic blood pressure measurement in the diagnosis of hypertension: an introductory report. Ann Transplant. 2014;19:188–98.
Miller LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant. 2002;2(9):807–18.
Azancot MA, Ramos N, Moreso FJ, Ibernon M, Espinel E, Torres IB, et al. Hypertension in chronic kidney disease: the influence of renal transplantation. Transplantation. 2014;98(5):537–42.
Agena F, Prado Edos S, Souza PS, da Silva GV, Lemos FB, Mion D, et al. Home blood pressure (BP) monitoring in kidney transplant recipients is more adequate to monitor BP than office BP. Nephrol Dial Transplant. 2011;26(11):3745–9.
Covic A, Segall L, Goldsmith DJ. Ambulatory blood pressure monitoring in renal transplantation: should ABPM be routinely performed in renal transplant patients? Transplantation. 2003;76(11):1640–2.
Ferreira SR, Moises VA, Tavares A, Pacheco-Silva A. Cardiovascular effects of successful renal transplantation: a 1-year sequential study of left ventricular morphology and function, and 24-hour blood pressure profile. Transplantation. 2002;74(11):1580–7.
Jacobi J, Rockstroh J, John S, Schreiber M, Schlaich MP, Neumayer HH, et al. Prospective analysis of the value of 24-hour ambulatory blood pressure on renal function after kidney transplantation. Transplantation. 2000;70(5):819–27.
Wadei HM, Amer H, Taler SJ, Cosio FG, Griffin MD, Grande JP, et al. Diurnal blood pressure changes one year after kidney transplantation: relationship to allograft function, histology, and resistive index. J Am Soc Nephrol. 2007;18(5):1607–15.
Haydar AA, Covic A, Agharazili M, Jayawardene S, Taylor J, Goldsmith DJ. Systolic blood pressure diurnal variation is not a predictor of renal target organ damage in kidney transplant patients. Am J Transplant. 2003;4(2):244–7.
Ibernon M, Moreso F, Sarrias X, Sarrias M, Grinyo JM, Fernandez-Real JM, et al. Reverse dipper pattern of blood pressure at 3 months is associated with inflammation and outcome after renal transplantation. Nephrol Dial Transplant. 2012;27(5):2089–95.
Anderson CF, Velosa JA, Frohnert PP, Torres VE, Offord KP, Vogel JP, et al. The risks of unilateral nephrectomy: status of kidney donors 10 to 20 years postoperatively. Mayo Clin Proc. 1985;60(6):367–74.
Torres VE, Offord KP, Anderson CF, Velosa JA, Frohnert PP, Donadio JV, et al. Blood pressure determinants in living-related renal allograft donors and their recipients. Kidney Int. 1987;31(6):1383–90.
Talseth T, Fauchald P, Skrede S, Djøseland O, Berg KJ, Stenstrøm J, et al. Long-term blood pressure and renal function in kidney donors. Kidney Int. 1986;29(5):1072–6.
Ommen ES, Schröppel B, Kim JY, Gaspard G, Akalin E, de Boccardo G, et al. Routine use of ambulatory blood pressure monitoring in potential living kidney donors. Clin J Am Soc Nephrol. 2007;2(5):1030–6.
DeLoach SS, Meyers KE, Townsend RR. Living donor kidney donation: another form of white coat effect. Am J Nephrol. 2012;35(1):75–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Peixoto, A.J. (2016). Assessment of Hypertension in Chronic Kidney Disease. In: Singh, A., Agarwal, R. (eds) Core Concepts in Hypertension in Kidney Disease. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-6436-9_2
Download citation
DOI: https://doi.org/10.1007/978-1-4939-6436-9_2
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-6434-5
Online ISBN: 978-1-4939-6436-9
eBook Packages: MedicineMedicine (R0)