Abstract
Neuroendocrine tumor (NET) is a family of neoplasms that arises from nervous (neuro-) system and hormonal (endocrine) cells of multiple organs and functions. NET cells in the GI tract share common histological characteristics typically associated with neuroendocrine differentiation. Immunohistochemically, NET cells produce common polypeptide hormones and a variety of biogenic amines related to modulating the biological functions of the organs where these tumors arise. The immunophenotypical profiles of these neoplasms are relatively specific: they are positive for markers of neuroendocrine differentiation and may produce various polypeptides and hormones unique to their subtypes. Immunohistochemical examination of these neuroendocrine markers, such as synaptophysin, chromogranin, CD56, and other markers such as neuron-specific enolase (NSE), as well as proliferative markers (Ki-67 labeling index), should be carried out for the proper classification and grading of any NET. Additionally, in this era of molecular medicine and personalized precision medicine, the molecular background and the involved molecules are gaining more recognition. The current knowledge on the molecular genetics of neuroendocrine tumors will be introduced at the end of this chapter. In short, this chapter will focus on immunophenotypical profile and currently understood molecular genetics of gastrointestinal neuroendocrine tumors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Neuroendocrine tumor
- Immunophenotypical profile
- Molecular genetics
- Neuroendocrine markers
- Gastrointestinal tract
- Pancreatobiliary
Introduction
Neuroendocrine tumors (NETs) are a family of neoplasms that arises from nervous (neuro-) system and hormonal (endocrine) cells. These tumors may originate from a variety of organs [1]. In the gastrointestinal (GI) tract, NET has been identified in each organ and site, mostly in the small intestine and appendix, followed by the stomach and colon. The esophagus and anal region are less involved than the rest of the GI tract [1]. Neuroendocrine cells in the GI tract may be viewed as the largest endocrine organ in the human body. NET frequently occurs in the luminal parts of the GI tract but also in the pancreatobiliary system, where they often involve the pancreas. It has been well recognized that most NETs are low grade, while a minority of neuroendocrine neoplasms demonstrate an aggressive behavior and are classified as neuroendocrine carcinoma (NEC) [1].
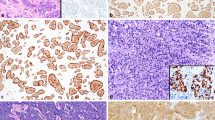
Histologically, the cells of NET share common characteristics, typically associated with neuroendocrine differentiation: medium to abundant amount of slightly eosinophilic, finely granular cytoplasm, containing neurosecretory granules (as it can be visualized by electron microscopy). The nuclei are oval to round and pericentrally or centrally located, which cytologically exhibit the “salt-pepper-like” chromatin pattern [1–4]. These morphological features are demonstrated in Fig. 1a, b, where a well-differentiated NET involving the terminal ileum is shown. Despite the absence of necrosis and the low mitotic rate (less than 2 mitoses per 10 high-power fields), the tumor infiltrated the nests and nodules of the perienteric adipose tissue. This tumor was classified as pathological stage T3. Perineural invasion and lymphovascular invasion were both present (photos not shown here). Immunohistochemically, NET cells in the GI tract produce common polypeptide hormones and multiple biogenic amines. These substances modulate the biological functions of the organs where these tumors arise [1, 2, 5–7]. Accordingly, the immunophenotypical profiles of these neoplasms are relatively specific: they are positive for markers of neuroendocrine differentiation and may produce various polypeptides and hormones unique to their subtypes [1, 2, 5–7]. NETs from a particular anatomical origin usually have similar behavior; occasionally, individual tumor within the same group may display distinct biological and biochemical characteristics [2]. There are also certain histochemical differences among various NETs, depending on their location and the grade of differentiation [4] [4]. Specifically, foregut and hindgut NETs are predominantly argentaffin negative, while midgut NETs are mostly argentaffin positive [2, 6, 8, 9]. However, for practical purposes, the workup of GI NETs does not routinely include argentaffin staining. Immunohistochemical examination of common neuroendocrine markers (usually synaptophysin, chromogranin, CD56, neuron-specific enolase) and of cell proliferation 0 markers (Ki-67 or MIB-1 labeling index) are regularly performed [2, 6, 8, 9].
Morphological futures and immunoprofile of a well-differentiated neuroendocrine tumor of the terminal ileum. (a, b) Hematoxylin and eosin-stained sections. (c–f) Cytokeratin AE1/3 and CAM5.2 and neuroendocrine (synaptophysin and chromogranin A) labeling in this grade 1 well-differentiated neuroendocrine tumor. (g, h) Ki-67 nuclear index illustrated by Ki-67 and cytokeratin (AE1/3) double staining method. Ki-67 dark brown, AE1/3 red. Less than 2 % Ki-67 index is seen by this double staining
Immunophenotypical Profiles of Neuroendocrine Tumors
Immunohistochemical Markers of Epithelial Differentiation and Neuroendocrine Tumors
When dealing with an unknown, newly identified epithelioid neoplasm exhibiting histological features reminiscent of neuroendocrine cells (more frequently NET instead of NEC), one essential step, prior to testing the neuroendocrine nature of the lesion, is to prove that the lesional cells are epithelial in nature, usually an immunostain for cytokeratin(s) (AE1/AE3 or CAM5.2). The utility of these two markers has been displayed in a terminal ileum NET shown in Fig. 1c, d. The neuroendocrine markers are generally tested immunohistochemically at the same time or right after the cytokeratin examination. Positive synaptophysin and chromogranin A immunostains are represented by moderately to darkly labeled cytoplasm where the fine neuroendocrine granules within the cytoplasm will be highlighted by one or both markers (Fig. 1e, f).
Immunohistochemical Markers of Neuroendocrine Differentiation
Synaptophysin, a protein encoded by the SYP gene, also named the major synaptic vesicle protein p38, is a 313 amino acid synaptic vesicle glycoprotein with a molecular weight of 38 kDa [10]. Synaptophysin is extensively detected in a variety of neuroendocrine cells in the human body and was believed to play a key role in synaptic transmission. Still, the exact function of this protein remains incompletely understood. Recent research has implicated synaptophysin in the development of the nervous system and has suggested a potential modulator function of this protein in biological behavioral regulation [10, 11]. Nonetheless, due to its almost universal distribution in NETs, positive immunohistochemical staining of synaptophysin has been used as the standard for diagnosing these tumors. A diffuse unequivocal labeling for synaptophysin indicates neuroendocrine differentiation (see Fig. 1e which shows an example of synaptophysin expression in an NET of the terminal ileum. Both well-differentiated NETs and poorly differentiated NECs express synaptophysin; the latter includes small cell carcinoma and large cell neuroendocrine carcinoma [1, 5–7]. In addition, synaptophysin can be also detected in the neuroblastoma, pheochromocytoma, and medullary thyroid carcinoma, which should be included in the differential diagnosis of an NET.
Another most frequently used marker of NETs is chromogranin A. This protein is the product of CHGA gene, which is also called parathyroid secretory protein 1, being a member of the granin family of neuroendocrine proteins [12, 13]. Chromogranin A is located within the neurosecretory vesicles of neuroendocrine cells such as those of pancreatic islet beta cell and enterochromaffin-like (ECL) cells in the luminal GI mucosa. Chromogranin A seems to be involved in the modulation of the autocrine and paracrine function of neuroendocrine cells, and it has been recognized as the precursor of multiple functional peptides implicated in neuroendocrine functions [14, 15]. As a specific marker for NETs, chromogranin A can be detected in both low and malignant (high-grade) neuroendocrine neoplasms [1, 5–7]. Both synaptophysin and chromogranin A positivity are represented by a moderate to strong staining of the cytoplasm corresponding to the visualization of the neurosecretory granules (demonstrated in Fig. 1f).
In addition to immunoreactivity for the abovementioned neuroendocrine markers, GI NETs regularly secrete a spectrum of peptides and hormones that have been acknowledged as NET markers. For example, the majority of NETs show cellular immunoreactivity for several molecules involved in neuroendocrine functions, such as neuron-specific enolase (NSE), UCH-L1, and CD56; the latter two being regarded as relatively sensitive but less specific markers of neuroendocrine differentiation [6, 8, 16, 17]. Although historically these molecules had been holding their status as neuroendocrine markers, currently they are not routinely utilized for immunohistochemical examination.
Immunohistochemistry for Secretory Proteins and Receptors
A number of secretory molecules are important because of their site-specific expression and their involvement in the so-called carcinoid syndrome. Serotonin, for example, is mainly produced by serotonin-producing enterochromaffin cells in NETs. These NETs are most frequently identified in the small intestine (mostly in the terminal ileum and jejunum). These tumors grow in nested, insular, pseudoglandular, and rosette-like patterns [5, 6]. Approximately 90 % of small intestinal NETs express high levels of serotonin and substance P. They also occasionally express prostatic acid phosphatase. These tumors are always argentaffinic and argyrophilic. Frequently, these neuroendocrine tumors possess high levels of hormone receptors, especially somatostatin receptors, and uptake this hormone and its analogues strongly [5, 6, 18]. With the availability of newer somatostatin receptor analogues, it is becoming more relevant to evaluate the expression of various somatostatin receptors (SSTRs 1-5) in NE tumor tissues. Investigators from our institute have shown differential levels of expression of the five SSTRs in intestinal and pancreatic endocrine tumors [19]. Clinical validation and utility of such advanced immunohistochemical approaches can further contribute to the success of newer somatostatin analogues. Through their unique receptor profiles, the avidity of NETs for various hormones has been widely utilized in the studies and diagnostic workup of both primary and metastatic NETs and also in the clinical management of patients with advanced-stage GI NETs which have shown vulnerability to hormone receptor antagonists [6, 20].
Location of Neuroendocrine Neoplasms and Choice of Immunohistochemical Markers
It should be noted that sometimes the utilization of these NET markers could be restricted depending on the primary site of the neoplasm. In other words, NETs identified in the foregut (also including the pancreas, thymus, airway, and lung), midgut, and hindgut will show distinct immunoprofiles. For example, foregut NETs express serotonin and are argentaffin negative; they frequently produce histamine and are often associated with carcinoid syndrome. Hindgut NETs are also argentaffin negative, but they seldom secrete serotonin or any vasoactive peptides [1, 6, 20]. In contrast, midgut NETs are argentaffin positive; they often secrete high levels of serotonin, kinins, prostaglandins, substance P (SP), and vasoactive peptides [1, 6, 20]. Usually aware that these hormones and protein markers are not routinely examined when studying and diagnosing GI NETs. We must be aware that occasionally small intestinal NETs express only synaptophysin but not chromogranin A, and that rare cases have even shown positivity for chromogranin B but not chromogranin A.
Epithelial Versus Site-specific Immunohistochemical Markers
The majority of NETs stain with pan-cytokeratin AE1/AE3 and CAM5.2 and diffusely and strongly for neuroendocrine marker(s). In addition, depending on the site of origin, NETs may be positive for relatively specific “site of origin”-related markers. For example, NETs originating in the GI tract are usually positive for CDX-2 [21], although this is not always the case [20, 22]. NETs originating in the pancreas are usually immunohistochemically positive for nuclear PAX-8 [23]. Similarly, pulmonary NETs are often positive for nuclear TTF-1 [22]. However, be aware that, when dealing with a high-grade neuroendocrine carcinoma (NEC), a positive nuclear labeling of TTF-1 does NOT necessarily indicate a lung origin [24], and positivity for TTF-1 has been frequently seen in NECs from a wide spectrum of primary sites [25, 26].
Importantly, one must keep in mind that occasionally NETs may be cytokeratin negative. In the presence of such a case, one should consider to perform S-100 immunostaining to rule out a pheochromocytoma/paraganglioma. The latter is characterized by typical sustentacular cells which are highlighted by the S-100 stain. Careful histological examination will also identify the “zellballen” arrangement of the tumor cells and a rich fibrovascular background.
Immunohistochemistry and Grade of Neuroendocrine Neoplasms
Compared with grade 1 and grade 2 well-differentiated NETs, small cell carcinoma (SCC) and large cell neuroendocrine carcinoma (LCNEC) may show similar or weaker and focal staining for neuroendocrine markers. In some occasions, they may actually be negative or display only very focal areas of positivity for certain but not all neuroendocrine markers (Fig. 2). Hence, immunohistochemical negativity for the common neuroendocrine markers in a poorly differentiated tumor with histological features resembling a neuroendocrine carcinoma does not necessarily exclude such diagnosis. When possible, alternative marker(s) should be attempted to determine neuroendocrine differentiation. At the same time, one needs to pay attention to the histological features. In a large cell NEC, the abundant cytoplasm, the vesicular nuclei with coarse chromatin distribution and prominent nucleoli, and the high mitotic rates will be storytelling. In a small cell carcinoma, features such as scant cytoplasm, smaller cell size, extensive necrosis, frequent single cell apoptosis, fine chromatin distribution, characteristic nuclear molding, and a high Ki-67 proliferative index will direct to the correct diagnosis, even in the absence of ancillary stains.
Morphological features and immunoprofiles of a metastatic small cell carcinoma (SCC) involving the liver. (a, b) Hematoxylin and eosin-stained sections. (c) AE1/3 cytokeratin immunostain. (d, e) Relatively weak synaptophysin and chromogranin A labeling in this SCC. (f) Ki-67 nuclear index illustrated by Ki-67 immunostaining: more than 90 % SCC cells nuclei were labeled positive, reflecting its highly aggressive nature
Role of Immunohistochemistry in Differentiating Neuroendocrine from Other Malignancies
At times poorly differentiated adenocarcinoma may mimic neuroendocrine carcinoma both morphologically and immunophenotypically, causing a diagnostic dilemma. These rare tumors may occasionally form ill-formed glandular and tubular structures, with subtle, intracytoplasmic, or extracellular mucinous vacuoles on H&E-stained sections. Sometimes the mucinous vacuoles are near invisible by H&E staining, but they can be highlighted by a PAS-diastase or mucicarmine special stains. Even when these tumors are positive for synaptophysin, chromogranin A, and/or CD56, the presence of positive PAS-D or mucicarmine stain should raise the suspicion of a poorly differentiated adenocarcinoma. These rare tumors are classified as either mixed adenoneuroendocrine carcinoma (MANEC) or poorly differentiated adenocarcinoma with neuroendocrine differentiation. The WHO guidelines have clear criteria on the classification of these tumors, and only when both neuroendocrine and adenocarcinoma components represent at least 30 % of the total tumor it can be classified as MANEC. In our era of precision medicine and personalized therapy, the molecular background of a tumor is becoming integral part of the data required by the clinicians for the proper management of a patient. With the recent progress in our understanding of the molecular genetics of neuroendocrine tumors, we will now provide a brief overview of these developments to the extent that they can impact the practice of diagnostic neuroendocrine pathology in the twenty-first century.
Molecular Genetics of Neuroendocrine Tumors
Regarding the molecular profile and genetic background of NETs, it has been well recognized that although most NETs are sporadic, certain patients present with multiple simultaneous NETs and/or carry a strong family history of NETs, indicating molecular and/or genetic susceptibility to NET.
One main group of disorders is represented by multiple endocrine neoplasia (MEN) types 1 and 2, which are rare hereditary cancer syndromes expressing a wide spectrum of neuroendocrine neoplasms. The current investigations on the molecular and clinical genetics associated with these entities demonstrate that the gene responsible for MEN1 is a tumor suppressor gene, which acts as a regulator of the nuclear transcriptional machinery in tumor cells [27–29]. The newly acquired genetic and diagnostic modalities will be able to identify neoplastic lesions at an earlier stage, potentially improving outcome and quality of life in those patients. Similarly, our knowledge on genotype-phenotype correlations is illustrated by MEN2, caused by a mutation in the RET oncogene [27, 30]. Novel genetic and diagnostic methods have enabled us to identify NETs lesions at much earlier stages. This advancement has led to improved outcome and quality of life in a significant number of patients [30, 31], which also facilitates more individualized treatment strategies for these patients. For example, MEN1 encodes a nuclear protein named menin, which binds to and modulates directly the transcriptional function of Jun-D. In particular, menin’s tumor suppressor function involves the inhibition of Jun-D-activated transcription [31]. Predominant germline or somatic mutations in MEN1 gene are truncation or absence of the encoded product [29]; similarly, 11q13 loss of heterozygosity in tumors predicts inactivation of the other MEN1 copy. MEN1 somatic mutation is prevalent in nonhereditary, MEN1-like tumor types [29].
Other hereditary disorders in which patients may be affected with an NET include the von Hippel-Lindau (VHL)-related syndrome, von Recklinghausen’s disease or neurofibromatosis type 1 (NF-1), and tuberous sclerosis (so-called TSCs, including TSC1 and TSC2) [32]. In these patients, the genetic abnormalities play a significant role in the development of NETs, which are frequently multifocal. The pathological features of familial/hereditary NETs are generally similar to the sporadic form, with subtle differences [33]. For example, VHL disease has an incidence of one in near 36,000 births. There is over 90 % penetrance by the age of 65. Age at diagnosis ranges from as young as a few years to late 70s, with an average patient age at clinical diagnosis of 26 [34]. NF-1 or TSC1/2 mutations result in loss of function of the corresponding protein products neurofibromin and tuberin, respectively [35]. Specifically, the intact proteins of these NET-related genes suppress the function of a common target, namely, mammalian target of rapamycin (mTOR) [35], which is a key regulator of cell growth and integrates a wide variety of cellular inputs, such as growth factors, nutrients, energy status, and hypoxia-induced stress; thus, it is regarded as a potential therapeutic target for NETs in the pancreas [36, 37]. Recent investigation has indicated that everolimus significantly prolonged progression-free survival among patients with advanced stages of pancreatic neuroendocrine tumors and was associated with a low rate of severe adverse events [37].
In sporadic cases, GI NETs show predominantly genetic alterations concentrated on chromosome 18; and losses of the entire chromosome as well as smaller deletions have both been documented. The most frequently identified mutation in GI NETs is that of beta-catenin, with overexpression of cyclin D1 and c-Myc. Of notice, a set of genes including NAP1L1, MAGE-2D, and MTA1 has been correlated with prognosis [38]. In parallel, in sporadic pancreatic NETs, the newly updated WHO 2010 classification scheme adopts a proliferation-based grading system together with the classical histopathological diagnostic criteria for NETs [39]. Molecular genetics and comparative genomic hybridization (CGH) indicate that the chromosomal losses occur more frequently than the gains, whereas amplifications are uncommon. Especially, losses of chromosome 1 and 11q as well as gains of 9q appear to be early events in the development of pancreatic tumors [40, 41]. These findings demonstrate that chromosomal instability and alterations of tumor suppressor pathways are crucial mechanisms associated with NET progression and behavior. In addition, gains of chromosome 4 and losses of 6q have been found in about 50 % of functioning tumors, among which, the majority were confirmed to be insulinomas [42]. Recent genome-wide single nucleotide polymorphism (SNP) analysis observed that about up to one-third of pancreatic endocrine tumors had genetic imbalances and chromosomal aberrations [43]. Furthermore, homozygous deletion and hypermethylation of p16/MTS1 or a deletion of the p16INK4a tumor suppressor gene on chromosome 9p21 has also been demonstrated in sporadic gastrinomas, while both benign and malignant insulinomas possess high LOH rates for chromosome 22q [38]. Moreover, cyclin D1 overexpression was identified by both protein and nucleic acid analyses in 43 % of NETs [44]. Lastly, high-grade pancreatic NETs display a marked fraction of genetic abnormalities usually seen with conventional cancers, the most frequently observed abnormality being the cell cycle key regulatory TP53.
In pancreatic NETs, whole exome sequencing has shown nonsynonymous mutations that were far fewer in numbers than those in ductal pancreatic adenocarcinoma [45]. The genes commonly involved in pancreatic NETs include MEN1, DAXX, and ATRX, and genes coding for various members of the mammalian target of rapamycin (mTOR) pathway [46]. DAXX (death-domain-associated protein) and ATRX (α thalassemia/mental retardation syndrome, X-linked) are chromatin remodeling genes, and one of these genes is somatically mutated in 45 % of sporadic pancreatic NETs. Mutations in these genes are associated with the alternative lengthening of telomeres (ALT) phenotype, a telomerase-independent mechanism for telomere maintenance [47].
Pancreatic NETs with mutant MEN1, DAXX, and ATRX genes have better prognosis than wild-type tumors. As such, somatic mutations in DAXX and ATRX genes are late events in pancreatic NETs, evidenced by their absence in microadenomas [48]. Mutations in mTOR (mammalian target of rapamycin) pathway, including PIK3CA, PTEN, and TSC2 are present in 14 % of pancreatic NETs, and be targeted by mTOR pathway inhibitors [37, 45]. Similarly, the VHL/HIF pathway is also important in the genesis of pancreatic NETs [49], and pancreatic neuroendocrine microadenomas are present in about three-fourths of patients with vHL syndrome. Sporadic pancreatic NETs can rarely harbor somatic VHL gene mutation, but promoter hypermethylation and deletion of VHL occur in up to 25 % sporadic pancreatic NETs and are associated with adverse outcome [50].
Accumulating evidence suggests that a wide spectrum of molecular and genetic abnormalities could be involved in the initiation and progression of NETs. Therefore, it is conceivable that such new information may be used to predict tumor behavior and prognosis in a given case of NET. Recent work from our institution has identified several novel molecular markers of tumor progression, including RUNX1T1, palladin, and others, in localized pancreatic endocrine tumors [51, 52]. Molecular and immunohistochemical evaluation of these and other progression genes/proteins in otherwise low-grade NETs may identify subsets within otherwise indolent NETs that may benefit patients by more aggressive monitoring and evaluation of newer therapies. The epigenetic modifications and differential microRNA expression patterns examined in the aberrant signaling pathways of NETs are still being investigated; the relevant findings hopefully will shed light on predicting a given NET behavior and improving patient management. While NETs remain a group of intriguing diseases continuously posing diagnostic and management challenges [39], with the availability of higher quality immunohistochemical markers and molecular genetic approaches, we not only hope to improve diagnostic standards in the practice of neuroendocrine pathology, but we, as modern-age pathologists, will also contribute more actively to the management of NET patients by our clinical oncology colleagues.
In conclusion, immunohistochemistry plays an important role, not only in the diagnosis, differential diagnosis, and grading of neuroendocrine neoplasms, it is also relevant in the localization of various peptides secreted by the NET cells. More recently, the applications of immunohistochemistry are becoming even broader and will continue to serve the anatomic pathologists interested in cost-effective and reliable evaluation of newer protein targets, as we implement more personalized approaches to diagnose and classify NETs in the age of molecular advancement. Advances in the molecular genetics of NETs have already contributed a large body of useful knowledge regarding pathogenesis, clinical behavior, and response of NETs to the emerging targeted therapies. In future, with the advancements in genetic technologies and cost-effectiveness, it is hoped that anatomic and molecular pathologists will be able to contribute even more effectively to the management of patients with neuroendocrine malignancies.
References
Rindi G, Petrone G, Inzani F. 25 Years of neuroendocrine neoplasms of the gastrointestinal tract. Endocr Pathol. 2014;25(1):59–64.
Hirabayashi K, Zamboni G, Nishi T, Tanaka A, Kajiwara H, Nakamura N. Histopathology of gastrointestinal neuroendocrine neoplasms. Front Oncol. 2013;3:2.
Maillard MH, Hiroz P, Wagner D, Prior J, Boubaker A, Pralong F, et al. Gastrointestinal neuroendocrine tumors: pleomorphic and often ignored. Rev Med Suisse. 2012;8(352):1658–63.
Williams GT. Endocrine tumours of the gastrointestinal tract-selected topics. Histopathology. 2007;50(1):30–41.
Kloppel G, Rindi G, Perren A, Komminoth P, Klimstra DS. The ENETS and UICC TNM classification of neuroendocrine tumors of the gastrointestinal tract and the pancreas: comment. Pathologe. 2010;31(5):353–4.
Anthony LB, Strosberg JR, Klimstra DS, Maples WJ, O’Dorisio TM, Warner RR, et al. The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39(6):767–74.
Amarapurkar DN, Juneja MP, Patel ND, Amarapurkar AD, Amarapurkar PD. A retrospective clinico-pathological analysis of neuroendocrine tumors of the gastrointestinal tract. Trop Gastroenterol. 2010;31(2):101–4.
Oberg KE. Gastrointestinal neuroendocrine tumors. Ann Oncol. 2010;21 Suppl 7:vii72–80.
Leja J, Essaghir A, Essand M, Wester K, Oberg K, Totterman TH, et al. Novel markers for enterochromaffin cells and gastrointestinal neuroendocrine carcinomas. Mod Pathol. 2009;22(2):261–72.
Sudhof TC, Lottspeich F, Greengard P, Mehl E, Jahn R. The cDNA and derived amino acid sequences for rat and human synaptophysin. Nucleic Acids Res. 1987;15(22):9607.
Schmitt U, Tanimoto N, Seeliger M, Schaeffel F, Leube RE. Detection of behavioral alterations and learning deficits in mice lacking synaptophysin. Neuroscience. 2009;162(2):234–43.
Helman LJ, Ahn TG, Levine MA, Allison A, Cohen PS, Cooper MJ, et al. Molecular cloning and primary structure of human chromogranin A (secretory protein I) cDNA. J Biol Chem. 1988;263(23):11559–63.
Gazdar AF, Helman LJ, Israel MA, Russell EK, Linnoila RI, Mulshine JL, et al. Expression of neuroendocrine cell markers L-dopa decarboxylase, chromogranin A, and dense core granules in human tumors of endocrine and nonendocrine origin. Cancer Res. 1988;48(14):4078–82.
Nikou GC, Lygidakis NJ, Toubanakis C, Pavlatos S, Tseleni-Balafouta S, Giannatou E, et al. Current diagnosis and treatment of gastrointestinal carcinoids in a series of 101 patients: the significance of serum chromogranin-A, somatostatin receptor scintigraphy and somatostatin analogues. Hepatogastroenterology. 2005;52(63):731–41.
Cetin Y, Aunis D, Bader MF, Galindo E, Jorns A, Bargsten G, et al. Chromostatin, a chromogranin A-derived bioactive peptide, is present in human pancreatic insulin (beta) cells. Proc Natl Acad Sci U S A. 1993;90(6):2360–4.
Schott M, Kloppel G, Raffel A, Saleh A, Knoefel WT, Scherbaum WA. Neuroendocrine neoplasms of the gastrointestinal tract. Dtsch Arztebl Int. 2011;108(18):305–12.
Modlin IM, Moss SF, Oberg K, Padbury R, Hicks RJ, Gustafsson BI, et al. Gastrointestinal neuroendocrine (carcinoid) tumours: current diagnosis and management. Med J Aust. 2010;193(1):46–52.
Burke AP, Thomas RM, Elsayed AM, Sobin LH. Carcinoids of the jejunum and ileum: an immunohistochemical and clinicopathologic study of 167 cases. Cancer. 1997;79(6):1086–93.
Nasir A, Stridsberg M, Strosberg J, Su PH, Livingston S, Malik HA, et al. Somatostatin receptor profiling in hepatic metastases from small intestinal and pancreatic neuroendocrine neoplasms: immunohistochemical approach with potential clinical utility. Cancer Control. 2006;13(1):52–60.
Rothenstein J, Cleary SP, Pond GR, Dale D, Gallinger S, Moore MJ, et al. Neuroendocrine tumors of the gastrointestinal tract: a decade of experience at the Princess Margaret Hospital. Am J Clin Oncol. 2008;31(1):64–70.
Erickson LA, Papouchado B, Dimashkieh H, Zhang S, Nakamura N, Lloyd RV. Cdx2 as a marker for neuroendocrine tumors of unknown primary sites. Endocr Pathol. 2004;15(3):247–52.
Srivastava A, Hornick JL. Immunohistochemical staining for CDX-2, PDX-1, NESP-55, and TTF-1 can help distinguish gastrointestinal carcinoid tumors from pancreatic endocrine and pulmonary carcinoid tumors. Am J Surg Pathol. 2009;33(4):626–32.
Sangoi AR, Ohgami RS, Pai RK, Beck AH, McKenney JK, Pai RK. PAX8 expression reliably distinguishes pancreatic well-differentiated neuroendocrine tumors from ileal and pulmonary well-differentiated neuroendocrine tumors and pancreatic acinar cell carcinoma. Mod Pathol. 2011;24(3):412–24.
Du EZ, Goldstraw P, Zacharias J, Tiffet O, Craig PJ, Nicholson AG, et al. TTF-1 expression is specific for lung primary in typical and atypical carcinoids: TTF-1-positive carcinoids are predominantly in peripheral location. Hum Pathol. 2004;35(7):825–31.
Verset L, Arvanitakis M, Loi P, Closset J, Delhaye M, Remmelink M, et al. TTF-1 positive small cell cancers: don’t think they’re always primary pulmonary! World J Gastrointest Oncol. 2011;3(10):144–7.
Agoff SN, Lamps LW, Philip AT, Amin MB, Schmidt RA, True LD, et al. Thyroid transcription factor-1 is expressed in extrapulmonary small cell carcinomas but not in other extrapulmonary neuroendocrine tumors. Mod Pathol. 2000;13(3):238–42.
Carling T. Multiple endocrine neoplasia syndrome: genetic basis for clinical management. Curr Opin Oncol. 2005;17(1):7–12.
Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276(5311):404–7.
Agarwal SK, Lee Burns A, Sukhodolets KE, Kennedy PA, Obungu VH, Hickman AB, et al. Molecular pathology of the MEN1 gene. Ann N Y Acad Sci. 2004;1014:189–98.
Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86(12):5658–71.
Agarwal SK, Guru SC, Heppner C, Erdos MR, Collins RM, Park SY, et al. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell. 1999;96(1):143–52.
Rindi G, Wiedenmann B. Neuroendocrine neoplasms of the gut and pancreas: new insights. Nat Rev Endocrinol. 2012;8(1):54–64.
Frankel WL. Update on pancreatic endocrine tumors. Arch Pathol Lab Med. 2006;130(7):963–6.
Kim JJ, Rini BI, Hansel DE. Von Hippel Lindau syndrome. Adv Exp Med Biol. 2010;685:228–49.
Ehehalt F, Saeger HD, Schmidt CM, Grutzmann R. Neuroendocrine tumors of the pancreas. Oncologist. 2009;14(5):456–67.
Wiedenmann B, Pavel M, Kos-Kudla B. From targets to treatments: a review of molecular targets in pancreatic neuroendocrine tumors. Neuroendocrinology. 2011;94(3):177–90.
Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–23.
Oberg K. Genetics and molecular pathology of neuroendocrine gastrointestinal and pancreatic tumors (gastroenteropancreatic neuroendocrine tumors). Curr Opin Endocrinol Diabetes Obes. 2009;16(1):72–8.
Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39(6):707–12.
Lubensky IA, Zhuang Z. Molecular genetic events in gastrointestinal and pancreatic neuroendocrine tumors. Endocr Pathol. 2007;18(3):156–62.
Bloomston M, Durkin A, Yang I, Rojiani M, Rosemurgy AS, Enkmann S, et al. Identification of molecular markers specific for pancreatic neuroendocrine tumors by genetic profiling of core biopsies. Ann Surg Oncol. 2004;11(4):413–9.
Arnold C. Neuroendocrine tumors of the gastrointestinal tract. Praxis (Bern 1994). 2007;96(1-2):19–28.
Kimdo H, Nagano Y, Choi IS, White JA, Yao JC, Rashid A. Allelic alterations in well-differentiated neuroendocrine tumors (carcinoid tumors) identified by genome-wide single nucleotide polymorphism analysis and comparison with pancreatic endocrine tumors. Genes Chromosomes Cancer. 2008;47(1):84–92.
Chung DC, Brown SB, Graeme-Cook F, Seto M, Warshaw AL, Jensen RT, et al. Overexpression of cyclin D1 occurs frequently in human pancreatic endocrine tumors. J Clin Endocrinol Metab. 2000;85(11):4373–8.
Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331(6021):1199–203.
Hessman O, Lindberg D, Einarsson A, Lillhager P, Carling T, Grimelius L, et al. Genetic alterations on 3p, 11q13, and 18q in nonfamilial and MEN 1-associated pancreatic endocrine tumors. Genes Chromosomes Cancer. 1999;26(3):258–64.
Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333(6041):425.
de Wilde RF, Heaphy CM, Maitra A, Meeker AK, Edil BH, Wolfgang CL, et al. Loss of ATRX or DAXX expression and concomitant acquisition of the alternative lengthening of telomeres phenotype are late events in a small subset of MEN-1 syndrome pancreatic neuroendocrine tumors. Mod Pathol. 2012;25(7):1033–9.
Speisky D, Duces A, Bieche I, Rebours V, Hammel P, Sauvanet A, et al. Molecular profiling of pancreatic neuroendocrine tumors in sporadic and Von Hippel-Lindau patients. Clin Cancer Res. 2012;18(10):2838–49.
Schmitt AM, Schmid S, Rudolph T, Anlauf M, Prinz C, Kloppel G, et al. VHL inactivation is an important pathway for the development of malignant sporadic pancreatic endocrine tumors. Endocr Relat Cancer. 2009;16(4):1219–27.
Nasir A, Helm J, Turner L, Chen DT, Strosberg J, Hafez N, et al. RUNX1T1: a novel predictor of liver metastasis in primary pancreatic endocrine neoplasms. Pancreas. 2011;40(4):627–33.
Henderson-Jackson EB, Helm J, Strosberg J, Nasir NA, Yeatman TJ, Kvols LK, et al. Palladin is a marker of liver metastasis in primary pancreatic endocrine carcinomas. Anticancer Res. 2011;31(9):2957–62.
Abbreviations
ATRX α Thalassemia/mental retardation syndrome X-linked
DAXX Death-domain-associated protein
NET Neuroendocrine tumor
NSE Neuron-specific enolase
GI Gastrointestinal
SCC Small cell carcinoma
LCNEC Large cell neuroendocrine carcinoma
MANEC Mixed adenoneuroendocrine carcinoma
VHL Von Hippel-Lindau
MEN Multiple endocrine neoplasms
SSTRs Somatostatin receptors (1-5)
mTOR Mammalian target of rapamycin
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Jiang, K., Sheikh, U., Nasir, A., Coppola, D. (2016). Role of Immunohistochemistry and Molecular Genetics in Neuroendocrine Tumors. In: Nasir, A., Coppola, D. (eds) Neuroendocrine Tumors: Review of Pathology, Molecular and Therapeutic Advances. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3426-3_4
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3426-3_4
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-3424-9
Online ISBN: 978-1-4939-3426-3
eBook Packages: MedicineMedicine (R0)