Abstract
Although exercise among girls and women is beneficial for overall health and well-being, the development of an energy deficiency as a result of inadequate energy intake to compensate for exercise energy expenditure can lead to menstrual dysfunction. It has been established that it is not the stress of exercise that causes reproductive dysfunction; rather, in an attempt to conserve energy, metabolic adaptations triggered by an energy deficiency alter the normal production and pulsatility of reproductive hormones at all levels of the hypothalamic–pituitary–ovarian (HPO) axis. As such, estrogen and progesterone concentrations decline, resulting in a spectrum of exercise-associated menstrual disturbances (EAMD). The spectrum of EAMD includes the severe menstrual disturbances, amenorrhea and oligomenorrhea, which are easily detected by the absence of menses for at least 3 months or long and inconsistent cycles of 36–90 days, respectively. Less severe EAMD include luteal phase defects and anovulation which typically occur within regular intermenstrual intervals, thereby causing these disturbances to often remain undetected. Suppressed follicular growth and oocyte maturation, poor endometrial quality, spontaneous abortion, and infertility are all clinical reproductive consequences of EAMD. However, EAMD can be prevented by maintaining a healthy body weight and an energy replete state. Likewise, effective nonpharmacological treatment of EAMD includes an increase in caloric intake and weight gain to reverse the energy deficiency and promote recovery of normal menstrual function. Upon recovery and/or maintenance of an energy replete state, regular exercise among girls and women is encouraged.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Exercise-associated menstrual disturbances
- Amenorrhea
- Oligomenorrhea
- Anovulation
- Luteal phase defects
- Energy deficiency
- Infertility
- Prevalence
- Treatment
Introduction
Regular physical activity and exercise among girls and women is beneficial for overall health and well-being; however, increases in exercise energy expenditure that are not compensated for by energy intake can lead to perturbations in the reproductive axis, resulting in infertility and numerous skeletal and cardiovascular health consequences [1–4]. Exercise-associated menstrual disturbances (EAMD) have been commonly reported in exercising women , with prevalence estimates of EAMD approaching 50 % [5]. Severe forms of EAMD such as the absence of menses (amenorrhea) or long intermenstrual intervals (oligomenorrhea) are clinically recognizable and typically identified based on menstrual history and self-report. However, other forms of EAMD such as luteal phase defects (LPD) and anovulation are not readily apparent and are often masked within cycles of regular length, yet are arguably the most prevalent [5, 6]. Therefore, these subtle menstrual disturbances in exercising women are not easily diagnosed, silently indicating an energy deficit and potentially contributing to infertility . Due to the frequency and, at times, silent nature of EAMD, awareness of the symptoms and consequences of an energy deficiency in exercising women is vital for the health and well-being of physically active girls and women.
The challenge that is imposed on physiological systems during an energy deficiency causes energy to be repartitioned toward processes that are necessary for survival, such as thermoregulation, locomotion, and cellular maintenance, and away from processes that are not critical for survival, that is, reproduction and growth [7]. As such, an energy deficiency often promotes a cascade of metabolic alterations in an effort to conserve energy, and these energy-conserving mechanisms in turn contribute to disruptions in the hypothalamic–pituitary–ovarian (HPO) axis [7]. Beginning with the “generator” of reproductive function in the hypothalamus and ending with ovarian steroid secretion by the follicles, alterations in the production and secretion of reproductive hormones occur at each level of the reproductive axis. As such, the purpose of this chapter is to explore the impact of physical activity and exercise on reproductive function and fertility in adolescent girls and women by examining both subtle and severe menstrual disturbances and the changes that occur within the HPO axis to induce reproductive dysfunction. The effects of exercise and, more specifically, an energy deficiency on reproductive function and fertility will be explored by assessing the presence and quality of key reproductive events such as ovulation, the normal cyclicity of reproductive hormone concentrations, and endometrial proliferation.
Normal Menstrual Cycle and HPO Axis Activity
To adequately understand the effects of exercise on reproductive function, it is imperative to begin with a description of a normal menstrual cycle and optimal reproductive function, which serve as the healthy reference point to which the changes that are observed in both subtle and severe menstrual disturbances are compared. The menstrual cycle, which is defined as the period from the onset of menses to the day before the next onset of menses, typically lasts about 28 days and is divided into two phases, the follicular phase and the luteal phase [8]. The follicular phase begins at the onset of menses and the luteal phase begins the day after ovulation occurs (Fig. 11.1). Therefore, ovulation is a mid-cycle event that separates the follicular and luteal phases.
Profile of daily urinary excretion of reproductive hormones for a representative eumenorrheic, ovulatory menstrual cycle. Classic characteristics include the E1G peak in the late follicular phase, the LH surge following the E1G peak, and rising PdG concentrations during the luteal phase. E1G estrone-1-glucuronide, PdG pregnanediol glucuronide, LH luteinizing hormone
The cascade of events surrounding the menstrual cycle commences with secretion of gonadotropin-releasing hormone (GnRH) from the arcuate nucleus and preoptic area of the hypothalamus which in turn stimulates the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from gonadotroph cells of the anterior pituitary gland, the two gonadotropins that stimulate the production of estrogen and progesterone from the ovaries [9] (Fig. 11.2).
HPO axis sequence of events related to the menstrual cycle. Neurons in the arcuate nucleus and preoptic area of the hypothalamus secrete GnRH which, in turn, stimulates the release of FSH and LH from the gonadotroph cells of the anterior pituitary gland. FSH and LH increase the production of estrogens progestins, and androgens by follicular granulosa and theca cells in the ovaries. During the luteal phase, the corpus luteum formed by the dominant follicle produces progesterone and estrogens. Typically, the ovarian hormones exert negative feedback on the anterior pituitary and hypothalamus, causing a decrease in the secretion of the gonadotropins. The negative feedback is depicted by the blue dashed lines. However, during the late follicular phase, rapidly rising estrogen concentrations exert positive feedback on the anterior pituitary and hypothalamus resulting in the LH surge. The positive feedback is depicted by the green solid lines. HPO hypothalamic–pituitary–ovarian, GnRH gonadotropin-releasing hormone, FSH follicle-stimulating hormone, LH luteinizing hormone
GnRH is known as the “master hormone” of reproduction due to its role as regulator of LH and FSH pulsatility [10]. Evidence from classic experiments conducted in rhesus monkeys [11, 12] demonstrated the rhythmic and acute secretory actions of GnRH. Typically, GnRH release occurs every 60–90 min, and, consequently, gonadotropin secretion occurs approximately once per hour from the anterior pituitary, paralleling the release of GnRH [8, 9, 13]. During the follicular phase, GnRH pulsatility and, therefore, LH and FSH pulsatility maintain a relatively high frequency [13]. Near the end of the follicular phase, the frequency and amplitude of GnRH pulses and, subsequently, LH and FSH pulses increase in response to the positive feedback of high estradiol concentrations [13]. However, at the end of the luteal phase, GnRH and LH pulsatility declines in response to negative feedback from progesterone [9, 13]. In turn, LH and FSH bind to receptors on the granulosa and theca cells of the developing ovarian follicle during the follicular phase and luteal cells during the luteal phase to produce estrogens, androgens, and progesterone [14].
During the late luteal and early follicular phase, FSH production increases, stimulating follicular growth and recruitment of the dominant follicle within the ovaries [13]. Production of the estrogen, estradiol, by the synergistic efforts of the theca and granulosa cells of the ovarian follicles also increases [13]; thus, during the follicular phase, estradiol concentrations gradually increase, upregulating the number of FSH receptors in the mature follicles and consequently increasing the action of FSH and the production of estradiol [13, 14]. During the mid-follicular phase, negative feedback by estradiol on the anterior pituitary prevents further increases in FSH and LH [9, 15]. Near the late mid-follicular phase to the end of the follicular phase, one follicle has achieved dominance and rapidly increases its production of estradiol while the other less-dominant follicles undergo atresia [13]. The rapidly rising concentrations of estradiol exert positive feedback in the preoptic area of the hypothalamus and on the gonadotroph cells of the anterior pituitary, sensitizing the cells to GnRH and stimulating the release of a bolus of LH after plasma estradiol concentrations exceed a threshold for at least 36 h [9, 11, 13, 15].
Therefore, the LH surge typically occurs 24–36 h after attainment of peak estradiol secretion and lasts for approximately 24–48 h [13]. In turn, the LH surge prompts proteolytic enzymes to digest the follicular wall, allowing the release of the oocyte from the dominant follicle and initiating ovulation, the event that separates the follicular and luteal phases [13]. Under the influence of LH, luteinization of the erupted follicle occurs, resulting in the formation of a corpus luteum, consisting of theca-lutein and granulosa-lutein cells [13]. These luteinized cells produce progesterone and, to a lesser extent, estradiol, which inhibit both the release of gonadotropins from the anterior pituitary and subsequent folliculogenesis [13]. In the absence of pregnancy-induced concentrations of human chorionic gonadotropin (hCG), the corpus luteum degenerates forming the corpus albicans and progesterone production declines at the end of the luteal phase, thereby removing the negative feedback on the anterior pituitary and hypothalamus [13]. FSH concentrations begin to increase again, recruiting another cohort of follicles for the subsequent cycle [13].
Therefore, in summary, a normal menstrual cycle demonstrates slowly increasing concentrations of FSH during the luteal-follicular transition and early follicular phase. Rising estradiol concentrations during the follicular phase exert negative feedback on the anterior pituitary and hypothalamus, resulting in no further increases of FSH and LH during the mid-follicular phase [9, 13, 15]. The peak in estradiol concentration mid-cycle triggers the LH surge, leading to ovulation and commencement of the luteal phase. The luteal phase is characterized by rising progesterone concentrations that decline near the end of the cycle as the corpus luteum degenerates (Fig. 11.1).
Within the uterus, the proliferative and secretory phases of the uterine cycle coincide with the follicular and luteal phases of the ovarian cycle. The proliferative phase involves a rebuilding of the functional layer of the endometrium after it has been shed during menses [16]. The cells of the zona basalis, that is, basal stromal cells, proliferate in response to rising concentrations of estradiol from the developing follicles [13]. During the late proliferative phase, hyperplasia of endometrial cells results in thickening of the endometrial wall such that the endometrium may increase in thickness from 0.5 to 5 mm [13]. Stimulated by progesterone from the corpus luteum, the secretory phase involves the glandular secretion of glycogen and increased vascularization to support the implantation of an embryo in the event that fertilization occurred [13, 16].
Decidual cells formed from stromal cells produce secretions in concert with the endometrial glands and create the zona compacta, the dense layer of upper endometrial cells [13]. The zona spongiosa, that is, the mid-layer of epithelial cells which consists of prominent endometrial glands, also becomes apparent during this phase [13]. In the absence of fertilization, progesterone and estrogen concentrations decline and the endometrium is deprived of hormonal support, causing the spiral arteries to constrict and destruction of the functional layer of the endometrium, i.e., the zona compacta and the zona spongiosa [13]. Eventually, as the upper two thirds of the endometrium degenerates, the arteries relax and menses begins [16]. As such, the menstrual phase of the uterine cycle is characterized by the loss of the functional layer of the endometrium as a result of ischemia and necrosis of endometrial tissues [13]. The innermost layer of the endometrium, the zona basalis, is all that remains at the end of the menstrual phase [13].
Thus, it is evident that both the ovarian and uterine cycles rely on proper functioning of the HPO axis and, subsequently, adequate hormonal concentrations, for normal menstrual function . Among exercising women with reproductive disturbances, the metabolic environment alters the HPO axis, leading to disruptions in the menstrual cycle that affect both the ovarian and uterine cycles and, in turn, influence reproductive potential.
Types of EAMD
EAMD occur along a spectrum ranging from mild to severe (Fig. 11.3). The least severe presentations of menstrual dysfunction include subtle menstrual disturbances, also known as subclinical menstrual disturbances that occur without a change in cycle length and are, therefore, frequently undetected; these subtle menstrual disturbances include LPD and anovulation . Severe menstrual disturbances, also known as clinical menstrual disturbances, exist at the pathological endpoint of the continuum and are characterized by long intermenstrual intervals (oligomenorrhea) or the absence of menstruation for more than 90 days which is referred to clinically as functional hypothalamic amenorrhea (FHA) .
Subtle Menstrual Disturbances: LPD
LPD are characterized by adequate ovulatory function despite poor implantation and poor endometrial quality [1, 17, 18]. More specifically, LPD cycles are characterized by ovulatory cycles with normal and repeatable intermenstrual intervals but luteal phase dysfunction; they are defined by a short luteal phase length of < 10 days and/or inadequate progesterone production during the luteal phase [17, 19] (Fig. 11.4a). It has been suggested that a critical 3- or 5-day sum of mid-luteal progesterone concentrations can be used to identify inadequate progesterone exposure associated with LPD [19, 20].
Profile of daily urinary excretion of reproductive hormones for subtle menstrual disturbances. a Representative menstrual cycle with a short and inadequate luteal phase defect. Classic characteristics include a luteal phase < 10 days in length and suppressed progesterone production during the luteal phase. b Representative anovulatory menstrual cycle. Classic characteristics include the lack of both a mid-cycle E1G peak and LH surge and the failure of PdG to rise during the latter part of the cycle, indicating the absence of ovulation. E1G estrone-1-glucuronide, PdG pregnanediol glucuronide, LH luteinizing hormone
As such, previous reports have used either a urinary pregnanediol glucuronide (PdG) peak of < 5 µg/ml or the sum of a 3-day mid-luteal PdG peak of < 10 µg/ml as indicators of an inadequate luteal progesterone production [5, 20]. Typically, women with LPD demonstrate a prolonged follicular phase in concert with the shortened luteal phase; thus, for example, an individual with a 28-day cycle and a 7-day luteal phase will have a 21-day follicular phase with an LH peak occurring on day 21 compared to women with normal ovulatory cycles in whom the LH peak and presumably ovulation occurs mid-cycle (days 12–14) for a 28-day cycle [1, 19].
The etiology of LPD has been proposed to be impaired folliculogenesis and oocyte maturation that results from disruptions of the reproductive axis [1, 17, 19]. Estrogen exposure during the follicular phase is suppressed among LPD cycles of exercising women compared to ovulatory cycles with normal luteal function [6]. Likewise, there is a delayed rise in FSH concentrations during the end of the preceding luteal phase, often referred to as the luteal-follicular transition, which is a critical time period for successful follicle recruitment [6]. A reduction in the concentration of the LH peak has also been reported in LPD cycles [5, 21]. Each of these hormonal alterations may contribute to abnormal function of the corpus luteum and, subsequently, suppressed progesterone concentrations [5, 6, 17, 21].
The determination of LPD in exercising women relies on the measurement of mid-cycle LH and daily progesterone concentrations in the luteal phase via daily urine or a timed serum sample during a single cycle; however, the monitoring of multiple consecutive cycles is advised for detection of LPD due to the inconsistency with which LPD cycles are observed in exercising women [5, 6]. For example, women may present with a normal, ovulatory cycle one month followed by an LPD or anovulatory cycle the next month. Inconsistent presentations of LPD and anovulation during consecutive cycles may also occur in the same individual. In fact, it has been reported that almost half (46 %) of exercising women present with inconsistent menstrual status; therefore, monitoring only one cycle may underestimate the incidence of menstrual disturbances among exercising women by 38 % [6]. Procedures for daily urine or serum sampling are costly and often not feasible; therefore, the detection of LPD in exercising women is difficult and the majority of women with LPD are often unaware of the presence of this subclinical menstrual perturbation. Notably, self-report and/or assessment of menstrual history alone will not detect LPD, thereby further contributing to the underestimation of the prevalence of menstrual disturbances among exercising women.
Subtle Menstrual Disturbances: Anovulation
Anovulation represents a subtle menstrual disturbance that is more severe than LPD. The hallmark characteristic of anovulation is the failure of follicular estrogen to rise concomitant with the lack of the mid-cycle LH surge and the subsequent failure to ovulate [5] (Fig. 11.4b). Similar to women with LPD, women with anovulatory cycles are, for the most part, experiencing regular intermenstrual intervals, making the identification of anovulation difficult. Due to the absence of ovulation and, consequently, the failure to produce a corpus luteum, progesterone concentrations do not increase during the latter part of the cycle. Therefore, anovulation has also been defined as the lack of an increase in urinary PdG from a 5-day follicular phase baseline or a peak PdG value < 2.49 µg/ml [5]. Both estrogen and progesterone concentrations have been reported to be lower in anovulatory cycles of exercising women compared to ovulatory cycles of exercising women, suggesting that disruptions in FSH and LH pulsatility contribute to anovulation [5, 6]. Adequate estrogen concentrations, however, allow for degeneration of the functional layer of the endometrium upon withdrawal of hormonal support at the end of the luteal phase, thereby resulting in normal menses [13].
Severe Menstrual Disturbances: Oligomenorrhea
Oligomenorrhea represents cycles with long and inconsistent intermenstrual intervals of 36–90 days that are often accompanied by 3–6 menses events per year [1, 22, 23] (Fig. 11.5a). This severe menstrual disturbance is perhaps the least understood and most difficult perturbation to interpret due to its inconsistent hormonal characteristics. An oligomenorrheic cycle may be ovulatory or anovulatory, and estrogen concentrations often produce erratic profiles during the extended cycle as follicles seek dominance [1].
Profile of daily urinary excretion of reproductive hormones for severe menstrual disturbances. a Representative oligomenorrheic, anovulatory menstrual cycle. Classic characteristics include a cycle 36–90 days in length and an erratic hormonal profile. b Representative amenorrheic 28-day monitoring period. Classic characteristics include chronic suppression of E1G and PdG. E1G estrone-1-glucuronide, PdG pregnanediol glucuronide, LH luteinizing hormone
The etiology of oligomenorrhea in exercising women may or may not be hypothalamic in nature [22]. Oligomenorrhea can be associated with prolactin-secreting tumors, thyroidtoxicosis and other endocrinopathies, but most often, oligomenorrhea is associated with hyperandrogenism [22, 24–27]. Hyperandrogenism is often secondary to polycystic ovarian syndrome (PCOS) [28] , which is causally linked to infertility in women [29].
In exercising women, oligomenorrhea has been often associated with hyperandrogenism, but may also occur secondary to an energy deficit. Investigators have observed hyperandrogenism concomitant with elevated LH/FSH ratio and free androgen index, two additional markers of PCOS , among athletes with menstrual dysfunction [24–26, 30]. Rickenlund et al. [24] identified that a distinct group of athletes with menstrual dysfunction presented with hyperandrogenism, and upon comparison of the oligo-amenorrheic athletes with hyperandrogenemia (H-OAM) to oligo-amenorrheic athletes with normal androgen profiles (N-OAM), the H-OAM group demonstrated a higher LH/FSH ratio than the N-OAM group, indicating that the profile of reproductive hormones differed between the two groups.
Of interest, however, is that circulating concentrations of triiodothyronine (TT3), a marker of energy deficiency , were significantly lower in both the H-OAM and N-OAM groups compared to a control group of sedentary women, suggesting that both groups may have been in an energy-deficient state [24].
On the other hand, when assessing athletes based on type of menstrual disturbance, Rickenlund et al. [25] observed that 24-h diurnal secretion of testosterone was significantly elevated among oligomenorrheic athletes compared to amenorrheic and regularly menstruating athletes. In addition, amenorrheic athletes demonstrated reduced LH pulsatility, a surrogate marker of GnRH inhibition at the hypothalamus, compared to regularly-menstruating controls, whereas oligomenorrheic athletes demonstrated an LH pulse pattern similar to that observed in regularly-menstruating controls [25].
Therefore, oligomenorrheic athletes did not display the normal hormonal pattern typical of hypothalamic inhibition due to an energy deficiency as was observed in amenorrheic athletes, suggesting that other factors such as hyperandrogenism could be a mechanism underlying oligomenorrhea in athletes. As such, the etiology of oligomenorrhea among exercising women with hyperandrogenemia is ambiguous, thereby complicating the treatment of menstrual dysfunction among this subgroup of exercising women. Careful screening of oligomenorrheic exercising women is necessary to determine if the long, inconsistent cycles are due to an energy deficit or PCOS [22] .
Severe Menstrual Disturbances: FHA
At the extreme end of the menstrual disturbance continuum is FHA, the most severe menstrual disturbance that is associated with severe estrogen deficiency and typically defined as the absence of menses for at least 90 days [1, 23], although definitions have varied [23, 31]. FHA is typically classified as either primary or secondary in nature [27]. Primary amenorrhea is defined as the failure to menstruate by 15 years of age in girls with secondary sex characteristics [27]; whereas, secondary amenorrhea is the abnormal cessation of the menstrual cycle after menarche [27].
FHA among exercising women refers to menstrual dysfunction that is caused by disruptions in the hypothalamus due to energy conservation and is unrelated to other causes of FHA associated with the four-compartment model [27, 32]. Exercising women with FHA present with chronically suppressed estrogen and progesterone concentrations [5, 33, 34] (Fig. 11.5b). This suppression is most likely the result of impaired GnRH, LH, and FSH pulsatility that are, therefore, inadequate to stimulate ovulation from the ovary as well as appropriate proliferation and removal of the functional layer of the endometrium.
As such, the ovaries and uterus of amenorrheic women are largely quiescent with minimal production of reproductive hormones. FHA is associated with the most severe clinical sequelae such as low bone mineral density (BMD) [35, 36], poor bone quality [37], and cardiovascular consequences, including a poor lipid profile and endothelial dysfunction [38–40].
Prevalence of EAMD
The prevalence of menstrual disturbances among exercising women has been reported to range from 0 to 60 %, a large range that encompasses the prevalence of both subtle and severe menstrual disturbances [41]. The range in prevalence rates in exercising women is large because of the variations in definitions used and methods of assessment in exercising women [5, 6, 42–53]. The prevalence estimates, however, frequently exceed that observed in the general population of nonathletic women that is as low as 3–5 % [5, 54–56] .
Prevalence of Subtle Menstrual Disturbances
Due to the burdensome nature of investigating the presence of subtle menstrual disturbances, only a few investigators have reported their prevalence among exercising women [5, 6, 42–44] despite LPD and anovulation together representing the most common menstrual disturbances linked to exercise training [5, 6]. The prevalence of subtle menstrual disturbances is alarmingly high given that these disturbances are masked by regular intermenstrual intervals. Prevalence estimates range from 5.9 to 43.0 % [5, 6, 42, 44] and 12.0 to 30.0 % [5, 6, 44] for LPD and anovulation, respectively. Indeed, the ideal method of identifying subtle menstrual disturbances requires the measurement of daily urinary excretion of reproductive hormones over multiple consecutive cycles.
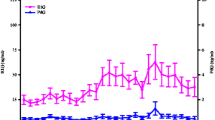
Based on reports from our lab which has undertaken the task of assessing multiple cycles among exercising women, we observed that 27 and 25 % of exercising women with self-reported eumenorrheic cycles (i.e., 26–35 days in length) presented with an LPD or anovulatory cycle, respectively [5]. Therefore, over half of exercising women presented with a subtle menstrual disturbance, compared to only 5 % of sedentary women (Fig. 11.6).
Prevalence of subtle menstrual disturbances among sedentary and exercising women. a Proportion of sedentary women categorized as having ovulatory or abnormal (LPD or anovulatory) cycles. b Proportion of exercising women categorized as having ovulatory or abnormal (LPD or anovulatory) cycles. Exercising women compared with sedentary women: † indicates p = 0.050; § indicates p < 0.001. Reprinted with permission of Oxford University Press from De Souza et al. [5]
Similarly, upon evaluation of individual menstrual cycles among exercising women monitored for 1–3 menstrual cycles, 21 and 29 % of the cycles demonstrated evidence of an LPD and anovulation, respectively, representing 50 % of the 120 cycles assessed in exercising women [5]. Only 4 % of the cycles of sedentary women had a subtle menstrual disturbance, all of which were characterized by an LPD [5] (Fig. 11.7).
Prevalence of subtle menstrual disturbances among individual cycles of sedentary and exercising women. a Proportion of cycles displaying subtle menstrual disturbances among sedentary women. b Proportion of cycles displaying subtle menstrual disturbances among exercising women. Cycles of exercising women compared with cycles of sedentary women: ‡ indicates p < 0.050; § indicates p < 0.001. Reprinted with permission of Oxford University Press from De Souza et al. [5]
Therefore, these results clearly demonstrate the high prevalence with which these largely underdiagnosed menstrual disturbances occur, most often indicative of an energy-deficient state and other underlying health concerns. The strength of our study lies in the daily urinary assessment of reproductive hormones, which provides a complete picture of the hormonal fluctuation throughout the cycle. Therefore, this methodology allows for a more accurate estimate of the prevalence of EAMD than can be obtained from relying solely on self-report measures that often underestimate EAMD prevalence. The frequency at which these “hidden” menstrual disturbances present in exercising women is cause for concern due to the negative impact of an energy deficit and menstrual disturbances on health outcomes and lack of symptomatic indicators that such disturbances are present.
Prevalence of Severe Menstrual Disturbances
Several investigators have evaluated the prevalence of the severe menstrual disturbances (FHA and oligomenorrhea) in female athletes, including both high school [48, 57–59] and adult women [5, 6, 45–47, 60–65]. The earliest prevalence estimates of clinical menstrual disturbances were evaluated in long-distance runners [49–53, 66], dancers [67, 68], and gymnasts [69], and in general, severe menstrual disturbances are documented at much higher rates in premenopausal exercising women than in sedentary women [54–56].
Based on these reports in female athletes and exercising women, the prevalence of primary and secondary amenorrhea ranged from 0 to 56.0 % (determined in 13 studies) [41] and 1 to 60.0 % (determined in 35 studies) [41], respectively; whereas, the range in prevalence of oligomenorrhea was 0.9–52.5 % (determined in 23 studies) [41]. In a recent report that assessed menstrual status in recreationally active women based on daily urinary steroid excretion, investigators observed that 7 % of exercising women presented with oligomenorrhea; whereas, 37 % were amenorrheic [5]. No sedentary women in the sample, however, presented with either oligomenorrhea or amenorrhea [5]. Therefore, menstrual disturbances among exercising women are relatively frequent, highlighting the need for awareness of the problem and its associated consequences in an effort to promote healthy exercise habits.
Changes in HPO Activity Associated with EAMD
Chronic energy deficiency targets the pulsatile secretion of GnRH from the arcuate nucleus of the hypothalamus . Disruptions in GnRH pulsatility often lead to changes in the frequency and amplitude of LH and FSH pulses, longer cycle length (particularly a longer follicular phase), reductions in average and peak luteal phase progesterone concentrations, and suppressed estradiol and progesterone [70]. It is believed that GnRH pulsatility, as governed by the pulse generator located in the hypothalamus [8], is sensitive to changes in the metabolic environment that are characteristic of an energy deficiency [7, 71]. (For a detailed description of metabolic adaptations that affect reproductive function , refer to Chap. 12). Thus, an energy deficit disrupts GnRH pulsatility, beginning the cascade of alterations in FSH and LH secretion, estrogen and progesterone production, and ultimately reproductive dysfunction.
Effects of Energy Deficiency on LH Secretion
One of the characteristics of the HPO axis in response to an energy deficit that is created by either energy intake restriction or increased energy expenditure or both is the disruption of LH pulsatility. In an environment of chronic energy deficiency , a reduction in LH frequency has been observed [72, 73].
Loucks et al. [72] compared LH secretory dynamics among regularly menstruating athletes (CA), amenorrheic athletes (AA), and regularly menstruating sedentary women (CS). The frequency of LH pulses was significantly lower in the AA group compared to both CA and CS groups [72]. In addition, relative quiescence of 24-h LH pulsatility was observed in AA, as evidenced by no significant changes in the wake and sleep values of LH in AA compared to the slowing of LH pulse frequency and increase of pulse amplitude that were observed during the sleep hours versus awake hours in both CA and CS women [72] .
Likewise, in a similarly designed study, Veldhuis et al. [73] also observed decreased LH pulse frequency among amenorrheic/oligomenorrheic athletes when compared with sedentary, regularly menstruating control women. In response to GnRH administration, however, the amenorrheic/oligomenorrheic athletes demonstrated greater LH secretion than the control women [73], a result that was replicated by Loucks et al. [72]. These results suggest that the reduced LH pulsatility among amenorrheic athletes is likely due to metabolically induced alterations in GnRH pulsatility rather than diminished pituitary responsiveness to GnRH [72, 73].
Causal evidence of the effect of a chronic energy deficiency derived from either dietary energy restriction or increased energy expenditure or both on LH pulsatility was demonstrated by Scheid et al. [74] in a longitudinal model and by Loucks et al. [75] and Williams et al. [76] in an acute model.
Scheid et al. [74] exposed previously sedentary women to a 3-month longitudinal model of energy deficiency that consisted of dietary restriction (−30 to −60 % of baseline energy needs) combined with regular aerobic exercise (70–80 % of maximum heart rate) performed 5 days per week. Before and after the 3-month intervention, women underwent 24-h repeated blood sampling to determine the impact of a long-term energy deficiency on LH pulsatility [74]. After completion of the intervention, a significant decline in 24-h LH pulse frequency was observed among the women exposed to the energy deficit; whereas, women in a control group who neither exercised nor restricted dietary intake demonstrated no change in LH pulse characteristics [74]. This decrease in LH pulse frequency is evident in Fig. 11.8 which depicts individual 24-h profiles of LH pulsatility pre- and post-intervention .
Examples of individual 24-h profiles of LH pulsatility before (pre) and after (post) a 3-month intervention consisting of dietary restriction and aerobic exercise. a Subject #1 was in the Control group, lost 0.1 kg body weight, and increased LH pulse frequency by 0.02 pulses/h. b Subject #2 was in the Energy Deficit group, lost 3.3 kg body weight, and decreased LH pulse frequency by 0.75 pulses/h. c Subject #3 was in the Energy Deficit group, lost 6.3 kg body weight, and decreased LH pulse frequency by 0.89 pulses/h. * represents LH pulse determined using cluster analysis software. LH luteinizing hormone. Reprinted with permission of The American Physiological Society from Scheid et al. [74]
An acute energy deficiency has also been demonstrated to impact LH pulsatility [75, 76]. In a classic study, the induction of low energy availability (EA) (≤ 20 kcal/kg LBM/day) for 5 days in sedentary, regularly menstruating women resulted in suppressed LH pulse frequency and increased LH pulse amplitude compared to a replete EA (45 kcal/kg LBM/day) [75]. Similarly, restriction of energy intake by 60 % for 7 days concomitant with a short-term increase in exercise training volume for 3 days in premenopausal women resulted in a significant decrease in LH pulse frequency compared with an experimental condition characterized by a 3-day increase in exercise volume with 7 days of eucaloric intake [76]. In fact, LH pulsatility appears to rapidly respond, even within minutes, to shifts in EA in the animal model [7]. Taken together, these observations provide evidence that both chronic and acute energy deficits driven either by dietary energy restriction or increased energy expenditure or both lead to disruption of LH secretory patterns, likely due to energetically-driven alterations in GnRH pulsatility.
Energy Deficiency and FSH Secretion
Although it is well established that energy deficiency alters LH pulsatility, likely via disruptions in GnRH pulsatility, the role of FSH in menstrual disturbances has not been well characterized [75] . It appears, however, that FSH secretion is indeed impacted by an energy deficiency and contributes to menstrual disturbances.
Among exercising women with LPD and a low EA , De Souza et al. [6] observed significantly lower FSH concentrations during the luteal-follicular transition (last 5 days of the cycle) compared with cycles of sedentary ovulatory women, suggesting that a decline in FSH concentrations may contribute to the suppressed ovarian function that is observed among women with EAMD . This decrease in FSH secretion during the luteal-follicular transition is believed to impact follicular recruitment and maturation, thereby contributing to the low estrogen concentrations that were also observed in this group of exercising women during days 6–12 of the cycle [6]. However, the imposition of low EA for 5 days among regularly menstruating women did not result in changes in FSH concentrations despite changes in LH pulsatility and estrogen concentrations compared with an adequate EA [75]. These findings suggest that FSH may respond differently to acute and chronic energy deficits.
Energy Deficiency and Ovarian Hormone Production
Disruptions in LH and FSH secretion, in turn , lead to suppression of estrogen and progesterone production from the ovaries, a hallmark characteristic of EAMD. Reductions in FSH during the luteal-follicular transition may cause delayed follicular maturation and, thus, a decrease in estrogen production from the developing follicles during the follicular phase.
Anovulatory cycles of exercising women demonstrated significantly lower estrogen excretion and area under the curve during the follicular phase compared to ovulatory cycles of both sedentary and exercising women [5]. Likewise, mean estrogen concentration and estrogen exposure during a 28-day monitoring period among amenorrheic exercising women has been observed to be significantly lower than that observed during a monitored cycle of exercising ovulatory women [34]. Disruption of LH pulsatility and the LH surge may contribute to lower progesterone production as has been demonstrated in LPD, anovulatory, and amenorrheic cycles of exercising women compared with ovulatory cycles of sedentary and exercising women [5, 34]. As such, urinary and serum measurements of ovarian hormone concentrations in exercising women with menstrual disturbances indicating a chronic energy deficit or regularly menstruating women with an acute energy deficit have revealed declines in estrogen and progesterone concentrations [5, 33, 34, 75, 77].
The suppression in ovarian hormone concentrations varies with the severity of the menstrual disturbance, with the degree of suppression increasing from the least severe menstrual disturbance of LPD to the most severe menstrual disturbance of amenorrhea [5] . In fact, a characteristic hormonal profile of amenorrhea is a chronic suppression of estrogen and progesterone, with the normal peaks of these ovarian hormones notably absent (Fig. 11.5b). Therefore, taken together, menstrual dysfunction associated with exercise, of which the underlying etiology is an energy deficit, is the result of a sequence of reproductive hormone changes, beginning in the hypothalamus and affecting each level of the HPO axis.
Stress Hypothesis Versus EA Hypothesis
Although exercising women frequently present with menstrual dysfunction, it is imperative to highlight that it is not the exercise per se that leads to menstrual dysfunction and the cascade of subsequent health consequences; rather, it is the energy deficiency caused by inadequate caloric intake to compensate for energy expenditure that leads to the altered metabolic and hormonal environment typical of women with FHA. The “exercise stress hypothesis” postulates that the stress of exercise, defined as everything related to exercise except the energy cost, upregulates the hypothalamic–pituitary–adrenal (HPA) axis, disrupting reproductive function at the level of the hypothalamus by altering GnRH secretion [78].
On the other hand, the “energy availability hypothesis” suggests that markers of energy status alter reproductive function by influencing the GnRH pulse generator and subsequently LH pulsatility [78] (refer to Chap. 12 for a detailed description of the metabolic markers that alter reproductive function).
These hypotheses were tested by Loucks et al. [78] using a carefully designed experimental protocol that included both exercising and nonexercising treatment groups among sedentary women. For 4 days on two separate occasions, the exercising treatment group engaged in 30 kcal/kg LBM/day of exercise and were provided with either (1) a 75 kcal/kg LBM/day diet to set EA at 45 kcal/kg LBM/day (balanced) or (2) a diet of 40 kcal/kg LBM/day to set EA at 10 kcal/kg LBM/day (deprived). The nonexercising treatment group did not expend energy via exercise but were provided with either (1) a diet of 45 kcal/kg LBM/day (balanced) or (2) a diet of 10 kcal/kg LBM/day (deprived). It was previously demonstrated that an EA of 30 kcal/kg LBM/day was adequate to maintain optimal TT3 concentrations and LH pulsatility [75, 79]; whereas, at an EA below 25–30 kcal/kg LBM/day, a cascade of negative metabolic and hormonal adaptations occurred, indicating an energy-deficient state [75, 79, 80].
In the exercise treatment group, the deprived condition demonstrated a significant reduction in TT3 concentrations, a hormone that is a key marker of energy status and is typically suppressed among amenorrheic exercising women [79], as well as a reduction in LH pulse frequency compared to the balanced condition [78]. Within the nonexercise treatment group, these results were mimicked with the exception of LH pulse frequency which showed a much larger reduction in the deprived condition. However, upon comparison of the exercise treatment group with the nonexercise treatment group, the stress of exercise did not suppress TT3 concentrations or LH pulse frequency [78, 79]. Taken together, these results indicate that exercise itself, apart from its energy cost, does not disrupt the reproductive axis. On the contrary, alterations in LH pulsatility that translate to EAMD are due to the energetic cost of exercise, in particular, when energy intake is inadequate for energy expenditure, creating an energy deficit.
These findings have been supported by studies in monkeys that demonstrated that the induction of amenorrhea was associated with an increase in exercise energy expenditure that was not combined with compensatory increases in energy intake, thus creating an energy deficit [81]. Among eight monkeys that began exercise training (progressive increase to 12.3 ± 0.9 km/d of running) without changes in energy intake, each monkey developed amenorrhea (defined as absence of menses for at least 100 days) 7–24 months into the intervention, coinciding with suppressed estrogen, progesterone , LH, and FSH concentrations [81]. Four of the monkeys were then fed supplemental calories (138–181 % of energy intake during amenorrhea) without changes in exercise training [70]. These monkeys resumed normal menstrual cycles in response to the adequate energy intake, corresponding with increases in LH, FSH, and estrogen during the follicular phase and increases in progesterone during the luteal phase [70]. Accordingly, the recovery of menses correlated with energy intake during refeeding and an increase in TT3 [70]. As such, these results provide further support for the hypothesis that EA rather than the stress of exercise is primarily responsible for regulating the HPO axis in exercising women.
Reproductive Potential and Clinical Relevance
In terms of reproductive and clinical significance, the impact of menstrual dysfunction at any point along the continuum in exercising women has a profound impact on fertility. The acute effects of FHA on reproductive potential are clear in that the complete absence of the menstrual cycle precludes pregnancy. Likewise, the inconsistent presentation of oligomenorrhea with varied cycle lengths and the occurrence of both ovulatory and anovulatory cycles may create an unstable environment for both unwanted and wanted conception due to the uncertain status of the cycle at any given time. LPD are a unique menstrual disturbance in that, unlike other disturbances, the irregular cycles are masked in the presence of regular length and ovulation . However, LPD are associated with infertility and spontaneous abortion due to the inadequate progesterone environment [17]; the low progesterone production inhibits proper maturation of the endometrium, leading to poor endometrial quality that cannot support blastocyst implantation and causes embryonic loss [18–20, 82]. Therefore, in summary, women with LPD associated with inadequate progesterone production often experience disruptions in follicular growth, suppressed oocyte maturation, and endometrial dysfunction, which may lead to compromised fecundity, spontaneous abortion, and infertility [17, 19, 20].
An important consideration for reproductive potential among exercising women, particularly those presenting with apparently regular menstrual cycles, is the consistency at which ovulatory and abnormal cycles occur. De Souza et al. [5] reported that A greater proportion of sedentary women (Sed) presented with consistently ovulatory cycles compared to exercising women (Ex) (Sed: 95 % vs. Ex: 32 %); whereas, exercising women presented with more consistently abnormal cycles (Ex: 32 % vs. Sed: 0 %) and more inconsistent cycles than sedentary women (Ex: 36 % vs. Sed: 6 %) [5]. These findings indicate that exercising women are more likely to experience abnormal cycles over the course of several consecutive cycles [5]. The inconsistent presentations of normal and abnormal cycles in exercising women introduce challenges for exercising women with respect to fertility.
Although the impact of EAMD on reproductive potential is unfavorable, consequences of EAMD on reproductive function appear to be acute and do not cause permanent damage [83, 84]. Upon recovery of an optimal energy status, exercising women with menstrual dysfunction can recover normal menstrual function [83–86] , allowing proper follicular, ovarian, and endometrial function. It is believed that as the improvement in energy status progresses, menstrual disturbances may also move along the spectrum from the most severe (i.e., amenorrhea) to the least severe (i.e., LPD) before attaining eumenorrheic, ovulatory cycles [83, 88] thus, complete restoration of energy status is essential for optimal reproductive function . For example, an amenorrheic woman who resumes normal menses may still present with anovulatory or LPD cycles, identifying that a small degree of energy deficiency may still be present. Continued improvement in energy status would be necessary to prevent infertility and spontaneous abortion.
Effective nonpharmacological treatment strategies for EAMD include an increase in caloric intake and weight gain. Dueck et al. [85] and Kopp-Woodroffe et al. [86] described case studies of five amenorrheic recreationally active women and female athletes who participated in a diet and training intervention to reverse amenorrhea. Caloric intake was increased by approximately 360 kcal/day and training was reduced by 1 day/week for 12–20 weeks, contributing to a weight gain of 1–3 kg [85, 86]. During the intervention, three of the five women resumed menses [85, 86]. Of the two that did not resume menstruation during the intervention, one woman maintained the increased caloric intake and resumed menses 3 months after completion of the intervention [85]. The other woman withdrew from the study to begin oral contraceptives; therefore, it cannot be determined if resumption of menses would have eventually occurred [86].
Other case reports in recreationally active women with amenorrhea have revealed that an increase in caloric intake of approximately 300 kcal and a weight gain of 3–4 kg contributes to resumption of menses [88]. Although the actual time to resumption of menses may vary for women depending on the severity of the energy deficiency and menstrual disturbance, the amount of weight that is typically gained leading to resumption of menses is not exorbitant, thereby alleviating some concerns that exercising women may have about weight gain.
In addition, weight gain that leads to resumption of menses is also associated with improvement of other clinical sequelae characteristic of exercise-associated amenorrhea [87]. Miller et al. [87] reported that resumption of menses occurred in anorexic women who gained 4 kg of body mass, on average, and the combined effects of weight gain and resumption of menses contributed to significant improvements in lumbar spine and hip BMD. As such, relatively small increases in body mass can lead to a cascade of beneficial health outcomes among women with menstrual dysfunction associated with an energy deficiency.
Conclusion
Adolescent girls and premenopausal women engaging in regular exercise, including both recreational and competitive physical activity , are at risk of developing a menstrual disturbance if energy intake is inadequate to compensate for energy expenditure, resulting in an energy deficit. EAMD include both clinical presentations (i.e., FHA and oligomenorrhea ) and subclinical presentations (i.e., LPD and anovulation); therefore, awareness of the importance of adequate energy intake among exercising girls and women is essential. Each menstrual disturbance is linked to spontaneous abortion or infertility , thereby having a profound effect on fecundity. However, with the maintenance of a replete energy state, EAMD that is commonly observed among exercising women can be avoided. Nonpharmacological strategies to prevent and reverse EAMD include consuming adequate kilocalories on a daily basis to support healthy body weight and menstrual function . Although energy intake requirements vary among individuals, regular estimations of daily energy expenditure and energy intake and subsequent calculations of energy balance (energy intake–energy expenditure) will promote maintenance of a positive energy balance that should ultimately result in favorable outcomes for both reproductive and overall health.
Abbreviations
- BMD:
-
Bone Mineral Density
- EA:
-
Energy availability
- EAMD:
-
Exercise-associated menstrual disturbances
- FHA:
-
Functional hypothalamic amenorrhea
- FSH:
-
Follicle-stimulating hormone
- GnRH:
-
Gonadotropin-releasing hormone
- hCG:
-
Human chorionic gonadotropin
- HPA:
-
Hypothalamic–pituitary–adrenal
- HPO:
-
Hypothalamic–pituitary–ovarian
- LBM:
-
Lean body mass
- LH:
-
Luteinizing hormone
- LPD:
-
Luteal phase defects
- PCOS:
-
Polycystic ovarian syndrome
- PdG:
-
Pregnanediol glucuronide
- TT3:
-
Triiodothyronine
References
De Souza MJ, Williams NI. Physiological aspects and clinical sequelae of energy deficiency and hypoestrogenism in exercising women. Hum Reprod Update. 2004;10(5):433–48.
O’Donnell E, De Souza MJ. The cardiovascular effects of chronic hypoestrogenism in amenorrhoeic athletes: a critical review. Sports Med. 2004;34(9):601–27.
O’Donnell E, Harvey PJ, De Souza MJ. Relationships between vascular resistance and energy deficiency, nutritional status and oxidative stress in oestrogen deficient physically active women. Clin Endocrinol (Oxf). 2009;70(2):294–302.
Keen AD, Drinkwater BL. Irreversible bone loss in former amenorrheic athletes. Osteoporos Int. 1997;7(4):311–5.
De Souza MJ, Toombs RJ, Scheid JL, O’Donnell E, West SL, Williams NI. High prevalence of subtle and severe menstrual disturbances in exercising women: confirmation using daily hormone measures. Hum Reprod. 2010;25(2):491–503.
De Souza MJ, Miller BE, Loucks AB, Luciano AA, Pescatello LS, Campbell CG, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83(12):4220–32.
Wade GN, Schneider JE, Li HY. Control of fertility by metabolic cues. Am J Physiol. 1996;270(1 Pt 1):E1–19.
Yen SSC. The human menstrual cycle: neuroendocrine regulation. In: Yen SSC, Jaffe RB, Barbieri RL, editors. Reproductive endocrinology: physiology, pathology, and clinical management. 4th ed. Philadelphia: Saunders; 1999. p. 191–217.
Knobil E. The wisdom of the body revisited. News Physiol Sci. 1999;14:1–11.
Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J. 2009;56(6):729–37.
Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G. Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science. 1980;207(4437):1371–3.
Plant TM, Krey LC, Moossy J, McCormack JT, Hess DL, Knobil E. The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta). Endocrinology. 1978;102(1):52–62.
Jones EE, DeCherney AH. Chapter 54: the female reproductive system. In: Boron WF, Boulpaep EL, editors. Medical physiology. 2nd ed. Philadelphia: Elsevier Saunders; 2005. p. 1141–66.
Rhoades R, Pflanzer R. Chapter 30: reproductive physiology. Human physiology. 2nd ed. Philadelphia: Saunders; 1992. p. 986–98.
Karsch FJ, Dierschke DK, Weick RF, Yamaji T, Hotchkiss J, Knobil E. Positive and negative feedback control by estrogen of luteinizing hormone secretion in the rhesus monkey. Endocrinology. 1973;92(3):799–804.
Marieb EN. Chapter 27: the reproductive system. Human anatomy and physiology. 6th ed. San Francisco: Pearson Benjamin Cummings; 2004. p. 1064–108.
Jones GS. The luteal phase defect. Fertil Steril. 1976;27(4):351–6.
Jones GS. Some newer aspects of the management of infertility. JAMA. 1949;141(16):1123–9.
De Souza MJ. Menstrual disturbances in athletes: a focus on luteal phase defects. Med Sci Sports Exerc. 2003;35(9):1553–63.
McNeely MJ, Soules MR. The diagnosis of luteal phase deficiency: a critical review. Fertil Steril. 1988;50(1):1–15.
Ayabe T, Tsutsumi O, Momoeda M, Yano T, Mitsuhashi N, Taketani Y. Impaired follicular growth and abnormal luteinizing hormone surge in luteal phase defect. Fertil Steril. 1994;61(4):652–6.
Awdishu S, Williams NI, Laredo SE, De Souza MJ. Oligomenorrhoea in exercising women: a polycystic ovarian syndrome phenotype or distinct entity? Sports Med. 2009;39(12):1055–69.
Loucks AB, Horvath SM. Athletic amenorrhea: a review. Med Sci Sports Exerc. 1985;17(1):56–72.
Rickenlund A, Carlstrom K, Ekblom B, Brismar TB, von Schoultz B, Hirschberg AL. Hyperandrogenicity is an alternative mechanism underlying oligomenorrhea or amenorrhea in female athletes and may improve physical performance. Fertil Steril. 2003;79(4):947–55.
Rickenlund A, Thoren M, Carlstrom K, von Schoultz B, Hirschberg AL. Diurnal profiles of testosterone and pituitary hormones suggest different mechanisms for menstrual disturbances in endurance athletes. J Clin Endocrinol Metab. 2004;89(2):702–7.
Hagmar M, Berglund B, Brismar K, Hirschberg AL. Hyperandrogenism may explain reproductive dysfunction in Olympic athletes. Med Sci Sports Exerc. 2009;41(6):1241–8.
Practice Committee of the American Society for Reproductive Medicine. Current evaluation of amenorrhea. Fertil Steril. 2006;86(5 Suppl 1):148–55.
Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–7.
Usadi RS, Legro RS. Reproductive impact of polycystic ovary syndrome. Curr Opin Endocrinol Diabetes Obes. 2012;19(6):505–11.
Constantini NW, Warren MP. Menstrual dysfunction in swimmers: a distinct entity. J Clin Endocrinol Metab. 1995;80(9):2740–4.
Drinkwater BL, Bruemner B, Chesnut CH, 3rd. Menstrual history as a determinant of current bone density in young athletes. JAMA. 1990;263(4):545–8.
Berga S, Naftolin F. Neuroendocrine control of ovulation. Gynecol Endocrinol. 2012;28(Suppl 1):9–13.
De Souza MJ, Lee DK, VanHeest JL, Scheid JL, West SL, Williams NI. Severity of energy-related menstrual disturbances increases in proportion to indices of energy conservation in exercising women. Fertil Steril. 2007;88(4):971–5.
Scheid JL, Toombs RJ, Ducher G, Gibbs JC, Williams NI, De Souza MJ. Estrogen and peptide YY are associated with bone mineral density in premenopausal exercising women. Bone. 2011;49(2):194–201.
Christo K, Prabhakaran R, Lamparello B, Cord J, Miller KK, Goldstein MA, et al. Bone metabolism in adolescent athletes with amenorrhea, athletes with eumenorrhea, and control subjects. Pediatrics. 2008;121(6):1127–36.
Rencken ML, Chesnut CH, 3rd, Drinkwater BL. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA. 1996;276(3):238–40.
Ackerman KE, Nazem T, Chapko D, Russell M, Mendes N, Taylor AP, et al. Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. J Clin Endocrinol Metab. 2011;96(10):3123–33.
Rickenlund A, Eriksson MJ, Schenck-Gustafsson K, Hirschberg AL. Amenorrhea in female athletes is associated with endothelial dysfunction and unfavorable lipid profile. J Clin Endocrinol Metab. 2005;90(3):1354–9.
O’Donnell E, Harvey PJ, Goodman JM, De Souza MJ. Long-term estrogen deficiency lowers regional blood flow, resting systolic blood pressure, and heart rate in exercising premenopausal women. Am J Physiol Endocrinol Metab. 2007;292(5):E1401–9.
O’Donnell E, Goodman JM, Harvey PJ. Clinical review: cardiovascular consequences of ovarian disruption: a focus on functional hypothalamic amenorrhea in physically active women. J Clin Endocrinol Metab. 2011;96(12):3638–48.
Gibbs JC, Williams NI, De Souza MJ. Prevalence of individual and combined components of the female athlete triad. Med Sci Sports Exerc. 2013;45(5):985–96.
Broocks A, Pirke KM, Schweiger U, Tuschl RJ, Laessle RG, Strowitzki T, et al. Cyclic ovarian function in recreational athletes. J Appl Physiol. 1990;68(5):2083–6.
Ellison PT, Lager C. Moderate recreational running is associated with lowered salivary progesterone profiles in women. Am J Obstet Gynecol. 1986;154(5):1000–3.
Winters KM, Adams WC, Meredith CN, Loan MD, Lasley BL. Bone density and cyclic ovarian function in trained runners and active controls. Med Sci Sports Exerc. 1996;28(7):776–85.
Beals KA, Manore MM. Disorders of the female athlete triad among collegiate athletes. Int J Sport Nutr Exerc Metab. 2002;12(3):281–93.
Cobb KL, Bachrach LK, Greendale G, Marcus R, Neer RM, Nieves J, et al. Disordered eating, menstrual irregularity, and bone mineral density in female runners. Med Sci Sports Exerc. 2003;35(5):711–9.
Beals KA, Hill AK. The prevalence of disordered eating, menstrual dysfunction, and low bone mineral density among US collegiate athletes. Int J Sport Nutr Exerc Metab. 2006;16(1):1–23.
Nichols JF, Rauh MJ, Barrack MT, Barkai HS, Pernick Y. Disordered eating and menstrual irregularity in high school athletes in lean-build and nonlean-build sports. Int J Sport Nutr Exerc Metab. 2007;17(4):364–77.
Glass AR, Deuster PA, Kyle SB, Yahiro JA, Vigersky RA, Schoomaker EB. Amenorrhea in Olympic marathon runners. Fertil Steril. 1987;48(5):740–5.
Shangold MM, Levine HS. The effect of marathon training upon menstrual function. Am J Obstet Gynecol. 1982;143(8):862–9.
Dale E, Gerlach DH, Wilhite AL. Menstrual dysfunction in distance runners. Obstet Gynecol. 1979;54(1):47–53.
Sanborn CF, Martin BJ, Wagner WW Jr Is athletic amenorrhea specific to runners? Am J Obstet Gynecol. 1982;143(8):859–61.
Feicht CB, Johnson TS, Martin BJ, Sparkes KE, Wagner WW Jr Secondary amenorrhoea in athletes. Lancet. 1978;2(8100):1145–6.
Pettersson F, Fries H, Nillius SJ. Epidemiology of secondary amenorrhea. I. Incidence and prevalence rates. Am J Obstet Gynecol. 1973;117(1):80–6.
Singh KB. Menstrual disorders in college students. Am J Obstet Gynecol. 1981;140(3):299–302.
Bachmann GA, Kemmann E. Prevalence of oligomenorrhea and amenorrhea in a college population. Am J Obstet Gynecol. 1982;144(1):98–102.
Nichols JF, Rauh MJ, Lawson MJ, Ji M, Barkai HS. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160(2):137–42.
Hoch AZ, Pajewski NM, Moraski L, Carrera GF, Wilson CR, Hoffmann RG, et al. Prevalence of the female athlete triad in high school athletes and sedentary students. Clin J Sport Med. 2009;19(5):421–8.
Barrack MT, Rauh MJ, Nichols JF. Prevalence of and traits associated with low BMD among female adolescent runners. Med Sci Sports Exerc. 2008;40(12):2015–21.
Pollock N, Grogan C, Perry M, Pedlar C, Cooke K, Morrissey D, et al. Bone-mineral density and other features of the female athlete triad in elite endurance runners: a longitudinal and cross-sectional observational study. Int J Sport Nutr Exerc Metab. 2010;20(5):418–26.
Thompson SH. Characteristics of the female athlete triad in collegiate cross-country runners. J Am Coll Health. 2007;56(2):129–36.
Rauh MJ, Nichols JF, Barrack MT. Relationships among injury and disordered eating, menstrual dysfunction, and low bone mineral density in high school athletes: a prospective study. J Athl Train. 2010;45(3):243–52.
Reinking MF, Alexander LE. Prevalence of Disordered-Eating Behaviors in Undergraduate Female Collegiate Athletes and Nonathletes. J Athl Train. 2005;40(1):47–51.
Meyer NL, Shaw JM, Manore MM, Dolan SH, Subudhi AW, Shultz BB, et al. Bone mineral density of Olympic-level female winter sport athletes. Med Sci Sports Exerc. 2004;36(9):1594–601.
Vardar SA, Vardar E, Altun GD, Kurt C, Ozturk L. Prevalence of the female athlete triad in Edirne, Turkey. J Sport Sci Med. 2005;4(4):550–5.
Wakat DK, Sweeney KA, Rogol AD. Reproductive system function in women cross-country runners. Med Sci Sports Exerc. 1982;14(4):263–9.
Abraham SF, Beumont PJ, Fraser IS, Llewellyn-Jones D. Body weight, exercise and menstrual status among ballet dancers in training. Br J Obstet Gynaecol. 1982;89(7):507–10.
Calabrese LH, Kirkendall DT. Nutritional and medical considerations in dancers. Clin Sports Med. 1983;2(3):539–48.
Robinson TL, Snow-Harter C, Taaffe DR, Gillis D, Shaw J, Marcus R. Gymnasts exhibit higher bone mass than runners despite similar prevalence of amenorrhea and oligomenorrhea. J Bone Miner Res. 1995;10(1):26–35.
Williams NI, Helmreich DL, Parfitt DB, Caston-Balderrama A, Cameron JL. Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J Clin Endocrinol Metab. 2001;86(11):5184–93.
De Souza MJ, Leidy HJ, O’Donnell E, Lasley B, Williams NI. Fasting ghrelin levels in physically active women: relationship with menstrual disturbances and metabolic hormones. J Clin Endocrinol Metab. 2004;89(7):3536–42.
Loucks AB, Mortola JF, Girton L, Yen SS. Alterations in the hypothalamic-pituitary-ovarian and the hypothalamic-pituitary-adrenal axes in athletic women. J Clin Endocrinol Metab. 1989;68(2):402–11.
Veldhuis JD, Evans WS, Demers LM, Thorner MO, Wakat D, Rogol AD. Altered neuroendocrine regulation of gonadotropin secretion in women distance runners. J Clin Endocrinol Metab. 1985;61(3):557–63.
Scheid JL, De Souza MJ, Hill BR, Leidy HJ, Williams NI. Decreased luteinizing hormone pulse frequency is associated with elevated 24-hour ghrelin after calorie restriction and exercise in premenopausal women. Am J Physiol Endocrinol Metab. 2013;304(1):E109–16.
Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab. 2003;88(1):297–311.
Williams NI, Young JC, McArthur JW, Bullen B, Skrinar GS, Turnbull B. Strenuous exercise with caloric restriction: effect on luteinizing hormone secretion. Med Sci Sports Exerc. 1995;27(10):1390–8.
Williams NI, Reed JL, Leidy HJ, Legro RS, De Souza MJ. Estrogen and progesterone exposure is reduced in response to energy deficiency in women aged 25–40 years. Hum Reprod. 2010;25(9):2328–39.
Loucks AB, Verdun M, Heath EM. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol. 1998;84(1):37–46.
Loucks AB, Heath EM. Induction of low-T3 syndrome in exercising women occurs at a threshold of energy availability. Am J Physiol. 1994;266(3 Pt 2):R817–23.
Ihle R, Loucks AB. Dose-response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res. 2004;19(8):1231–40.
Williams NI, Caston-Balderrama AL, Helmreich DL, Parfitt DB, Nosbisch C, Cameron JL. Longitudinal changes in reproductive hormones and menstrual cyclicity in cynomolgus monkeys during strenuous exercise training: abrupt transition to exercise-induced amenorrhea. Endocrinology. 2001;142(6):2381–9.
Balasch J, Vanrell JA. Corpus luteum insufficiency and fertility: a matter of controversy. Hum Reprod. 1987;2(7):557–67.
Bullen BA, Skrinar GS, Beitins IZ, von Mering G, Turnbull BA, McArthur JW. Induction of menstrual disorders by strenuous exercise in untrained women. N Engl J Med. 1985;312(21):1349–53.
Beitins IZ, McArthur JW, Turnbull BA, Skrinar GS, Bullen BA. Exercise induces two types of human luteal dysfunction: confirmation by urinary free progesterone. J Clin Endocrinol Metab. 1991;72(6):1350–8.
Dueck CA, Matt KS, Manore MM, Skinner JS. Treatment of athletic amenorrhea with a diet and training intervention program. Int J Sport Nutr. 1996;6(1):24–40.
Kopp-Woodroffe SA, Manore MM, Dueck CA, Skinner JS, Matt KS. Energy and nutrient status of amenorrheic athletes participating in a diet and exercise training intervention program. Int J Sport Nutr. 1999;9(1):70–88.
Miller KK, Lee EE, Lawson EA, Misra M, Minihan J, Grinspoon SK, et al. Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab. 2006;91(8):2931–7.
Mallinson RJ, Williams NI, Olmsted MP, Scheid JL, Riddle ES, De Souza MJ. A case report of recovery of menstrual function following a nutritional intervention in two exercising women with amenorrhea of varying duration. J Int Soc Sports Nutr. 2013;10:34.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Mallinson, R., Gibbs, J., De Souza, M. (2016). Impact of Physical Activity and Exercise on Female Reproductive Potential. In: Vaamonde, D., du Plessis, S., Agarwal, A. (eds) Exercise and Human Reproduction. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3402-7_11
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3402-7_11
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-3400-3
Online ISBN: 978-1-4939-3402-7
eBook Packages: MedicineMedicine (R0)