Abstract
Comparison of international standards for titanium and titanium alloys (Refs. 1, 2)
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1c.1 Composition
1c.3 Processing of cp-Ti and Ti Alloys
Provided that the following characteristics of titanium are taken into consideration, almost all processing procedures are possible:
-
1.
High affinity to oxygen, nitrogen, and hydrogen gases
-
2.
High reactivity to all metals to produce intermetallic compounds
-
3.
Relatively low Young’s modulus and therefore backspringing
-
4.
Relatively low thermal conductivity
-
5.
Tendency to stick to tools
1c.3.1 Hot Working and Heat Treatment
Titanium and titanium alloys are fabricated into semifinished products by conventional methods such as forging, rolling, pressing, and drawing. When Ti materials are heated, care must be taken to avoid an excessive adsorption of oxygen, nitrogen, and hydrogen. Therefore, heating and annealing should take place in a neutral or slightly oxidizing atmosphere. During heating in a gas-fired furnace, direct contact with the flame must be avoided because of the risk of hydrogen pickup and local overheating. In a short heating period, oxygen pickup is restricted to the surface area. This surface zone must be removed by chemical or mechanical methods. Hydrogen is able to penetrate the matrix rapidly; therefore, a reducing atmosphere must be avoided. The hot working temperature depends on the alloy composition and should be selected to obtain the best mechanical properties and grain structure (A1–A9 according to ETTC2, Ref. 17). Table 1c.8 summarizes the deformation temperatures for the various Ti materials. Table 1c.9 lists the temperature ranges and recommended annealing times for stress relieving, soft annealing, and solution treating with age hardening. When the cross section is very small, annealing is favorably carried out in a high-vacuum furnace. Prior to this annealing treatment, the oxide film must be removed from the surface to avoid diffusion of oxygen into the material.
1c.3.2 Working of Sheet
At room temperature cp-Ti grades 1 and 2 can be worked very well, and grades 3 and 4 only moderately well. Titanium alloys, because of their high yield/tensile strength ratio, can be worked only under certain conditions.
For deep drawing, special coatings in the form of polymer foils have proved to be effective. At high temperatures colloidal graphite and common hot press greases with graphite or molybdenum disulfide additives have been successful. The Fe-, Ni-, and Cr-contents should be limited to 0.05, 0.1, and 0.33 wt%, respectively, to allow during a short annealing treatment (1–5 min at 750–850 °C) a grain growth producing an average grain size of 50–70 μm. Due to this grain growth a deformation by twinning, with a resulting increased deep drawability, occurs (Ref. 19).
Superplastic forming is a material-saving and cost-reducing process for manufacturing parts of a complicated shape because it can be carried out together with diffusion welding in a single operation. Fine-grained alloys like Ti6Al4V and Ti5Al2.5Fe can be used for superplastic deformation. Other special deformation processes such as stretch forming, spinning, or explosion forming are also possible.
1c.3.3 Descaling
The average thickness of the oxide layer on the surface of cp-Ti as a function of temperature can be found in Table 1c.10 and the composition of the oxide layer on cp-Ti and Ti alloys can be found in Table 1c.11. The oxide layer on the surface of thick-walled pieces generated during deformation and/or heat treatment is removed by sandblasting and/or pickling. The workpiece is treated in an aqueous solution of 20 wt% HNO3 and 2 wt% HF.
Thin-walled pieces are merely pickled in an electrolytic solution or salt bath. It is important that not only the surface layer of oxide but also the underlying oxygen enriched diffusion zone is removed. Otherwise, the machinability and the service life of turning and milling tools would be negatively affected.
1c.3.4 Machining
The machining of titanium materials presents no difficulties provided that the following properties are taken into account:
-
1.
Relatively low thermal conductivity which may cause high thermal stresses at the cutting edge of the tool
-
2.
Low Young’s modulus which applies pressure to the tool
-
3.
Tendency to stick to the tool
Titanium materials must be machined at low cutting speeds, at a relatively high feed rate, and with an ample supply of coolant (sulfur-containing oil; mixture of tetrachloride carbon, molybdenum sulfide, and graphite; 5 % aqueous solution of sodium nitrite, 5–10 % aqueous solution of water-soluble oil or sulfurized chlorinated oil). The cutting tools should be sharp and mounted as rigidly as possible. Recommended parameters for turning and milling are given in Table 1c.12. Since titanium dust and chips can easily catch fire, safety precautions must be taken. Threads should be cut on a lathe, as thread-cutting discs are subject to seizure.
Sawing causes no difficulties if a high blade pressure is used and the coolant supply is sufficient. Coarse-toothed blades (4 teeth per inch) are recommended.
For grinding, aluminum oxide (5–10 m/s) and silicon carbide (20–30 m/s) can be used.
1c.3.5 Soldering and Brazing
Immediately before soldering and brazing, the oxide layer present on the surface of titanium material must be removed. For direct applications using a torch, aluminum–zinc and tin–zinc solders are suitable. The higher temperatures required for brazing present the difficulty of avoiding the formation of intermetallic phases. As with almost all metals, titanium forms brittle intermetallic phases in the fusion zone. The only exception is silver, so that this metal forms one of the main constituents of brazers. The sources of heat used for brazing and soldering are the acetylene torch, high-frequency induction coils, infrared radiation, an inert-gas-shielded arc with graphite or tungsten electrodes, furnaces with an argon atmosphere (min. 99.99 % and/or a dew point below −50 °C), and high-vacuum furnaces. If brazing is not performed under vacuum or in a controlled atmosphere, fluxes are necessary to dissolve the oxide layers and prevent a pickup of gases.
1c.3.6 Welding
The inert-gas-shielded arc processes (TIG and MIG) are mainly used for fusion welding. In special cases resistance, ultrasonic, electron beam, diffusion, and laser welding are applied. With the cp-Ti grades the weld attains mechanical properties approximating those of the base metal. A slight decrease in ductility may occur with high tensile grades. Under passivating conditions, titanium welds have the same corrosion resistance as the base metal. On the contrary, in reducing media the weld may be subjected to a more severe corrosive attack than the base metal. During the welding operation, the weld, the heat-affected zone, and the underside of the weld are shielded from the atmosphere. The filler rod used is an uncoated wire of the same grade or of a grade with a lower hardness than the base metal. Careful preparation of the joint is necessary; that is, surface impurities must be removed by grinding or pickling in order to avoid porosity. Even fingermarks can produce a hardening of the weld. A single layer can weld sheets up to 2.5 mm thick. In order to avoid local oxygen concentrations oxidation products, such as those found at the tip of the electrode, must be cut off. The effectiveness of the inert gas is responsible for the welding rate. The optimum argon flow rate has proved to be about 6–8 l/mm. After welding, the appearance of a dark blue or gray oxide layer indicates an insufficient inert gas shielding and an embrittlement of the weld due to oxygen and/or nitrogen pickup. The hardness of a good weld may exceed that of the fully recrystallized base metal by a maximum of 50 VHN. If, after a slight grinding of the surface, a hardness test should give a higher value, the weld must be completely removed because of embrittlement.
Electron beam welding is particularly suitable for titanium materials. It offers many advantages such as very narrow seams and small heat-affected zones, weldability of thick diameters, high welding speed, and reproducibility of even complex welds.
Titanium materials can be spot welded without any particular preparation under similar conditions to those of stainless steel. Using flat-tipped electrodes, spot welding can be performed without inert gas. A hardening of the zone by up to 50 VHN compared with the base metal is regarded as normal and does not diminish the strength of the joint. Seam and flashbutt welding are also possible if an argon atmosphere is used.
Diffusion welding is of particular importance for titanium materials because these materials are more amenable to a homogeneous band in the solid state than other metals. After welding, the joint zone shows a higher temperature under high vacuum or, in an inert atmosphere, a microstructure very similar to that of the base metal.
1c.4 Mechanical Properties
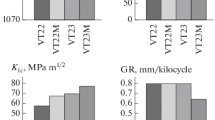
1c.5 Fatigue
1c.6 Corrosion and Wear
References
Cverna F, Horesh J, Whittle S, Yuko I (2001) Worldwide guide to equivalent nonferrous metals and alloys, 4th edn. ASM International, Materials Park, OH
Donachie MJ (2000) Titanium: a technical guide, 2nd edn. ASM International, Materials Park, OH
Standard specification for unalloyed titanium, for surgical implant applications (UNS R50250, UNS R50400, UNS R50550, UNS R50700) (2006) ASTM International, West Conshohocken, PA
Brunette DM, Tengvall P, Textor M, Thomsen P (2001) Titanium in medicine: material science, surface science, engineering, biological responses and medical applications, 1st edn. Springer, New York
Zhou YL, Niinomi M, Akahori T (2004) Effects of Ta content on Young’s modulus and tensile properties of binary Ti-Ta alloys for biomedical applications. Mater Sci Eng A 371:283–290
Semlitsch M, Staub F, Weber H (1985) Development of a vital, high-strength titanium aluminium-niobium alloy for surgical implants. In: Proc. fifth European CONF. ON BIOMATERIALS, Paris, 4–6 Sept 1985
Mausli P-A, Steinemann SG, Simpson JP (1984) Properties of surface oxides on titanium and some titanium alloys. In: Proc. of the sixth world conference on titanium, vol 3, pp. 1759–1764
Material properties of titanium and titanium alloys (DIN 17869) (1990) Beuth Veriag, Germany
Microstructure standard for titanium alloy bars, Technical Committee of European Titanium Producers, Publication ETTC2 (1979)
Supra Alloys, Inc. (2013) Aerospace materials specifications for titanium and titanium alloys. http://www.supraalloys.com/specs.php
MatWeb Material Property Data. Titanium, Ti. http://www.matweb.com/search/DataSheet.aspx?MatGUID=66a15d609a3f4c829cb6ad08f0dafc01
Eliaz N (2012) Degradation of implants, 2012th edn. Springer, New York
Titanium alloys—Ti6Al7Nb properties and applications. AZO materials. http://www.azom.com/properties.aspx?ArticleID=2064
MatWeb Material Property Data. Titanium IMI 367 (Ti-6Al-7Nb). http://www.matweb.com/search/datasheet.aspx?matguid=71fff43e6722453c8c9783d017d66977&ckck=1
Breme J, Wadewitz V, Burger K (1988) Verbund Titanlegierungen/Al2O3-Keramik fur dentale Implantate—Entwicklung geeigneter Legierungen. In: Ondracek G (ed) Verbundwerkstoffe—Stoffverbunde. DGM, pp. 123–130
Breme J, Biehl V, Schulte W, d'Hoedt B, Donath K (1993) Development and functionality of isoelastic dental implants of titanium alloys. Biomaterials 14(12):887–892
Akahori T, Niinomi M, Fukui H, Ogawa M, Toda H (2005) Improvement in fatigue characteristics of newly developed beta type titanium alloy for biomedical applications by thermo-mechanical treatments. Mater Sci Eng C 25:248–254
Kim SE, Jeong HW, Hyun YT, Lee YT, Jung CH, Kim SK, Song JS, Lee JH (2007) Elastic modulus and in vitro biocompatibility of Ti-xNb and Ti-xTa alloys. Met Mater Int 13(2):145–149
Zwicker U (1974) Titan und Titanlegierungen. Springer, New York
Lee W, Lin C (1998) High-temperature deformation behaviour of Ti6Al4V alloy evaluated at high strain-rate compression tests. J Mater Process Technol 75:127–136
Titanium and titanium wrought alloys forgings (hammer and drop forgings)—technical specification (DIN 17864) (1990) Beuth Veriag, Germany
Long M, Rack HJ (2008) Titanium alloys in total joint replacement—a materials science perspective. Biomaterials 19:1621–1639
Ramsdell JD, Hull ED (1960) Characteristics of cold-rolled and annealed Ti. Bureau of Mines Rep. of Inv. 5656
Breme J, Wadewitz V (1989) Comparison of Ti-Ta, Ti-Nb alloys. J Oral Max Implants 4(2):113–118
Niinomi M (1998) Mechanical properties of biomedical titanium alloys. Mater Sci Eng A243:231–236
Shuh A, Bigoney J, Hönle W, Zeiler G, Holzwarth U, Forst R (2007) Second generation (low modulus) titanium alloys in total hip arthroplasty. Materwiss Werkstofftech 38(12):1003–1007
Stemlitsch M, Staub F, Weber H (1985) Development of a vital, high-strength titanium aluminum-niobium alloy for surgical implants. In: Proc. fifth European conf. on biomaterials, Paris, 4–6 Sept 1985
Breme J, Zhou Y, Groh L (1995) Development of a titanium alloy suitable for an optimized coating with hydroxyapatite. Biomaterials 16:239–244
Titanium and titanium alloy strip, sheet and plate—technical conditions of delivery (DIN 17860) (1990) Beuth Veriag, Germany
Lanagan J (1988) Properties of plasma nitrided titanium alloys. In: Proc. of the sixth world conf. on titanium, vol 4, pp. 1957–1962
Borowy KH, Kramer KH (1984) On the properties of a new titanium alloy (TiA15Fe2.5) as implant material. In: Proc. of the fifth world conf. on Ti, vol 2, pp. 1381–1386
Thull R (1979) Eigenschaften von Metallen, fur orthopadische Implantate und deren Priifung. Orthopädie 7:29
Breme J, Schmidt H-J (1990) Criteria for the bioinertness of metals for osseo-integrated implants. In: ed. Heimke G (ed) Osseo-integrated implants, CRC press, vol 1, pp. 31–80
Semlitsch M, Weber H (1992) Titanlegierungen fur zementlose Huftprothesen. In: Hipp E, Gradinger R, Asherl R (eds) Die zementlose Hiiftprothese. Demeter Verlag, Grafelfingen, pp 18–26
Semlitsch M, Panic B (1994) 15 years of experience with test criteria for fracture-proof anchorage stems of artificial hip joints. In: Buchhorn GH, Willert H-G (eds) Technical principles, design and safety of joint implants. Hogrefe & Huber Publishers, Seattle, pp 23–36
Papakyriacou M, Mayer H, Pypen C, Plenk H Jr, Stanzl-Tschegg S (2000) Effects of surface treatments on high cycle corrosion fatigue of metallic implant materials. Int J Fatigue 22:873–886
Narayan R (2009) Biomedical materials, 2009th edn. Springer, New York
Kunze E (1988) Vergleichende Untersuchungen zum Langzeit-Ermii-dungsverhalten von Hiiftgelenkprothesen an Luft und in NaCl-Losung. Metall 2:140–145
RMI-Titanium (1969) Reactive Metals Inc., Niles, OH
Weinberg IG, Hanna IE (1957) An evaluation of the fatigue properties of Ti and Ti-alloys. TML Rep. 77
Mote WM, Frost RB (1958) The engineering properties of commercial Ti-alloys. TML Rep. 92
Weltzin RB, Koves G (1968) Impact fatigue testing of Ti alloys. J Mater 3:469–480
Properties and selection of metals. In: ASM metals handbook, vol 1. ASM International (1961)
Dittmar CB, Bauer GW, Evers D (1957) The effect of microstructural variables and interstitial elements on the fatigue behavior of Ti and commercial Ti alloys, Mallory, Sharen Titanium Corp. AD-110726WADC-TR 56–304, 95
Liitjering G, Gysler A (1984) Fatigue. In: Proc. of the fifth conf. on Ti, pp. 2065–2083
Hempel M, Hillnhagen E (1966) Dauerschwingfestigkeit der technischen Titanlegierungen TiA15Sn2.5 und TiA16V4. Arch Eisenhiittenwesen 37:263–277
Riidinger K, Fischer D (1984) Relationship between primary alpha content, tensile properties and high cycle fatigue behaviour of Ti6Al4V. In: Proc. of the fifth conf. on Ti, pp. 2123–2130
Wagner L, Gerdes C, Liitjering G (1984) Influence of surface treatment on fatigue strength of Ti6Al4V. In: Proc. of the fifth conf. on Ti, vol 4, pp. 2147–2154
Liu HW (1956) Effect of surface finish on the fatigue strength of TiA16V4- alloy. Dept. of Theoretical and Applied Mechanics, University of Illinois, T.U.A.M. Rep. 533
Geis-Gerstorfer J, Weber H, Breme J (1988) Electrochemische Untersuchung auf die Korrosionsbestandigkeit von Implantatlegierungen. Z Zahnarztl Implantol 4(1):31–36
Titanium alloys; chemical composition (DIN 17851) (1990) Beuth Veriag, Germany
Luiz de Assis S, Wolynec S, Costa I (2006) Corrosion characterization of titanium alloys by electrochemical techniques. Electrochim Acta 51:1815–1819
Kesteven J, Kannan MB, Walter R, Khakbaz H, Choe H (2015) Low elastic modulus Ti–Ta alloys for load-bearing permanent implants: enhancing the biodegradation resistance by electrochemical surface engineering. Mater Sci Eng C 46:226–231
Cai Z, Nakajima H, Woldu M, Berglud A, Bergman M, Okabe T (1999) In vitro corrosion resistance of titanium made using different fabrication methods. Biomaterials 20:183–190
Breme J (1988) Titanium and titanium alloys, biomaterials of preference. In: Proc. of the sixth world conf. on Ti, vol 1, pp. 57–68
Fraker AC, Ruff AW et al (1983) Surface preparation and corrosion. Behaviour of titanium alloys for surgical implants. In: Luckey HA, Kubli F (eds) Titanium alloys in surgical implants. ASTM STP796, pp. 206–219
Breme J, Wadewitz V, Ecker D (1986) Untersuchungen zum Einfluf* des Geftigezustandes auf das Korrosionverhalten der Implantatlegierung TiA15Fe2.5. Z./Zahnarztl. Implantologie, Bd. II 32–37
Gagg C (1988) Corrosion characteristics of Til5V3Cr3Sn3Al (Til5-3) alloy in a physiological environment
Mareci D, Ungureanu G, Aelenei DM, Mirza Rosca JC (2007) Electrochemical characteristics of titanium based biomaterials in artificial saliva. Mater Corros 58(11):848–856
Wadewitz V, Breme J (1989) Titan-Legierungen fur dentale Implantate. Z Zahnarztl Implantol V:116–120
Maurer A, Brown SA, Merrit K (1992) Effects of surface treatments on fretting corrosion of Ti6A14V. In: Proc. of the fourth world biomaterials congress, p. 200
Streicher RM, Weber H, Schon R, Semlitsch M (1991) Wet behaviour of different ceramic surfaces in comparison to TiN and OHD treated Ti6A14V alloy paired with polyethylene. In: Vincenzini P (ed) Ceramics in substitutive and reconstructive surgery. Elsevier, Amsterdam, pp 197–205
Fellah M, Assala O, Labaïz M, Dekhil L, Iost A (2013) Friction and wear behavior of Ti-6Al-4V and Ti-6Al-7Nb biomaterial alloy. J Biomater Nanobiotechnol 4:374–384
Hu Y-S, Dai D-H, Dong Y-L (1988) The ion nitriding of titanium materials and its applications. In: Proc. of the sixth world confer, on titanium, vol 4, pp. 1801–1804
Mears DC (1977) Metals in medicine and surgery. Int Met Rev, Review 218, 119–155
Hildebrand HF (1993) Biologische Aspekte beim Einsatz von Implantatwerkstoffen. DGM Hochschulseminar, Saarbriicken, Germany
Easter TL, Graham RM, Jacobs JJ, Black J, LaBerge M (1994) Clinical performance of Ti6A14V femoral components: wear mechanisms vs. surface profile. In: Proc. of the 20th annual meeting of the society for biomaterials, p. 185
Ebel B (1990) Zur Biokompatibilitat von Implantatwerkstoffen. KfK 4476
Tautzenberger P, Stockel D (1987) Vergleich der Eigenschaften von Thermobimetallen und Memory-Elementen, 1, 26–32
Nitinol Technical Properties. Johnson Matthey Medical Components (2015) http://jmmedical.com/resources/221/Nitinol-Technical-Properties.html
Thompson SA (2000) An overview of nickel-titanium alloys used in dentistry. Int Endod J 33:297–310
Trépanier C, Tabrizian M, Yahia L, Bilodeau L, Piron DL (1997) Effect of modification of oxide layer on NiTi stent corrosion resistance; Nitinol: Freemont, CA. http://www.nitinol.com/media/reference-library/033.pdf
Rocher P, El Medawar L, Hornez JC, Traisnel M, Breme J, Hildebrand HF (2004) Biocorrosion and cytocompatibility assessment of NiTi shape memory alloys. Scr Mater 50:255–260
El Medawar L, Rocher P, Hornez JC, Traisnel M, Breme J, Hildenbrand HF (2002) Electrochemical and cytocompatibility assessment of NiTiNOL memory shape alloy for orthodontic use. Biomol Eng 19(2–6):153–160
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Breme, H., Biehl, V., Reger, N., Gawalt, E. (2016). Chapter 1c Metallic Biomaterials: Titanium and Titanium Alloys. In: Murphy, W., Black, J., Hastings, G. (eds) Handbook of Biomaterial Properties. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3305-1_16
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3305-1_16
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-3303-7
Online ISBN: 978-1-4939-3305-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)