Abstract
In 2012 the USPSTF published its findings, stating there was no evidence of benefit for PSA screening, citing increased harm secondary to overdiagnosis and treatment. The task force recommended against routine screening in the general population. While there has been a decrease in the use of PSA for prostate cancer screening, other modalities to enhance the specificity of PSA are being explored.
Shared decision-making has been recommended for many years, yet compliance on the part of physicians has been poor. In order to employ shared decision-making, physicians would individualize testing by educating patients about their specific risk factors (age, family history, race, and lifestyle) and understanding patients’ expectations.
“…we primary care clinicians must ensure there is no more routine, indiscriminate PSA screening and no washing our hands of responsibility once the patient is referred to a specialist for prostate-cancer treatment. We owe it to our patients to provide them with the kind of guidance about this screening test that they need and deserve. That's the way to help put the [PSA] controversy to rest . . . one man at a time.” [1]
Mary F. McNaughton-Collins, M.D., M.P.H., and Michael J. Barry, M.D.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
It is clear that prostate cancer represents a major public health concern . With almost 200,000 new cases annually and nearly 30,000 deaths each year, the human toll is substantial [2].
When one recognizes that approximately one man in four who undergoes prostate biopsy is found to have prostate cancer, we can estimate that 800,000 prostate biopsies are being performed annually, subjecting many men to significant morbidity as well. This too has been a major healthcare concern.
Although the detection of prostate cancer has increased since the introduction of PSA screening, more cancers tend to be localized as well as low grade. In the pre-PSA era , 11 % of men were diagnosed with prostate cancer; however, most of these were clinically symptomatic at the time of diagnosis and 75 % eventually died as consequence of prostate cancer. In the current PSA era , more men are diagnosed with prostate cancer; however, only 3 % die from the consequences of prostate cancer. In the pre-PSA era only 27 % of prostate cancers were localized, while today, 98 % of men diagnosed via PSA screening have localized prostate cancer [3]. Currently, it is estimated that 80 % of men diagnosed with prostate cancer will never develop symptoms of the disease [4]. Indeed, these diagnoses may be considered false positives, carrying with them unnecessary and enormous costs—physically, emotionally, and economically.
The morbidity associated with treatment is significant; this toll on our male population is even more disturbing when we consider that most men would eventually die from other causes without treatment and die with their diagnosis but not from it.
Based on the review of several trials, the US Preventive Services Task Force concluded in the fall of 2011 that PSA-based screening is associated with detection of more prostate cancers; small to no reduction in prostate cancer-specific mortality after about 10 years; and harms related to false-positive test results, subsequent evaluation, and therapy, including overdiagnosis and overtreatment. They recommended against routine screening of prostate cancer with a D statement [5]. This grade D recommendation applies to healthy men of all ages, regardless of race or family history. The task force’s grade D recommendation is intended to “discourage the use of this service.”
Tragically, a study done shortly before the USPSTF investigation showed that up until 2010, PSA screening occurred in 69.8 % in the low-risk group, 65.3 % in the intermediate risk, and 56 % in the high risk [6]. This means that doctors were not adherent to the criteria of screening, which had always recommended against screening any person who had less than a 10-year life expectancy. The study showed that 56 % of men screened by PSA were at highest risk and therefore ineligible for screening. (Imagine how many of this group with abnormal PSA went on to have biopsy or worse treatment for prostate cancer!)
13.2 How Have We Justified Screening for Prostate Cancer in the Past?
Preventive healthcare strategies are typically divided into three categories: primary, secondary, and tertiary prevention. Primary prevention involves steps toward avoiding occurrence of disease or trauma. Examples include vaccinations, healthy diet and lifestyle, the use of seat belts, and not smoking. Secondary prevention involves the screening or detection of disease prior to the appearance of symptoms. The objective is to diagnose a problem early, when it is most treatable. Examples included cancer screening programs (PSA, mammography, PAP, and colonoscopy) and measurement of cholesterol to prevent cardiovascular diseases. Tertiary prevention is intended to reduce the negative impact of a symptomatic disease. Examples would include antibiotics to treat pneumonia and surgery to stop the spread or progression of a disease.
Localized prostate cancer exists almost always without symptoms. PSA was introduced in 1986 as a means of screening and detecting cancer early. Although it is frequently recommended that men with obstructive voiding symptoms consider an evaluation for possible prostate cancer, this would be an unusual presenting complaint. The reason: prostate cancer generally arises in the peripheral zone of the prostate and rarely will lead to urethral obstruction. It is only when metastases to bone (pain) or to regional lymph nodes (lower extremity lymphedema or deep vein thrombosis) or to the retroperitoneal lymph nodes (ureteral obstruction, flank pain, and uremia) occur that symptoms develop. I have, however, diagnosed prostate cancer in a supposedly “asymptomatic” 52-year-old man, with minimal PSA elevation, who had noted erectile dysfunction over the previous 4 years. His prostate was a concrete mass, and not surprising, the pathology showed Gleason 10. Unfortunately, he had not had screening for sexual dysfunction nor a digital rectal examination to screen for prostate cancer prior to his urological consultation. The neurovascular bundles involved in erectile function had been clearly destroyed by the malignancy.
Since the advent of prostate-specific antigen (PSA) testing, in combination with digital rectal examination (DRE), prostate cancer diagnosis has been changed in a revolutionary manner. Approximately 10 % of men tested with PSA will be found to have a value >4.0 ng/mL and between 3 and 10 % will have an abnormal DRE . Of men undergoing biopsy, cancer detection rates have been increasing and vary from 25 to 50 %. With PSA screening, more than 97 % of prostate cancer cases diagnosed are clinically confined to the prostate [7].
Clearly, the outcome of treatment is related to the extent of disease. Failures are greater if nodal disease, seminal vesicle involvement, or extracapsular disease is present. For tumors that are confined to the prostate, presumed to be detected early, the progression-free probability at 10 year exceeds 90 % [8]. However, lead time bias may be an important factor in this interpretation.
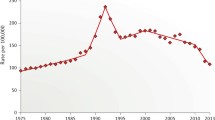
PSA screening in the USA began in earnest in the late 1980s. In association with this was a dramatic increase in the detection of disease. After a period of almost exponential rise in detection, the incidence rate fell to relatively stable rates. Beginning in 1991, and virtually every year since, a fall in the rate of metastatic disease has been seen [9].
Since the early 1990s when the rate of metastatic disease began to fall, there has been a gradual decrease in prostate cancer mortality [10]. This was in the face of gradually increasing mortality rates in the late 1980s. The increased use of hormonal therapies as injectable LHRH agonists and oral antiandrogens may have also played a role in decreased mortality rates in patients with metastatic disease.
13.3 But Routine Prostate Cancer Screening Went Too Far!
A combination of autopsy data and national statistics suggests that although almost 70 % of men will develop histological evidence of prostate cancer and 16 % will be diagnosed, only 3–4 % will die of the disease. These statistics can be translated to real-life scenario in the recent ERSSPC results , which showed that it was necessary to screen 1410 men [several hundred biopsies] and to treat 48 men to save one life [11].
13.4 We Relied on PSA, Which Lacks Predictive Value
Despite the fact that the combination of regular PSA and DRE testing significantly reduces the stage of prostate cancer at diagnosis, they are not perfect. In those men in the Washington University series who were diagnosed with prostate cancer at the time of their initial PSA, 37 % had clinically or pathologically advanced disease. Among those with serial PSA measurements, 29 % still had advanced disease [12]. These observations suggest that, with time, even those men who are screened repetitively with PSA will be at risk for treatment failure and demise from their disease.
PSA thresholds may also provide false sense of security for men with low levels, while causing anxiety with repeated, often unnecessary, biopsies in those with higher levels.
PSA and histology data , reviewed from 36,316 patients, revealed that prostate cancer incidence among men with PSA 2.5–4.0 ng/mL was the same as in men with PSA levels between 4.0 and 10 ng/mL [13]. Other investigators likewise demonstrated the risk of having prostate cancer with PSA as low as 0.5 ng/mL or less, which was 6.6 % [14].
Others have reported the prevalence of prostate cancer among men with PSA levels <2.0. This led to proposal of lower PSA threshold of 2.6 in 1995, which yielded 22 % cancer detection rate via sextant biopsy [15]. Among men with PSA levels < 4.0, other investigators diagnosed 15.2 % of men with prostate cancer, observing that 14.9 % had Gleason 7 score or higher [16]. However, another way of interpreting this result is by acknowledging the fact that 85 % of patients had low-grade cancers, most of which may be clinically insignificant.
In a review conducted over a 15-year period at the Cleveland Clinic, data from 5570 biopsy cases were analyzed and comparisons were made with regard to race and PSA. PSA differences between Blacks and Whites diminished over time. Also, as the levels of PSA declined over time, the association with cancer detection weakened for both races. In most years after 2000, the association between PSA and cancer was not significant—with areas under the ROC curve close to 0.5 [17]. In other words, PSA is as good as a coin toss, simply providing an excuse rather than a valid reason to biopsy men. It is well known that PSA functions well as a BPH or prostate volume surrogate [18, 19]. Therefore, [positive] PSA screening may simply reflect the increase incidence of both BPH and cancer with age.
13.5 Diagnosis Through Transrectal Ultrasound-Guided Prostate Biopsy Carries with It Significant Risks and Complications
As stated by Neulander and colleagues in their letter to editor, “…prostate biopsy is the only means for prostate cancer detection. It is an invasive procedure through a septic cavity and any casual approach to this operation should be avoided…” [20].
Prostate biopsy is associated with risk of infection, sepsis, hematuria, hematospermia, pain, and rectal bleeding, which can be fatal in extremely rare cases. Prostate biopsy has a relatively high false negative, up to 45 % depending on the number of cores obtained [3]. To avoid this problem, 12–14 cores are recommended for initial biopsy, further increasing the risk of complications.
In a group of 2023 men who underwent TRUS prostate biopsy, the number of cores was correlated to risk of infection. The overall sepsis rate was 3.06 % or 62/2023 patients; but when analyzed according to number of cores taken, the incidence of sepsis was 2.74 % vs. 4.21 % among men who had undergone 8–10-core biopsy vs. 12-core biopsy, respectively (p < 0.001) [21]. Nam and colleagues also reported a fourfold increase in hospitalizations due to post-prostate biopsy infection or bleeding, which was related to the increase in number of cores taken during one biopsy session, i.e., sextant biopsy compared with 8-, 10-, or 18-core biopsy [22].
In 2006, Jones and colleagues compared two cohorts of men undergoing initial biopsy. One group, consisting of 139 men, underwent 24-core saturation biopsy and the other group of 87 men underwent 10-core prostate biopsy. To the investigators’ surprise, saturation biopsy afforded no advantage in the cancer detection rate and did not significantly increase the rate of complication such as infection [23].
There is growing evidence and concern regarding the overall increasing incidence of post biopsy infection and the increasing proportion of infections caused by organisms resistant to fluoroquinolones. At the 30th Annual Congress of the European Urological Association, researchers presented data demonstrating significant rise in the post biopsy infection rate over the past 10 years. In the USA, there is increasing rate of infection requiring hospitalization [24].
Gross hematuria is a relatively common complication of prostate biopsy, occurring in up to 58 % of patients. While gross hematuria is very distressing to patients, the majority of episodes are short-lived and do not require medical intervention nor hospitalization. When considering adverse effects, some investigators only included gross hematuria that persisted for more than 2 weeks or rare instances that required hospitalization, with reported rate of 6.5 % [25].
Reported incidence of hematospermia is quite variable, ranging from 0.2 % to 84%! The study reporting the highest incidence of hematospermia (84 %, with mean duration of 3.5 weeks) was the only study designed prospectively to research this specific problem and therefore much more compelling to me as a men’s health specialist. The researchers were also sensitive to anticipate this problem among men who were able to be sexually active [26]. The otherwise, significantly lower incidence of hematospermia reported in the literature may reflect study design, age, and comorbid conditions of patients, as well as cultural differences between regions and study centers.
Hematochezia is observed in 1.5–37 % of men after TRUS biopsy. Some researchers believe the prevalence of rectal bleeding after TRUS-guided prostate biopsy is underestimated in many cases because the bleeding episode is clinically irrelevant or ceases within a couple of days [27]. Fortunately, severe bleeding is rare but can be fatal.
Other adverse effects of prostate biopsy include pain, psychological stress, and the ongoing risk of all complications secondary to the possibility of requiring repeated biopsies for persistently elevated PSA, PCA3, or other abnormal screening parameters (false-negative screening tests).
13.6 Another Risk of Prostate Biopsy Is the High Rate of “False Positives”
I am defining false positives as the burden of a “cancer” diagnosis, in cases of clinically insignificant malignancy. This may represent approximately 75 % of all cancers detected in the PSA era!
False positives, or the detection of low-grade low-volume cancers, frequently lead to secondary physical harm by way of overtreatment as well as the complications associated with therapies. Options for localized prostate cancer include, generally, radiotherapy with external beam, brachytherapy, and surgery (radical prostatectomy). Each of these carries with it a unique spectrum of complications, including erectile dysfunction, urinary incontinence, urinary obstruction, radiation injury to the rectum or bladder, urethral strictures, and need for secondary therapies. These complications are challenging to justify especially with the recent observations published by ERRSSPC cited earlier, indicating that 48 men need to be treated in order to save one man’s life.
Diagnosis of prostate cancer is occurring at much lower PSA levels with much greater likelihood of localized and low-grade cancers. As observed in the Prostate Cancer Prevention Trial, where all men were biopsied for study purposes, regardless of PSA level, high-grade tumors (Gleason 7 or greater) were detected in 10–27 % of men diagnosed with prostate cancer who had PSA levels less than 4.0 ng/mL [13]. Again, using the reciprocal, one can conclude that 73–90 % of cancers are low grade and, therefore, eligible for active surveillance.
Sadly, some experts have found that only 36 % of men surveyed who were diagnosed with low-volume, low-grade disease were offered active surveillance by their urologists, when a doctor’s counseling about AS may be the most important factor in a man’s decision to pursue this management option [28]. Additional concerns have been raised regarding the pressure hospitals have in promoting robotic prostatectomy in order to pay off the $2 million equipment used for this procedure. Robotics are used in 75 % of US prostatectomy cases, and the rate of prostatectomies has been shown to increase significantly and dramatically, soon after hospitals acquire the robotics. This was even true in regions where the overall incidence of prostate cancer had diminished [29].
Complications of prostatectomy include bladder irritability, incontinence, and erectile dysfunction. Despite improved surgical techniques, the reported stress urinary incontinence rates range between 5 % and 48.0 % [10]. A literature review revealed high prevalence of de novo voiding dysfunction confirmed urodynamically in post-prostatectomy patients. Detrusor overactivity was reported in 2–77 % of patients. Impaired bladder compliance was present in 8–39 % of patients and was de novo in about half. Impaired detrusor contractility was found de novo in 47 % but was recovered in about half of these men after 1 year [30].
The incidence of post-prostatectomy erectile dysfunction is common. Potency rates, as cited by Tal and Mulhall, range from 21 to 62 % of patients after unilateral nerve-sparing prostatectomy and 54 to 86 % of patients after bilateral nerve-sparing prostatectomy. The data reviewed includes patients who underwent radical, laparoscopic, and robotic procedures and were defined as patients with full or partial erections sufficient for intercourse. In some studies, patients were taking medication, such as PDE-5 inhibitors, to treat post-prostatectomy erectile dysfunction. According to the SEER registry, however, 80 % of patients were not able to achieve erections sufficient for intercourse after prostatectomy [31].
In a meta-analysis (26 articles = 8302 patients) comparing quality of life among prostatectomy patients and radiation therapy patients, all treatments were associated with short-term or long-term reductions in urinary, bowel, and sexual domains. Bowel quality of life and bladder irritation were worse for radiation patients. A greater decline in sexual function was observed in surgery patients compared to radiation patients; however, surgery patients had higher levels of sexual functioning at baseline [32]. Using the CaPSURE database , investigators compared the health-related quality life of patients who underwent prostate cancer treatment. Among 3294 men, 1139 (34 %) underwent nerve-sparing radical prostatectomy (NSRP) , 860 (26 %) underwent non-NSRP, 684 (21 %) underwent brachytherapy, 386 (12 %) underwent external beam radiotherapy, 161 (5 %) underwent primary androgen deprivation therapy, and only 64 (2 %) pursued watchful waiting/active surveillance. Median follow-up was 74 months. Most treatments resulted in early declines in quality of life, with some recovery over the next 1–2 years and a plateau in scores thereafter. Radiation had the strongest effect on bowel function. Not surprising, surgery had the largest impact on sexual function and bother and on urinary function. Androgen deprivation therapy had the strongest effect on physical function [33]. Rectourethral fistulas are rare but devastating consequences of radiation therapy, specifically combined brachytherapy and external beam regimens. Early- or late-onset bladder or rectal bleeding occurs secondary to mucosal and vascular damage. Unfortunately these cannot be predictive; however, lower dose therapies may decrease this risk.
The European Randomized Study of Screening for Prostate Cancer (ERSSPC) , initiated in the early 1990s, showed PSA screening reduced the rate of death due to prostate cancer by 20 %. But what does a 20 % reduction really mean? If an average middle-aged man has a 3 % risk of dying of prostate cancer, would screening decrease this risk to 2.4 %? The study also showed that overdiagnosis and overtreatment are probably the most important adverse effects of prostate cancer screening and are more common than in screening for breast, colorectal, or cervical cancer [34]. Additionally, population observations regarding changes in mortality are subject to numerous confounds and do not clearly demonstrate an effect of screening or treatment.
In another prospective study from Sweden, 20,000 men age 50 to 64 were followed for 14 years. One-half of the men were screened using PSA every 2 years, while the others were not screened. All cause mortality was the same for both groups; however, the prostate cancer mortality was reduced by 44 % in the screened group; however, absolute reduction was determined to be only .34 percentage points. From a public health standpoint, this difference may be too costly given the larger number of men who must submit to biopsy, are over diagnosed, and undergo unnecessary therapy (along with all the comorbidities of treatment) [35].
13.7 What Can Be Done to Enhance the Predictive Value of PSA to Decrease Unnecessary Biopsies and Ultimately Unnecessary Therapies?
PSA coupled with MRI may help to decrease the number of unnecessary biopsies (30th Annual Congress of the EAU). At the 30th Annual Congress of the European Association of Urology (March, 2015), data was presented demonstrating the use of lower PSA thresholds in combination with MRI, resulting in fewer men requiring diagnostic needle biopsy. While decreasing the overdetection of low-grade low-volume prostate cancers is a priority, one immediate benefit of this approach is the decreased risk of TRUS biopsy , which is currently associated with higher rates of infection requiring hospitalization as cited earlier. Significant increases over the past 10 years in infection rates associated with TRUS biopsy have also been reported in Europe.
MRI–TRUS fusion involves prostate ultrasound, as performed for the past several decades. While viewing the prostate, the MRI of that prostate, which is performed beforehand and stored in the device, is fused with real-time ultrasound using a digital overlay, allowing the target(s), previously delineated by a radiologist, to be brought into the aiming mechanism of the ultrasound machine. The fusion results in the creation of a three-dimensional reconstruction of the prostate, and on the reconstructed model, the aiming and tracking of biopsy sites occur. The results are very promising in that more specific targeting and therefore better yield of the procedure are done without increasing number of cores taken. However, differentiating low grade from high grade has not been proven [36]. Other investigators observe that MRI targeted biopsies are more sensitive for detection of prostate cancer than TRUS-guided, systematic biopsies and detect more significant prostate cancers and fewer insignificant cancers than conventional biopsies. However, the same group believes that a negative MRI scan should not defer biopsy [37], which leads us back to the significant risk of over biopsy and overdiagnosis, not to mention higher costs of MRI.
PSA blood tests can be coupled with urine Prostate Cancer Assay 3 (PCA3) . It is unclear, however, that PCA3 can help in differentiating clinically significant malignancies among prostate biopsy naïve patients or if it has a role as part of initial screening.
A group of 859 men (mean age, 62 years) from 11 centers scheduled for a diagnostic prostate biopsy between December 2009 and June 2011 were enrolled to test performance of PCA3. Using a score of >60, positive predictive value (PPV) of PCA3 was tested in men undergoing a biopsy for the first time. Negative predictive value was tested using a score <20 among men undergoing repeat biopsy [38]. The addition of PCA3 to individual risk estimation models (which included age, race/ethnicity, prior biopsy, PSA, and digital rectal examination) improved the stratification of cancer and of high-grade cancer. In the setting of repeat biopsy, use of the PCA3 could reduce the number of biopsies performed by half! This reduction could be associated with a 3 % risk of missing high-grade disease; however, as noted in editorial comment, the incidence of high-grade prostate cancer in the setting of low PCA3 is higher at 13 % [39].
For the purposes of initial screening, however, PCA3 may perform too well, predicting all cancers, with an overall detection rate of 80 % [37]. It is unable to differentiate, therefore, leaving us with similar challenges as PSA in overdiagnosis of low-grade disease. So while it has a promising role in preventing the over biopsy and overdiagnosis of those undergoing repeat biopsy, PCA3 may not be helpful for initial screening.
The prostate health index (PHI) may potentially decrease the rate of prostate biopsy by 30 %. Loeb and colleagues used PHI , which is comprised of 3 parameters (total PSA, free PSA and p2PSA, an isoform of free PSA identified as most specific to prostate cancer) to compare predictive value of each screening parameter alone. Among 658 men (median age of 63), PHI was correlated to biopsy results. Clinically significant cancer was defined by Epstein criteria (Gleason 7 or greater, 3 or more positive cores, and >50 % involvement of any core). The PHI outperformed free PSA alone in differentiating clinically significant cancer from indolent cancer or negative biopsies; therefore, PHI may afford a 30 % decrease in number of men requiring biopsy, compared to possible avoidance of biopsy of 21 % with free PSA [40].
13.8 Shared Decision-Making
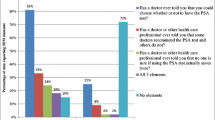
For decades, family doctors were guided by the American College of Physicians and the American Academy of Family Physicians to counsel men older than 50 years about the “known risks and unknown benefits” of PSA screening and to obtain informed consent from those who wish to proceed with screening [41].
We as primary care physicians have not adhered to this policy of shared decision-making for prostate cancer screening. In one study, 47 % of physicians endorsed “shared decision-making” for prostate cancer screening [42]. However, most doctors surveyed cited lack of time, fear of malpractice, and other barriers as the cause for continued PSA testing without informed or shared decision-making [42, 43], hence the inappropriate counseling and testing of so many men, elderly, infirmed, or just uninformed. And once Pandora’s box is opened, in the form of a patient having an abnormal PSA result, it is more challenging to assuage the fear of the patient and to avoid further evaluation even when screening had been inappropriate from the start.
PSA testing decreased significantly after the USPSTF recommendations came out. One group of researchers examined overall trends by facility locations (urban, suburban, or rural), by patient age, and by provider type (primary care or urology). Although decreased PSA screening occurred across all specialties over time, the greatest reductions in such testing were seen among urologists, among patients in the intermediate age group (aged 50–59 years), and at an urban teaching hospital [44]. While this is a favorable trend, consistent with the objectives implicit of the task force recommendation, it is this author’s hope that this reflects shared decision-making rather than a simple paucity in the discussion of screening during patient visits.
The American Cancer Society (ACS) recently updated its guideline for the early detection of prostate cancer (http://www.cancer.org/cancer/prostatecancer/moreinformation/prostatecancerearlydetection/prostate-cancer-early-detection-acs-recommendations), recommending that asymptomatic men who have at least a 10-year life expectancy be given an opportunity to make an informed decision with their healthcare provider about screening for prostate cancer. Informed decision implies a discussion about the uncertainties, risks, and potential benefits associated with screening. The ACS asserts that prostate cancer screening should not occur without an informed decision-making process. For far too long, PSA was simply added to a battery of tests which were ordered for any patient when they reached a certain age and annually thereafter. The serious implications of this test were overshadowed by the simplicity and accessibility of the blood test.
The push back by the USPSTF and others was not just the misuse of the blood test, but the further abuse of the results leading to, perhaps, due indiscriminate biopsies followed by costly and morbid radiation therapy or surgery to treat low-grade disease.
Improved education and counseling about watchful waiting and active surveillance may help prevent the conversion of overdiagnosis to overtreatment, mitigating the harms of screening that are so accurately portrayed by the task force.
The grade D recommendation made by the task force once again removes the patient from the decision-making process while swinging the pendulum from broad, uninformed, indiscriminate use of screening to unilateral inaccessibility to screening. Either way, “adherence” to either standard seems like a cop out on the part of primary care doctors and urologists.
This author agrees with McNaughton-Collins and Barry in proposing a modification to the task force’s recommendation, from Grade D to a C recommendation. The Grade C recommendation would include the suggestion that physicians “offer/provide this service only if other considerations support offering or providing the service in an individual patient.” In keeping with the ACS guidelines, the C recommendation would allow the patient to be involved in the decision of screening by means of digital rectal examination and/or other modalities such as PSA, MRI, or PCA3. The pros and cons would be equally presented and discussed. The patient could then provide his perspective on how he views the trade-off, in short, a genuine shared decision-making process.
We must also accept that shared decision-making will have as many different formulas as there are patient personalities. This, perhaps, may seem the most challenging to already time-restricted and overworked doctors. Ultimately, it will be up to the patient to define the balance of decision-making and the physician to provide the reciprocal or compensatory counterbalance through patient education and compassion.
13.9 Conclusion
Dr. Ian Thompson, a leading oncological urologist, was recently quoted about the PSA dilemma: “….better diagnostic techniques , such as biopsies guided by magnetic resonance imaging tests, along with personalized risk assessment and more informed decision making by men and their doctors [is] a better way to use the PSA test than to screen every man of a certain age.”
In treating each patient as individuals, we must set a goal to achieve a reduction in the mortality and morbidity of prostate cancer, while minimizing the risks, cost, complications, and emotional burdens of screening, diagnosing, and overtreating this disease.
References
McNaughton-Collins MF, Barry MJ. One man at a time – resolving the PSA Controversy. N Engl J Med. 2011;365:1951–3.
Greenlee RT, Hill-Harmon MB, Murray T, et al. Cancer statistics 2001. CA Cancer J Clin. 2001;5:15–36.
Shteynshlyuger A, Andriole G. Prostate cancer: to screen or not to screen? Urol Clin North Am. 2010;37:1–9.
Yao SL, Lu-Yao G. Understanding and appreciating overdiagnosis in the PSA era. J Natl Cancer Inst. 2002;94(13):958–60.
Moyer VA. U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–34.
Royce T: MS4 Affiliation: University of North Carolina at Chapel Hill, Chapel Hill, NC. Does Patient Life Expectancy Affect Receipt of Routine Cancer Screening in the United States? A Population-Based Study. Reported by: Abigail Berman, MD Affiliation: The Abramson Cancer Center of the University of Pennsylvania Last Modified: October 31, 2012.
Smith DS, Catalona WJ. The nature of prostate cancer detected through prostate specific antigen based screening. J Urol. 1994;152:1732–6.
Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002;167:528–34.
Hankey BF, Feuer EJ, Clegg LX, et al. Cancer surveillance series: interpreting trends in prostate cancer—Part I: evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999;91:1017–24.
Potosky AL, Feuer EJ, Levin DL. Impact of screening on incidence and mortality of prostate cancer in the United States. Epidemiol Rev. 2001;23:181–6.
Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:11320.
Catalona WJ, Smith DS, Ratliff TL, Basler JW. Detection of organ-confined prostate cancer is increased through prostate-specific antigen-based screening. JAMA. 1993;270:948–54.
Gilbert SM, Cavallo CB, Kahane H, et al. Evidence suggesting a PSA cutpoint of 2.5 ng/mL for promoting prostate biopsy: a review of 36,316 biopsies. Urology. 2005;65:549–53.
Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239–46.
Krumholtz JS, Carvalhal GF, Ramos CG, Smith DS, Thorson P, Yan Y, Humphrey PA, Roehl KA, Catalona WJ. Prostate-specific antigen cutoff of 2.6 ng/mL for prostate cancer screening is associated with favorable pathologic tumor features. Urology. 2002;60(3):469–73.
Thompson IM, Ankerst DP, Chi C, et al. Operating characteristics of prostate-specific antigen in men with initial PSA level of 3.0 ng/mL or lower. JAMA. 2005;294:66–70.
Potts J, Lutz L, Walker E, Modlin C, Klein E. Trends in PSA, age and prostate cancer detection among black and white men from 1990-2006 at a tertiary care center. Cancer. 2010;116(16):3910–5.
Roehrborn CG. The utility of serum prostatic-specific antigen in the management of men with benign prostatic hyperplasia. Int J Impot Res. 2008;20 Suppl 3:S19–26.
Stamey TA, Johnstone IM, McNeal JE, et al. Preoperative serum prostate specific antigen levels between 2 and 22 ng./ml. correlate poorly with post-radical prostatectomy cancer morphology: prostate specific antigen cure rates appear constant between 2 and 9 ng./ml. J Urol. 2002;167(1):103–11.
Neulander EZ, Yusim I, Kaneti J. Letter Re: increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy: R. K. Nam, R. Saskin, Y. Lee, Y. Liu, C. Law, L. H. Klotz, D. A. Loblaw, J. Trachtenberg, A. Stanimirovic, A. E. Simor, A. Seth, D. R. Urbach and S. A. Narod J Urol 2010; 183: 963-969. J Urol. 2010;184(5):2216–7.
Simsir A, Kismali E, Mammadov R, Gunaydin G, Cal C. Is it possible to predict sepsis, the most serious complication in prostate biopsy?. Urol Int. 2010;84(4):395–9.
Nam RK, Saskin R, Lee Y, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2010;183(3):963–8.
Jones JS, Patel A, Schoenfield L, et al. Saturation technique does not improve cancer detection as an initial prostate biopsy strategy. J Urol. 2006;175(2):485–8.
Nam RK, Saskin R, Lee Y, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2013;189(1 Suppl):S12–7. discussion S17–8.
Ecke TH, Gunia S, Bartel P, Hallmann S, et al. Complications and risk factors of transrectal ultrasound guided needle biopsies of the prostate evaluated by questionnaire. Urol Oncol. 2008;26(5):474–8.
Manoharan M, Ayyathurai R, Nieder AM. Soloway MS Hemospermia following transrectal ultrasound-guided prostate biopsy: a prospective study. Prostate Cancer Prostatic Dis. 2007;10(3):283–7.
Kilciler M, Erdemir F, Demir E, et al. The effect of rectal Foley catheterization on rectal bleeding rates after transrectal ultrasound-guided prostate biopsy. J Vasc Interv Radiol. 2008;19(9):1344–6.
Gorin MA, Soloway CT, Eldefrawy A, Soloway MS. Factors that influence patient enrollment in active surveillance for low-risk prostate cancer. Urology. 2011;77(3):588–91.
Neuner JM, See WA, Pezzin LE, et al. The association of robotic surgical technology and hospital prostatectomy volumes: increasing market share through the adoption of technology. Cancer. 2012;118(2):371–7.
Porena M, Mearini E, Mearini L, et al. Voiding dysfunction after radical retropubic prostatectomy: more than external urethral sphincter deficiency. Eur Urol. 2007;52(1):38–45.
Tal R, Alphs HH, Krebs P, Nelson CJ, Mulhall JP. Erectile function recovery rate after radical prostatectomy: a meta-analysis. J Sex Med. 2009;6(9):2538–46.
Lee TK, Breau RH, Mallick R, Eapen L. A systematic review of expanded prostate cancer index composite (EPIC) quality of life after surgery or radiation treatment. Can J Urol. 2015;22(1):7599–606.
Punnen S, Cowan JE, Chan JM, Carroll PR, Cooperberg MR: Long-term health-related quality of life after primary treatment for localized prostate cancer: results from the CaPSURE Registry. Eur Urol. 2014 Sep 18. pii: S0302-2838(14)00844-6.
Andriole GL, Crawford ED, Grubb III RL, Buys SS, Chia D, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9.
Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, et al. Mortality results from the Göteborg randomized population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–32.
Marks L, Young S, Natarajan S. MRI–ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol. 2013;23(1):43–50.
Stephenson SK, Chang EK, Marks LS. Screening and detection advances in magnetic resonance image-guided prostate biopsy. Urol Clin North Am. 2014;41(2):315–26.
Wei JT, Feng Z, Partin A, et al. Can urinary PCA3 supplement PSA in the early detection of prostate cancer? J Clin Oncol. 2014;32(36):4066–72.
Vickers AJ. Markers for the early detection of prostate cancer: some principles for statistical reporting and interpretation. J Clin Oncol. 2014;32(36):4033–4.
Loeb S, Sanda MG, Broyles DL, et al. The prostate health index selectively identifies clinically significant prostate cancer. J Urol. 2015;193(4):1163–9.
Gates TJ. Screening for cancer: evaluating the evidence. Am Fam Physician. 2001;63(3):513–22.
Davis K, Haisfield L, Dorfman C, et al. Physicians’ attitudes about shared decision making for prostate cancer screening. Fam Med. 2011;43(4):260–6.
Dunn AS, Shridharani KV, Lou W, et al. Physician-patient discussions of controversial cancer screening tests. Am J Prev Med. 2001;20(2):130–4.
Aslani A, Minnillo BJ, Johnson B, Cherullo EE, Ponsky LE, Abouassaly R. The impact of recent screening recommendations on prostate cancer screening in a large health care system. J Urol. 2014;191(6):1737–42.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Potts, J.M. (2016). Prostate Cancer Screening. In: Potts, J. (eds) Men's Health. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3237-5_13
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3237-5_13
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-3236-8
Online ISBN: 978-1-4939-3237-5
eBook Packages: MedicineMedicine (R0)