Abstract
Osteoporosis is among the most devastating side effects of glucocorticoid (GC) therapy for the management of inflammatory and auto-immune diseases. Evidence from both humans and mice indicate deleterious skeletal effects within weeks of pharmacological GC administration, both related and unrelated to a decrease in bone mineral density (BMD). Osteoclast numbers and bone resorption are also rapidly increased, and together with osteoblast inactivation and decreased bone formation, these changes lead the fastest loss in BMD during the initial disease phase. Bone resorption then decreases to sub-physiological levels, but persistent and severe inhibition of bone formation leads to further bone loss and progressively increased fracture risk, up to an order of magnitude higher than that observed in untreated individuals. Bone forming osteoblasts are thus considered the main culprits in GC-induced osteoporosis (GIO). Accordingly, we focus this review primarily on deleterious effects on osteoblasts: inhibition of cell replication and function and acceleration of apoptosis. Mediating these adverse effects, GCs target pivotal regulatory mechanisms that govern osteoblast growth, differentiation and survival. Specifically, GCs inhibit growth factor pathways, including Insulin Growth Factors, Growth Hormone, Hepatocyte Growth/Scatter Factor and IL6-type cytokines. They also inhibit downstream kinases, including PI3-kinase and the MAP kinase ERK, the latter attributable in part to direct transcriptional stimulation of MAP kinase phosphatase 1. Most importantly, however, GCs inhibit the Wnt signaling pathway, which plays a pivotal role in osteoblast replication, function and survival. They transcriptionally stimulate expression of Wnt inhibitors of both the Dkk and Sfrp families, and they induce reactive oxygen species (ROS), which result in loss of ß-catenin to ROS-activated FoxO transcription factors. Identification of dissociated GCs, which would suppress the immune system without causing osteoporosis, is proving more challenging than initially thought, and GIO is currently managed by co-treatment with bisphosphonates or PTH. These drugs, however, are not ideally suited for GIO. Future therapeutic approaches may aim at GC targets such as those mentioned above, or newly identified targets including the Notch pathway, the AP-1/Il11 axis and the osteoblast master regulator RUNX2.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Glucocorticoid-Induced Osteoporosis (GIO)
The Clinical Problem
Since 1950’s Nobel laureates P. Hench, E. Kendall and T. Reichstein introduced glucocorticoids (GCs) for the treatment of rheumatoid arthritis, these drugs have been widely used in the management of a myriad autoimmune and inflammatory diseases. GCs mainly act through the NR3C1-encoded glucocorticoid receptor (GR). Once bound, the activated GR translocates to the nucleus and regulates transcription of target genes either as a homodimer or a monomer that can interact with other transcription factors. Physicians frequently prescribe synthetic GCs to treat patients with advanced rheumatoid arthritis, asthma, multiple sclerosis, systemic lupus erythematosus, inflammatory bowel disease, and, in combination with other drugs, patients with hematologic and other malignancies and after organ transplantation. The high efficacy of synthetic GCs as immune suppressants is blemished, however, by deleterious side effects, in particular rapid bone loss leading to GC-induced osteoporosis (GIO). The GR is ubiquitously expressed, including in the various cell types present in bone, such as bone forming osteoblasts, matrix embedded osteocytes, and bone resorbing osteoclasts. GIO results from effects of GCs on all of these, as well as additional cell types, of which the effects on osteoblasts are generally considered the most important in mediating the progressive bone loss in patients chronically treated with GCs.
Following bone loss in post-menopausal women and in aging individuals of both sexes, GIO is the third most-common etiology of pathological bone loss, approaching by some estimates 20 % of all patients with osteoporosis [1]. Several large population-based and cohort studies have evaluated the relationship between oral GC and bone mineral density (BMD) as well as fracture risk, and the largest of these, the General Practice Research Database (GPRD) in the UK, has shown that even daily doses as low as 2.5 mg of prednisone are associated with increased risk for vertebral and hip fracture [2, 3]. BMD decreases as a function of cumulative exposure [4] and fracture risk depends additionally on the maximum daily dose [4]. The highest risk is observed in the spine, where the incidence of fracture compared to controls increases by more than fivefold with daily doses equivalent to or greater than 7.5 mg prednisolone [3]. Within the first year after treatment initiation, patients on oral GCs show an average of 54 % increased fracture risk, which far outpaces the decrease seen in BMD [3]. The apparent BMD-independent fracture risk may reflect effects of GCs on neurons and muscle cells, as well as microanatomical changes to the bone microarchitecture and/or material properties not captured by conventional imaging techniques [5]. Nonetheless, a decrease in BMD is readily detectable by the 12th week of GC administration and rapid bone loss continues through the 24th week of treatment. Thereafter, the BMD loss rate appears to slow, although fracture risk continues to accumulate [4, 6, 7]. Bone is lost from both the cortical and the trabecular compartments, and even though the latter is affected more severely, the trabecular thinning is usually not associated with perforations [4, 8, 9].
In addition to oral GC administration, many patients with severe obstructive respiratory diseases such as asthma and COPD are treated with high-dose inhaled corticosteroids. While there has been some controversy over whether or not inhaled GCs lead to an increased risk of fracture, or whether increased fracture rates were secondary to the underlying disease, the EOLO (Evaluation of Obstructive Lung Disease and Osteoporosis) Study group has recently shown that inhaled GCs at the highest doses, >1500 μg/day, have a 1.4-fold increased risk of fracture compared with controls and patients on lower doses of inhaled steroids. Additionally, univariate and multivariate analyses did not show any independent association between obstructive lung disease and fracture risk [10, 11].
Several recent reviews provide excellent coverage of clinical manifestations and management of GIO [12, 13]. When applicable, local GC administration should be preferred over systemic treatment, and chronic treatment with oral GCs should be considered a last resort. Once prescribed, oral GCs should be accompanied with prophylactic therapy to minimize deleterious side effects. This includes cases where high dose GCs are prescribed for short periods of time to control “flares” (exacerbations) of common inflammatory diseases because, as mentioned above, GC increase fracture risk within a few weeks of administration. Specialists treating autoimmune and inflammatory diseases often fail to take the necessary measures to prevent osteoporosis, and GIO-related fractures are frequently the basis for successful litigation [12]. The American College of Rheumatology advocates management of patients initiating ≥3-month treatment with a daily dose of ≥7.5 mg of prednisone equivalents with anti-resorptive therapy (e.g., bisphosphonates) to ameliorate GIO [14]. Recently, however, the bone anabolic peptide PTH(1-34) has been shown to counteract deleterious effects of GCs on osteoblasts in vitro and in mice [15], and a clinical trial suggested that it was in fact superior to anti-resorptive therapy for GIO [16]. The results of this clinical trial are consistent with the central role of osteoblasts in GIO (section “Cellular Mechanisms of GIO: Osteoblasts at the Center Stage”), and provide the prospect that further improvement of patient care may be achieved through better understanding of the underlying cellular and molecular disease mechanisms. Most of the published work on cellular and molecular mechanisms underlying GIO has focused on osteoblasts and their precursors, which is reviewed in detail in this chapter. We open, however, with a brief review of mouse models, which also highlight the less well-investigated roles for GCs in osteocytes and osteoclasts.
The Mouse as a Model
The adult mammalian skeleton undergoes continuous remodeling throughout life. Bone resorbing osteoclasts, bone forming osteoblasts and matrix-embedded osteocytes that derive from osteoblasts are the major cell types responsible for this process. Early studies with several animal models resulted in paradoxical observations, which impeded progress with in vivo investigation of GIO, but recent work shows that principal GIO mechanisms can be usefully modeled in mice of certain strains, including Swiss-Webster [17], Balb/c [18] and FVB/N [19]. Similar to the human disease, the chronic phase of decreased osteoblastogenesis and bone formation in these mouse models is preceded by an early phase dominated by exaggerated osteoclast-mediated bone resorption, resulting in the highest rates of bone loss early on after commencement of GC administration [17, 20].
Mechanistic investigation of GIO in vivo, including in mice, is limited because results of molecular analyses, for example gene expression data, are typically obtained at the tissue level and not from individual cell types at specific differentiation stages. Mouse genetics, however, has proven invaluable for functional assessment, in vivo, of the significance of various molecular aspects of GR signaling in particular cell types. For example, a direct role in GIO has been unequivocally assigned to osteoblasts using two mouse models where GC signaling was abrogated specifically in this cell type. In one model, GC signaling was ablated by knocking out the GR gene in cells that express Cre recombinase under the control of Runx2 regulatory sequences [19]. In an earlier study, GC signaling in osteoblasts was abrogated by over-expression of the GC inactivating enzyme 11ß-HSD2 under the control of the Osteocalcin Gene 2 (OG2) promoter [21]. In both mouse models, administration of prednisolone resulted in less severe GIO compared to that observed in control mice, indicating that GC signaling in osteoblasts plays a critical role in GIO [19, 21] (section “Cellular Mechanisms of GIO: Osteoblasts at the Center Stage” below). Interestingly, the OG2-HSD2 transgenic mice had no bone phenotype at the basal state [21]. In contrast, some skeletal deficiencies at the basal state were observed in transgenic mice expressing 11ß-HSD2 in osteoblasts under the control of the Collagen α1(I) promoter [22, 23] and in mice lacking the GR in the osteoblast lineage [19], indicating that endogenous GC signaling plays a bone anabolic role during early stages of osteoblast differentiation.
Another mouse model was used to assess the contribution of GR homodimerization to GIO. Classically, transcriptional stimulation in response to GCs occurs through binding of GR homodimers to palindromic GC response elements (GREs) at target gene enhancers, and for many years this was considered the predominant mechanism underlying GIO. A paradigm shift, however, was offered by administration of prednisolone to so-called GRdim mice, harboring a GR mutant with an impaired dimerization interface [24, 25]. After 2 weeks of GC treatment, these mice had reduced osteoblast colony forming units (CFU-OBs) in the bone marrow, reduced osteoblast numbers on the bone surface, lower bone formation rates, and decreased bone mass, all similar to GC-treated wild type mice [19]. Thus, dimerization-independent mechanisms, such as binding of GR monomers to non-palindromic DNA response elements [26, 27], appear to be critical for the development of GIO. The significance of this paradigm shift to the future of GIO research is discussed in section “Glucocorticoids Without Osteoporosis?”.
Finally, although this chapter addresses the contribution of osteoclastogenesis to GIO only briefly, such contribution appears quite sizable, especially at the early phase of GIO. Indeed, GCs promote osteoclast survival and function in vivo [20, 28] and the GC-induced bone loss (albeit without loss of bone strength) in the aforementioned OG2-11ß-HSD2 mice that lack GC signaling in osteoblasts [21] could result from persistent activation of osteoclasts in the presence of GCs. GC-stimulated bone resorption likely occurs through their receptors in cells of the osteoblast lineage (see section “Involvement of Cells Other than Osteoblasts in GIO”), although involvement of osteoclast GR in increased resorption has been suggested based on evidence from mice with conditional GR inactivation in the monocytic lineage [29, 30].
Cellular Mechanisms of GIO: Osteoblasts at the Center Stage
The multifaceted and complex mechanisms underlying GIO have been extensively reviewed [12, 13, 31–33]. Early anecdotal evidence suggested indirect effects of GCs on bone through their actions in the gonads and in calcium-regulating organs (kidney, intestine). However, more recent clinical observations and in vivo investigation of mouse models argue against such indirect effects as primary pathogenic mechanisms in GIO [12, 13, 44]. Instead, it is now widely accepted that GIO is caused primarily through direct effects of GCs in bone cells.
Bone loss in the chronic state of GIO is mostly attributable to decreased bone formation by osteoblasts [13], secondary to impaired osteoblast cell replication (section “Glucocorticoids Inhibit Osteoblast Cell Cycle” below), diminished osteoblast differentiation and function (section “Glucocorticoids Inhibit Osteoblast Differentiation and Function” below), and accelerated osteoblast and osteocyte apoptosis (section “Glucocorticoids Promote Osteoblast Apoptosis” below). Additional considerations will be briefly reviewed in the section “Involvement of Cells Other than Osteoblasts in GIO”.
Glucocorticoids Inhibit Osteoblast Cell Cycle
Reports on GC-mediated inhibition of osteoblast proliferation in vitro date back to the 1970s [35]. Definitive in vivo evidence for inhibition of osteoblastic cell proliferation was demonstrated in GC-treated mice, where a dramatic decrease was observed in the number of bone marrow-derived CFU-Ob representing mesenchymal progenitors capable of bone formation [17, 19].
While acting as anti-mitogens in a variety of cell types, including fibroblasts, lymphocytes, hepatocytes, and lung alveolar cells, GCs engage different cell cycle regulatory mechanisms in a context-dependent manner. Even among osteoblast models, effects of GCs on cell cycle progression and the underlying molecular mechanisms vary as a function of the particular culture system and the differentiation stage. Treatment of mouse calvaria-derived osteoblasts with dexamethasone (dex) resulted in up to ~50 % reduction in the proportion of cells traversing through the active cell cycle phases (S/G2/M), but this inhibition occurred only at and after, not before, a well-defined developmental stage marked by a commitment to terminal differentiation [36, 37]. This differentiation stage-related anti-mitogenic effect of GCs was demonstrable in both the MC3T3-E1 immortalized cell line [36] and primary osteoblast cultures derived from newborn mouse calvariae [37], and in both cases inhibition of cell cycle progression was most strongly associated with suppression of cyclin A expression [36, 37]. In MC3T3-E1 cells, inhibition of cell cycle progression (as well as promotion of apoptosis) was also associated with activation of p53 [38]. In primary human osteoblast culture models, dex decreased thymidine incorporation into newly synthesized DNA and the proportion of cells traversing through the S/G2/M phases of the cell cycle [39]. In this system, as well as in human osteosarcoma cell lines, the anti-mitogenic effect of GCs is associated with either down-regulation of cell cycle stimulators such as CDK2, 4 and 6, cyclin D, c-Myc, and E2F-1, or upregulation of the cyclin-dependent inhibitors p21 and p27 [39, 40].
GC-mediated inhibition of thymidine incorporation was also demonstrated in the murine MBA-15.4 and the human MG-63 cell lines [41]. Co-treatment with sodium orthovanadate, a protein tyrosine phosphatase inhibitor, reversed the anti-mitogenic effect of dex, implicating tyrosine phosphatase(s) in the anti-mitogenic activity of GCs in these cells (see section “Additional Signaling Pathways”). Additional GC-regulated mechanisms involved in their anti-mitogenic effect in osteoblasts include the Wnt signaling pathway, Akt and MAPK signaling, as well as transcription factors such as FoxO3 (see section “Molecular Targets of Glucocorticoids in Osteoblasts”). Although the relative contribution of the aforementioned mechanisms to the inhibition of osteoblast replication remains to be determined, they all likely require the GC receptor (GR), as indicated by their sensitivity to GR antagonism by RU486 [36, 41].
Glucocorticoids Inhibit Osteoblast Differentiation and Function
Mesenchymal progenitors that reside in the bone marrow give rise to osteoblasts for the life-long process of filling eroded surfaces with new bone matrix. The early progenitors, commonly referred to as bone marrow stromal pluripotent cells, or mesenchymal stem cells, can select one of several developmental fates with appreciable plasticity, including a well documented reciprocal relationship between osteoblastogenesis and adipogenesis [42–47]. Many in vitro studies suggest that GCs influence this competitive cell fate choice, promoting the differentiation of bone marrow stromal cells into adipocytes at the expense of osteoblasts. Treatment of ST2 bone marrow-derived pluripotent cells with cortisol strongly stimulated the adipocytic genes PPARγ, C/EBPα, C/EBPδ and Adipsin [43] and inhibited RUNX2 (See also section “RUNX2”). Stimulation of C/EBPα and C/EBPδ was also observed in a microarray-based global gene expression study of dex-treated MC3T3-E1 pre-osteoblasts [48]. Similar to the commitment stage described in primary osteoblast cultures [37] and in MC3T3-E1 cells [49], GCs drive ST2 cells towards the adipocyte lineage and away from the osteoblastic cell fate only when administered early; once cells undergo commitment to the osteoblast lineage, the bone phenotype continues to develop normally even in the presence of GCs [50]. The idea that GIO is mediated in part by favoring differentiation of bone marrow pluripotent mesenchymal cells into adipocytes at the expense of osteoblasts is circumstantially supported by the increased marrow adiposity observed in GC-treated mice and humans [44], although direct in vivo evidence is lacking. Recent advances in the identification of skeletal stem cells may facilitate lineage tracing experiments to overcome this shortcoming [51, 52].
GCs at pharmacological concentrations suppress fundamental osteoblast functions, which can be partially traced to their influence, as described above, on cell fate at the osteoblast/adipocyte decision fork. Most significantly, GCs inhibit the biosynthesis of type I collagen, the predominant organic component of the bone matrix, in vitro [53] and in vivo [54]. Contributing to this inhibition, GCs first inhibit Procollagen α1(I) transcription; this was demonstrated in primary rat calvarial osteoblast cultures using nuclear run-off assays, where strong inhibition was evident within as little as two hours of treatment with 1 μM cortisol [55]. Secondly, GCs destabilize the Procollagen α1(I) transcript [55]. Thirdly, GCs inhibit collagen accumulation in a manner independent on Procollagen α1 mRNA; this was demonstrated, for example, by Sirius red-based assay of collagen accumulation in BMP2-treated MC3T3-E1 cultures. Co-treatment of these cultures with 0.1 or 1 μM dex resulted in a ~4-fold decline in the collagen accumulation rate even though Procollagen α1(I) mRNA levels did not decrease, but in fact increased [56]. The mRNA-independent decrease in collagen accumulation is attributable to inhibition of collagen translation, secretion, assembly, and/or accelerated collagen breakdown by GC-inducted collagenases [57].
Beside collagen accumulation, investigators in the GIO field often rely on assays of alkaline phosphatase (ALP) activity and mineral deposition to assess GC-mediated inhibition of osteoblast differentiation and function in culture models. Indeed, GCs at pharmacological concentrations inhibit both of these outcome parameters [19, 36, 53, 58–61]. The clinical relevance of ALP and mineralization assays to GIO, however, is not trivial because unlike inhibition of osteoblast proliferation and collagen synthesis, the results of ALP and mineralization assays can be species-dependent. In rodent osteoblast cultures, GCs at pharmacological levels usually inhibit ALP activity and mineralization, whereas physiological GC concentrations usually have stimulatory effects in these models [53, 62]. In human osteoblast cultures, on the other hand, GCs, often at low concentration, but sometimes even at pharmacological concentrations have been shown to stimulate ALP and mineral deposition [63, 64], and such stimulatory effects were also observed in murine cultures under specific experimental conditions [48, 65]. These observations initially led to a concept that GC might drive precocious osteoblast differentiation and thus exhaustion of a mesenchymal stem cell pool that otherwise continues to supply osteoblasts for bone formation throughout life. However, the paradoxical stimulation of osteoblast differentiation in vitro is typically observed at physiological rather than pharmacological GC concentrations [65], and most investigators no longer consider this a significant in vivo mechanism contributing to GIO. Among considerations arguing against this paradigm, regain of bone mass is observed shortly following GC withdrawal [6].
It is generally assumed that GC-mediated inhibition of ALP activity and mineralization, which are most reproducible in murine osteoblast cultures, capture a GC-sensitive phase of osteoblast differentiation. An alternative interpretation, however, is that some of the inhibitory effects of GCs in murine osteoblast cultures (e.g., on mineralization) are not as relevant to the human disease, and that other inhibitory effects (e.g., on cell replication and collagen synthesis) better model GIO in humans. Another cautionary note for interpreting results of ALP and mineralization assays in the context of GIO is that the two are not always coupled, and therefore inhibition of any one or even both of them by GCs may ultimately prove irrelevant to GIO. This is suggested, for example, by the good correlation between mineralization and the activity SMAD-BMPs, but not between mineralization and ALP activity in MC3T3-E1 cultures treated with dex along with recombinant BMP-2 or BMP-4 [67]. In addition ALP and mineralization assays can provide data that does not parallel collagen accumulation [68]. That cell proliferation and collagen accumulation assays are likely more relevant to GIO than mineralization assays is suggested by the lack of evidence for poorly mineralized osteoid in GIO in vivo. In section “Molecular Targets of Glucocorticoids in Osteoblasts”, we review several signaling pathways adversely affected by exposure of osteoblast cultures to GCs; some of these pathways may ultimately provide the basis for assays most relevant to molecular mechanisms underlying GIO and to developing bone-sparing GC-based anti-inflammatory therapies. Some of these assays may capture mechanisms that reflect control of both osteoblast replication and maturation, two processes that classically represent opposite aspects of cell growth and differentiation, but may in fact operate hand-in-hand during development of the osteoblast phenotype [36, 69–71].
Glucocorticoids Promote Osteoblast Apoptosis
Many studies demonstrated GC-driven apoptosis of osteoblasts and osteocytes, both in vivo and in vitro [13, 33, 41, 72]. A milestone study invigorated this research avenue in 1998, showing by TUNEL staining increased apoptosis of osteoblasts and osteocytes in bone specimens from GC-treated mice and humans [17]. GC-mediated promotion of osteoblast apoptosis is a cell autonomous effect because apoptosis is not observed in transgenic mice with osteoblast-specific overexpression of the GC inactivating enzyme 11β-HSD2 [21].
Modeling osteoblast apoptosis in GIO, dex induced apoptosis in both the MC3T3-E1 and UMR-106 osteoblastic cell lines, and in both culture systems this was associated with activation of Caspase 3, a common downstream effector of multiple apoptotic signaling pathways [38, 73]. Apoptosis in the MC3T3-E1 culture model was also linked to activation of p53 [38]. GCs dose-dependently induced apoptosis in cultures of primary human osteoblasts derived from surgical bone chips, and this was attributable to increased mRNA and protein expression of Bak, as well as decreased mRNA and protein expression of Bcl-XL [39]. Annotations of genes differentially expressed in GC-treated versus control primary osteoblast cultures were highly enriched for apoptosis-related functions [74]. The same gene-set was also enriched for oxidative stress-related genes, hinting to one mechanism underlying GC-induced osteoblast apoptosis [74]. Related mechanisms of GC-induced apoptosis include inhibition of major survival pathways, such as Wnt, PI3K and ERK (section “Molecular Targets of Glucocorticoids in Osteoblasts”). Inhibition of these pathways by GCs leaves unopposed pro-apoptotic pathways that are activated by Fas, TNF, TRAIL [75] and Reactive Oxygen Species [ROS; sections “Pyk2, JNK and p66shc” and “FoxO Proteins”].
Several open questions related to the role of osteoblast and osteocyte apoptosis in GIO should be noted. In the aforementioned milestone study [17], less than 1 % of murine osteoblasts in vivo were TUNEL-positive, and GCs increased this value threefold. The significance of these observations to the debilitation of bone formation, by up to ~80 %, in GIO remains a matter of debate. Under-estimation of the magnitude of basal and/or GC-induced apoptosis could result from technical difficulties with demonstration and quantitation of osteoblast apoptosis in vivo. Alternative techniques and/or approaches may shed light on this controversy. Furthermore, GC did not appear to induce apoptosis in osteoblasts during certain developmental stages [75] and in mice of certain strains [19]. Additionally, prevention of GC-induced osteoblast apoptosis did not rescue the low bone formation rates or the loss of spinal BMD in the aforementioned OG2-11ß-HSD2 mice (although the decrease in vertebral compression strength was prevented; see section “Involvement of Cells Other than Osteoblasts in GIO” below) [21]. Despite the uncertainty as to the relative contributions of effects of GCs on pre-osteoblast proliferation, impaired osteoblast function and increased apoptosis, the combined effect on all of these aspects together appears to create a “perfect storm” that leaves osteoblasts incapable of balancing bone resorption in GIO.
Involvement of Cells Other than Osteoblasts in GIO
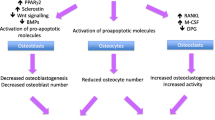
Osteocytes. Not all osteoblasts undergo apoptosis after depositing new bone material at sites that have just been resorbed. Some give rise to flat lining cells that remain on the bone surface and many others incorporate into the newly formed matrix, where they continue to live for lengthy periods of time, contributing to the largest subpopulation of bone cells—the osteocytes. Through neuronal-like processes embedded in a highly interconnected canalicular system, osteocytes serve as biological relays, which stimulate osteoclasts, osteoblasts and their precursors in response to microdamage and mechanical loading. In fact, osteocytes are a major source of RANKL, a quintessential osteoclastogenic factor [77, 78], and their role in bone homeostasis is increasingly appreciated [79]. Thus, skeletal effects of GCs through osteocytes are both direct (as discussed immediately below) and indirect via osteoclastogenesis (see ‘osteoclasts’ thereafter).
There is significant evidence that high-dose GCs increase fracture risk not only by decreasing bone mass, but also by compromising bone material quality [13]. One of several explanations for this phenomenon entails GC-induced osteocyte apoptosis [17]. Contrasting osteocyte autophagy induced by GCs at physiological concentrations, which may protect these cells against stress [80], osteocyte apoptosis in response to high-dose GCs may deprive osteoclasts and osteoblasts the input, based on which they would otherwise respond to biomechanical needs. GC-induced osteocyte apoptosis is attributable to inhibition of survival mechanisms including Wnt signaling, Akt, and Pyk2 (section “Molecular Targets of Glucocorticoids in Osteoblasts” below). Additionally, recent evidence suggests that osteocytes directly modify the bone matrix in which they are embedded and that GCs interfere with a post-osteoblast mineralization process, whereby osteocytes regulate their immediate microenvironment [5]. This novel effect of GCs, hypomineralization of peri-osteocytic bone material, was demonstrated using a nanoindentation technique assisted by atomic force microscopy, and cannot be detected by conventional imaging or histmorphometric methods [5].
Osteoclasts. The early and most destructive phase of GIO is driven not only by the inhibition of osteoblastic bone formation as described above, but also by simultaneous stimulation of osteoclastic bone resorption. After prolonged treatment, however, bone resorption is suppressed to sub-physiological levels [28], contributing to the overall low bone turnover rates typical of GIO.
Stimulation of osteoclastogenesis by GCs at the early disease phase is attributable to both cell autonomous and paracrine mechanisms. Because osteoclastogenesis is a process that usually requires a few days for completion, the fast increase in osteoclast number observed immediately after GC administration has been attributable to extended life span of pre-existing osteoclasts. GC-mediated extension of the osteoclast life-span appears to be cell-autonomous because it occurred in isolated osteoclasts in vitro [28], and because osteoclast number was lower in wild type GC-treated mice compared to GC-treated transgenic mice over-expressing the GC-inactivating enzyme 11β-HSD2 is osteoclasts [30]. Additionally, in vitro data suggest enhancement of osteoblast-driven osteoclastogenesis by GCs, potentially contributing to the increased bone resorption observed in the early phase of GIO [29]. In particular, GCs dramatically suppress OPG mRNA and protein expression in human osteoblast cultures [18]. It remains to be clarified whether GC administration also stimulates bone resorption through osteocyte-borne RANKL [77, 78] and/or other osteoclastogenenic factors, and whether GC-induced osteocyte apoptosis facilitates the release of such factors to promote osteoclastogenesis [81].
The decreased osteoclastic bone resorption observed after prolonged GC treatment periods is generally considered secondary to attenuation of cell number and function in the osteoblast lineage as described above. There is intriguing evidence in vivo, however, suggesting that the GR in myeloid progenitor cells decreases the resorptive activity of osteoclasts, and that this is mediated by their failure to form cytoskeletal structures necessary for attachment to and resorption of the bone matrix [29].
Non-bone cells. In addition to mineralized tissue, the bone as an organ consists of soft tissues, such as cartilage, bone marrow, vessels and nerves. Potential contributions of these tissues to GIO are largely unknown. Furthermore, GCs may have indirect negative effects on bone through their actions in the neuroendocrine system, the gonads, the intestine, and the kidney (reviewed in [13]). Finally, adverse effects of GCs on motor function and muscle strength may contribute to fracture risk by increasing fall rates.
Molecular Targets of Glucocorticoids in Osteoblasts
The Wnt Signaling Pathway
Glucocorticoids Inhibit Wnt Signaling in Osteoblasts
As with several other cell types, Wnt signaling in osteoblasts increases cell proliferation by promoting cell cycle progression and by inhibiting apoptosis [82]. Because Wnt ligands are known to promote asymmetric stem cell division [83], they can be expected to allow early pre-osteoblast pool expansion to supply cells for the formative arm of bone remodeling, while preserving a mesenchymal stem cell pool in the bone marrow microenvironment. The central role of Wnt signaling in bone biology was initially indicated by the inactivating mutations in LRP5, coding a Wnt co-receptor, in patients with the familial bone disease osteoporosis-pseudoglioma [84]. The opposite, high bone mass (HBM), is observed in patients carrying LRP5 activating mutations [85, 86]. Alterations to many additional Wnt-related genes have been linked to bone mass control, and experiments manipulating such genes in mice solidified the notion that Wnt signaling plays a pivotal role in bone metabolism and bone mass control (reviewed in [82]). Accordingly, experimental stimulation of Wnt signaling in osteoblasts results in increased proliferation, increased expression of differentiation markers, decreased apoptosis, as well as attenuated osteoblast-driven osteoclastogenesis [82].
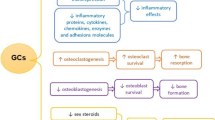
Bioinformatic analysis of global gene expression in bone tissue of GC-treated versus control mice linked the differentially expresed genes to the Wnt pathway, with indications for reduced signaling due, in part, to low expression of Wnt ligands [5]. GCs also decrease Wnt signaling in osteoblast cultures as indicated by decreased expression of Wnt targets, both endogenous genes and reporter constructs [37, 60, 87–90]. Mechanisms underlying inhibition of Wnt signaling in GIO are reviewed below and schematically summarized in Fig. 8.1.
GCs stimulate GSK3ß and inhibit Wnt signaling. Filled (colored) shapes represent components of the canonical Wnt pathway and empty (white) shapes represent signaling molecules that intersect with the Wnt pathway. GSK3ß is positioned at an interesection between the Wnt pathway and protein tyrosine kinase signaling. Lightning bolts indicate phosphorylation. Inhibitory and stimulatory effects of GCs are depicted by red and green circled G’s, respectively. GCs inhibit growth factors (GF) and the downstream PI3K/Akt pathway. Consequently, the inhibitory phosphoryltion of GSK3ß on Ser9 (octagon) is attenuated and GSK3ß is thus activated. Outside the canonical Wnt pathway, active GSK3ß phophorylates c-Myc on Thr58, resulting in c-Myc degradation. Within the canonical Wnt pathway, GSK3ß phosphorylates ß-catenin, resulting in its degradation. This adds to increased rates of ß-catenin degradation due to stimulation of Wnt inhibitors of the SFRP and DKK families, as well as inhibition of Wnt ligands. Inactivation of the Wnt receptor complex stabilizes the ß-catenin destruction complex, where ß-catenin is phosphorylated, tagging it for degradation. Accumulation of ß-catenin is thus inhibited. Because ß-catenin is a critical co-activator for LEF/TCF transcription factors, their target genes (i.e., Wnt target genes) are suppressed. Additionally, GCs attenuate expression of some LEF/TCF transcription factors and stimulate that of HDAC proteins, which inhibit both ß-catenin and LEF/TCF. Abbreviations: APC adenomatous polyposis coli, CK-Iα casein kinase-Iα, DVL disheveled, GSK glycogen synthase kinase, HDAC histone deacetylase, LRP5 low density lipoprotein-related protein 5, SFRP secreted freezled-related protein
Role of Dkks in GIO
DKK1, encoding dickkopf-1 (DKK1), a secreted antagonist of canonical WNT signaling, is one of few examples available to date for classical direct transcriptional stimulation by GCs in osteoblasts. Treatment of human osteoblasts with ≥10 nM dex resulted in robust stimulation of DKK1 mRNA expression, attributable to a glucocorticoid response element (GRE) located 0.8 kb upstream of the DKK1 transcription start site [91]. In a microarray study of primary human osteoblast cultures, DKK1 was among the genes most strongly upregulated by GCs [74]. GCs also stimulated Dkk1 expression in primary rat calvarial osteoblast cultures [92]. A role for GC-induced Dkk1 in inhibiting Wnt signaling in osteoblasts is indicated by reversal of the suppressive effect of dex on Wnt signaling upon addition of anti-Dkk1 antibody to the osteoblast culture medium [88].
Members of the Dkk family inhibit Wnt signaling through interaction with LRP5/LRP6 and with Kremen1/Kremen2 in the Wnt receptor complex [93, 94]. The critical role of DKKs in regulating Wnt signaling in osteoblasts and consequently controlling bone mass is demonstrated by the fact that the G171V mutation in LRP5 that leads to a HBM phenotype [85] is associated with abrogation of its binding to Dkk-1 [95, 96]. The HBM phenotype observed in patients with the G171V LRP5 mutation is therefore indicative of high sensitivity of the Wnt pathway and bone mass to reduced DKK1 activity upon LRP5 in osteoblasts. This is also demonstrated by the strong anabolic response and increased bone mass in DKK1 hypomorphic or heterozygous mice [97, 98]. The opposite effect, decreased bone mass, can therefore be expected to follow increased activity of DKK1 on osteoblasts’ LRP5 in GIO. Indeed, GC-mediated suppression of Wnt/β-catenin signaling and osteoblast differentiation [86] was attenuated when stimulation of Dkk1 was prevented by siRNA-mediated Dkk1 knockdown [60]. In addition to the stimulation of Dkk1 in human and rat cells, GCs have also been shown to stimulate expression of the closely related Dkk2 in primary mouse calvarial osteoblast cultures [37], potentially resulting in the observed inhibition of Wnt signaling in osteoblasts through a similar mechanism, again leading to decreased osteoblast function and loss of bone mass.
Consistent with a potential role for DKK1 in GIO, its levels were elevated in the sera of children with 21-hydroxylase deficiency and chronically treated with high-dose glucocorticoids (10–25 mg/m2 of hydrocortisone). The patients’ sera inhibited osteoblast differentiation in vitro (although the inhibition—of ALP activity—was small), and the effect was ameliorated after administration of anti-DKK1 antibodies [99]. Additional data obtained from these patients suggested that the excess DKK1 could be partly biosynthesized by leukocytes, and that it played a role in regulating RANKL levels in these patients [99]. Dkk1 mRNA was also elevated in mouse bones in vivo after 56 days of prednisolone treatment [20]; this study, however, raises the question of whether GC-mediated stimulation of Dkk1 is a primary event in the mouse because the early time point in vivo (7-days) indicated decreased, not increased DKK1 mRNA levels [20]. Further questioning the role of DKK1 in GIO, its levels decreased, not increased in a prospective study with patients initiating GC therapy [100]. Additional work is therefore needed, for example using conditional knockout mice, to rigorously test the potential role of DKK1 in GIO.
Role of Secreted Frizzled-Related Proteins (SFRPs) in GIO
The first step in activating Wnt signaling is the binding of Wnt ligands to frizzled family receptors. A group of decoy receptors, the secreted frizzled-related proteins (SFRPs) compete with membrane bound frizzled receptors for Wnt binding (Fig. 8.1), thus attenuating both canonical and non-canonical Wnt signaling [93, 101]. Sfrp1 knockout mice have increased trabecular bone mineral density [102] and injection of rats with recombinant SFRP1 decreased bone mineral density [62]. Both canonical and non-canonical Wnt signaling in osteoblasts have been implicated in the regulation of osteoblast proliferation, differentiation and apoptosis by SFRP1 [102].
Dex at concentrations ≥0.1 μM dramatically stimulated Sfrp1 mRNA expression in primary rat bone marrow stromal cell cultures, and this was independent of new protein synthesis [62]. Dex also stimulated Sfrp1 expression in mouse primary calvarial osteoblast cultures [92]. Furthermore, siRNA knock down of SFRP1 led to increased β-catenin accumulation, enhanced Runx2 activity and high levels of ALP and osteocalcin expression, culminating in robust nodule formation even at high dex concentrations [62]. Hence, SFRP could serve as a therapeutic target, inhibition of which may ameliorate GIO.
Additional Wnt-Related GC Targets
Wnt ligands. GCs regulate the expression of some Wnt ligands, potentially contributing to inhibition Wnt signaling in osteoblasts. For example, corticosterone at 100 nM inhibited by ~50 % expression of Wnt 7b and Wnt 10b in mature green fluorescent protein (GFP)-expressing osteoblasts of Col2.3-GFP mice. Interestingly, 10 nM corticosterone had the opposite effect, potentially accounting for paradoxical anabolic effects often observed with low GC doses [92]. Loss of the autocrine/paracrine activity of Wnt ligands at high GC concentrations may amplify the aforementioned anti-Wnt effects of Dkk1, which were confirmed in the Col2.3-GFP-expressing cells [92].
GSK3ß . GC-treated osteoblasts from both human and mouse origin display a decrease in the inhibitory phosphorylation of GSK3β on its Ser9 residue, resulting in increased enzyme activity [46, 49]. The role of GSK3β in the anti-mitogenic effect of GCs was demonstrated by the rescue of cell cycle progression in GC-arrested MC3T3-E1 osteoblasts co-treated with lithium chloride, a GSK3β inhibitor [49]. GC-stimulated GSK3β attenuates cell cycle progression both by inhibiting ß-catenin/LEF-mediated transcription [87] and by phosphorylation of c-MYC on Thr58, which marks the protein for proteasomal degradation [49]. The phosphorylation of GSK3β represents an important point of intersection between growth factor signaling and the canonical Wnt pathway (Fig. 8.1). Specifically, following the activation of PI3K by receptor tyrosine kinases [section “Akt”], Akt phosphorylates GSK3β’s serine9 residue, which results in loss of GSK3β activity upon its targets, such as β-catenin and c-Myc. Accordingly, pharmacological and molecular inhibition of PI3K/Akt in GC-treated MC3T3-E1 osteoblasts is associated with decreased phosphorylation of GSK3β’s Ser9 as well as c-MYC’s Thr58 [49]. Thus, GC-mediated stimulation of the inhibitory kinase GSK3β results in (i) attenuation of β-catenin/LEF-driven transcription, adding to other inhibitory effects of GCs within the canonical Wnt pathway; and (ii) abrogation of GSK3β functions outside the canonical Wnt pathway (Fig. 8.1).
ß-catenin. Ligand-bound GR has been shown to physically interact with β-catenin itself in U2OS/GR cells [103]. This could contribute to inhibition of LEF/TCF-mediated cyclin D transcription and to GIO in vivo, even though GCs did not inhibit cell cycle progression in the U2OS/GR cell culture model [103]. Additionally, GCs may inhibit Wnt signaling by translocating β-catenin from the cell nucleus to the cytoplasmic membrane, which is mediated though interactions of GR with calreticulin. Indeed, silencing of calreticulin abolished dex-mediated inhibition of cyclin D1 expression [104]. Finally, as will be described in section “FoxO Proteins”, GCs interfere with canonical Wnt signaling at the level of β-catenin by generating reactive oxygen species, resulting in activation of FoxO transcription factors, which interact with β-catenin at the expense of LEF/TCF transcription factors.
Recent work suggests that GC-mediated suppression of Wnt/β-catenin signaling is mediated in part through inhibition of mir-29a [105]. In murine calvarial osteoblasts, both primary and MC3T3-E1 cells, mir-29a promotes bone phenotypic properties by suppressing expression of HDAC4, a β-catenin deacetylase [105]. GC-mediated downregulation of mir-29a, and the subsequent deacetylation and inactivation of ß-catenin by HDAC4 appear critical for suppression of the bone phenotype because anti-sense-mediated silencing of HDAC4 rendered the cultures resistant to GCs. Consistent with these findings, GC-mediated inhibition of cell cycle progression in MC3T3-E1 cultures was partially negated in the presence of the HDAC inhibitor trichostatin A [87].
LEF/TCF. Signals elicited by binding of Wnt ligands to their cell surface receptors ultimately lead to changes in gene expression by the binding of activated β-catenin to transcription factors of the LEF/TCF family (Fig. 8.1). In newborn mouse calvarial osteoblast cultures, 1 μM dex decreased the expression of Lef1, Tcf1 and Tcf4 (but not Tcf3) mRNA [37]. Interestingly, the effect of dex on Lef1 and Tcf1 expression depended on the developmental stage with respect to a commitment stage defined based on resistance that these cultures develop on day 6–7 to GC-mediated attenuation of mineral deposition. Specifically, dex inhibited Lef1 only before the commitment stage, whereas the inhibition of Tcf1 was most robust after that stage [37].
Axin2. As discussed in section “Glucocorticoids Inhibit Osteoblast Differentiation and Function”, GCs drive osteoblast precursors towards adipogenesis at the expense of osteogenesis [46, 90, 106]. In murine MC3T3-E1 pre-osteoblasts and ROB-C26 rat mesenchymal progenitor cells, this was attributable in part to a dex-mediated 3-fold increase in Axin2 mRNA expression [90, 107]. Indeed, dex also abrogated ß-catenin activation and this was no longer obvious after depletion of Axin2 in ROB-C26 cells [90]. Consistently, knockdown of Axin2 antagonized dex-mediated adipogenesis, although inhibition of ALP by dex persisted in Axin2-depleted ROB-C26 cultures [90].
Additional Signaling Pathways
In addition to the well documented role of the Wnt signaling pathway in bone pathophysiology in general, and GIO in particular, GCs affect several other pathways in osteoblasts, any of which may ultimately prove an effective target for therapeutic intervention. We briefly review here evidence for the involvement of Notch and BMP signaling, as well as several growth factor pathways, in GIO.
Notch Signaling
Glucocorticoids strongly stimulate transcription of Notch1 and Notch2 in osteoblasts, resulting in several-fold increased mRNA expression within hours of treatment [108]. The activated Notch Intracellular Domain (NICD) is known to inhibit osteoblast differentiation by targeting RUNX2 both directly and indirectly [109, 110]. Although manipulation of Notch signaling in vivo results in a complex skeletal phenotype that depends on age, sex and bone tissue type [110–111], GC-mediated stimulation of Notch signaling likely plays an important role in GIO, which may be mediated in part by inhibition of RUNX2 [section “RUNX2”].
BMP Signaling
Comprehensive gene expression analysis in GC-arrested MC3T3-E1 osteoblast cultures indicated a threefold increase in the expression of Follistatin and Dan mRNAs, encoding inhibitors of BMP signaling [49]. In the same culture model, GCs also strongly inhibited Bmp2 gene expression, and recombinant BMP2 reversed the inhibitory effects of GCs on mineral deposition, ALP activity, osteocalcin expression, as well as (transiently) cell cycle progression [56, 68]. These, however, remain indirect lines of evidence for a role that BMP signaling may play in GIO. In fact, dex did not inhibit the activity of a SMAD-BMP reporter in cultures of MC3T3-E1 cells [67], and some investigators even demonstrated stimulation of BMP signaling by GCs in osteoblasts [32]. Paradoxically, stimulation of BMP signaling by GCs may contribute to GIO through inhibition of Wnt signaling [112], although this conjuncture remains to be tested. Another interesting speculation is that GCs concomitantly stimulate and inhibit BMP signaling in a target gene-dependent manner. Be that as it may, global inhibition of BMP-SMAD signaling does not appear to occur in GIO, or at least not in the MC3T3-E1 culture model; high expression levels of other BMPs, in particular BMP-4, could have sustained activity of the BMP-SMAD reporter in the presence of GCs [67]. Still, decreased BMP2 levels may contribute to GIO when alternative osteogenic BMP genes are not expressed. Under such circumstances (and not in MC3T3-E1 cells), inhibition of Bmp2 transcription, through regulatory sequences located >50-kb downstream of the transcription start site [67], might reduce BMP-SMAD signaling below a threshold necessary for development of the osteoblast phenotype.
Growth Factors
GCs inhibit the biosynthesis of hepatocyte growth factor (HGF, a.k.a. Scatter Factor) in human osteoblasts [113, 114], which could interrupt an autocrine mechanism whereby HGF stimulates osteoblast proliferation by binding to its c-Met receptor on the osteoblast membrane [115]. Indeed, inhibition of HGF signaling mimicked the anti-mitogenic effect of GCs in human osteoblast-like cultures, and GCs no longer inhibited cell proliferation in the presence of added recombinant HGF [114]. These results suggest that HGF may play an important role in GIO, although direct evidence to this effect is lacking.
Growth hormone (GH) and insulin-like growth factors (IGFs) influence bone metabolism through both endocrine and paracrine/autocrine mechanisms, of which the latter are more likely to play a role in GIO [116]. Indeed, osteoblast proliferation and collagen synthesis, probably the two most important functions inhibited in GIO, are stimulated by IGF-I and IGF-II, and GCs have been shown to inhibit IGF signaling at several levels. First, GCs inhibit IGF-I transcription and secretion in primary rat calvarial osteoblast cultures [117, 118], which again may be related to the promotion of the alternative, adipocyte cell fate decision. Indeed, the GC-induced adipogenic factors C/EBPα and C/EBPδ appear to bind Igf-1 regulatory sequences and block transcriptional initiation or elongation [119]. Second, GCs inhibit expression of IGF-binding protein-3 (IGFBP-3) and IGFBP-5 in human osteoblast cultures [120]. The strong inhibition of Igfbp5, which occurs at the transcriptional level in rat calvarial osteoblast cultures [121], is of particular interest because IGFBP5 has an additional, autonomous effect on human osteoblast proliferation [122] and a selective bone anabolic effect in mice in vivo [123–125].
Downstream Kinases and Phosphatases
ERK
In addition to kinase activity directly associated with the receptors reviewed above, downstream kinases with roles in osteoblast growth and differentiation have been implicated in GIO. Chief among them, ERK is a central hub downstream of a variety of osteogenic stimuli elicited by interaction of growth factors and extracellular matrix proteins with their respective receptor tyrosine kinases and integrin receptors [126–129]. Activated ERK executes much of its function in the osteoblast nucleus, where it associates with specific DNA elements to activate key regulators of cell growth and differentiation, including the osteoblast master regulator RUNX2 [130, 131]. Accordingly, stimulation and inhibition of ERK results in enhancement and impediment, respectively, of osteoblast differentiation and bone formation in vivo and in vitro [41, 132, 133].
High dose GCs inhibit ERK signaling and its downstream effectors, and these inhibitory activities are similar to those observed after treatment of osteoblasts with the MEK/ERK inhibitor U0126 [41]. Both dex and U0126 decreased thymidine incorporation into newly synthesized DNA in serum- and TPA-stimulated MBA-15.4 and MG-63 osteoblastic cells [41]. Attenuated ERK activity in GC-treated osteoblasts is likely related to many aspects of GIO, including inhibition of osteoblast proliferation, differentiation and survival [75, 129]. Protection of ERK from GCs has the potential of partially reversing GIO [134].
Akt
Activation of the PI3K/Akt pathway by hormones and growth factors, including PTH and IGFs, is required for the differentiation of mesenchymal pluripotent cells into osteoblasts, as well as survival of the committed cells [135–137]. Mechanisms of action of Akt in regulating canonical signaling pathways such as Wnt (through GSK3β) and mTOR are well established [138, 139]. Additionally in osteoblasts, Akt promotes RUNX2 activity [140], in part through post-translational regulation of Smurf2 [141], and may also activate transcription factors directly within the cell nucleus [139]. GCs attenuate the activity of Akt in osteoblasts by decreasing growth factor availability [see section “Growth Factors”], and by compromising the cellular response to such growth factors [87], which is partially attributable to oxidative stress [89, 143].
Pyk2, JNK and p66shc
In MLO-Y4 osteocyte-like cells, the two highly homologous kinases, FAK and Pyk2, play opposing roles with regard to interaction with the extracellular matrix (ECM). Whereas FAK promotes ECM attachment and cell survival, Pyk2 activation results in loss of cellular processes and anoikis, i.e., detachment-mediated programmed cell death [144]. GCs activates Pyk2 in a manner independent of either RNA or protein synthesis; the underlying mechanism of action, likely employing membrane-associated GR, involves stimulation of JNK [144]. Indeed, GCs no longer induce anoikis in Pyk2-depleted cells or in cells that express either an inactive Pyk2 mutant or a dominant negative JNK [143, 144]. GC-mediated detachment of cells from the ECM through the Pyk2/JNK axis may play a role in the apoptosis of both osteocytes and osteoblasts in vivo [17]. JNK also plays a role in GC-mediated ROS-driven apoptosis through activation of FoxO transcription factors [see section “FoxO Proteins”]. Indeed, GC-mediated FoxO-driven transcription and apoptosis was associated with activation of JNK, and both were severely compromised in fibroblasts derived from JNK1/2 double-knockout mouse embryos [89].
GCs also activated the p66shc kinase in bone in vivo and in cultured osteoblasts in vitro, leading to accumulation of reactive oxygen species (ROS) [89]. Indeed, GCs no longer stimulate ROS accumulation in osteoblast cultures in which p66shc is absent, or in which PKCß, the kinase that phosphorylates p66shc, is pharmacologically inhibited [89]. ROS have many deleterious effects in osteoblasts. As described above they activate JNK, which results in apoptosis through FoxO-dependent and independent mechanisms, and they inhibit Wnt signaling through activation of FoxO transcription factors [section “FoxO Proteins” below] and inhibition of Akt [section “Akt” above].
MKP-1/DUSP
Protein tyrosine phosphatases (PTPs) have been strongly implicated in GC-mediated inhibition of ERK signaling and osteoblast function, as they are the dominant active phosphatase class in the lineage [41]. Inhibition of PTP with sodium orthovanadate restored ERK activity and osteoblast proliferation in dex-inhibited osteoblast cultures [134] and in methylprednisolone-treated Sprague–Dawley rats [145].
The main phosphatase implicated in GC-mediated inhibition of ERK is MAPK phosphatase-1 (MKP-1), also named dual specificity phosphatase-1 (DUSP1), which colocalizes with ERK and limits its effects on target gene transcription [146–149]. Although JNK and p38 can also be inactivated by MKP-1 [150] and even though MKP-3 is most efficient in inactivating ERK1/2 [148], studies in osteoblasts clearly indicate that GCs stimulate MKP-1 [41, 151, 152] while inhibiting MKP-3 [41], and that MKP-1 inactivates ERK [152]. In addition, knockdown of MKP-1 with siRNA in human MG-63 osteosarcoma cells prevented dex-mediated ERK dephosphorylation [152].
Stimulation of MKP-1 expression by GCs is rapid and robust. MKP-1 mRNA levels increased by >10-fold within 30 min of treatment of mouse MBA-15.4 and human MG-63 pre-osteoblasts with dex, an effect that lasted >24 h [41, 152]. This was associated with a similar >10-fold induction of MKP-1 protein, which precisely correlated with inhibition of ERK phosphorylation [41, 152]. GC-induced expression of MKP-1 was confirmed in a global microarray analysis of dex-treated MC3T3-E1 osteoblast-like cells [48], as well as in fibroblast-like synoviocytes [147]. Whereas GR occupancy at the MKP-1 locus in osteoblasts has not been mapped systematically, it has been shown to associate, either directly or through a tethering mechanism, with a GRE-C/EBP composite element located ~1.3-kb upstream of the DUSP transcription start site in A549 human lung adenocarcinoma cells [153, 154]. GCs have also been shown to increase MKP-1 stability in mast cells and fibroblasts [151], although this mechanism is less likely relevant to bone cells because GCs no longer increase MKP-1 levels in cyclohexamide-treated MG-63 cells [152].
Does inhibition of MKP-1 offer a realistic approach for the management of GIO? Despite the pivotal roles of ERK in osteoblast growth and differentiation, side effects of global MKP-1 inhibition are more than likely to occur, which would necessitate specific targeting to bone. Additionally, the efficacy of prospective anti-MKP-1 approaches for GIO has been questioned by observations in MKP-1 knockout mice treated for 28 days with methylprednisolone [155]. The absence of MKP-1 did not negate the GC-mediated decrease in bone formation, suggesting that inhibition of MKP-1 alone is insufficient for protection against GIO [155].
Transcription Factors
FoxO Proteins
The FoxO (forkhead box O) family, consisting of FoxO1, FoxO3a, FoxO4, and FoxO6 [156], play important roles in cellular responses to ROS as well as regulation of cell cycle progression and apoptosis [157]. As in other cells, FoxO family members defend osteoblasts against ROS [89, 158]. The balance between protective and deleterious effects of ROS and FoxO transcription factors is therefore key to for bone health [143]. GCs severely impair this balance by super-activating FoxO transcription factors. This results in the concomitant inhibition of Wnt signaling [143, 159, 160], thus compromising osteoblast proliferation and differentiation (see sections “Glucocorticoids Inhibit Osteoblast Cell Cycle”–“Glucocorticoids Promote Osteoblast Apoptosis”).
Similar to the transcriptional and post-transcriptional regulation of FoxO3 in non-bone cells [161], GCs stimulate FoxO transcription factors in osteoblasts through several independent mechanisms. First, FoxO mRNA levels are upregulated by GCs. Indeed, FoxO3a and FoxO1a were two of the mRNAs most strongly upregulated in a microarray study of GC-treated primary human osteoblasts [74]. Second, GC-induced ROS stimulate the PKCβ/p66shc axis, resulting in activation of JNK and the subsequent phosphorylation of FoxO [143] [see section “Pyk2, JNK and p66shc”]. Third, GCs inhibit Akt [see section “Akt”], which results in the activation of FoxO proteins at the expense of LEF/TCF transcription factors [89].
When treated with pharmacologic doses of GCs, activated FoxO proteins bind and compete for a limited supply of ß-catenin [143, 162, 163]. Direct interaction between ß-catenin and FoxO3a was demonstrated by co-immunoprecipitation of assays in C2C12 cells [143]. Serving as a co-activator, ß-catenin stimulates expression of FoxO target genes at the expense of Wnt/TCF target genes [163]. Indeed, mimicry of GC treatment by over-expression of FoxO3a in Wnt3a-treated C2C12 cultures abrogated development of the osteoblast phenotype, illustrated by a decrease in ALP activity [143]. Furthermore, overexpression of ß-catenin partially overcame FoxO-mediated suppression of TCF/LEF-driven transcription, again suggesting that a limited pool of ß-catenin is shared for the activation of LEF/TCF and FoxO target genes [143]. Furthermore, unlike osteoblasts isolated from WT mice, Wnt3a-driven LEF/TCF activity was not inhibited by GCs in osteoblasts isolated from mice lacking FoxO1, FoxO2 and FoxO3, illustrating the critical role of FoxO proteins in GC-mediated inhibition of Wnt signaling [89].
AP-1
Much of the anti-inflammatory activity of GCs is attributable to both direct and indirect interactions between the GR and other transcription factors. Direct interactions occur both at cis-acting regulatory DNA elements and away from DNA. Indirect interactions involve, for example, regulation of phosphorylation and competition for common co-activators [164]. Perhaps the most important GR-interacting proteins in the context of immune suppression are AP1 (FOS/JUN) and NF-κB transcription factors. Of these, interaction with NF-kB does not appear to play a role in GIO because GCs suppress ALP activity in primary osteoblast cultures even when the cells are impaired for NF-κB activation [19]. In contrast, interactions of the GR with AP-1 family members, which are well documented in contexts other than osteoblasts [164–169], appear to play a role in GIO. This notion is consistent with the pivotal roles that FOS and JUN family members play in osteoblast growth and differentiation [170–172]. Indeed, GRdim mice, in which classical transcriptional stimulation by GR dimers is impaired but inhibition of AP-1 is preserved, developed GIO [19]. Furthermore, abrogation of AP-1-depedent expression of IL-11 [174], a cytokine critical for bone formation in vivo [175], has been implicated in GIO, and administration of IL-11 restored ALP activity, mineralization, and RUNX2 expression in GC-inhibited murine primary calvarial osteoblast cultures [19]. Intriguingly, PTH administration overcame GC-mediated suppression of IL11 expression by stimulating Smad1 and the FOS family member delta FosB [176], a mechanism that could be exploited to combat GIO.
RUNX2
RUNX2 is the most powerful osteoblast lineage specifying factor known to date. Runx2 ablation in the mouse resulted in absence of osteoblasts and a general failure to form any mineralized tissue [177, 178]. Accordingly, manipulation of RUNX2 in cell culture models led to corresponding gain or loss of osteoblast phenotypic properties [179–181]. Because functional osteoblasts are needed not only for embryonic bone development, but also to balance the resorptive activity of osteoclasts at bone multicellular units (BMUs) throughout life, suppression of RUNX2 in adults can be expected to result in bone loss [182] [183]. Therefore, the inhibition of Runx2 expression by GCs, observed in several osteoblast culture systems and in vivo, may play an important role in GIO [19, 43, 184, 185].
Because RUNX2 activity is strongly regulated post-translationally by covalent modifications and protein-protein interactions [186, 187], data on its mRNA and even protein expression levels can be misleading. It is therefore important to note that, in addition to inhibition RUNX2 expression, GCs have been reported to suppress the expression of RUNX2 targets, both endogenous genes such as osteocalcin and artificial constructs designed to specifically report on RUNX activity [61, 184]. Furthermore, a recent study demonstrated that GCs inhibit the activity of RUNX2 even when constitutively expressed from an exogenous lentiviral vector [61]. The implied post-translational inhibition of RUNX2, without a decrease in its mRNA or protein levels [61], is likely the primary effect leading secondarily to changes in the expression levels of endogenous RUNX2 because endogenous Runx2 is subjected to auto-regulation [188]. Indeed, exceptional cases where endogenous Runx2 expression is not inhibited by GCs [185] may represent culture models, in which RUNX2 autoregulatory loops are not operative. Alternatively, inhibition of RUNX2 activity by GCs may be specific for differentiation stages represented by some and not other tissue culture models [56, 185].
Like several other steroid hormone receptors [189–191], the GR physically interacts with RUNX2 [61, 192], possibly inhibiting its DNA-binding and/or transcriptional activation activity. Such inhibition may vary depending on the target gene, including the topographic relationships between local sites occupied by RUNX2, GR and possibly other transcription factors [61, 193]. Additionally, GCs may inhibit RUNX2 indirectly, by suppressing Wnt [section “The Wnt Signaling Pathway”], ERK [section “ERK”] and Akt signaling [section “Akt”], all of which have been implicated in stimulating RUNX2 [130, 131, 140, 141, 194], or by stimulating Notch signaling, which inhibits RUNX2 [109, 110] [section “Notch Signaling”]. A comprehensive understanding of GC-mediated regulation of RUNX2 activity may ultimately lead to the development of novel therapeutic approaches for the management of GIO.
Additional Transcription Factors
The suppression of RUNX2 discussed in the previous section may constitute a critical mechanism underlying GC-mediated inhibition of osteocalcin, both a clinical marker of bone formation and a classical model for osteoblast-specific gene expression. The inhibition of osteocalcin expression by GCs, reproducibly observed both in vitro and in vivo, both in humans and mice, has been investigated for decades, with initial reports focusing on GR binding to osteocalcin proximal promoter elements [20, 195–201]. The inhibition of RUNX2 itself, however, is likely much more relevant to GIO than the inhibition of Osteocalcin, because Osteocalcin does not play any critical role in bone formation [202]. Still, an additional mechanism of osteocalcin transcriptional repression has been discovered using the MC3T3-E1 cell line, in which GCs do not inhibit Runx2 [56, 185]. In these cells, GCs inhibit osteocalcin transcription by strongly repressing expression of Krox20 [48, 203], which has been implicated in embryonal bone development in vivo [204]. Recent studies, however, have raised a doubt regarding the role of Krox20 in osteoblast suppression in GIO because its main function in the adult mouse skeleton in vivo appears to be inhibition of osteoclastogenesis and bone resorption [205, 206].
Microarray-assisted profiling of gene expression in GC-arrested MC3T3-E1 osteoblast cultures [48] confirmed the GC-mediated stimulation of the adipogenic regulators C/EBPß and C/EBPδ and the inhibition of Krox20 (see section “Glucocorticoids Inhibit Osteoblast Differentiation and Function” and previous paragraph, respectively). Together with Krox20, another zinc finger transcription factor gene, the Kruppel-like factor 10 gene (Klf10; a.k.a TGFß-inducible growth response, or Tieg), displayed the strongest suppression (6-fold) in the GC-arrested as compared to control cultures [48]. The relevance of these repressed transcription factor genes to GIO, as well as that of GC-stimulated transcription factors including Klf 13, Period circadian clock 1 (Per1) [48] and Glucocorticoid-Inducible Leucine Zipper (Gilz) [207], is less certain. Unexpectedly, some of the GC-upregulated genes play positive roles in osteoblast differentiation [207] and may explain paradoxical anabolic effects of GCs. Alternatively, these genes may play a role in GIO by abrogating a finely tuned circadian rhythm of gene expression [208, 209], and thus mediate the impact of GCs on proliferation and differentiation of osteoblasts.
Glucocorticoids Without Osteoporosis?
The current standard of care for GIO management is administration of bisphosphonates, which suppresses osteoclast activity. In contrast to high turnover osteoporosis (e.g., after estrogen loss), the use of bisphosphonates for patients undergoing long-term GC therapy is questionable, because it does not address osteoblast suppression and abrogation of bone formation, the hallmark of GIO. In fact, the outcome of bisphosphonate therapy for GIO is a further decrease of the bone turnover rate that is already low due to GC administration [15]. In this sense, intermittent treatment with recombinant PTH appears better suited for the management of GIO because it increases bone mass through stimulation of osteoblast function, directly counteracting adverse effects of GCs in osteoblasts [15, 210]. However, PTH therapy is costly, limited to 18–24 months, and requires daily subcutaneous injections, which together warrant efforts for the development of solutions more suitable for the prevention and treatment of GIO.
At least two further strategies can be envisioned for the development of bone-sparing GC-based therapy. One option is to develop GR ligands, which promote its anti-inflammatory properties without eliciting adverse effects in osteoblasts. This could be accomplished as a selective GR modulator monotherapy, or by taking advantage of bone targeting strategies [211]. Alternatively, rather than targeting GR itself, one can envision the development of combined therapies, whereby conventional GCs are administered along with an anabolic compound, preferably one that restores mechanisms that are compromised in osteoblasts and osteocytes exposed to GCs.
Dissociated Glucocorticoids
Transactivation versus Transrepression: A Model Too Simplistic for the Development of Osteoblast-Forgiving, Bone-Sparing GCs
A strategy used by pharmaceutical companies to reduce side effects of steroid therapy such as osteoporosis has been the design of ligands that avoid GR dimerization-dependent transactivation, but still transrepress inflammatory genes [212]. Limitations of this strategy, however, have quickly come to attention as compounds that specifically promote transrepression were not necessarily successful in avoiding bone loss [213]. Furthermore, such compounds demonstrated limited efficacy, and only a few have been tested in clinical trials [214]. Part of the problem with this approach was that first generation steroidal SGRMs were identified in in vitro high throughput screens, but appeared to be metabolized in vivo into non-dissociated compounds. It remains to be seen if second generation non-steroidal SGRMs will avoid such shortcomings.
The limited clinical success in identifying bone-sparing GR ligands also reflects under-appreciation of the complexity of GR-mediated transcriptional control. Basically, it was assumed that GR employs fundamentally different mechanisms in mediating immune suppression versus side effects. Specifically, an over-simplistic paradigm predominated, which associated side effects with GR homodimers at palindromic GREs, while ascribing the repression of pro-inflammatory genes to a tethering mechanism whereby the GR is recruited to pro-inflammatory genes by transcription factors such as NF-κB, AP-1 and IRF3 [215]. Recent progress, however, revealed a more complex picture. Specifically, chromatin immunoprecipitation (ChIP)-based techniques are currently allowing investigators to map transcription factor locations, as well as capture large-scale chromatin landscapes genome wide. The results highlight cell type-dependent GR-mediated mechanisms that are specific for activation states of cells and individual genes. Based on the emerging paradigm, the limited success of prior efforts is not surprising because both the immune suppression property and the side effects of GCs employ similar fundamental mechanisms of action.
According to the new paradigm, cell type-specific effects of GCs are based on the principle that GR binding is dependent to a large extent on chromatin accessibility, which itself is tissue-specific [216]. Furthermore, direct binding of GR monomers to DNA seems to be more common than initially anticipated and treatment with pharmacological GCs favors binding of GR dimers to classical palindromic DNA elements [26, 27]. Tissue-specific accessibility, in turn, depends on histone modifications and nucleosome positioning within gene regulatory regions [217]. Transcription factors such as C/EBP in liver and fat cells [218, 219], Stat3 in pituitary cells, and AP-1 in mammary epithelial cells [220] often determine accessibility. They are often lineage-specific master regulators, and serve as pioneering transcription factors. Thus, the view of interactions between GR and other transcription factors is changing: early studies of individual genes were mostly interpreted as tethering of the GR by other transcription factors via direct protein-protein interaction. The more recent, genome wide analyses, however, suggest a more complex picture. For example the combinatorial activation of GR and NF-κB leads to the creation of novel binding sites in addition to those occupied after activation of either GR alone or NF-κB alone [221]. Moreover NF-κB target genes sometimes bear GR DNA binding sites close to the NF-κB sites, leading to gene activation or repression dependent on the inflammatory state of innate immune cells [222]. These complex regulatory mechanisms explain, in retrospect, why early attempts to develop bone-sparing dissociating GR ligands were unsuccessful; they relied on transactivation/transrepression of a small number of genes and reporter constructs, which miss the big picture, where GR-mediated transcriptional control is heavily context-dependent. It greatly varies as a function of the individual target gene and it strongly depends on the cellular milieu, which itself depends on the cell type, on whether it is cycling or quiescent, and on the stage of differentiation.
New Requirements for Selective GR Agonists
New principles are sought after towards the identification of bone-sparing glucocorticoids, which do not depend on dissociating dimerization-dependent transactivation from dimerization-independent transrepression. Candidate dissociating ligands will have to be assessed for their bone-sparing property in primary cell systems. That such efforts may be fruitful is demonstrated by the discovery of the plant-derived GR ligand compound A (CpdA). CpdA does not compromise osteoblast differentiation [223]. In contrast to the classical ligand dex, CpdA does not antagonize AP-1-dependent IL-11 expression [223], a pivotal mechanism leading to inhibition of osteoblast differentiation [19]. Consequently, the anti-inflammatory activity of CpdA in arthritis [224, 225], experimental autoimmune encephalomyelitis (EAE) [226, 227] and asthma [225], which is attributable to inhibition of NF-κB [225] and thus decreased levels of cytokines such as IL-6 [223], is not associated with a decrease in bone mass [229]. Future cell-based screens may result in the discovery of additional GR ligands that spare osteoblasts, and some of these ligands may serve as lead compounds for the development of novel bone-sparing anti-inflammatory GC drugs.
Aiming at GR Targets
In addition to employing novel strategies for identification and optimization of novel GR ligands, parallel efforts are warranted towards the development of improved combination therapies for GIO. An optimal combination therapy would replace bisphosphonates or PTH with a treatment modality best suited to counteract pathogenic mechanisms of GIO. Several examples are provided below.
IL11 (interleukin-11), LIF1 (leukemia inhibitory factor 1). As described in section “AP-1”, GCs inhibit AP-1-mediated stimulation of Il11 in osteoblasts in vitro and in vivo. The GC-mediated decrease in IL11, as well as LIF1 expression, appears to significantly contribute to the inhibition of osteoblast differentiation [19, 176]. Supplementation of LIF and IL11 in animal models counteracts GIO at least partially (unpublished observations), consistent with data from transgenic mouse models indicating an important role for IL-11 in bone formation and bone mass control in vivo [230]. However, the safety profile of IL-11, as well as LIF, can be problematic; due to their pleiotropic effects, they might induce hematologic and other complications [231].
DKK1, sclerostin (SOST). We reviewed in section “Glucocorticoids Inhibit Wnt Signaling in Osteoblasts” evidence for the critical role of the Wnt pathway in osteoblast replication, differentiation and survival, and cited many lines of evidence implicating inhibition of Wnt signaling in GIO. One of the most appealing lines of evidence is the direct stimulation of Dkk1, a Wnt inhibitor, by GCs (section “Role of Dkks in GIO”). Regardless of whether or not GCs stimulate Dkk-1 expression in vivo as they do in vitro (see section “Role of Dkks in GIO”), restoration of Wnt signaling is an attractive option for the management of GIO. This can be achieved, for example, by neutralizing DKK1 using anti-DKK1 antibodies. Another potential approach to restore Wnt signaling in GC-treated patients is co-administration of antibodies against sclerostin, another Wnt antagonist. An advantage of the latter is that the sclerostin-coding gene Sost is primarily expressed in osteocytes [232], and its neutralization is therefore less likely to have undesirable extraskeletal effects. Encouraging results from a recent preclinical study, in which mice were co-treated with GCs and anti-sclerostin antibodies, warrant further efforts towards the development of Wnt-targeting strategies for the management of GIO [233].
microRNAs. Restoration of Wnt signaling in the presence of GCs may also be achieved by manipulation of microRNAs, an emerging therapeutic modality that remains to be exploited in the context of GIO. We reviewed in section “Additional Wnt-Related GC Targets” the suppression of HDAC4 by mir29-a and the consequential stimulation of β-catenin and the osteoblast phenotype. Because GCs inhibit mir29-a expression, its pharmacological stimulation appears an attractive avenue towards shielding osteoblasts from adverse effects of GCs.
Unbiased screens. With the advent of high throughput screening technologies, identification of lead compounds, steroidal or otherwise, for the management of GIO may be achieved in an agnostic fashion. Synthetic steroids or large chemical libraries can be screened in the presence of dex, for example, using cell-based assays that report on cellular features such as proliferation, differentiation and/or apoptosis [234]. Alternatively, the assay, typically fluorescent, may report on a distinct aspect of osteoblast differentiation. For example, the readout may reflect the activity of ALP, RUNX2, or a molecular pathway such as Notch or Wnt signaling [235]. Establishing such assays in primary mesenchymal stem cells, pre-osteoblastic cells or osteocytes may be challenging, but worth the effort. With recent advancements in the understanding of cellular and molecular mechanisms underlying GIO, the identification of target genes and compounds for combating this devastating iatrogenic disease is increasingly within reach.
References
Soen S, Tanaka Y. Glucocorticoid-induced osteoporosis: skeletal manifestations of glucocorticoid use and 2004 Japanese Society for Bone and Mineral Research-proposed guidelines for its management. Mod Rheumatol. 2005;15:163–8.
van Staa TP, et al. A simple score for estimating the long-term risk of fracture in patients using oral glucocorticoids. QJM. 2005;98:191–8.
Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993–1000.
van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–87.
Lane NE, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466–76.
Laan RF, et al. Low-dose prednisone induces rapid reversible axial bone loss in patients with rheumatoid arthritis. A randomized, controlled study. Ann Intern Med. 1993;119:963–8.
McKenzie R, et al. Decreased bone mineral density during low dose glucocorticoid administration in a randomized, placebo controlled trial. J Rheumatol. 2000;27:2222–6.
Aaron JE, Francis RM, Peacock M, Makins NB. Contrasting microanatomy of idiopathic and corticosteroid-induced osteoporosis. Clin Orthop Relat Res. 1989;243:294–305.
Laan RF, et al. Differential effects of glucocorticoids on cortical appendicular and cortical vertebral bone mineral content. Calcif Tissue Int. 1993;52:5–9.
Maggi S, et al. Osteoporosis risk in patients with chronic obstructive pulmonary disease: the EOLO study. J Clin Densitom. 2009;12:345–52.
Gonnelli S, et al. Effect of inhaled glucocorticoids and beta(2) agonists on vertebral fracture risk in COPD patients: the EOLO study. Calcif Tissue Int. 2010;87:137–43.
Weinstein RS. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am. 2012;41:595–611.
Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–28.
Grossman JM, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res. 2010;62:1515–26.
Weinstein RS, Jilka RL, Almeida M, Roberson PK, Manolagas SC. Intermittent parathyroid hormone administration counteracts the adverse effects of glucocorticoids on osteoblast and osteocyte viability, bone formation, and strength in mice. Endocrinology. 2010;151:2641–9.
Gluer CC, et al. Comparative effects of teriparatide and risedronate in glucocorticoid-induced osteoporosis in men: 18-month results of the EuroGIOPs trial. J Bone Miner Res. 2013;28:1355–68.
Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–82.
Hofbauer LC, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382–9.
Rauch A, et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010;11:517–31.
Yao W, et al. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum. 2008;58:1674–86.
O’Brien CA, et al. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–41.
Sher LB, et al. Transgenic expression of 11beta-hydroxysteroid dehydrogenase type 2 in osteoblasts reveals an anabolic role for endogenous glucocorticoids in bone. Endocrinology. 2004;145:922–9.
Yang M, et al. Col3.6-HSD2 transgenic mice: a glucocorticoid loss-of-function model spanning early and late osteoblast differentiation. Bone. 2010;47:573–82.
Reichardt HM, et al. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO J. 2001;20:7168–73.
Reichardt HM, et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–41.
Lim HW et al. Genomic redistribution of GR monomers and dimers mediates transcriptional response to exogenous glucocorticoids in vivo. Genome Research 2015 May 8 [Epub ahead of print].
Schiller BJ, Chodankar R, Watson LC, Stallcup MR, Yamamoto KR. Glucocorticoid receptor binds half sites as a monomer and regulates specific target genes. Genome Biol. 2014;15:3181.
Weinstein RS, et al. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest. 2002;109:1041–8.
Kim HJ, et al. Glucocorticoids suppress bone formation via the osteoclast. J Clin Invest. 2006;116:2152–60.
Jia D, O’Brien CA, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592–9.
Alesci S, De Martino MU, Ilias I, Gold PW, Chrousos GP. Glucocorticoid-induced osteoporosis: from basic mechanisms to clinical aspects. Neuroimmunomodulation. 2005;12:1–19.
Moutsatsou P, Kassi E, Papavassiliou AG. Glucocorticoid receptor signaling in bone cells. Trends Mol Med. 2012;18:348–59.
Baschant U, Lane NE, Tuckermann J. The multiple facets of glucocorticoid action in rheumatoid arthritis. Nat Rev Rheumatol. 2012;8:645–55.
Weinstein RS, et al. The skeletal effects of glucocorticoid excess override those of orchidectomy in mice. Endocrinology. 2004;145:1980–7.
Chen TL, Aronow L, Feldman D. Glucocorticoid receptors and inhibition of bone cell growth in primary culture. Endocrinology. 1977;100:619–28.
Smith E, et al. Glucocorticoids inhibit developmental stage-specific osteoblast cell cycle. Dissociation of cyclin A-cyclin-dependent kinase 2 from E2F4-p130 complexes. J Biol Chem. 2000;275:19992–20001.
Gabet Y, Noh T, Lee C, Frenkel B. Developmentally regulated inhibition of cell cycle progression by glucocorticoids through repression of cyclin A transcription in primary osteoblast cultures. J Cell Physiol. 2011;226:991–8.
Li H, et al. Glucocorticoid receptor and sequential P53 activation by dexamethasone mediates apoptosis and cell cycle arrest of osteoblastic MC3T3-E1 cells. PLoS One. 2012;7:e37030.
Chang JK, et al. Anti-inflammatory drugs suppress proliferation and induce apoptosis through altering expressions of cell cycle regulators and pro-apoptotic factors in cultured human osteoblasts. Toxicology. 2009;258:148–56.
Rogatsky I, Trowbridge JM, Garabedian MJ. Glucocorticoid receptor-mediated cell cycle arrest is achieved through distinct cell-specific transcriptional regulatory mechanisms. Mol Cell Biol. 1997;17:3181–93.
Engelbrecht Y, et al. Glucocorticoids induce rapid up-regulation of mitogen-activated protein kinase phosphatase-1 and dephosphorylation of extracellular signal-regulated kinase and impair proliferation in human and mouse osteoblast cell lines. Endocrinology. 2003;144:412–22.
Lecka-Czernik B, et al. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J Cell Biochem. 1999;74:357–71.
Pereira RC, Delany AM, Canalis E. Effects of cortisol and bone morphogenetic protein-2 on stromal cell differentiation: correlation with CCAAT-enhancer binding protein expression. Bone. 2002;30:685–91.
Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43.
Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66:236–53.
Naito M, Omoteyama K, Mikami Y, Takahashi T, Takagi M. Inhibition of Wnt/beta-catenin signaling by dexamethasone promotes adipocyte differentiation in mesenchymal progenitor cells, ROB-C26. Histochem Cell Biol. 2012;138:833–45.
Berendsen AD, Olsen BR. Osteoblast-adipocyte lineage plasticity in tissue development, maintenance and pathology. Cell Mol Life Sci. 2014;71:493–7.
Leclerc N, et al. Gene expression profiling of glucocorticoid-inhibited osteoblasts. J Mol Endocrinol. 2004;33:175–93.
Smith E, Coetzee GA, Frenkel B. Glucocorticoids inhibit cell cycle progression in differentiating osteoblasts via glycogen synthase kinase-3beta. J Biol Chem. 2002;277:18191–7.
Mikami Y, Lee M, Irie S, Honda MJ. Dexamethasone modulates osteogenesis and adipogenesis with regulation of osterix expression in rat calvaria-derived cells. J Cell Physiol. 2011;226:739–48.
Worthley et al. Gremlin 1 Identifies a Skeletal Stem Cell with Bone, Cartilage, and Reticular Stromal Potential Cell. 2015;160:269–284.
Chan et al. Identification and Specification of the Mouse Skeletal Stem Cell. Cell. 2015;160:285–298.
Canalis E. Effect of glucocorticoids on type I collagen synthesis, alkaline phosphatase activity, and deoxyribonucleic acid content in cultured rat calvariae. Endocrinology. 1983;112:931–9.
Harris C, et al. Large increases in adipose triacylglycerol flux in Cushingoid CRH-Tg mice are explained by futile cycling. Am J Physiol Endocrinol Metab. 2013;304:E282–93.
Delany AM, Gabbitas BY, Canalis E. Cortisol downregulates osteoblast alpha 1 (I) procollagen mRNA by transcriptional and posttranscriptional mechanisms. J Cell Biochem. 1995;57:488–94.
Luppen CA, et al. Brief bone morphogenetic protein 2 treatment of glucocorticoid-inhibited MC3T3-E1 osteoblasts rescues commitment-associated cell cycle and mineralization without alteration of Runx2. J Biol Chem. 2003;278:44995–5003.
Delany AM, Jeffrey JJ, Rydziel S, Canalis E. Cortisol increases interstitial collagenase expression in osteoblasts by post-transcriptional mechanisms. J Biol Chem. 1995;270:26607–12.
Lian JB, et al. Species-specific glucocorticoid and 1,25-dihydroxyvitamin D responsiveness in mouse MC3T3-E1 osteoblasts: dexamethasone inhibits osteoblast differentiation and vitamin D down-regulates osteocalcin gene expression. Endocrinology. 1997;138:2117–27.
Chen TL, Fry D. Hormonal regulation of the osteoblastic phenotype expression in neonatal murine calvarial cells. Calcif Tissue Int. 1999;64:304–9.
Butler JS, et al. Silencing Dkk1 expression rescues dexamethasone-induced suppression of primary human osteoblast differentiation. BMC Musculoskelet Disord. 2010;11:210.
Koromila T, et al. Glucocorticoids antagonize RUNX2 during osteoblast differentiation in cultures of ST2 pluripotent mesenchymal cells. J Cell Biochem. 2014;115:27–33.
Wang FS, et al. Secreted frizzled-related protein 1 modulates glucocorticoid attenuation of osteogenic activities and bone mass. Endocrinology. 2005;146:2415–23.
Subramaniam M, et al. Glucocorticoid regulation of alkaline phosphatase, osteocalcin, and proto-oncogenes in normal human osteoblast-like cells. J Cell Biochem. 1992;50:411–24.
Cheng SL, Yang JW, Rifas L, Zhang SF, Avioli LV. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277–86.
Shalhoub V, et al. Glucocorticoids promote development of the osteoblast phenotype by selectively modulating expression of cell growth and differentiation associated genes. J Cell Biochem. 1992;50:425–40.
Ishida Y, Heersche JN. Glucocorticoid-induced osteoporosis: both in vivo and in vitro concentrations of glucocorticoids higher than physiological levels attenuate osteoblast differentiation. J Bone Miner Res. 1998;13:1822–6.
Luppen CA, Chandler RL, Noh T, Mortlock DP, Frenkel B. BMP-2 vs. BMP-4 expression and activity in glucocorticoid-arrested MC3T3-E1 osteoblasts: Smad signaling, not alkaline phosphatase activity, predicts rescue of mineralization. Growth Factors. 2008;26:226–37.
Luppen CA, Smith E, Spevak L, Boskey AL, Frenkel B. Bone morphogenetic protein-2 restores mineralization in glucocorticoid-inhibited MC3T3-E1 osteoblast cultures. J Bone Miner Res. 2003;18:1186–97.
Smith E, et al. Expression of cell cycle regulatory factors in differentiating osteoblasts: postproliferative up-regulation of cyclins B and E. Cancer Res. 1995;55:5019–24.
Smith E, et al. Post-proliferative cyclin E-associated kinase activity in differentiated osteoblasts: inhibition by proliferating osteoblasts and osteosarcoma cells. J Cell Biochem. 1997;66:141–52.
Carcamo-Orive I, et al. Regulation of human bone marrow stromal cell proliferation and differentiation capacity by glucocorticoid receptor and AP-1 crosstalk. J Bone Miner Res. 2010;25:2115–25.
Gu G, Hentunen TA, Nars M, Harkonen PL, Vaananen HK. Estrogen protects primary osteocytes against glucocorticoid-induced apoptosis. Apoptosis. 2005;10:583–95.
Liu Y, et al. Prevention of glucocorticoid-induced apoptosis in osteocytes and osteoblasts by calbindin-D28k. J Bone Miner Res. 2004;19:479–90.
Hurson CJ, et al. Gene expression analysis in human osteoblasts exposed to dexamethasone identifies altered developmental pathways as putative drivers of osteoporosis. BMC Musculoskelet Disord. 2007;8:12.
Conradie MM, et al. Vanadate prevents glucocorticoid-induced apoptosis of osteoblasts in vitro and osteocytes in vivo. J Endocrinol. 2007;195:229–40.
Zalavras C, Shah S, Birnbaum MJ, Frenkel B. Role of apoptosis in glucocorticoid-induced osteoporosis and osteonecrosis. Crit Rev Eukaryot Gene Expr. 2003;13:221–35.
Nakashima T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–4.
Xiong J, et al. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–41.
Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell … and more. Endocr Rev. 2013;34:658–90.
Jia J, et al. Glucocorticoid dose determines osteocyte cell fate. FASEB J. 2011;25:3366–76.
Gu G, Mulari M, Peng Z, Hentunen TA, Vaananen HK. Death of osteocytes turns off the inhibition of osteoclasts and triggers local bone resorption. Biochem Biophys Res Commun. 2005;335:1095–101.
Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492:1–18.
Habib SJ, et al. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339:1445–8.
Gong Y, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–23.
Little RD, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–9.
Boyden LM, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–21.
Smith E, Frenkel B. Glucocorticoids inhibit the transcriptional activity of LEF/TCF in differentiating osteoblasts in a glycogen synthase kinase-3beta-dependent and -independent manner. J Biol Chem. 2005;280:2388–94.
Ohnaka K, Tanabe M, Kawate H, Nawata H, Takayanagi R. Glucocorticoid suppresses the canonical Wnt signal in cultured human osteoblasts. Biochem Biophys Res Commun. 2005;329:177–81.
Almeida M, Han L, Ambrogini E, Weinstein RS, Manolagas SC. Glucocorticoids and tumor necrosis factor alpha increase oxidative stress and suppress Wnt protein signaling in osteoblasts. J Biol Chem. 2011;286:44326–35.
Naito M, Mikami Y, Takagi M, Takahashi T. Up-regulation of Axin2 by dexamethasone promotes adipocyte differentiation in ROB-C26 mesenchymal progenitor cells. Cell Tissue Res. 2013;354:761–70.
Ohnaka K, Taniguchi H, Kawate H, Nawata H, Takayanagi R. Glucocorticoid enhances the expression of dickkopf-1 in human osteoblasts: novel mechanism of glucocorticoid-induced osteoporosis. Biochem Biophys Res Commun. 2004;318:259–64.
Mak W, Shao X, Dunstan CR, Seibel MJ, Zhou H. Biphasic glucocorticoid-dependent regulation of Wnt expression and its inhibitors in mature osteoblastic cells. Calcif Tissue Int. 2009;85:538–45.
Cruciat CM, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol. 2013;5:a015081.
Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–6.
Patel MS, Karsenty G. Regulation of bone formation and vision by LRP5. N Engl J Med. 2002;346:1572–4.
Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–55.
Morvan F, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21:934–45.
MacDonald BT, et al. Bone mass is inversely proportional to Dkk1 levels in mice. Bone. 2007;41:331–9.
Brunetti G, et al. High dickkopf-1 levels in sera and leukocytes from children with 21-hydroxylase deficiency on chronic glucocorticoid treatment. Am J Physiol Endocrinol Metab. 2013;304:E546–54.
Gifre L, et al. Effect of glucocorticoid treatment on Wnt signalling antagonists (sclerostin and Dkk-1) and their relationship with bone turnover. Bone. 2013;57:272–6.
Jones SE, Jomary C. Secreted frizzled-related proteins: searching for relationships and patterns. Bioessays. 2002;24:811–20.
Bodine PV, et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18:1222–37.
Takayama S, Rogatsky I, Schwarcz LE, Darimont BD. The glucocorticoid receptor represses cyclin D1 by targeting the Tcf-beta-catenin complex. J Biol Chem. 2006;281:17856–63.
Olkku A, Mahonen A. Calreticulin mediated glucocorticoid receptor export is involved in beta-catenin translocation and Wnt signalling inhibition in human osteoblastic cells. Bone. 2009;44:555–65.
Ko J-Y, et al. MicroRNA-29a ameliorates glucocorticoid-induced suppression of osteoblast differentiation by regulating β-catenin acetylation. Bone. 2013;57:468–75.
Nuttall ME, Patton AJ, Olivera DL, Nadeau DP, Gowen M. Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J Bone Miner Res. 1998;13:371–82.
Hayashi K, et al. BMP/Wnt antagonists are upregulated by dexamethasone in osteoblasts and reversed by alendronate and PTH: potential therapeutic targets for glucocorticoid-induced osteoporosis. Biochem Biophys Res Commun. 2009;379:261–6.
Pereira RM, Delany AM, Durant D, Canalis E. Cortisol regulates the expression of Notch in osteoblasts. J Cell Biochem. 2002;85:252–8.
Engin F, et al. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med. 2008;14:299–305.
Hilton MJ, et al. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med. 2008;14:306–14.
Zanotti S, Canalis E. Notch1 and Notch2 expression in osteoblast precursors regulates femoral microarchitecture. Bone. 2014;62:22–8.
Kamiya N, et al. BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development. 2008;135:3801–11.
Skrtic S, Ohlsson C. Cortisol decreases hepatocyte growth factor levels in human osteoblast-like cells. Calcif Tissue Int. 2000;66:108–12.
Tsunashima Y, Kondo A, Matsuda T, Togari A. Hydrocortisone inhibits cellular proliferation by downregulating hepatocyte growth factor synthesis in human osteoblasts. Biol Pharm Bull. 2011;34:700–3.
Grano M, et al. Hepatocyte growth factor is a coupling factor for osteoclasts and osteoblasts in vitro. Proc Natl Acad Sci U S A. 1996;93:7644–8.
Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–59.
McCarthy TL, Centrella M, Canalis E. Cortisol inhibits the synthesis of insulin-like growth factor-I in skeletal cells. Endocrinology. 1990;126:1569–75.
Delany AM, Canalis E. Transcriptional repression of insulin-like growth factor I by glucocorticoids in rat bone cells. Endocrinology. 1995;136:4776–81.
Delany AM, Durant D, Canalis E. Glucocorticoid suppression of IGF I transcription in osteoblasts. Mol Endocrinol. 2001;15:1781–9.
Chevalley T, Strong DD, Mohan S, Baylink D, Linkhart TA. Evidence for a role for insulin-like growth factor binding proteins in glucocorticoid inhibition of normal human osteoblast-like cell proliferation. Eur J Endocrinol. 1996;134:591–601.
Gabbitas B, Pash JM, Delany AM, Canalis E. Cortisol inhibits the synthesis of insulin-like growth factor-binding protein-5 in bone cell cultures by transcriptional mechanisms. J Biol Chem. 1996;271:9033–8.
Andress DL, Birnbaum RS. Human osteoblast-derived insulin-like growth factor (IGF) binding protein-5 stimulates osteoblast mitogenesis and potentiates IGF action. J Biol Chem. 1992;267:22467–72.
Richman C, Baylink DJ, Lang K, Dony C, Mohan S. Recombinant human insulin-like growth factor-binding protein-5 stimulates bone formation parameters in vitro and in vivo. Endocrinology. 1999;140:4699–705.
Miyakoshi N, et al. Evidence that IGF-binding protein-5 functions as a growth factor. J Clin Invest. 2001;107:73–81.
Salih DA, et al. Insulin-like growth factor-binding protein-5 induces a gender-related decrease in bone mineral density in transgenic mice. Endocrinology. 2005;146:931–40.
Chaudhary LR, Avioli LV. Activation of extracellular signal-regulated kinases 1 and 2 (ERK1 and ERK2) by FGF-2 and PDGF-BB in normal human osteoblastic and bone marrow stromal cells: differences in mobility and in-gel renaturation of ERK1 in human, rat, and mouse osteoblastic cells. Biochem Biophys Res Commun. 1997;238:134–9.
Xiao G, et al. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res. 2002;17:101–10.
Xiao G, Jiang D, Gopalakrishnan R, Franceschi RT. Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J Biol Chem. 2002;277:36181–7.
Greenblatt MB, Shim JH, Glimcher LH. Mitogen-activated protein kinase pathways in osteoblasts. Annu Rev Cell Dev Biol. 2013;29:63–79.
Xiao G, et al. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem. 2000;275:4453–9.
Li Y, Ge C, Franceschi RT. Differentiation-dependent association of phosphorylated extracellular signal-regulated kinase with the chromatin of osteoblast-related genes. J Bone Miner Res. 2010;25:154–63.
Lai CF, et al. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J Biol Chem. 2001;276:14443–50.
Ge C, Xiao G, Jiang D, Franceschi RT. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J Cell Biol. 2007;176:709–18.
Hulley PA, Gordon F, Hough FS. Inhibition of mitogen-activated protein kinase activity and proliferation of an early osteoblast cell line (MBA 15.4) by dexamethasone: role of protein phosphatases. Endocrinology. 1998;139:2423–31.
Xian L, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18:1095–101.
Yamamoto T, et al. Parathyroid hormone activates phosphoinositide 3-kinase-Akt-Bad cascade in osteoblast-like cells. Bone. 2007;40:354–9.
Mukherjee A, Rotwein P. Akt promotes BMP2-mediated osteoblast differentiation and bone development. J Cell Sci. 2009;122:716–26.
Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–45.
Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004.
Fujita T, et al. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J Cell Biol. 2004;166:85–95.
Choi YH, et al. Akt enhances Runx2 protein stability by regulating Smurf2 function during osteoblast differentiation. FEBS J. 2014;281(16):3656–66.
Borgatti P, et al. Translocation of Akt/PKB to the nucleus of osteoblast-like MC3T3-E1 cells exposed to proliferative growth factors. FEBS Lett. 2000;477:27–32.
Almeida M, Han L, Martin-Millan M, O’Brien CA, Manolagas SC. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem. 2007;282:27298–305.
Plotkin LI, Manolagas SC, Bellido T. Glucocorticoids induce osteocyte apoptosis by blocking focal adhesion kinase-mediated survival. Evidence for inside-out signaling leading to anoikis. J Biol Chem. 2007;282:24120–30.
Hulley PA, Conradie MM, Langeveldt CR, Hough FS. Glucocorticoid-induced osteoporosis in the rat is prevented by the tyrosine phosphatase inhibitor, sodium orthovanadate. Bone. 2002;31:220–9.
Clark AR, Lasa M. Crosstalk between glucocorticoids and mitogen-activated protein kinase signalling pathways. Curr Opin Pharmacol. 2003;3:404–11.
Toh ML, Yang Y, Leech M, Santos L, Morand EF. Expression of mitogen-activated protein kinase phosphatase 1, a negative regulator of the mitogen-activated protein kinases, in rheumatoid arthritis: up-regulation by interleukin-1beta and glucocorticoids. Arthritis Rheum. 2004;50:3118–28.
Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16.
Slack DN, Seternes OM, Gabrielsen M, Keyse SM. Distinct binding determinants for ERK2/p38alpha and JNK map kinases mediate catalytic activation and substrate selectivity of map kinase phosphatase-1. J Biol Chem. 2001;276:16491–500.
Franklin CC, Kraft AS. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem. 1997;272:16917–23.
Kassel O, et al. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 2001;20:7108–16.
Horsch K, et al. Mitogen-activated protein kinase phosphatase 1/dual specificity phosphatase 1 mediates glucocorticoid inhibition of osteoblast proliferation. Mol Endocrinol. 2007;21:2929–40.
Johansson-Haque K, Palanichamy E, Okret S. Stimulation of MAPK-phosphatase 1 gene expression by glucocorticoids occurs through a tethering mechanism involving C/EBP. J Mol Endocrinol. 2008;41:239–49.
Shipp LE, et al. Transcriptional regulation of human dual specificity protein phosphatase 1 (DUSP1) gene by glucocorticoids. PLoS One. 2010;5:e13754.
Conradie MM, et al. MKP-1 knockout does not prevent glucocorticoid-induced bone disease in mice. Calcif Tissue Int. 2011;89:221–7.
Katoh M, Katoh M. Human FOX gene family (Review). Int J Oncol. 2004;25:1495–500.
Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–60.
Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–8.
Essers MA, et al. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802–12.
Iyer S, et al. FOXOs attenuate bone formation by suppressing Wnt signaling. J Clin Invest. 2013;123:3409–19.
Lutzner N, Kalbacher H, Krones-Herzig A, Rosl F. FOXO3 is a glucocorticoid receptor target and regulates LKB1 and its own expression based on cellular AMP levels via a positive autoregulatory loop. PLoS One. 2012;7:e42166.
Essers MA, et al. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–4.
Hoogeboom D, et al. Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. J Biol Chem. 2008;283:9224–30.
De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522.
Jonat C, et al. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–204.
Yang-Yen HF, et al. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990;62:1205–15.
Subramaniam N, Cairns W, Okret S. Studies on the mechanism of glucocorticoid-mediated repression from a negative glucocorticoid response element from the bovine prolactin gene. DNA Cell Biol. 1997;16:153–63.
Miner JN, Yamamoto KR. The basic region of AP-1 specifies glucocorticoid receptor activity at a composite response element. Genes Dev. 1992;6:2491–501.
Tuckermann JP, et al. The DNA binding-independent function of the glucocorticoid receptor mediates repression of AP-1-dependent genes in skin. J Cell Biol. 1999;147:1365–70.
McCabe LR, et al. Developmental expression and activities of specific fos and jun proteins are functionally related to osteoblast maturation: role of Fra-2 and Jun D during differentiation. Endocrinology. 1996;137:4398–408.
Sabatakos G, et al. Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat Med. 2000;6:985–90.
Wagner EF. Functions of AP1 (Fos/Jun) in bone development. Ann Rheum Dis. 2002;61 Suppl 2:ii40–2.
Roohk DJ, et al. Dexamethasone-mediated changes in adipose triacylglycerol metabolism are exaggerated, not diminished, in the absence of a functional GR dimerization domain. Endocrinology. 2013;154:1528–39.
Matsumoto T, Kuriwaka-Kido R, Kondo T, Endo I, Kido S. Regulation of osteoblast differentiation by interleukin-11 via AP-1 and Smad signaling. Endocr J. 2012;59:91–101.
Sims NA, et al. Interleukin-11 receptor signaling is required for normal bone remodeling. J Bone Miner Res. 2005;20:1093–102.
Kuriwaka-Kido R, et al. Parathyroid hormone (1-34) counteracts the suppression of interleukin-11 expression by glucocorticoid in murine osteoblasts: a possible mechanism for stimulating osteoblast differentiation against glucocorticoid excess. Endocrinology. 2013;154:1156–67.
Komori T, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts [see comments]. Cell. 1997;89:755–64.
Otto F, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development [see comments]. Cell. 1997;89:765–71.
Banerjee C, et al. Runt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone-specific complex. J Cell Biochem. 1997;66:1–8.
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation [see comments]. Cell. 1997;89:747–54.
Komori T. Signaling networks in RUNX2-dependent bone development. J Cell Biochem. 2011;112:750–5.
Ducy P, et al. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev. 1999;13:1025–36.
Estrada K, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44:491–501.
Chang DJ, et al. Reduction in transforming growth factor beta receptor I expression and transcription factor CBFa1 on bone cells by glucocorticoid. J Biol Chem. 1998;273:4892–6.
Prince M, et al. Expression and regulation of Runx2/Cbfa1 and osteoblast phenotypic markers during the growth and differentiation of human osteoblasts. J Cell Biochem. 2001;80:424–40.
Bae SC, Lee YH. Phosphorylation, acetylation and ubiquitination: the molecular basis of RUNX regulation. Gene. 2006;366:58–66.
Li X, Decker M, Westendorf JJ. TEThered to Runx: novel binding partners for runx factors. Blood Cells Mol Dis. 2010;45:82–5.
Drissi H, et al. Transcriptional autoregulation of the bone related CBFA1/RUNX2 gene. J Cell Physiol. 2000;184:341–50.
Paredes R, et al. Bone-specific transcription factor Runx2 interacts with the 1alpha,25-dihydroxyvitamin D3 receptor to up-regulate rat osteocalcin gene expression in osteoblastic cells. Mol Cell Biol. 2004;24:8847–61.
Khalid O, et al. Modulation of Runx2 activity by estrogen receptor-alpha: implications for osteoporosis and breast cancer. Endocrinology. 2008;149:5984–95.
Baniwal SK, et al. Repression of Runx2 by androgen receptor (AR) in osteoblasts and prostate cancer cells: AR binds Runx2 and abrogates its recruitment to DNA. Mol Endocrinol. 2009;23:1203–14.
Ning YM, Robins DM. AML3/CBFalpha1 is required for androgen-specific activation of the enhancer of the mouse sex-limited protein (Slp) gene. J Biol Chem. 1999;274:30624–30.
Little GH, et al. Differential effects of RUNX2 on the androgen receptor in prostate cancer: synergistic stimulation of a gene set exemplified by SNAI2 and subsequent invasiveness. Cancer Res. 2014;74:2857–68.
Gaur T, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–40.
Ekenstam E, Stalenheim G, Hallgren R. The acute effect of high dose corticosteroid treatment on serum osteocalcin. Metabolism. 1988;37:141–4.
Heinrichs AA, et al. Identification of multiple glucocorticoid receptor binding sites in the rat osteocalcin gene promoter. Biochemistry. 1993;32:11436–44.
Cosman F, Nieves J, Herbert J, Shen V, Lindsay R. High-dose glucocorticoids in multiple sclerosis patients exert direct effects on the kidney and skeleton. J Bone Miner Res. 1994;9:1097–105.
Morrison N, Eisman J. Role of the negative glucocorticoid regulatory element in glucocorticoid repression of the human osteocalcin promoter. J Bone Miner Res. 1993;8:969–75.
Morrison NA, et al. 1,25-dihydroxyvitamin D-responsive element and glucocorticoid repression in the osteocalcin gene. Science. 1989;246:1158–61.
Stromstedt PE, Poellinger L, Gustafsson JA, Carlstedt-Duke J. The glucocorticoid receptor binds to a sequence overlapping the TATA box of the human osteocalcin promoter: a potential mechanism for negative regulation. Mol Cell Biol. 1991;11:3379–83.
Meyer T, Gustafsson JA, Carlstedt-Duke J. Glucocorticoid-dependent transcriptional repression of the osteocalcin gene by competitive binding at the TATA box. DNA Cell Biol. 1997;16:919–27.
Ducy P, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–52.
Leclerc N, Noh T, Khokhar A, Smith E, Frenkel B. Glucocorticoids inhibit osteocalcin transcription in osteoblasts by suppressing Egr2/Krox20-binding enhancer. Arthritis Rheum. 2005;52:929–39.
Levi G, et al. Defective bone formation in Krox-20 mutant mice. Development. 1996;122:113–20.
Gabet Y, et al. Gender-specific control of peak bone mass by the Wnt pathway: androgen signaling protects against lef1 haploinsufficiency-induced bone loss. Bone. 2008;42:S50 (meeting abstract).
Kim HJ, et al. Early growth response 2 negatively modulates osteoclast differentiation through upregulation of Id helix-loop-helix proteins. Bone. 2012;51:643–50.
Shi X, et al. A glucocorticoid-induced leucine-zipper protein, GILZ, inhibits adipogenesis of mesenchymal cells. EMBO Rep. 2003;4:374–80.
Fu L, Patel MS, Karsenty G. The circadian modulation of leptin-controlled bone formation. Prog Brain Res. 2006;153:177–88.
Wu X, et al. Circadian mechanisms in murine and human bone marrow mesenchymal stem cells following dexamethasone exposure. Bone. 2008;42:861–70.
Jilka RL, et al. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–46.
Whyte MP, et al. Enzyme-replacement therapy in life-threatening hypophosphatasia. N Engl J Med. 2012;366:904–13.
Schäcke H, Berger M, Rehwinkel H, Asadullah K. Selective glucocorticoid receptor agonists (SEGRAs): novel ligands with an improved therapeutic index. Mol Cell Endocrinol. 2007;275:109–17.
Belvisi MG, et al. Therapeutic benefit of a dissociated glucocorticoid and the relevance of in vitro separation of transrepression from transactivation activity. J Immunol. 2001;166:1975–82.
Schäcke H, et al. Characterization of ZK 245186, a novel, selective glucocorticoid receptor agonist for the topical treatment of inflammatory skin diseases. Br J Pharmacol. 2009;158:1088–103.
Kassel O, Herrlich P. Crosstalk between the glucocorticoid receptor and other transcription factors: molecular aspects. Mol Cell Endocrinol. 2007;275:13–29.
John S, et al. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat Genet. 2011;43:264–8.
Miranda TB, Morris SA, Hager GL. Complex genomic interactions in the dynamic regulation of transcription by the glucocorticoid receptor. Mol Cell Endocrinol. 2013;380:16–24.
Grøntved L, et al. C/EBP maintains chromatin accessibility in liver and facilitates glucocorticoid receptor recruitment to steroid response elements. EMBO J. 2013;32:1568–83.
Siersbæk R, et al. Extensive chromatin remodelling and establishment of transcription factor ‘hotspots’ during early adipogenesis. EMBO J. 2011;30:1459–72.
Biddie SC, et al. Transcription factor AP1 potentiates chromatin accessibility and glucocorticoid receptor binding. Mol Cell. 2011;43:145–55.
Rao NAS, et al. Coactivation of GR and NFKB alters the repertoire of their binding sites and target genes. Genome Res. 2011;21:1404–16.
Uhlenhaut NH, et al. Insights into negative regulation by the glucocorticoid receptor from genome-wide profiling of inflammatory cistromes. Mol Cell. 2013;49(1):158–71.
Rauch A, et al. An anti-inflammatory selective glucocorticoid receptor modulator preserves osteoblast differentiation. FASEB J. 2011;25:1323–32.
Rauner M, et al. Effects of the selective glucocorticoid receptor modulator compound A on bone metabolism and inflammation in male mice with collagen-induced arthritis. Endocrinology. 2013;154(10):3719–28.
De Bosscher K, et al. Selective modulation of the glucocorticoid receptor can distinguish between transrepression of NF-κB and AP-1. Cell Mol Life Sci. 2013. doi:10.1007/s00018-013-1367-4.
Wüst S, et al. Peripheral T cells are the therapeutic targets of glucocorticoids in experimental autoimmune encephalomyelitis. J Immunol. 2008;180:8434–43.
Van Loo G, et al. Antiinflammatory properties of a plant-derived nonsteroidal, dissociated glucocorticoid receptor modulator in experimental autoimmune encephalomyelitis. Mol Endocrinol. 2010;24(2):310–22.
Reber LL, et al. A dissociated glucocorticoid receptor modulator reduces airway hyperresponsiveness and inflammation in a mouse model of asthma. J Immunol. 2012;188(7):3478–87.
Thiele S, et al. Selective glucocorticoid receptor modulation maintains bone mineral density in mice. J Bone Miner Res. 2012;27:2242–50.
Sims NA, Walsh NC. GP130 cytokines and bone remodelling in health and disease. BMB Rep. 2010;43:513–23.
Metcalf D, Nicola NA, Gearing DP. Effects of injected leukemia inhibitory factor on hematopoietic and other tissues in mice. Blood. 1990;76:50–6.
van Bezooijen RL, ten Dijke P, Papapoulos SE, Lowik CW. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev. 2005;16:319–27.
Marenzana M, et al. Sclerostin antibody treatment enhances bone strength but does not prevent growth retardation in young mice treated with dexamethasone. Arthritis Rheum. 2011;63:2385–95.
Bickle M. The beautiful cell: high-content screening in drug discovery. Anal Bioanal Chem. 2010;398:219–26.
Borchert KM, et al. High-content screening assay for activators of the Wnt/Fzd pathway in primary human cells. Assay Drug Develop Technol. 2005;3:133–41.
Acknowledgements
We thank Gillian H. Little, Michael R. Stallcup (University of Southern California) and Tamas Röszer (University of Ulm) for insightful comments. BF, who holds the J. Harold and Edna L. LaBriola Chair in Genetic Orthopaedic Research, acknowledges support from the National Institutes of Health (RO1 DK071122). JT acknowledges support from Deutsche Forschungsgemeinschaft Immunbone (SPP 1468 Tu220/6-2 INST 40/492 SFB 1149, Collaborative Research Centre 1149) and from KaroBioScience Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Frenkel, B., White, W., Tuckermann, J. (2015). Glucocorticoid-Induced Osteoporosis. In: Wang, JC., Harris, C. (eds) Glucocorticoid Signaling. Advances in Experimental Medicine and Biology, vol 872. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2895-8_8
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2895-8_8
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2894-1
Online ISBN: 978-1-4939-2895-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)