Abstract
Unlike other steroid hormone receptors, the glucocorticoid receptor (GR) is not considered an oncogene. In breast cancer, the estrogen receptor (ER) drives cell growth, proliferation, and metastasis, and the androgen receptor (AR) plays a similar role in prostate cancer. Accordingly, treatment of these diseases has focused on blocking steroid hormone receptor function. In contrast, glucocorticoids (GCs) work through GR to arrest growth and induce apoptosis in lymphoid tissue. Glucocorticoids are amazingly effective in this role, and have been deployed as the cornerstone of lymphoid cancer treatment for decades. Unfortunately, not all patients respond to GCs and dosage is restricted by immediate and long term side effects. In this chapter we review the treatment protocols that employ glucocorticoids as a curative agent, elaborate on what is known about their mechanism of action in these cancers, and also summarize the palliative uses of glucocorticoids for other cancers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Glucocorticoids in Cancer
Unlike other highly related nuclear hormone receptors, the glucocorticoid receptor (GR) is not considered an oncogene. In breast cancer, the estrogen receptor (ER) drives cell growth, proliferation and metastasis. The androgen receptor (AR) plays a similar role in prostate cancer. Accordingly, treatment of these diseases has focused on blocking estrogen or testosterone production, or directly blocking steroid binding to their respective receptors. In contrast, glucocorticoids (GCs) work through GR to perform a variety of functions, including arresting growth or inducing apoptosis in lymphocytes. Glucocorticoids are so effective in this role that they are the cornerstone of treatment for all lymphatic cancers, though often hampered by a panoply of off-target consequences. In this chapter, we will review the treatment protocols that employ glucocorticoids as a curative agent, elaborate on what is known about the mechanism of how they function in those cancers, and also summarize the palliative uses of glucocorticoids in treatment of a variety of cancers, and the implications of that use.
Lymphoid Cancers
Glucocorticoids in Childhood Leukemia
Much of what is known about glucocorticoids as a chemotherapeutic, and indeed many other cytotoxic agents, was learned from treatment of childhood leukemia. The predominant role of glucocorticoids in cancer is in the treatment of lymphoid malignancies. Building on an observation that there is an inverse relationship between the size of the adrenal cortex and thymus [1], Dougherty and White demonstrated that administration of ACTH reduced the size of most lymphoid tissue (excluding the spleen) [2]. Around the same time, cells from a tumor of unknown origin, later described to be “lymphosarcoma,” were injected into mice and were found to form tumors. These tumors did not spontaneously regress, but instead shrunk when exposed to Compound E, otherwise known as cortisone [3]. A similar effect was observed in rats [4]. The increased catabolism observed at the time was thought to be responsible for the consumption of the tumor. These findings were rapidly moved to the clinic where is was reported in 1949 that either ACTH or cortisone acetate dramatically reduced the size of lymphoid tumors or leukemias, but not other carcinomas [5]. Emboldened by these findings, much larger studies were undertaken to explore the effect of cortisone and ACTH on hematological disorders. Patients were recruited with lymphoid malignancies, including CLL, lymphoma, Hodgkin’s, acute lymphoblastic leukemia, and multiple myeloma (plasma cell myeloma), as well as the myeloid derived AML and CML. Beneficial effects were observed specifically in lymphoid, but not myeloid disease, ranging from symptomatic relief (multiple myeloma) to complete, but temporary remission in childhood ALL [6]. Despite the effectiveness of GCs in reducing lymphoid disease, as single agents they did not produce durable remissions, much less a cures.
Combination Therapy: Glucocorticoids Have a Central Role
Arround the same time, in the early 1950s, other agents were being tested for their anti-tumorigenic properties. Folic acid had been shown to increase the growth of lymphoid tumors, likely by upregulating amino acid and purine biosynthetic pathways. Much as with GCs blocking these pathways with anti-folates, such as the early aminopterin and later methotrexate, produced, a dramatic, but temporary remission in lymphoid malignancies. A dark, but serendipitous, observation brought another agent to the fore. Exposure to mustard gases in World War I was shown to deplete bone marrow and lymph nodes. The alkylating properties of these gasses were developed into drugs, such as cyclophosphamide, that proved to be potent anti-tumor agents in rodent models and later in human trials. Inhibition of nucleotide synthesis was effective as well, with 6-mercaptopurine showing promise both in acute leukemias and other cancers [7]. Interestingly, although each of these classes of drugs was shown to reduce or even clear the cancerous disease, none of the remissions proved durable.
Three treatment breakthroughs came in the early 1960s. First, vinca alkaloids (later vincristine), isolated from plants, were found to disrupt microtubules and have potent anti-tumorogenic properties [7]. Next, it was discovered that leukemic blasts are unable to make their own asparagine, and thus needed to absorb it from their medium to survive. To exploit this, a component of guinea pig serum, l-asparaginase, was isolated that converts free asparagine to aspartic acid, effectively starving the cells [8]. Perhaps most importantly, in the early 1960s intrepid cancer physicians took the radical step of administering agents in combination. The first successful protocol, called VAMP (vincristine, amethopterin, 6-mercaptopurine, and prednisone), improved. The 5 year survival of children with leukemia from 25 % with single agents to over 60 % [7].
For childhood leukemia, these basic components have evolved over the years to today’s treatment, which is administered in three phases: remission induction, intensification (consolidation), and maintenance. Glucocorticoids can be administered during all three phases, but are used most intensely during remission induction, with the goal of eliminating greater than 99 % of the disease tissue (minimum residual disease, MRD) [9]. During this phase a glucocorticoids are administered with vincristine and asparaginase and are 98 % effective in inducing remission in childhood B-cell leukemias. In intensification, mercaptopurine is combined with polyethylene glycol conjugated asparaginase (pegasparaginase), and methotrexate. Intensification may also include cyclophosphamide or cytarabine (araC) or cycles of reinduction, using the same agents described above. GCs may again be administered during maintenance therapy, though at a lower dose, and less frequently [9]. These advances in treatment have led to cure rates approaching 90 % in children with both B and T-cell acute lymphoblastic leukemia, making it one of the most treatable cancers. In addition, the success of these trials have informed the treatment of other lymphoid cancers, discussed below.
GC Response Predicts Treatment Response
Though only effective as a curative agent in combination, GCs are central to the effectiveness of treatment. This has been best elucidated in European studies from the Berlin-Frankfurt-Muenster (BFM) group, who have developed treatment protocols in parallel with those in the US. This consortium showed that the initial response of infants and children with leukemia to prednisone alone was the best predictor of eventual outcome to full treatment [10, 11]. Importantly, other groups showed that patient response to prednisone, and overall response, could also be predicted by treatment of leukemic blasts ex vivo with GCs [12, 13]. This suggested that the function of GCs in inducing leukemic blast cell death was not necessarily dependent on the environment, but the cell autonomous program initiated by the drug.
The importance of GCs in treatment of leukemias is perhaps best highlighted by comparisons of patient response to dexamethasone and prednisone. Dex and pred are both derivatives of cortisol, with dex differing by addition of a fluorine at the 9α and a methyl at C16. These two differences make dex more specific for GR, with little to no MR activity, and about 10–16× more potent according to established indices [14]. In clinical trials, substituting dex for pred in high risk ALL patients improves outcome by over 10 % (81–94 % overall survival) (COG AALL0232), despite each inducing indistinguishable MRD after induction. This indicates that response to GCs in not only predictive of eventual outcome, but is a major determinant of outcome.
Side and Late Effects
Unfortunately, despite the clear benefits of using high-dose potent GCs in disease treatment, the side effects and potential late effects limit what can be administered. Although dex is much more effective than pred in children, it is only well-tolerated in children under ten. For children over ten, and adults, dex is significantly more likely to cause avascular necrosis (AVN), psychiatric issues, muscle wasting, and mortality [14, 15]. The late effects, or effects that arise years after cessation of treatment, are also a concern. The muscle wasting, osteoporosis and metabolic effects of GCs can persist after treatment ends, and the eventual neuro-psychiatric effects are of concern. More recently it has been shown that pulsing dex during maintenance can produce the same outcomes with more acceptable side effects. Nonetheless, physicians are currently challenged with the choice of which GC to use, what dose, and for how long. A more complete understanding of how GCs function in leukemic blasts and other tissues will help inform these choices.
Mechanism of Action
The biological function of GCs in hematopoietic cells and why they induce cell death of lymphoid cells is not clear. However, seminal work performed by John Ashwell at the NIH indicates that glucocorticoids serve as a negative signal in lymphoid development. By knocking out GR in developing thymocytes he showed that the mice were immunocompromised due to a reduction in T cell repertoire. He further showed that intact glucocorticoid signaling was important for proper T cell selection, perhaps pointing to a role for endogenous GCs in developing lymphocytes [16]. More recently, experiments in mice indicate that GCs may have a similar role in B cells [17].
Although non-genomic effects of GR have been proposed, cell death appears to require GC-induced gene regulation. General blocks of transcription or translation by actinomycin D and cycloheximide, respectively, block GC-induced cell death [18]. In addition, a mutation that weakens DNA-induced GR dimerization and blunts activation also impairs GC-induced cell death. Further, the effect of GCs appears to proceed in two steps; an initial growth arrest that lasts about 24 h, and subsequent cell death. Continuous administration of GCs is required through arrest to induce cell death, which takes 2–3 days more [19]. The most frequently reported mechanism of cell death is apoptosis, though cases of necrosis [20] and necroptosis [21] have also been reported.
The GR-regulated genes that are required to induce apoptosis have not been well defined, but studies on both patient samples and cell lines have lent some insight. The clearest role is in driving apoptosis genes. Activation of GR has been shown to tip the balance of the BH3 domain containing Bcl2 family of apoptotic factors towards apoptosis through activation of pro-apoptotic BIM (BCL2L11), and down regulation of the anti-apoptotic BCL2 [22, 23]. Less directly, GCs consistently upregulate thioredoxin interacting protein (TXNIP), which results in accumulation of reactive oxygen species and apoptosis [24]. Part of the initial growth arrest likely involves regulation of cell-survival genes, such as repression of pro-growth c-MYC and Hexokinase II, and cell cycle genes [19]. Although blocking these pathways does not necessarily block GC induced apoptosis, they may contribute eventually to cell death [25]. There is some evidence that by inhibiting NFκB and growth signaling pathways (e.g. ERK, MEK) GR can arrest cells and induce cell death, though a clear direct mechanism has not been elaborated [24, 26]. In addition, there is now also evidence that GR suppresses miRNA expression, and that repression of miR-17-92 correlates with apoptosis. Thus, although GCs can alter apoptotic and cell survival pathways it is not clear how prevalent either mechanism is in inducing cell death, or whether these pathways are directly regulated by GR.
Glucocorticoid Resistance
Misregulation of apoptotic factors have been implicated in resistance. For example, resistant ALL patients exhibit higher expression levels of the anti-apoptotic genes BCL2 and MCL1 [25, 27, 28], which likely blunt the apoptotic signal affected by GCs. Overexpression of MCL1 has been linked to activation of mTor signaling, and can be alleviated by inhibition of this pathway with rapamycin. On the other side of the coin, activation of Akt stimulates the mTor pathway (as well as perhaps inhibiting GR directly, see below) and makes cells resistant [28]. It has also been observed that a failure of GCs to activate the pro-apoptotic BIM contributes to resistance [29]. Thus, in cells where the balance of Bcl2 family members cannot be sufficiently biased toward pro-apoptosis, GC-induced cell death is likely to be impaired.
In addition to apoptosis, pathway analysis of resistant samples compared to sensitive ones has implicated repression of cell cycle genes [30], and increased carbohydrate consumption through overexpression of associated genes, including carbonic anhydrase 4 (CA4), glucose transporter 3 (GLUT3/SLC2A3), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [27, 31, 32].
More general changes in transcription have been also been linked to GC resistance. For example, ALLs that have translocations in the MLL gene are more likely to not respond to treatment [33]. MLL encodes a histone methyl transferase that methylates lysine 4 of histone H3 and is a mark of active enhancers including response elements and active genes [34, 35]. Translocations that impair the methyltransferase activity of MLL show widespread changes in the chromatin state that are thought to reprogram, and generally downregulate gene expression [36]. In addition, mutational analysis of diagnostic and relapsed patients shows a significant enrichment for transcription factor mutations over other gene sets, again implicating transcriptional defects [37].
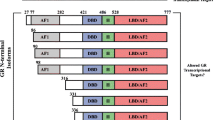
More recently, alterations in GR regulation have been implicated in resistance. Phosphorylation of S134 of GR was shown in to be associated with GC resistance in a T-ALL line (CEM). In a heterologous cell system, AKT phosphorylation of GR at this site appeared to impair translocation, providing a mechanism of resistance [38]. This finding, however, is at odds with another study showing that phosphorylation of S134 is able to translocate to the nucleus, but alters gene expression [39]. In other studies, a failure to increase GR levels through a positive feedback loop has been shown to impair GC induced apoptosis, though how this feedback is disrupted has not been established [40, 41]. Lastly, not all GR isoforms have the ability to induce apoptosis, suggesting that mechanisms that regulate isoform selection may play a role [42].
Adult Acute Lymphoblastic Leukemia
The treatment for adult ALL is modeled on the treatments that are so successful in children. Unfortunately, response rates in adults are significantly worse, with an 80–90 % initial response rate, but only 25–50 % disease free survival after 5 years. Why the response of adults is so much worse is not clear, but has been attributed to two factors. The first is that significantly more mutations accumulate in adult ALL blasts, though few have been directly attributable to treatment response. The second is that adults are not able to tolerate the treatment regimens as well as children. For example, with similar dosing of vincristine, daunorubicin, and dexamethasone during induction, treatment related death is almost 10 % in adults compared to ~1 % in children. To avoid some of these side effects, dex is given in pulses, rather than continuously during induction [43]. Because response rates are worse, bone marrow transplants are often the best route to a durable remission.
Intensification (consolidation) and maintenance are also similar to the children’s protocol. Typical consolidation includes methotrexate, cytarabine, cyclophosphamide, and asparaginase. Clinical trials using hyper CVAD (cyclophosphamide, vincristine, doxorubicin, and dex) have shown somewhat better responses [44]. Maintenance therapy consists of 6-mecaptopurine and methotrexate with monthly pulses of vincristine and prednisone.
Treatment of T-ALLs are similar [43], except for cases with mutation in the NOTCH pathway. Notch is a cell surface receptor, whose intracellular domain is liberated by cleavage with γ-secretase upon ligand binding. This domain translocates to the nucleus and acts as a coactivator of transcription. Activating mutations in NOTCH are found in ~50 % of T cell ALLs and correlate with GC resistance. Administration of γ-secretase inhibitors reverses this resistance, but causes gut toxicity. Fortunately, GCs protect against gut toxicity [45], allowing inhibitors to be used in clinical trials (NCT01088763).
Multiple Myeloma
Multiple Myeloma is the clonal expansion of plasma cells, the mature B cells that emerge from germinal centers. The resulting cells both overpopulate the bone marrow and secrete excessive immunoglobulin, resulting in impaired immune function, renal disease, and bone lesions. At this point, multiple myeloma is not curable, but can be managed with chemotherapy. Like ALL, chemotherapeutic regimens have dramatically improved prognosis, from months in 1950s to 7 years or more for standard risk patients today [46].
The treatment for multiple myeloma has, until recently, involved alkylating agents and glucocorticoids almost exclusively. Like ALL, mustard gases and their derivatives, such as malphalan, were initially used for treatment of the disease. They also induced remission, but at a lower rate (about 1/3 response), and also quickly relapsed. Prednisone also exhibited initial effectiveness, with an average complete response rate of 44 %. In 1969 these two agents were combined to produce a much more robust response rate of 60 %. Unlike ALL, however, these treatments did not fully cure the disease, but improved survival. In the early 1970s, multiple alkylating agents (carmustine, cyclophosphamide, and melphalan) were combined with GCs and vincristine to increase initial response rates, though survival was not improved. Subsequently, dex was combined with doxorubicin and vincristine under the VAD protocol, which was used for years as the main treatment [46]. While these formulations were being used in the clinic, researchers were exploring the use of thalidomide, the teratogenic treatment for nausea and morning sickness used in the early 1960s. In 1990s, thalidomide was shown to have anti-angiogenic properties, including specific action on multiple myeloma. Combination of thalidomide and its derivatives with GCs and cyclophosphamide proved effective in treatment of relapsed multiple myeloma and was installed as the main treatment for most patients [47].
Proteasome inhibitors have also emerged as having activity in myeloma and synergy with GCs [48]. Combination therapy of bortezomib with GCs began in 2003, with other proteasome inhibitors being developed since then that have proven effective both in initial and relapse treatment. These include carfilzomib, which target different proteolytic activities within the proteasome.
Mechanism
Although less is known about how GCs induce cell death in multiple myeloma, there are some clear parallels with their mechanism of action in ALL. First, they modulate the expression of Bcl2 family members, tipping the balance to apoptotis. Second, GCs also inhibit proliferation by suppressing c-MYC. Lastly, it has been shown that GCs also affect the redox balance of multiple myeloma cells, which makes them more susceptible to cell death. GC activation of the transcription factor GilZ has also been implicated in apoptosis. GilZ is regulated by GR in all known tissues, but, in contrast to other tissues, induces apoptosis in multiple myeloma [49].
Resistance to GCs show similarities, but also some differences. Like ALL, activation of Akt attenuates the cytotoxic effects of GCs. However, in multiple myeloma the disease microenvironment shows a clear effect. Secretion of IL6 by either the disease itself or the supporting tissue severely impairs the response of multiple myeloma to GCs and treatment in general, and appears to work though NFκB [50]. Like ALL, unfortunately, not enough is known about the GC-induced program of cell death to account for how resistance arises in most cases.
Hodgkin’s Disease
Hodgkin’s disease occurs within lymph nodes as the clonal expansion of mature B cells. The disease is characterized by starting in one node then spreading systemically. First described in 1832, it was initially treated with radiation. Response was poor, and the treatment was stopped in 1920s. Many of the agents that worked in ALL were tried for Hodgkin’s disease, including alkylating agents such as chlorambucil and cyclophosphamide, vincristine, and glucocorticoids. ACTH and cortisone used as single agents were found to induce remarkable, but temporary remission. It was not until 1967 that an effective combination therapy was formulated. The MOPP protocol included a mustard alkylator, vincristine (oncovin), procarbazine (another alkylator), and prednisone and achieved a 50 % cure rate. When combined with involved field radiation, MOPP produced even better results, with response rates as high as 70 % [43]. The MOPP protocol was unfortunately associated with a number of late effects, including nerve damage, infertility, and secondary malignancies such as acute leukemia. A better tolerated alternative called ABVD (Adriamycin, bleomycin, vinblastine, and dacarbazine) was developed in 1970s, and by 1990s had supplanted MOPP as more effective an better tolerated. Over the last few years, a new protocol, once again involving GCs, was developed called BEACOPP (bleomycin, etoposide, Adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone), and has demonstrated better initial response. This response comes at a cost. First, the BEACOPP treatment has a higher mortality rate, a higher secondary malignancy rate, and sterility risk. Second, the rate of recovery for those who fail to respond or relapse, called the “salvage rate” is lower. When taken into account, the rate of initial response plus the salvage rate for ABVD and BEACOPP are not significantly different [51]. The relative benefits of BEACOPP vs. ABVD are the subject of debate as of the writing of this chapter, though it is clear that ABVD is better tolerated by most patients.
Chronic Lymphocytic Leukemia
Chronic lymphocytic leukemia differs from ALL in that it is a disease of later stage B cells that accumulates over time, rather than as a result of hyperproliferation. CLL cells are refractory to apoptosis, and accumulate in the blood stream, lymph nodes, and bone marrow. The disease becomes pathological when CLL cells crowd out the production of other blood cells. In the early 1950s, the effect of ACTH or GCs on CLL was tested along with several other lymphoid malignancies [52]. When tested as a monotherapy, only ~11 % of patients had even a partial response [53]. Later, when used as part of combination therapies such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), GCs were found to have no effect on eventual outcome while still causing side effects, and were not included in treatment regimens. When high dose prednisone was tested in patients in 1990s, a better initial response was observed, but the response was not durable [53]. The most severe side effects in these studies were opportunistic infections, and have limited the usefulness of GCs in CLL.
More recently monoclonal antibodies (mAbs) have become an essential agent in treatment of CLL. The most common therapy for CLL is FCR which consists of: an alkylating agent, such as cyclophosphamide; flutarabine, a nucleoside analog; and rituximab, a mAb directed against the B cell specific CD20 cell surface marker. This combination therapy has a very high initial response rate (some reports as high as 90 %) with an overall response rate over 50 % [43]. Based on the success of rituximab, other mAbs have been developed, including Ofatumumab (also against CD20), and alemtuzumab (against CD52). Recently, high dose GCs have been combined with mAbs in clinical trials for CLLs refractory to standard therapy. Although overall response rates have been over 50 %, the median progression free survival is less than a year [53].
Use of GCs in CLL is still being considered because of their role not in cell death, but in lymphocyte redistribution. In the late 1940s and early 1950s, patients with CLL treated with ACTH or cortisone experienced a reduction in nodal or splenic tumor masses. Surprisingly, this was accompanied by an increase in the circulating leukocytes, called leukocytosis. It was thought that, in addition to probably modest cell death, GCs induce a redistribution of leukocytes to the blood stream that is reversed upon removal of GCs. This behavior mirrors the normal, circadian redistribution of B cells. Under non-pathological conditions, when GCs are low, B and T cell circulation is high, but when cortisol spikes in the morning, cells home to tissue locations. Why CLL cells would leave tumors is not clear, but GC induced expression of CXCR4 causes B and T cells to enter the bloodstream, and eventually migrate to environments, such as the bone marrow or lymph nodes, that express CXCR12 [54]. This window of time when lymphocytes are circulating provides an opportunity for other agents to attack.
Non-Hodgkin’s Lymphomas
Non-Hodgkin’s Lymphoma (NHL) is the most common hematological malignancy diagnosed in the US. It represents a collection of over 30 subtypes of lymphoid malignancies that are distinct, for the most part, from leukemias in that they are not circulating. The subtypes are distinguished by their lineage, developmental stage, or location. Despite this heterogeneity, most NHLs are treated with a similar protocol that involves GCs [55]. Follicular, Mantle cell, diffuse large B cell lymphoma (DLBCL), and T cell lymphomas are treated with the CHOP protocol, which comprises cyclophosphamide, doxorubicin (hydroxyduanomycin), vincristine (Oncovin), and prednisone. Outcome can be improved in most B cell lymphomas with the addition of the mAb rituximab targeted against the B-cell specific antigen CD20 (R-CHOP). For more advanced case of DLBCL, ACVBP (doxycycline, cyclophosphamide, vincristine, bleomycin, and prednisone) or R-CHOP with etoposide can be used. Higher grade, or more aggressive NHLs are can be treated with hyperCVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone), which is the same combination as CHOP, but with the more potent dexamethasone [43]. As observed in childhood ALL, the upgrade to Dex is clearly more effective, but harbors the risk of more side effects and late effects.
Solid Tumors
Prostate
Prostate cancer afflicts about one in five men in the US, making it their second most common cancer. The growth and proliferation of prostate cancer is driven by androgens, which work through the androgen receptor exclusively. Accordingly, an important part of treatment for prostate cancer is to block production of androgens, typically through castration. Though this is effective in blocking production of testosterone and inducing cancer regression, the cancer often returns in 2–3 years [56]. The relapsed tumor is able to grow in the apparent absence of testosterone, and is termed either castration resistant prostate cancer (CRPC) or Hormone Refractory Prostate Cancer (HRPC). Small molecule inhibitors that block testosterone binding to the androgen receptor have been developed, and can once again induce regression [57]. However, once resistance to these anti-androgens develops, the treatment options for CRPC are significantly less effective. Glucocorticoids are not used in initial therapy, but the glucocorticoid receptor has two opposing functions in anti-androgen refractory CRPC.
Prostate cancer is diagnosed and staged based on biopsies of the prostate gland and invasion to nearby tissues. Concomitant measurement of Prostate Specific Antigen (PSA), a gene driven specifically by AR in the prostate, serves as a marker for activity of AR in the prostate and a potential indicator of PC growth. Although the accuracy of PSA as an indicator of prostate cancer progression or aggressiveness is controversial, it is nonetheless a widely accepted metric of AR activity in the prostate [43]. After successful initial therapy, when the prostate is either removed or regressed by androgen deprivation therapy followed by radiation therapy, PSA levels drop precipitously (normal, though it can vary widely, is ~4 ng/mL in the blood) [58]. During subsequent monitoring, a PSA level doubling time of 15 months or more is not associated with poor outcome, whereas a doubling time of <3 months is an indicator of recurrent disease. Radiographic evidence is more definitive. In these recurrent patients, AR activity is still observed despite low levels (<50 ng/dL) of circulating testosterone. This behavior is indicative of androgen independent activity of AR, hypersensitivity of AR to even low levels of testosterone, or an AR independent mechanism [58].
The former of these two possibilities, androgen independence and hypersensitivity to androgens, often result from overexpression of AR and are difficult to distinguish. One common therapy for such patients is glucocorticoids either alone or combined with other chemotherapeutics, such as paclitaxel or mitoxantrone. The effectiveness of this therapy varies widely, with 20–79 % of patients exhibiting suppressed PSA levels [56].
How patients derive any benefit from glucocorticoids is not clear. The administration of GCs provides a negative feedback on the pituitary gland, suppressing production of adrenal testosterone [59]. Though the adrenal gland is thought to be a minor source of androgens, a decrease may nonetheless provide relief if the patient is androgen hypersensitive. Quite separate from this mechanism, GR may act as a tumor suppressor in PC itself. GR is highly expressed in normal prostate, but often suppressed in PC. Studies in cell line models of PC show that GCs can suppress pro-growth or tumorogenic pathways such as IL-6, NFκB, and MAP and ERK kinases, as well as induce growth arrest through upregulation of TGFβ, p21 and p27 [60, 61]. However, for as many examples of GR regulated genes that potentially block PC proliferation, there are examples of the opposite, such as downregulation of p53 or upregulation of anti-apoptotic S100P. A definitive mechanism for how GCs prevent PC growth and progression awaits further study in primary tissue.
For locally advanced PC, the androgen deprivation therapy in combination with competitive AR blockers and radiation therapy are often used. Goserelin, a gonadotropin releasing hormone (GnRH) agonist, blocks androgen production by interrupting the endogenous feedback loop in the pituitary gland [57]. Androgen analogs, such as bicalutamide and flutamide, then inhibit AR function by blocking the testosterone-binding domain without activating the receptor. Despite the success of this therapy, resistance can emerge, and for those patients second-generation AR competitive antagonists have been developed, including enzaludamide [58]. Two modes of resistance to these second generation inhibitors have been described: mutation of AR and surprisingly upregulation of GR. High GR levels have been observed in PC bone metastases of enzaludamide resistant patients. In a preclinical model of PC in which GR is overexpressed, it was shown that GR could substitute for AR by regulating some of the same genes, including SGK1, STK39, and the PSA gene (KLK3). In all, about 80 % of GR regulated genes overlapped with those regulated by AR. Further, it was shown that GCs could induce growth of these cells in the presence of AR antagonists, and that effect could be blocked by GR antagonists [62]. This recent work suggests that in cells conditioned with anti-androgens, the highly homologous AR and GR can complement each other. The cellular factors that allow this complementation have not been identified, but this dependence on GR function for PC growth suggests that combination therapy with anti-glucocorticoids, such as RU-486, may be useful in resistant PC.
These two examples highlight the potential dangers of using GC therapy in hormone dependent cancers in which the mechanism is not well understood. GCs have a clear though modest, effect on CRPC, with a >20 % response rate. However, GCs can also be AR-like in hormone resistant PCs. Since the cellular determinants of GC action in these two relapsed PCs have not been determined, administering GCs may carry substantial risk.
Kaposi Sarcoma
Kaposi’s Sarcoma is a virally induced cancer that is best known for being activated in patients with HIV/AIDS. Although mostly disfiguring, KS can have cause serious problems, including lymphedema, gastrointestinal blockage, and in rare cases, death. First-line treatment includes ABV (doxorubicin, bleomycin, and vincristine) among several formulations. These treatments manage the disease, but are not a cure.
A retrospective study concluded that the Hodgkin’s formulation of EVAD (etoposide, vincristine, doxorubicin and dexamethasone) (EVAD) was an effective treatment for advanced or relapsed Kaposi’s Sarcoma, though no mechanism was proposed [63].
Cancers Where Glucocorticoids Are Not Used as a Curative Agent
Glucocorticoids are often administered to help patients tolerate treatment, rather than as a chemotherapeutic that targets the cancer itself. Reflective of the biology of glucocorticoids described elsewhere in this book, their palliative effects are diverse. In some chemotherapeutic regimens, for example those that include cisplatin, GCs are first-line antiemetics (see below). For others, such as folate inhibitors, they are used to blunt hypersensitivity, which can result in severe skin rashes (see Table 1.1). Glucocorticoids are used for their anti-inflammatory properties to relieve bone pain other discomfort that may arise from metastatic disease and CNS compression due to metastatic disease. Though effective for these purposes, the use of glucocorticoids in patients with cancer caries some risk of protecting the tumor against chemotherapeutic agents, or even increasing proliferation rates.
Use of GCs as Antiemetics
Chemotherapeutics, radiation, and surgery, all of which are important tools in the fight against cancer, are often poorly tolerated by patients. In addition to their side effects, they can cause severe nausea and vomiting that result in weakness, dehydration, and an unwillingness of patients to continue with therapy. The relative emetic risk of chemotherapeutic agents have been categorized and published by American Society of Clinical Oncology [75]. The categories range from high likelihood (>90 %), with cisplatin being at the top of this range, to minimal likelihood (<10 %), which includes agents such as vincristine and rituximab. The virtual universal reaction of patients to cisplatin, which began being used in 1978, prompted the search for effective anti-emetic agents [76, 77].
There are a number of agents that are used to ameliorate these effects, including: dopaminergic blockers (e.g. metoclopramide); serotonin type 3 (5-HT3) receptor inhibitors antagonists; NK1 receptor inhibitors (aprepitant); and low dose glucocorticoids such as dexamethasone and methylprednisolone. The ASCO guidelines recommend how to administer these classes of drugs. For moderately emetogenic treatments, a combination of dex and 5-HT is recommended. For highly emetogenic agents, such as cisplatin, combinations of GCs, 5-HT, and NK1 inhibitors are recommended. Although the mechanism for dopaminergic blockers, 5-HT, and NK1 inhibitors are well established, how GCs work is not well understood [78].
Some mechanisms for how GCs work have been hypothesized. First, physiological levels of GCs appear to be required for general well-being. Low levels of GC in and of themselves have been linked to nausea and vomiting [79]. Second, the anti-inflammatory actions may be sufficient on some cases. Cyclooxegenase inhibitors or ibuprofen, both of which suppress inflammation, can ameliorate the effects of both radiation and some chemotherapeutic agents [80]. Third, GCs have been shown to reduce 5-HT production, perhaps effectively blocking serotonin receptors in the vagal nerve complexes that transmit the vomiting response [81]. Lastly, GCs inhibit production of prostaglandins and substance P, both of which have been implicated in the vomiting response [80]. Other mechanisms have been suggested as well, such as reducing pain and direct effects on brain centers, but further research needs to be done to uncover which GC effects are most beneficial. In addition GCs are used as appetite stimulants in patients with cancer cachexia [82].
The Future of GCs in Cancer Treatment
GCs are still a critical component of chemotherapy for hematopoietic malignancies. As described above, they are very effective in treatment of lymphoid malignancies, including leukemia, lymphomas, and multiple myeloma, and much work is being done to enhance their effect and overcome resistance. However, the use of GCs as chemotherapeutic agents is considerably limited by their side effects, most prominently osteonecrosis [14]. A good deal of effort has been invested in the development of selective GR modulators (SGRMs, also sometime referred to as SEGRMs)—compounds that work through GR to enhance the beneficial effect and minimize or eliminate the side effects [83]. The prevailing model has been that GCs exert their beneficial effects by repression of genes with side effects resulting from gene activation [84]. Accordingly, the search for SGRMs has been focused on development of what are known as dissociating compounds, or those that selectively only allow GR to repress, but not activate genes. As more has been learned about GR gene regulation in a variety of tissues and conditions, this model has proven too simplistic (though it should be noted that the general trend holds). BIM and BCL2, positive and negative regulators of apoptosis, respectively, are good examples of how this model fails. To induce efficient apoptosis in leukemic blasts, GCs activate BIM, but repress BCL2 [22, 85]. Thus in this tissue, both activation and repression are beneficial. Further, both BIM and BCL2 appear to be similarly regulated in bone, contributing to osteonecrosis [86]. Therefor, for leukemia, a SGRM that represses but doesn’t activate would be a less effective chemotherapeutic because BIM would not be activated. Further, even if a dissociating SGRM was developed to preserve regulation of BIM and BCL2, it would still have severe osteonecrotic side effects.
The future use of SGRMs for treatment of hematopoietic malignancies thus requires a model of GC function with tissue and gene-specific resolution. One method is to first identify genes that are regulated by GR specifically in lymphoid cells that contribute to cell death, but do not perform similar function in bone. Recently, KLF13 was identified as a GR regulated gene that helps coordinate B and T cell development and GC-induced cell death [85]. As this gene does not yet appear to have a function in bone, development of compounds that separate allow KLF13 regulation, but not BIM or BCL2, might allow GC-induced apoptosis in leukemic blasts but not bone. There are other strategies currently under investigation to develop activity and tissue specific GC function. One is to develop a deeper of understanding of not just which genes are regulated, but how they are regulated in different tissues to identify alternative targets that enhance specific GR functions. One key may be in understanding which GR cofactors are used in each tissue. For examples, the GR cofactors NCOA1, 2, and 3, are differentially expressed in tissues [87]. If one, such as NCOA2, is the primary GR cofactor in bone, but not B cells, then it could be targeted to block bone-cell death but still allow GC-induced apoptosis in leukemic blasts. Lastly, it may be possible to develop GCs whose chemical makeup or delivery method partition uptake specifically to lymphoid cells over bone. In this way, the drug would provide selective modulation of GR function at the tissue level, but not at the level of gene regulation. Each of these strategies are the subject of current research efforts.
References
Kendall EC. Hormones. Annu Rev Biochem. 1941;10:285–336.
Dougherty TF, White A. Effect of pituitary adrenotropic hormone on lymphoid tissue. Exp Biol Med. 1943;53(2):132–3. doi:10.3181/00379727-53-14219P.
Heilman FR, Kendall EC. The influence of 11-dehydr0-17-hydroxycorticosterone (compound E) on the growth of a malignant tumor in the Mouse1. Endocrinology. 1944;34(6):416–20.
Murphy JB, Sturm E. The effect of adrenal cortical and pituitary adrenotropic hormones on transplanted leukemia in rats. Science. 1944;99(2572):303. doi:10.1126/science.99.2572.303.
Pearson OH, Eliel LP, Rawson RW, Dobriner K, Rhoads CP. Acth‐ and cortisone‐induced regression of lymphoid tumors in man. A preliminary report. Cancer. 1949;2(6):943–5. doi:10.1002/1097-0142(194911)2:6<943::AID-CNCR2820020602>3.0.CO;2-P.
Pearson OH, Eliel LP. Use of pituitary adrenocorticotropic hormone (ACTH) and cortisone in lymphomas and leukemias. J Am Med Assoc. 1950;144(16):1349–53.
DeVita VT, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68(21):8643–53. doi:10.1158/0008-5472.CAN-07-6611.
Broome JD. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. J Exp Med. 1963;118:121–48.
Pui C-H, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–78. doi:10.1056/NEJMra052603.
Dördelmann M, Reiter A, Borkhardt A, et al. Prednisone response is the strongest predictor of treatment outcome in infant acute lymphoblastic leukemia. Blood. 1999;94(4):1209–17.
Lonnerholm G, Thorn I, Sundstrom C, et al. In vitro cellular drug sensitivity at diagnosis is correlated to minimal residual disease at end of induction therapy in childhood acute lymphoblastic leukemia. Leuk Res. 2009;33(1):46–53. doi:10.1016/j.leukres.2008.06.012.
Hongo T, Yajima S, Sakurai M, Horikoshi Y, Hanada R. In vitro drug sensitivity testing can predict induction failure and early relapse of childhood acute lymphoblastic leukemia. Blood. 1997;89(8):2959–65.
Kaspers GJ, Veerman AJ, Pieters R, et al. In vitro cellular drug resistance and prognosis in newly diagnosed childhood acute lymphoblastic leukemia. Blood. 1997;90(7):2723–9.
Inaba H, Pui C-H. Glucocorticoid use in acute lymphoblastic leukaemia. Lancet Oncol. 2010;11(11):1096–106. doi:10.1016/S1470-2045(10)70114-5.
Teuffel O, Kuster SP, Hunger SP, et al. Dexamethasone versus prednisone for induction therapy in childhood acute lymphoblastic leukemia: a systematic review and meta-analysis. Leukemia. 2011;25(8):1232–8. doi:10.1038/leu.2011.84.
Mittelstadt PR, Monteiro JP, Ashwell JD. Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J Clin Invest. 2012;122(7):2384–94. doi:10.1172/JCI63067.
Gruver-Yates AL, Quinn MA, Cidlowski JA. Analysis of glucocorticoid receptors and their apoptotic response to dexamethasone in male murine B cells during development. Endocrinology. 2014;155(2):463–74. doi:10.1210/en.2013-1473.
Smith LK, Cidlowski JA. Glucocorticoid-induced apoptosis of healthy and malignant lymphocytes. Prog Brain Res. 2010;182:1–30. doi:10.1016/S0079-6123(10)82001-1.
Ploner C, Schmidt S, Presul E, et al. Glucocorticoid-induced apoptosis and glucocorticoid resistance in acute lymphoblastic leukemia. J Steroid Biochem Mol Biol. 2005;93(2–5):153–60. doi:10.1016/j.jsbmb.2004.12.017.
Lambrou GI, Vlahopoulos S, Papathanasiou C, et al. Prednisolone exerts late mitogenic and biphasic effects on resistant acute lymphoblastic leukemia cells: relation to early gene expression. Leuk Res. 2009;33(12):1684–95. doi:10.1016/j.leukres.2009.04.018.
Bonapace L, Bornhauser BC, Schmitz M, et al. Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J Clin Invest. 2010;120(4):1310–23. doi:10.1172/JCI39987DS1.
Ploner C, Rainer J, Niederegger H, et al. The BCL2 rheostat in glucocorticoid-induced apoptosis of acute lymphoblastic leukemia. Leukemia. 2007;22(2):370–7. doi:10.1038/sj.leu.2405039.
Schmidt S, Rainer J, Ploner C, Presul E, Riml S, Kofler R. Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ. 2004;11:S45–55. doi:10.1038/sj.cdd.4401456.
Tissing WJE, den Boer ML, Meijerink JPP, et al. Genomewide identification of prednisolone-responsive genes in acute lymphoblastic leukemia cells. Blood. 2007;109(9):3929–35. doi:10.1182/blood-2006-11-056366.
Sionov RV, Spokoini R, Kfir-Erenfeld S, Cohen O, Yefenof E. Chapter 6 mechanisms regulating the susceptibility of hematopoietic malignancies to glucocorticoid‐induced apoptosis. Adv Cancer Res. 2008;101:127–248. doi:10.1016/S0065-230X(08)00406-5.
Schmidt S. Identification of glucocorticoid-response genes in children with acute lymphoblastic leukemia. Blood. 2006;107(5):2061–9. doi:10.1182/blood-2005-07-2853.
Holleman A, Cheok MH, den Boer ML, et al. Gene-expression patterns in drug-resistant acute lymphoblastic leukemia cells and response to treatment. N Engl J Med. 2004;351(6):533–42. doi:10.1056/NEJMoa033513.
Wei G, Twomey D, Lamb J, et al. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell. 2006;10(4):331–42. doi:10.1016/j.ccr.2006.09.006.
Bachmann PS. Dexamethasone resistance in B-cell precursor childhood acute lymphoblastic leukemia occurs downstream of ligand-induced nuclear translocation of the glucocorticoid receptor. Blood. 2005;105(6):2519–26. doi:10.1182/blood-2004-05-2023.
Cario G, Fetz A, Bretscher C, et al. Initial leukemic gene expression profiles of patients with poor in vivo prednisone response are similar to those of blasts persisting under prednisone treatment in childhood acute lymphoblastic leukemia. Ann Hematol. 2008;87(9):709–16. doi:10.1007/s00277-008-0504-x.
Hulleman E, Kazemier KM, Holleman A, et al. Inhibition of glycolysis modulates prednisolone resistance in acute lymphoblastic leukemia cells. Blood. 2009;113(9):2014–21. doi:10.1182/blood-2008-05-157842.
Buentke E, Nordström A, Lin H, et al. Glucocorticoid-induced cell death is mediated through reduced glucose metabolism in lymphoid leukemia cells. Blood Cancer J. 2011;1(7):e31–9. doi:10.1038/bcj.2011.27.
Chen CS, Sorensen PH, Domer PH, et al. Molecular rearrangements on chromosome 11q23 predominate in infant acute lymphoblastic leukemia and are associated with specific biologic variables and poor outcome. Blood. 1993;81(9):2386–93.
Briggs SD, Bryk M, Strahl BD, et al. Histone H3 lysine 4 methylation is mediated by Set1 and required for cell growth and rDNA silencing in Saccharomyces cerevisiae. Genes Dev. 2001;15(24):3286–95. doi:10.1101/gad.940201.
Milne TA, Briggs SD, Brock HW, et al. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol Cell. 2002;10(5):1107–17.
Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7(11):823–33. doi:10.1038/nrc2253.
Mullighan CG, Phillips LA, Su X, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008;322(5906):1377–80. doi:10.1126/science.1164266.
Piovan E, Yu J, Tosello V, et al. Direct reversal of glucocorticoid resistance by AKT inhibition in acute lymphoblastic leukemia. Cancer Cell. 2013;24(6):766–76. doi:10.1016/j.ccr.2013.10.022.
Galliher-Beckley AJ, Williams JG, Cidlowski JA. Ligand-independent phosphorylation of the glucocorticoid receptor integrates cellular stress pathways with nuclear receptor signaling. Mol Cell Biol. 2011;31(23):4663–75. doi:10.1128/MCB.05866-11.
Gruber G, Carlet M, Türtscher E, et al. Levels of glucocorticoid receptor and its ligand determine sensitivity and kinetics of glucocorticoid-induced leukemia apoptosis. Leukemia. 2009;23(4):820–3. doi:10.1038/leu.2008.360.
Schmidt S, Irving JAE, Minto L, et al. Glucocorticoid resistance in two key models of acute lymphoblastic leukemia occurs at the level of the glucocorticoid receptor. FASEB J. 2006;20(14):2600–2. doi:10.1096/fj.06-6214fje.
Wu I, Shin SC, Cao Y, et al. Selective glucocorticoid receptor translational isoforms reveal glucocorticoid-induced apoptotic transcriptomes. Cell Death Dis. 2013;4, e453. doi:10.1038/cddis.2012.193.
DeVita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s cancer: principles & practice of oncology, vol. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
Thomas DA. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004;104(6):1624–30. doi:10.1182/blood-2003-12-4428.
Real PJ, Tosello V, Palomero T, et al. γ-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med. 2008;15(1):50–8. doi:10.1038/nm.1900.
Rajkumar SV. Multiple myeloma. Curr Probl Cancer. 2009;33(1):7–64. doi:10.1016/j.currproblcancer.2009.01.001.
Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111(6):2962–72. doi:10.1182/blood-2007-10-078022.
Munshi NC, Anderson KC. New strategies in the treatment of multiple myeloma. Clin Cancer Res. 2013;19(13):3337–44. doi:10.1158/1078-0432.CCR-12-1881.
Grugan KD, Ma C, Singhal S, Krett NL, Rosen ST. Dual regulation of glucocorticoid-induced leucine zipper (GILZ) by the glucocorticoid receptor and the PI3-kinase/AKT pathways in multiple myeloma. J Steroid Biochem Mol Biol. 2008;110(3–5):244–54. doi:10.1016/j.jsbmb.2007.11.003.
Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374(9686):324–39. doi:10.1016/S0140-6736(09)60221-X.
Viviani S, Zinzani PL, Rambaldi A. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365(3):203–12. doi:10.1056/NEJMoa1100340.
Rosenthal MC, Saunders RH, Schwartz LI, Zannos L, Perez Santiago E, Dameshek W. The use of adrenocorticotropic hormone and cortisone in the treatment of leukemia and leukosarcoma. Blood. 1951;6(9):804–23.
Smolej L. The role of high-dose corticosteroids in the treatment of chronic lymphocytic leukemia. Expert Opin Investig Drugs. 2012;21(7):1009–17. doi:10.1517/13543784.2012.690393.
Burger JA, Montserrat E. Coming full circle: 70 years of chronic lymphocytic leukemia cell redistribution, from glucocorticoids to inhibitors of B-cell receptor signaling. Blood. 2013;121(9):1501–9. doi:10.1182/blood-2012-08-452607.
Connors JM. Non-Hodgkin lymphoma: the clinician’s perspective—a view from the receiving end. Mod Pathol. 2013;26(s1):S111–8. doi:10.1038/modpathol.2012.184.
Kassi E, Moutsatsou P. Glucocorticoid receptor signaling and prostate cancer. Cancer Lett. 2011;302(1):1–10. doi:10.1016/j.canlet.2010.10.020.
Agarwal N, Di Lorenzo G, Sonpavde G, Bellmunt J. New agents for prostate cancer. Ann Oncol. 2014;25(9):1700–9. doi:10.1093/annonc/mdu038.
Cookson MS, Roth BJ, Dahm P, et al. Castration-resistant prostate cancer: AUA guideline. J Urol. 2013;190(2):429–38. doi:10.1016/j.juro.2013.05.005.
Montgomery B, Mostaghel E, Cheng H, Drechsler J. Glucocorticoids and prostate cancer treatment: friend or foe? Asian J Androl. 2014;16(3):354–8. doi:10.4103/1008-682X.125392.
Yemelyanov A, Czwornog J, Chebotaev D, et al. Tumor suppressor activity of glucocorticoid receptor in the prostate. Oncogene. 2006;26(13):1885–96. doi:10.1038/sj.onc.1209991.
Cavarretta IT, Neuwirt H, Untergasser G, et al. The antiapoptotic effect of IL-6 autocrine loop in a cellular model of advanced prostate cancer is mediated by Mcl-1. Oncogene. 2006;26(20):2822–32. doi:10.1038/sj.onc.1210097.
Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155(6):1309–22. doi:10.1016/j.cell.2013.11.012.
Zhong DT, Shi CM, Chen Q, Huang JZ, Liang JG, Lin D. Etoposide, vincristine, doxorubicin and dexamethasone (EVAD) combination chemotherapy as second-line treatment for advanced AIDS-related Kaposi’s sarcoma. J Cancer Res Clin Oncol. 2011;138(3):425–30. doi:10.1007/s00432-011-1109-7.
Sweeney CJ. Phase II, study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J Clin Oncol. 2006;24(21):3451–7. doi:10.1200/JCO.2005.03.6699.
Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–51. doi:10.1200/JCO.2007.15.0375.
Hanna N. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22(9):1589–97. doi:10.1200/JCO.2004.08.163.
Herbst RS, O’Neill VJ, Fehrenbacher L, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol. 2007;25(30):4743–50. doi:10.1200/JCO.2007.12.3026.
Belani CP, Ramalingam S, Perry MC, et al. Randomized, phase III study of weekly paclitaxel in combination with carboplatin versus standard every-3-weeks administration of carboplatin and paclitaxel for patients with previously untreated advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(3):468–73. doi:10.1200/JCO.2007.13.1912.
Vogelzang NJ. Phase III, study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636–44. doi:10.1200/JCO.2003.11.136.
Ceresoli GL, Phase II. Study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J Clin Oncol. 2006;24(9):1443–8. doi:10.1200/JCO.2005.04.3190.
Gill PS, Tulpule A, Espina BM, et al. Paclitaxel is safe and effective in the treatment of advanced AIDS-related Kaposi’s sarcoma. J Clin Oncol. 1999;17(6):1876–83.
Perez EA, Lerzo G, Pivot X, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2007;25(23):3407–14. doi:10.1200/JCO.2006.09.3849.
Roche H, Yelle L, Cognetti F, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapy. J Clin Oncol. 2007;25(23):3415–20. doi:10.1200/JCO.2006.09.7535.
Thomas E, Tabernero J, Fornier M, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol. 2007;25(23):3399–406. doi:10.1200/JCO.2006.08.9102.
American Society of Clinical Oncology, Kris MG, Hesketh PJ, et al. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol. 2006;24(18):2932–47. doi:10.1200/JCO.2006.06.9591.
Aapro MS, Alberts DS. Dexamethasone as an antiemetic in patients treated with cisplatin. N Engl J Med. 1981;305(9):520.
Rich WM, Abdulhayoglu G, DiSaia PJ. Methylprednisolone as an antiemetic during cancer chemotherapy—a pilot study. Gynecol Oncol. 1980;9(2):193–8.
Chu C-C, Hsing C-H, Shieh J-P, Chien C-C, Ho C-M, Wang J-J. The cellular mechanisms of the antiemetic action of dexamethasone and related glucocorticoids against vomiting. Eur J Pharmacol. 2014;722(C):48–54. doi:10.1016/j.ejphar.2013.10.008.
Hursti TJ, Fredrikson M, Steineck G, Börjeson S, Fürst CJ, Peterson C. Endogenous cortisol exerts antiemetic effect similar to that of exogenous corticosteroids. Br J Cancer. 1993;68(1):112–4.
Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–23. doi:10.1056/NEJMra050541.
Mantovani G, Maccio A, Massa E, Lai P, Esu S. Cisplatin induces serotonin release from human peripheral blood mononuclear cells of cancer patients and methylprednisolone inhibits this effect. Oncol Rep. 1997;4(5):1051–3.
Inui A. Cancer anorexia-cachexia syndrome: current issues in research and management. CA Cancer J Clin. 2002;52(2):72–91.
Quax RAM, Peeters RP, Feelders RA. Selective glucocorticoid receptor modulators: future of glucocorticoid immunosuppressive therapy? Endocrinology. 2011;152(8):2927–9. doi:10.1210/en.2011-1258.
Clark AR, Belvisi MG. Maps and legends: the quest for dissociated ligands of the glucocorticoid receptor. Pharmacol Ther. 2012;134(1):54–67. doi:10.1016/j.pharmthera.2011.12.004.
Jing D, Bhadri VA, Beck D, et al. Opposing regulation of BIM and BCL2 controls glucocorticoid-induced apoptosis of pediatric acute lymphoblastic leukemia cells. Blood. 2014;125(2):273–83. doi:10.1182/blood-2014-05-576470.
Moutsatsou P, Kassi E, Papavassiliou AG. Glucocorticoid receptor signaling in bone cells. Trends Mol Med. 2012;18(6):348–59. doi:10.1016/j.molmed.2012.04.005.
McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108(4):465–74.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Pufall, M.A. (2015). Glucocorticoids and Cancer. In: Wang, JC., Harris, C. (eds) Glucocorticoid Signaling. Advances in Experimental Medicine and Biology, vol 872. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2895-8_14
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2895-8_14
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2894-1
Online ISBN: 978-1-4939-2895-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)