Abstract
The purpose of this chapter is to discuss the ignition characteristics of combustible liquids that are in widespread use as fuels and solvents and are encountered as process fluids in the chemical and process industries. Ignition leads to flaming combustion in which the fuel undergoes a change of state and is converted from liquid to vapor.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The purpose of this chapter is to discuss the ignition characteristics of combustible liquids that are in widespread use as fuels and solvents and are encountered as process fluids in the chemical and process industries. Ignition leads to flaming combustion in which the fuel undergoes a change of state and is converted from liquid to vapor.
Unlike the flaming combustion of solid fuels, this conversion does not involve any chemical change to the fuel molecules that simply evaporate from the exposed surface.Footnote 1 The flammable vapors mix with air to burn as a diffusion flame. When combustible solids exhibit flaming combustion, the change of state from solid to vapor involves chemical decomposition (see Chap. 7). Unlike liquids for which the process of evaporation is reversible (the evolved vapors can be converted back to the original liquid by cooling or by compression), the conversion is irreversible, breaking down the large polymeric molecules of which the solid is composed into fragments that are small enough to vaporize and enter the gas phase. Some solids, such as the thermoplastics (e.g., polypropylene and polystyrene), first soften and liquefy before producing molecular fragments that are small enough to vaporize. Others such as wood do not liquefy but release gases and vapors directly leaving behind an involatile carbonaceous char that, if permitted to do so, will undergo surface oxidation (smoldering) at a much slower rate. As a general rule, fires involving combustible liquids are associated only with flaming combustion, but there are exceptions that will be discussed later.
The underlying physics of the vaporization process for liquids provides a relatively simple key to understanding the conditions under which liquids can be ignited. The vapors from combustible liquids are flammable and exhibit exactly the same properties and behavior as the common flammable gases such as methane and propane (see Chap. 17). Thus, we can identify flammability limits, autoignition temperatures, minimum ignition energies, quenching distances, and so on. Of these, the most important are the flammability limits. If the concentration of vapor above a liquid surface is below the lower flammability limit, then the vapors cannot be ignited, flame will not propagate through the vapor-air mixture, and the liquid will not “burn.” The limiting condition of the liquid at which the vapors are at the lower flammability limit is known as the flashpoint. Experimentally, this can be measured in a closed cup apparatus in which the vapor-air mixture in the closed volume above the surface (the “headspace”) is at equilibrium with the liquid—the vapor will be at a pressure (the saturation vapor pressure) that is defined by the temperature of the liquid. This “closed cup flashpoint” provides us with a relatively simple method of ranking flammable liquids according to the hazard they present in everyday use. In principle, the concept of flashpoint can also be applied to combustible solids, but because the phase change (solid to vapor) is irreversible there is no corresponding simple method for classifying solids according to their ignition hazard. The ignition of solids depends on a large number of factors including the physical form of solid and the mode and intensity of the heat transfer process. Such issues are discussed in Chap. 21.
For combustible liquids, the flashpoint is closely linked to the flammability limits of the vapor. If the liquid is in an unconfined cup or present as a pool, the minimum liquid temperature at which the vapors can be ignited and burn is found to be higher than the “closed cup flashpoint” as defined above and is called the “open cup flashpoint.” The reason for this is simply that the vapors will diffuse away from the liquid surface and for successful ignition from a “pilot” (a small flame or a spark) the pilot must be located in a region where the mixture is flammable. In general, a higher liquid temperature is required to ensure that the pilot is in a flammable zone. However, the “flash” of flame that occurs as flame propagates through the flammable mixture is not necessarily followed by sustained burning of the liquid. A criticality must be exceeded before this will occur. It is only then that the liquid can properly be said to have been ignited to flaming combustion. This is known as the firepoint, which will be discussed in a later section entitled “Measurement of Flashpoint and Firepoint.”
Vaporization of Liquids
The liquids of general interest to the fire protection engineer are those that are stable at normal atmospheric temperatures and pressures (say, 10–30 °C and 101.3 kPa). These include common liquid fuels (such as gasoline and kerosene), many solvents (e.g., acetone, diethyl ether, etc.), some paints and varnishes, and so on. Most are blends, but for convenience and clarity in the following discussion, a one-component system (such as pure n-hexane) will be considered.
The classic phase diagram for a one-component system is shown schematically in Fig. 18.1. The variables are pressure and temperature and the so-called “phase space” is divided into three areas corresponding, respectively, to solid, liquid, and gas (vapor). For a pure compound at constant pressure (illustrated by the horizontal dashed line) we can identify the melting point (T M ) and the boiling point (T B ), which are uniquely defined at any given pressure. The values quoted in the literature refer to normal atmospheric pressure. The upper pair of lines that intersect at the point T in Fig. 18.1 represent equilibrium states between solid and liquid and between liquid and vapor, respectively.Footnote 2
The line (TC) defines how the vapor pressure of the liquid varies with temperature. Thus, for n-hexane at normal atmospheric pressure, T B = 69 °C, which corresponds to the temperature at which the (saturated) vapor pressure is 101.3 kPa. The variation of boiling point with pressure is best illustrated using pure water as the example. At sea level (101.3 kPa) it has a boiling point of 100 °C, but, as with all other liquids, this point decreases with elevation. In Banff, Scotland (at sea level), water boils at 100 °C but in Banff, Alberta (elevation 1463 m), it boils at about 95 °C. On the summit of Mount Everest (8848 m) where the pressure is approximately 33 kPa, or one-third of the value at sea level, it boils at about 72 °C. As will be seen, the flashpoints of combustible liquids also change with a change of atmospheric pressure but for a subtly different reason, as will be discussed later.
On the phase diagram, temperature and pressure may be varied independently provided that only one phase is present: there are two degrees of freedom (i.e., independent variables), which in this case are temperature and pressure. Thus, a gas can be compressed and heated at the same time and still remain a gas (no change in state). The ideal gas law encapsulates this in the equation
where
-
P = Pressure
-
V = Volume
-
T = Temperature (K)
-
n = Number of moles of gas present (mass divided by the molecular weight)
-
R = Ideal gas constantFootnote 3
However, when two phases are present and in equilibrium, corresponding to a point on one of the lines on the phase diagram, then P and T cannot be varied independently without changing the number of phases present. For example, at point B, liquid and vapor are in equilibrium, with the saturated vapor pressure of n-hexane equal to 101.3 kPa (760 mmHg, or 1 bar) at 69 °C. If we increase the temperature, the resulting vapor pressure is defined by the line BC and (unless atmospheric pressure is increased in step) complete conversion of liquid to vapor will occur, and the number of phases present is reduced from two to one as expressed in Gibbs’s phase rule:
where f is the number of degrees of freedom (independent variables), c is the number of components, and p is the number of phases present (e.g., see Moore [2] and Atkins and de Paula [3]). For the one-component system (e.g., pure n-hexane), c = 1, so that when p = 2 (liquid and vapor present) the number of degrees of freedom f = 1. That is, we can change either the temperature or the pressure, but we cannot change them independently without changing the number of phases present. (Note that the intersection of the three lines on the phase diagram marked T is known as the triple point, where the three phases are in equilibrium; that is, p = 3. The number of degrees of freedom is, therefore, zero so that this point is uniquely defined.)
In summary, the lines that divide the phases in Fig. 18.1 represent equilibrium states: the line that separates the liquid and gaseous phases defines how the saturated vapor pressure of the liquid varies with temperature. However, this line does not continue indefinitely but ceases at the critical point marked C. At temperatures and pressures above the critical point, only one phase exists—the distinction between the liquid and gas disappears. Some values of critical temperatures and pressures are given in Table 18.1. A gas such as propane (boiling point −42 °C) is below its critical temperature at ambient temperatures and can be liquefied by pressurization. However, the so-called “permanent gases,” which include oxygen and nitrogen, are above their respective critical temperatures and cannot exist as liquids at ambient temperature (e.g., 25 °C) regardless of the pressure. They are stored under pressure as gases in cylinders, typically at 140 bar. A single phase then exists within the cylinder. The only way that a permanent gas can be stored as a pressurized liquid is to cool it below its critical temperature. Large quantities of natural gas (mainly methane) can be stored economically as a refrigerated (cryogenic) liquid: its critical temperature is −82.3 °C and its normal boiling point is −164 °C as given in Table 18.1.
If a liquid is in an enclosed space, such as a can, tank, or bottle, the vapor will be contained within the headspace and quickly reach equilibrium (i.e., the saturated vapor pressure will be reached). This value is predicted in the phase diagram and is a function of temperature (see Equations 18.3, 18.4, and 18.5). It represents a dynamic state in which vaporization continues but at a rate that is balanced exactly by condensation of vapor back to the liquid state (see Fig. 18.1). For this reason, if the liquid is unconfined (e.g., forming a pool in the open), the liquid will eventually undergo complete evaporation as vapor continuously diffuses away from the surface of the liquid. Consequently, the vapor pressure at the surface will be less than the saturated vapor pressure and equilibrium cannot be achieved. The rate of mass loss by evaporation will be determined by the temperature of the liquid, the exposed area of the pool, and any air movement over the liquid surface (see, for example, Wade [4] and Clancy [5]). Boiling occurs when the vapor pressure is equal to atmospheric pressure, as discussed above.
However, if the liquid is in a closed (sealed) container capable of withstanding high internal pressures, the two phases (liquid and vapor) will remain in equilibrium at temperatures well above the atmospheric boiling point. Thus, propane and butane (which have normal boiling points of −42.1 °C and −0.5 °C, respectively) can be stored as liquids at 25 °C at 9.6 bar and 2.3 bar (957 and 231 kPa), respectively, in appropriate pressure vessels. These pressures correspond to the saturated vapor pressures of these two hydrocarbons at 25 °C. The reduction in volume associated with condensation is very large, making liquefaction a particularly effective means of storing these and similar gases. They can be liquefied simply by compression, although this is not possible with the so-called permanent gases, as discussed above.
Clearly, methane, propane, and butane cannot exist as stable liquids at normal temperatures and pressures. If liquefied methane (at −163 °C) is released from a refrigerated tank and spilled on the ground, it will form a pool and boil vigorously until the surface of the ground has cooled to about −163 °C. Thereafter, it will behave as a stable liquid, evaporating at a rate dictated by the rate of heat transfer from the ground (see Thyer [6]).
Although methane is much lighter than air at ambient temperature, the vapor that evolves from the pool will be initially at −163 °C and much denser than the surrounding air. Consequently, it will spread horizontally until it gains sufficient heat from the surroundings to regain its buoyancy. Although propane is sometimes stored as a cryogenic liquid, propane and butane are more commonly stored in pressure vessels. Catastrophic release due to vessel failure gives rise to a BLEVE (boiling liquid expanding vapor explosion), a term originally coined for a pressure burst of a boiler containing superheated water (see Chap. 66). (It is defined by the Centre for Chemical Process Safety as “an explosion resulting from the failure of a vessel containing a liquid at a temperature significantly above its boiling point at normal atmospheric pressure.” [7]) The liquid boils throughout its volume once the pressure is released and a substantial quantity will convert to vapor. The heat of vaporization is taken from the remaining liquid so that the BLEVE produces a vapor cloud containing a significant proportion of the original mass as liquid droplets. These may fall to the ground, although if ignition occurs (as it will if the pressure burst has been the result of exposure of the vessel to fire), there will be a fireball that will burn out rapidly (see, for example, Abbassi and Abbassi [8]).
Calculation of Vapor Pressure
If the space above the liquid is enclosed (as in a bottle or other container), evaporation will take place until the vapor pressure reaches its saturation value. This equilibrium is described by a form of the Clapeyron-Clausius equation, which gives the saturated vapor pressure (p°) as a function of the temperature of liquid (T K).
where L v is the latent heat of evaporation of the liquid (kJ/kg) and R is the ideal gas constant. The derivation of this expression requires a number of approximations and may be found in most texts on physical chemistry [2, 3]. Integration of the equation gives the vapor pressure as a function of temperature; thus,
or
A plot of ln p° versus 1/T will be a line of slope –L v /R, although it is not strictly linear over an extended temperature range. However, it may be assumed to be linear within the range of temperatures with which we are concerned (i.e., we can assume that L v is constant). Values of L v for a range of liquids are given in Table 18.2.
The expression for vapor pressure is normally given in the form shown in Equation 18.5. The 53rd edition of the CRC Handbook of Chemistry and Physics [13] (and perhaps some later editions) gives an extensive table of data on p°(T), but in a modified form as follows:
where p° is given in mmHg. Values of A and B for some typical liquid fuels are given in Table 18.3 (converting the data from log10 to log e [i.e., ln] and from mmHg to kPa is a hazardous process that has not been attempted here). Vapor pressures may also be calculated from data in Yaws [12].
Example 1
Using the data in Table 18.3, calculate the pressure in a cylinder containing liquid isobutane at 25 °C. How can you determine how much fuel remains in the cylinder after drawing gas from it for a period of time?
Solution
For isobutane, A = 5416.2 K and B = 7.349085. T = 25 + 273 K = 298 K. Substituting these values in Equation 18.4 gives log10(p o) = 3.378, or p o = 2386.7 mmHg, or 3.14 bar. If the temperature remains 25 °C, this pressure will remain unchanged for as long as there is any liquid isobutane left in the container. Pressure is no guide to the amount of isobutane remaining. The only way to determine how much liquid is left is to weigh the container, assuming that you know the tare.
Example 2
Calculate the normal boiling point of n-hexane from the data in Table 18.3, assuming the atmospheric pressure is 760 mmHg.
Solution
Take p o = 760 mmHg, so that log10(760) = 2.881. For n-hexane, A = 7627.2 K and B = 7.717119, so by substitution and rearrangement:
which is about 2 K higher than the measured value quoted in Table 18.2.
Example 3
Calculate the temperature at which the vapor pressure of n-decane corresponds to the lower flammability limit for n-decane vapor. Assume that this vapor pressure is 0.75 % by volume (Table 18.3) and that the atmospheric pressure is 760 mmHg.
Solution
The vapor pressure corresponding to the lower flammability limit of n-hexane is 0.75 % of 760 mmHg, or 5.7 mmHg. The calculation is exactly the same as for the previous example, except that log10(p°) = log10(5.7) = 0.756. Thus
The closed cup flashpoint of n-decane is given in Table 18.2 as 317 K, or 44 °C. Sources of the difference between the calculated flashpoint and that measured in a standard test will be discussed below.
Vapor Pressure of Liquid Blends
Most commonly encountered fuels are in fact blends of different compounds. Gasoline, for example, contains several hundred individual hydrocarbons including a significant proportion of aromatics. The total vapor pressure is the sum of the partial vapor pressures of the individual components, which in turn depend on the concentration of the individual components in the blend. To illustrate how the vapor pressures of the components may be calculated, consider a mixture of two hydrocarbon liquids, A and B. At a given temperature, the partial vapor pressures of components A and B are given by Raoult’s Law [2, 3]:
where x A and x B are the mole fractions of A and B, respectively, given by
and n A and n B are the number of moles of A and B present (i.e., the mass of the component present divided by its molecular weight).
Suppose that A and B are n-hexane and n-decane, respectively, and the mixture is at a temperature of 25 °C. These hydrocarbons form an “ideal mixture” in that the molecules of A and B are so similar that they do not interact with one another, either physically or chemically (i.e., the interactions between A and B are no different from the interactions between A and A, or B and B).
As an example, consider a mixture containing 5 % hexane (by mass) in n-decane. Would its flashpoint be above or below 25 °C? (The flashpoints of n-hexane and n-decane are −29 °C and 44 °C, respectively.) This can be ascertained by calculating the partial vapor pressures of the two components at 25 °C and using Le Chatelier’s Principle (see Chap. 17) to discover if the total vapor pressure (p total = p n-hexane − p n-decane) is above or below the lower flammability limit. The principle states that a mixture of flammable vapors in air will be at the lower flammability limit if
where l i is the percentage composition (molar proportion) of component i in the vapor-air mixture and L i is the corresponding value for the lower flammability limit of component i. To calculate the equilibrium partial vapor pressures of n-hexane and n-decane above a 5 % hexane/95 % n-decane mixture (by mass), the respective mole fractions must be calculated; thus,
where the molecular weights are MW A = 86 and MW B = 142. According to Equation 18.6 and Table 18.3, the partial pressures of n-hexane and n-decane are 10.66 mmHg and 1.65 mmHg, respectively. Using Equation 18.8 with L n-hexane = 1.2 % and L n-decane = 0.75 % (see Table 18.2),
indicating that the mixture is above the lower flammability limit at 25 °C (i.e., the flashpoint of this mixture is below 25 °C).
Example 4
Determine by calculation whether n-decane containing 1 % n-pentane (by volume) would be classified as a Class 1C or a Class II flammable liquid according to the NFPA Standard. [14] (This is equivalent to posing the question, “Is the flashpoint above or below 37.8 °C?”)
Solution
This calculation is identical to that discussed previously, but the densities of the two liquids must be taken into account and the calculation carried out at 37.8 °C. The mixture can be taken as 0.01 × 626 kg of n-pentane + 0.99 × 730 kg of n-decane (where the densities of n-pentane and n-decane are 626 kg/m3 and 730 kg/m3, respectively). The mole fractions are
where now MW A = 72 (the molecular weight of pentane) and MW B = 142. These give x n-pentane = 0.0141 and x n-decane = 0.986. From Equation 18.6 and Table 18.3, the saturated vapor pressures of n-pentane and n-decane at 37.8 °C are 713.11 mmHg and 3.773 mmHg, respectively, so that the partial pressures are 10.05 mmHg and 3.72 mmHg. Applying the Le Chatelier Principle (with the lower flammability limit of n-pentane vapor as 1.4 %),
This is above the lower flammability limit and, consequently, the mixture has a flashpoint below 37.8 °C and is definitely not a Class II liquid. (Further calculation could be carried out to ascertain if the mixture is Class IB or IC; see below.)
This calculation reveals that the partial vapor pressure of the more volatile component can be disproportionately high and for this reason it will evaporate from the mixture much more rapidly than the less volatile component. Consequently, care must be taken when determining the flashpoints of such mixtures. The liquid to be tested should be kept in a closed container and a sample transferred to the flashpoint apparatus as quickly as possible to minimize evaporative loss. In some circumstances, it might be wise to refrigerate the liquid and chill the apparatus. The author has experience of assessing the flashpoint of a sample of crude oil that (without refrigeration) gave a flashpoint of 28 °C, but a flashpoint of 15 °C occurred if the liquid (and the apparatus) was cooled to 0 °C before opening the sample container. (The problem of evaporative loss is also encountered in the more extreme example of trying to identify traces of gasoline or other flammable liquids that may have been used in an arson attack.)
In the examples discussed above, the vapor pressure of liquid mixtures was calculated using Raoult’s Law (Equation 18.7), which applies only to ideal mixtures such as blends of hydrocarbons. It is important to note that many other liquid mixtures, such as alcohol and water, are not ideal as there is some interaction between the molecules of the different components (A and B). Instead of Equation 18.7, it is necessary to use Equation 18.14:
where α A is known as the activity of component A in the mixture, and p o A is the saturation vapor pressure of pure A, and so on. The activity coefficient α A is the product of the mole fraction of A (Equation 18.15) and the activity coefficient γ A :
where γ A is the activity coefficient of component A in the mixture (note that for a pure liquid, γ = 1). For a two-component mixture of A and B, the activity coefficients are given by the Van Laar equations:
Essentially the same set of calculations can be carried out to establish the flammability properties of nonideal mixtures, but the activity coefficients (Equations 18.15 and 18.16) must be calculated from Equations 18.16 using data such as those contained in Table 18.4.
Another more general data set than that given in Table 18.4 is given by Babrauskas [16].
Effect of Atmospheric Pressure on Flashpoint
The calculations that are provided above all refer to the standard atmosphere at sea level where the pressure is 101.3 kPa (760 mmHg), conventionally normalized as 1 bar. If the atmospheric pressure changes, this change has no significant effect on the vapor pressure, which is a function of the temperature of the liquid. At a constant temperature but a reduced pressure, the vapor-air ratio in the headspace will be increased (i.e., it will become richer in fuel). This has significant consequences for liquid fuels because it will reduce the flashpoint.
Consider the following argument. In Example 3, the temperature at which the saturated vapor pressure of n-decane corresponds to the lower flammability limit was shown by calculation to be 45.2 °C, which compares well with the measured value of the closed cup flashpoint (44 °C). At 45.2 °C, the vapor pressure was assumed to be 5.7 mmHg, which is 0.75 % of normal atmospheric pressure (760 mmHg). If the temperature remains the same (45.2 °C) but the pressure is reduced—say to the value appropriate to Denver, Colorado (at 1 mile high, 631 mmHg)—then the volumetric concentration of n-decane vapor in air becomes 5.7/631 = 0.009, or 0.9 %. It has been shown that the lower flammability limit is remarkably insensitive to a reduction in pressure until it falls below 200–300 mmHg (27–40 kPa) [17, 18]. Clearly, at 45 °C the saturated vapor pressure of n-decane is above the lower flammability limit. The effect on the flashpoint can be shown in the following example.
EXAMPLE 5
Calculate the flashpoint of n-decane if measured in Denver, Colorado, where the atmospheric pressure is 631 mmHg. Assume that the lower flammability limit of n-decane vapor is 0.75 %.
SOLUTION
The vapor pressure corresponding to the lower flammability limit of n-hexane is 0.75 % of 631 mmHg, or 4.73 mmHg. The calculation is exactly the same as in Example 3, except that log10(p°) = log10(4.73) = 0.675. Thus,
The value obtained in Example 3 at normal atmospheric pressure was 45.2 °C. The difference is not insignificant and could be very important for liquids close to the boundary between two classifications (see later discussion). The issue becomes more significant at higher altitudes such as Mexico City (2240 m) and Lhasa in Tibet (3650 m). In these cities, the flashpoint of n-decane would be approximately 39.4 °C and 35.9 °C, respectively.
An interesting consequence of this relates to the headspace in the fuel tanks of aircraft. The kerosene grades of commercial aviation fuel have closed cup flashpoints in the range of 35–63 °C [19], as measured at sea level. As an aircraft gains altitude after takeoff, the air pressure in the headspace will fall relatively rapidly, while the fuel will cool rather slowly. There is the potential for the vapor-air mixture in the headspace to become flammable. On long-haul flights, of course, the hazard will be relatively short-lived as the fuel loses heat and cools to below the local flashpoint, relevant to the pressure at cruising altitude. This phenomenon is discussed in NFPA’s Fire Protection Handbook [19].
Measurement of Flashpoint and Firepoint
There are a number of standard tests available for measuring the closed cup [20, 21] and open cup [22, 23] flashpoints (Fig. 18.2). The former measurement is directly related to the lower flammability limit of the fuel vapor and is used to classify liquids according to their ignition hazard [18]. Its relationship to equilibrium vapor pressure of the liquid is discussed in an earlier section.
Four of the commonly used apparatuses for determining flashpoints of flammable or combustible liquids [19]
Closed Cup Flashpoints
In the closed cup test, such as the Pensky-Martens apparatus [20] and the Tag tester [21], the flammability of the saturated (equilibrium) vapor-air mixture in the space above the liquid surface (i.e., the headspace) is tested by introducing a small pilot flame (see Fig. 18.2). The apparatus is designed to allow the miniature explosion within the headspace to vent through an aperture that is opened to admit the pilot ignition source, which also allows the “flash” of flame to be observed. The procedure involves raising the temperature of the liquid slowly from approximately 10–20 K below the anticipated flashpoint at a rate of 5–6 K/min, introducing the ignition source at intervals corresponding to about a 1 °C (1 K) temperature rise. The slow rate of heating is intended to allow enough time for equilibrium conditions to be reached within the headspace (see below). The lowest temperature at which a flash of flame is observed is recorded as the closed cup flashpoint. It is expected that it can be determined to an accuracy of better than ±1 °C for liquids with flashpoints below 100 °C. Values of the closed cup flashpoint for a range of liquids are given in Table 18.2. All refer to standard atmospheric pressure (101.3 kPa). If the closed cup flashpoint is measured when the atmospheric pressure differs from 760 mmHg, the value may be corrected using Equation 18.17:
where T is the measured flashpoint (°C) and P is the ambient atmospheric (barometric) pressure (mmHg). This is intended for relatively small excursions that are commonly experienced on a day-to-day basis. No guidance is given that is relevant to high-altitude locations.
In general, there is reasonable but not exact agreement between measured values and those calculated on the basis that the vapor pressure must correspond to the lower flammability limit. The reason for this may be that the lower flammability limit is based on the ability of a flame to propagate approximately 75 cm inside a vertical tube, 5 cm in diameter [24], whereas the flashpoint is observed as a localized ignition in the vicinity of the ignition source. Similar localized ignition occurs in the flammability limit apparatus but at a concentration of fuel in air that sustains only limited flame propagation. If this explanation is accurate, the “calculated” flashpoint would be expected to be greater than the measured one—as indeed the calculation above shows (Example 3).
Care should be taken when testing liquids of reduced flammability, such as certain chlorinated hydrocarbons. James and Tyler [25] investigated reports of fire and explosions that involved a commercial cleaning fluid, of which the principal component was methyl chloroform (1,1,1 trichloroethane, CCl3CH3). This compound does not give a flashpoint in the standard test, but a flashpoint of 12 °C was recorded in vessels of diameter greater than 12.4 cm [26]. Babrauskas [16] draws attention to a problem with blends containing halogenated components. If these are of high volatility, the blend may give a high flashpoint as a consequence of the inhibiting effect of the halogenated component. However, if this halogenated component is lost as a result of preferential evaporation over a period of time, the effective flashpoint can decrease, which is the reverse of the effect of the preferential loss of lighter hydrocarbons from fuel blends as discussed above.
The closed cup flashpoint is sometimes referred to as the “lower flashpoint.” Although not widely used, this term does emphasize the link to the lower flammability limit of the vapor and allows the concept of the “upper flashpoint” to be introduced. This term corresponds to the temperature at which the vapor concentration in the headspace is at the upper flammability limit, signifying that the mixture will not ignite when an ignition source is introduced, although a weak diffusion flame may exist briefly at the open aperture. Upper flashpoint is seldom measured, although Hasegawa and Takishi [27] have obtained some results in the Setaflash apparatus [16]. It is useful in identifying the temperature range within which the vapor-air mixture in the headspace is flammable. For example, at ambient temperatures, the vapor-air mixture in the headspace of a gasoline tank is well above the upper flammability limit and cannot be ignited. However, the upper flashpoint of the lower alcohols (in particular methanol and ethanol) appears to be in the mid-20s, only 10–15 K or so above the lower flashpoint. This means that at ambient temperatures (say, 15–20 °C) a partially full can of alcohol contains a flammable vapor-air mixture that can be easily ignited. This is a significant hazard that can give rise to serious consequences. For example, if an attempt is made to top-up a conventional flambé lamp directly from the fuel container before the flame has extinguished and if the temperature of the alcohol in the container is between the lower and upper flashpoints, flame will propagate into the container, perhaps causing it to burst or otherwise expel burning liquid. Such occurrences have led to a number of serious accidents in restaurants [28]. Provision of a flame arrester in the opening of the container would prevent such an occurrence.
Open Cup Flashpoints and Firepoints
Open cup flashpoints are not routinely available in the literature although they are clearly relevant to the ignition of open pools of liquid. They are determined using an open cup, the most common of which is the Cleveland apparatus [22] as shown in Fig. 18.2. Instead of the vapor accumulating immediately above the liquid surface, it is lost to the atmosphere by diffusion. Consequently, the concentration of vapor in air deceases with height above the liquid surface. In the standard test, the ignition source (a small diffusion flame at the end of a swivel arm) is moved across the top of the cup, no more than 2 mm above its rim, in a trajectory that carries the flame over the center. The process of heating the fuel is essentially the same as for the closed cup test, but in this case the result is more strongly apparatus dependent. A flash of flame is observed when the ignition source first encounters a mixture at the lower flammability limit. For this reason, the measured open cup flashpoint is very sensitive to the height of the ignition source above the surface. This was demonstrated very clearly by Glassman and Dryer [29], as shown in Fig. 18.3a. Clearly, this measurement is apparatus-specific and cannot provide information about the ignitability of the liquid that can properly be generalized—the flashpoint of an open pool of liquid will depend on the distance the vapor has to travel before meeting a suitable ignition source. Indeed, instead of observing a flashpoint, the liquid may catch fire and continue to burn (i.e., its temperature is above the firepoint [see Fig. 18.3a]).
Open cup flashpoint (o) and firepoint (•) of n-decane as a function of the height of the ignition source above the liquid surface. (a) Flashpoint, revealing how the onset of sustained burning occurs when the temperature of the liquid is above the firepoint (61.5 °C); (b) Firepoint as a function of the height of the ignition source, showing that it is relatively insensitive to heights less than about 9 mm. The arrow shows the height of the ignition source in the standard test [29]
This burning will occur when fuel vapors are being released at a high enough rate to support a diffusion flame. At the flashpoint (closed cup and open cup), the mixture is fuel lean and all the fuel vapor is consumed in the premixed flame. However, if the temperature of the liquid is high enough to produce a fuel-rich vapor-air mixture, a self-sustained diffusion flame becomes possible, as illustrated clearly in Fig. 18.3a, which reveals that a minimum fuel temperature must be achieved for this result to occur. This minimum temperature at which a self-sustaining diffusion flame becomes possible is known as the firepoint. Glassman and Dryer [29] found the firepoint to be much less sensitive to the height of the ignition source, as shown in Fig. 18.3b.
In general, firepoints are not routinely measured and there is not a good database. Some values quoted by Babrauskas [16] are given in Table 18.5. A different selection is given by Kanury [9], but these are all blends that are poorly defined. Typically the firepoint is 10–20 K above the closed cup flashpoint, but one cannot rely on this generalization. The difference appears to be erratic and can be much greater and more uncertain for high-flashpoint liquids (see Babrauskas [16]). The lower alcohols seem to behave in a very different manner. Glassman and Dryer [29] found the open cup flashpoints and the firepoints of methanol and ethanol were equal and—even more surprisingly—considerably less than the closed cup flashpoint. This anomaly disappeared if a spark ignition source was used instead of a flame in the open cup measurement: the open cup flashpoint and the firepoint remained equal but were now higher than the closed cup flashpoint (see Table 18.5). This observation has still to be explained satisfactorily, but clearly the behavior of the alcohols is not typical.
Several attempts have been made to define the firepoint of liquids (and indeed solids) in terms of the heat and mass transfer processes involved in the combustion of the fuel vapors close to the fuel surface. For a diffusion flame to become established at the surface of the liquid, the rate of evolution of flammable vapor must be greater than a certain critical value. It has been argued that it is determined by the need to establish a self-sustaining process whereby the energy required to maintain (and promote) the evolution of vapors comes from the flame by convective and radiative heat transfer. However, if the flow rate of vapors is too small, the flame will be too close to the surface and self-extinguish as a consequence of heat losses to the surface.
Valuable contributions to the definition of firepoint as a criticality have been made by Roberts and Quince [30], Rasbash [31], and Beyler [32]. In particular, they have used Spalding’s B-number, first used to describe the rate of burning of fuel droplets [33], to develop the concept of ignition [30, 31] and extinction [31, 32] criticalities. It is a dimensionless transfer number that can be used to express the conservation of heat (B H ) or mass (B M ), the values of which can be used to define the rates of heat and mass transfer, respectively. They can be expressed as follows:
and
where m og is the mass fraction of oxygen in the atmosphere, m fs is the mass fraction of fuel vapor immediately above the liquid surface, H is the heat of combustion of the fuel vapor, r is the stoichiometric ratio (mass of O2 required to burn unit mass of fuel), c is the specific heat, T g is the ambient air temperature, and T ls is the temperature of the surface of the liquid. B H and B M are assumed equal when the diffusivities of heat and mass are equal (the Lewis number is unity). However, this assumption carries with it the hidden assumption that radiative heat transfer can be ignored and only convection need be considered. For small flames—particularly those associated with the burning of small droplets for which this approach was developed—this approximation is reasonable.
The rate of burning can be expressed as a mass flux (ṁ″, the rate of mass transfer per unit surface area) in terms of the B-number using the following equation:
where h is the (convective) heat transfer coefficient. Following the argument developed by Roberts and Quince [9], which invokes the concept that there is a critical temperature below which a flame will extinguish (see Chap. 5), a critical B-number can be formulated as
where T f,max is the theoretical flame temperature assuming no heat losses to the surface of the liquid, T f,crit is the critical flame temperature below which the flame will extinguish, and T ls is the surface temperature of the liquid—the firepoint temperature. B crit can be calculated from Equation 18.19 for B M , substituting for m fs the mass concentration of fuel vapor above the liquid surface at the firepoint (calculated from the saturation vapor pressure derived from data similar to that contained in Table 18.3), allowing the critical temperature hypothesis to be tested. The theoretical temperature T f,max can be deduced from a heat balance at the surface, assuming that the flame loses no heat to the surface (i.e., it is adiabatic). For a range of fuels (identified in Table 18.2), T f,crit was found to have a mean value of 1350 °C (albeit ±100 K), which is not inconsistent with measured and predicted values for premixed flames close to the lower flammability limit (about 1300 °C) (see Chap. 5).
Observations of the firepoint temperatures of a number of fuels reveal that the saturated vapor pressure at the firepoint is above stoichiometric. Roberts and Quince [9] reported values from 1.33× to 1.92× stoichiometric. Clearly, the mixture immediately above the surface is rich by a significant margin but is still within the flammability range. (Zabetakis [24] has shown that the upper flammability limit is between 2.5× and 4× the stoichiometric concentration.) The firepoint represents a criticality, the rate of evolution of vapors being just sufficient to allow the establishment of a diffusion flame at the surface. It is closely linked to the “quenching distance,” a characteristic of premixed flames that are quenched (extinguished) within 1 or 2 mm of the surface due to heat losses and (probably) the loss of free radicals (see Chap. 12). The flow rate of vapors at the firepoint must be sufficient to allow a nascent diffusion flame to form far enough from the surface so that the quenching process does not occur. The critical flow rate of vapors at the firepoint will, therefore, be given by
ṁ ″ crit has not been determined for any liquid fuels but values have been reported for a range of solids (see Chap. 36).
Classification of Liquid Fuels
Although this chapter is entitled “Ignition of Liquids,” most of the emphasis has been on understanding the flashpoint, the minimum liquid temperature at which the vapor can be ignited. It is clear that it is the firepoint that determines whether or not sustained flaming of the liquid will occur, yet combustible liquids are classified—quite properly—in terms of their flashpoints. Measurement of the closed cup flashpoint provides a method of classifying flammable liquids according to the hazard they represent. Systems of classification have been developed in several countries, but they have as the common basis the need to identify and make provision for those liquids that can be easily ignited at ambient temperatures. Thus, in the United Kingdom under the Highly Flammable Liquids and Liquefied Petroleum Gases (HFL/LP-gas) Regulation 1972 [34], liquids with closed cup flashpoints less than 32 °C were classified as “highly flammable liquids.” NFPA 30, Flammable and Combustible Liquids Code [14], assigns liquids with flashpoints less than 37.8 °C (100 °F) to a similar category, known as Class I. Figure 18.4 compares the U.K. and U.S. systems and shows how the Class I liquids are subdivided into three subclasses A, B, and C. The boundary between I (A and B) and IC is set at 22.8 °C (73 °F), whereas Class IA liquids are distinguished from Class IB in having normal boiling points less than 37.8 °C (100 °F).
“Flammable liquids” (1972 Regulations, U.K.) and Class II liquids (U.S.) have a common upper bound of 60 °C. These are liquids that must be heated significantly above ambient temperature before the evolved vapor can be ignited, although it is clear that some caution is required if ambient temperatures above 32–37.8 °C are encountered. For example, strict application of the U.K. Highly Flammable Liquid Regulations or NFPA 30 would be highly inappropriate in Dubai where the average maximum temperature during the summer months is about 40 °C. Liquids with flashpoints above 60 °C are considered to be relatively “safe” in that significant temperature increases are required before they can be ignited. In the United Kingdom these are called “combustible liquids,” whereas in the United States they belong to Class III (which is further divided into Class IIIA and Class IIIB, as shown in Fig. 18.4).
Within the European Union (EU), new classification systems have evolved following the introduction of new regulations arising from EU Directives. These relate, rather confusingly, to the three main “endpoints” (i.e., storage, supply, or transport, each slightly differentFootnote 4). Over the years, the United Kingdom has modified the HFL/LP-gas regulations to conform to the EU system and as a consequence the 1972 U.K. Regulations have effectively been replaced by the Dangerous Substances and Explosive Atmospheres Regulations (DSEAR 2002) in which the classification system shown in Table 18.6 has been adopted.
This is not the place to attempt to describe the finer points of these classification systems (of which there are many), but it is relevant to draw the reader’s attention to the classification that has recently been adopted for transportation by the United Nations (UN) to facilitate international trade. It defines four categories of liquid, as shown in Table 18.7. This classification is the UN Globally Harmonized System (GHS) and is compared in Fig. 18.4 with the original U.K. (1972 Regulations) and U.S. systems of classification.
Sustained Ignition of Liquids
Provided that the temperature of a liquid is above its firepoint, ignition of the vapors above a pool will be followed by the establishment of a flame at the surface. If the temperature is only slightly above the firepoint, the initial diffusion flame will be weak but will strengthen as it transfers heat to the surface, causing the temperature to rise thus generating an increased rate of supply of fuel vapors. Eventually, a steady-state rate of burning will be achieved, controlled by the heat and mass balance of the surface of the fuel at a temperature close to the normal boiling point. [18] The standard open cup tests are designed to heat the liquid uniformly (bulk heating), but in practice such bulk heating is difficult to achieve unless the liquid is used in some process that requires an elevated temperature. An obvious example is the deep fat fryer and similar devices used in cooking, but oils and other high-flashpoint liquids may be used in heat exchangers and other devices at temperatures above their firepoints. If they are released, then in principle a small ignition source may ignite the vapors and cause a fire. This form of ignition is known as piloted ignition: spontaneous ignition, or “autoignition,” is discussed below.
A pool of a high-firepoint liquid at ambient temperature is difficult to ignite by means of a locally applied ignition source, such as a flame. If a flame is applied to the surface (e.g., using a blowtorch), convection currents are established that remove hot liquid from the point of flame application, replacing it with cooler (cold) liquid from below. This process occurs because of a surface tension–driven flow at the surface, first identified by Sirignano and Glassman [37] in their study of flame spread over liquid surfaces. It is a consequence of the fact that surface tension decreases with temperature so that there is a net force at the surface that draws the hotter liquid on the surface at the point of flame impingement toward the cooler regions. The movement at the surface created by this force effectively disperses the heat transferred to the surface and delays ignition. However, if the liquid is absorbed onto a porous substrate—a “wick”—then application of an ignition source in the form of a small flame will quickly raise the temperature of the liquid to its firepoint and burning will be established. There are two factors that contribute to this behavior: first, the liquid is held as a thin film on the material of the wick and surface tension–driven flows are suppressed; and, second, most wick materials have low thermal conductivities and insulate the liquid from heat loss through the wick (by conduction). The best-known example of the effectiveness of a wick in promoting ease of ignition is with the common candle. The wax melts at a relatively low temperature (about 100 °C), but the wick holds the molten wax in place, permitting easy ignition by a match flame. The amount of energy required has never been measured, but in principle it could be calculated assuming the liquid film acts as a thermally thin “solid.” However, the heat transfer characteristics of such ignition sources are difficult to quantify and the calculation would be rather academic.
Burgoyne and Roberts [38] studied the ignition of pools of high-flashpoint liquids from flames established on wicks that dipped into the liquid at one end of a 0.4-m-long tank. They found that ignition of the pool would occur after a delay (induction period), the length of which was determined by the depth of the pool. In this case, ignition of the pool comprised gradual heating of the surface layers by heat transfer from the flame on the wick until the firepoint temperature was achieved in the vicinity of the wick, after which the flame would spread over the surface of the pool. Minimum induction periods were observed for layers less than 5 mm, increasing by as much as an order of magnitude as the depth was increased to 8–10 mm, depending on the fuel. As an illustration, Fig. 18.5 shows the results obtained by Burgoyne and Roberts for three alcohols [38]. There appears to have been no further systematic studies of this mode of ignition, but it is reasonable to assume that the time to full ignition of a pool of liquid fuel will depend both on the firepoint of the liquid and the depth of the pool.
Spread of flame over the surface of a liquid from a flame established on a wick at one end of a pool contained in a long trough [38]. Effect of liquid depth on the duration of the induction period (the time taken for flame to begin to spread over the surface). The open cup flashpoints of hexanol, isopentanol (aka isoamyl alcohol) and butanol, are 74 °C, 46 °C and 43 °C, respectively. (The firepoints are not available)
It is appropriate to note at this point that high-flashpoint liquids (both “flammable” and “combustible” according to the U.K. classification system as shown in Fig. 18.4) can be ignited very easily if dispersed in air as a spray or mist. This behavior is analogous to a dust explosion, which involves dispersion of fine solid particles in air (see Chap. 70). The concept of flammability limits applies and in all other respects a mist of combustible liquid exhibits the same properties as a flammable vapor-air mixture. Spray ignition is discussed in the review by Aggarwal [39].
Autoignition
If the temperature of a flammable vapor-air mixture is increased sufficiently, it can ignite spontaneously without the introduction of a source of ignition—a “pilot”—such as a flame or spark. The concentration of fuel in air at which the autoignition temperature is a minimum corresponds to the stoichiometric vapor-air mixture, which is known to be the most reactive [24]. Values of the (minimum) autoignition temperatures (AIT) of a large number of liquid fuels (gases and liquids) are given in Table 18.2. These have been obtained in a standard test using the Setchkin apparatus [40, 41]. The measurement is made (for liquids) by dropping a small quantity of the liquid (0.1 ml) into a 500 ml spherical flask that has been heated to a carefully controlled temperature [18, 41]. If ignition is not observed within 10 min, the experiment is repeated at higher temperatures. The minimum autoignition temperature (AIT) is determined by repeated experiments, “bracketing” the final value. The values of AIT quoted in Table 18.2 were obtained in this way, but in fact these measurements are highly apparatus dependent. Lower values of AIT are found if the test is carried out in a larger vessel, specifically with a lower surface-to-volume ratio (Table 18.8). This is entirely consistent with the theory of spontaneous combustion (see Chap. 20).
However, the Setchkin test [40] provides data on autoignition under highly idealized conditions in which the liquid vaporizes inside an enclosed volume at a uniform temperature. A relatively uniform fuel/air mixture will be formed that will undergo autoignition if the temperature is high enough. However, this scenario is rarely encountered in practice: more commonly, a liquid fuel may come into contact with a hot surface, such as a bearing assembly or an exhaust manifold, which is exposed to the open atmosphere. The liquid evaporates from the hot surface and the vapor mixes with air at ambient temperature, or at a temperature that is significantly lower than that of the hot surface. Under these circumstances, the minimum AIT derived from the Setchkin test is not relevant as neither the temperature nor the concentration of vapor in air will be uniform. Logically one would expect the autoignition process to take place where the fuel concentration is at or close to stoichiometric (the most reactive mixture) and the local temperature is sufficiently high. To achieve this, the surface temperature will have to be much higher than the quoted literature value of the corresponding AIT.
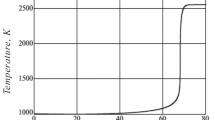
If the boiling point of the liquid is lower than the AIT (e.g., for n-decane, these temperatures are 174 °C and 201 °C, respectively), then spilling the liquid onto a hot, open surface cannot give rise to autoignition because the vapor cannot be at a temperature greater than the boiling point of the liquid. The situation would be different for a combustible liquid with a boiling point much higher than the AIT. Paraffin wax (a solid at room temperature but melting at temperatures less than 100 °C) boils at >370 °C, but the AIT is quoted as 245 °C. In principle, if the conditions are right, the hot vapor produced when paraffin wax is dropped onto a hot surface (>370 °C) may autoignite if a flammable vapor/air mixture is formed near the surface at a sufficiently high temperature (certainly >245 °C). However, the temperature of the surface at which autoignition will occur cannot be defined uniquely. Colwell and Resa [42] studied the autoignition of a range of liquid fuels and engine lubricants when single drops fell on to a flat, heated plate. It was necessary for them to present their results in terms of the probability of ignition, carrying out a large number of tests over a range of plate temperatures. Jet A fuel (similar to kerosene) did not autoignite at temperatures below 510 °C, despite the fact that the AIT (of kerosene) as measured in the Setchkin Test is quoted as 233 °C (see Table 18.8). If the plate temperature was 583 °C, the ignition probability was 50 %, increasing to 100 % above 650 °C. The probability distribution for kerosene and other fuels and lubricants, as determined by Colwell and Resa, is shown in Fig. 18.6. Similar studies, with similar conclusions, have been carried out by Shaw and Weckman [43] for diesel fuels and Davis et al. [44] for high performance fuels used in motorsports. Note that these results are specific to a flat surface, 0.579 m by 0.108 m which is horizontal and unconfined: the distribution will shift to lower temperatures if the surface is confined, or significantly profiled, or to higher temperatures if the surface is vertical or significantly smaller in area.
Ignition probability as a function of the surface temperature of a flat plate for aviation fluids [42]. With kind permission from Springer Science+Business Media B.V.
If a high-boiling point liquid (such as cooking oil) is overheated, autoignition can occur, with flame appearing spontaneously in the plume of hot vapor rising from the surface. As the liquid is already very hot (perhaps close to its boiling point), the fire that follows will immediately be burning at or close to its maximum rate. This type of behavior can be demonstrated using corn oil in a Cleveland open cup and is likely to be the cause of flaming fires in deep fat fryers. This cannot occur with low-boiling liquid fuels such as gasoline (as explained above), which accounts for the fact that if gasoline is spilled on to a hot exhaust, autoignition will not occur, although ignition will occur if there is a pilot source (e.g. a spark or flame) nearby. On the other hand, heavier fuels and lubricating oils (with high boiling points) may autoignite under these circumstances. There has been much interest in this issue [16].
Ignition of Liquids in Porous Materials
This chapter has so far dealt with the ignition of liquids, leading either to a transient premixed flame or to sustained, flaming combustion. However, under the right conditions, liquid fuels with high boiling points can undergo a form of smoldering combustion, although this form of combustion is normally associated with combustible solids that yield a solid char on heating (see Chap. 19). Clearly, a bulk liquid cannot undergo charring, but if it is dispersed as a thin film within a porous substrate (such as an insulating material) and provided it is of low volatility, it may undergo a slow exothermic reaction that leaves a charlike residue on the fibers of the substrate. The process starts insidiously, by a self-heating process in which the liquid reacts heterogeneously with oxygen from the air. If there is sufficient insulation, the rate of heat released by this process will be greater than the rate at which it can be removed and the temperature will rise. The phenomenon of self-heating in solids is discussed at length in Chap. 20.
The best-known example of self-heating (and spontaneous combustion) of liquids involves the so-called “drying oils,” such as linseed oil and tung oil. To quote Bowes [45], “The risk of self-heating and ignition in textile fibres impregnated with readily oxidisable oils has been recognised for a long time,” citing a paper by Hapke that refers to such incidents dating back to the eighteenth century. Self-heating of some of the vegetable oils that were used in wool blending in the nineteenth century were known to be the cause of fires and for this reason a test was devised in the United Kingdom to identify the oils that were particularly hazardous (the Mackey Test [45, 46]). The most hazardous are the unsaturated vegetable oils containing a high proportion of carbon–carbon double bonds, which are prone to oxidation. The proportion of these unsaturated linkages can be quantified in terms of the “iodine number,” which is effectively the percentage of iodine absorption due to the reaction of iodine at the carbon–carbon double bonds. The higher the iodine number, the greater the propensity of the oil to exhibit self-heating when dispersed onto rags, and so on. Typical ranges of values are shown in Table 18.9 (extracted from Bowes [45]).
The most hazardous oils can self-heat when dispersed onto quite small amounts of material—as low as 25 g has been reported. This means that rags used to apply linseed oil or tung oil–based finishes to wood surfaces can self-heat if they are not disposed of properly. The less reactive oils will present problems if dispersed on much larger quantities of porous material, such as bales of wool. Qualitative information on a wide range of liquids may be found in NFPA’s Fire Protection Handbook® [47], which provides a list of materials that are subject to spontaneous heating. This information has been extracted and is presented in Table 18.10.
Under the right conditions, self-heating of these dispersed liquids will lead to the onset of smoldering combustion. The self-heating process is a slow oxidation involving the carbon–carbon double bonds, resulting in changes to the structure of the absorbed liquid. The chemical mechanism has not been studied in detail, but the consequence appears to be that char is produced on the fibers, which can then oxidize more vigorously, producing a smolder that has the potential to undergo transition to flaming. The smolder will involve the substrate if it is combustible (e.g., cotton rags, wool, etc.), but it is not necessary for the substrate to be combustible, at least for the more reactive oils.
Another situation in which liquids can initiate self-heating, leading to spontaneous fires, is in the case of oil-soaked lagging [45, 48]. This situation is encountered in industrial plants where thermal insulation is provided around vessels and pipework to conserve heat and maintain process fluids at high temperatures. If the fluid leaks into the insulation, it will be dispersed through the open structure of the insulant. If the leak is too large, then the material will become logged with the fluid and self-heating will not occur. However, if the leak is small, then the fluid will become dispersed onto the fibers of the insulation material, creating a large area of fuel exposed to air within the interstices of the material—an ideal situation to promote self-heating, particularly as both the fluid and the insulation are already hot. The volatility of the fluid is important here: if at the relevant temperature its vapor pressure is too high, then the oxygen concentration in the pores of the insulation may be too low to allow the self-heating process to develop sufficiently.
There have been studies of the propensity of different fluids to undergo self-heating when dispersed on porous insulation materials and an empirical expression has been derived to assess the hazard. This is reported by Lindner and Seibring [49] and developed by Britton [50]. Self-heating may be an issue if
where AIT is the autoignition temperature and FP is the (closed cup) flashpoint. Although it may seem illogical to incorporate AIT (which refers to autoignition in the gas phase) into a correlation that deals with a heterogeneous process, the significance of AIT is that it is a measure of the reactivity of the fuel. FP is also associated with a gas-phase process, but in this expression, it is a surrogate for a measure of the volatility of the fuel as it correlates with the boiling point (see, for example, Babrauskas [16]). A “reactive fuel” (low AIT) of low volatility (high FP) will, therefore, give a high value of AIT/(AIT – FP)—consistent with the above inequality. However, this is at least two steps removed from understanding the process involved. The theory of self-heating is described in Chap. 20, but it is only recently that the parameters relevant to understanding lagging fires have been examined in detail [51].
Summary
Flammable and combustible liquids present a range of fire hazards in our everyday lives, whether it be in industry, commerce, or the home. In this chapter, an attempt has been made to outline the fundamental parameters that determine the flammability of liquids, bearing in mind that the hazard may present itself in a number of ways: as an unconfined pool of liquid, as a dispersion of droplets, or as liquid absorbed on a porous substrate. The simplest way of classifying the flammability of a liquid is in terms of its flashpoint, the lowest temperature at which the saturated vapor pressure of the liquid corresponds to the lower flammability limit of the vapor in air at normal atmospheric pressure. The classification schemes used in the United States and the United Kingdom are based simply on the flashpoint, but it is important to recognize that the flashpoint temperature decreases with atmospheric pressure, while the actual flashpoint is not relevant if the liquid is presented to an ignition source as a mist or spray. Indeed, liquids with flashpoints above 300 °C may be ignited by a small flame or a spark if they are dispersed in droplet form.
A distinction must be made between flashpoint, as defined above, and firepoint. The latter refers to the lowest temperature at which a liquid will continue to burn following ignition of the vapors by a pilot ignition source. It is higher than the flashpoint, generally by 10–20 K. Values of autoignition temperature (AIT) are quoted in the literature, but these are not related to either the flashpoint or the firepoint. The AIT represents the lowest temperature at which flame develops spontaneously in a uniformly heated closed vessel into which a small sample of liquid is introduced. This idealized configuration must be taken into account when “autoignition” of liquids in practical situations is considered (e.g., spilling gasoline onto a hot exhaust).
If a combustible liquid is absorbed into a porous medium, such as a rag or the insulation around a hot pipe, then it may undergo a self-heating process leading initially to a smoldering-type reaction. Reactive drying oils such as linseed will undergo this process at ambient temperatures and can lead to flaming combustion under suitable conditions. Similarly, hot process fluid contaminating the insulation around pipework in industrial plants can lead to “lagging fires,” which are difficult to control. The hazard associated with liquids under these conditions is poorly understood, which makes awareness of the problem even more important.
Notes
- 1.
There are exceptions to this generalization. High molecular weight liquids with high flashpoints (e.g., cooking oil, flashpoint 321 °C) will be undergoing some chemical decomposition at temperatures associated with vapor formation.
- 2.
The third line in Fig. 18.1, below the intersection at T, represents the equilibrium states between solid and vapor. Solid converts directly to vapor by the process of sublimation. It will not be considered further here.
- 3.
The numerical value of R depends on the units used for P and V (see Chap. 5).
- 4.
Storage: “The Highly Flammable Liquids and Liquefied Petroleum Gases Regulations (HFL/LP-gas)”; Supply: “The Chemical (Hazard Information and Packaging for Supply) Regulations (CHIPS)”; and Transport: “The Carriage of Dangerous Goods and Use of Transportable Pressure Equipment Regulations (CDG).”
References
D.R. Lide (ed.), Handbook of Chemistry and Physics, 80th ed., CRC, Boca Raton, FL (2001/2002).
W.J. Moore, Physical Chemistry, 5th ed., Longman, London, UK (1972).
P.W. Atkins and de Paula, Physical Chemistry, 8th ed., Oxford University Press, Oxford, UK (2006).
S.H. Wade, “Evaporation of Liquids in Currents of Air,” in Proceedings of the Institution of Chemical Engineers, Institution of Chemical Engineers, Rugby, UK (Jan. 1942).
V.J. Clancey, “The Evaporation and Dispersion of Flammable Liquid Spillages,” in Proceedings of the Symposium on Chemical Process Hazards, University of Manchester, Institution of Chemical Engineers, Rugby, UK (1974).
A.M. Thyer, “A Review of Data on Spreading and Vaporization of Cryogenic Liquids Spills,” Journal of Hazardous Materials, 99, pp. 31–40 (2003).
Center for Chemical Process Safety, “Guidelines for Evaluating the Characteristics of Vapor Cloud Explosions, Flash Fires and BLEVEs,” AIChE, New York (1994).
T. Abbassi and S.A. Abbassi, “The Boiling Liquid Expanding Vapor Explosion (BLEVE): Mechanism, Consequence, Assessment, Management,” Journal of Hazardous Materials, 141, pp. 489–519 (2007).
A.M. Kanury, “Ignition of Liquid Fuels,” in SFPE Handbook, 3rd ed., National Fire Protection Association, Quincy, MA, pp. 2-188–2-199 (2002).
E.W. Washburn (ed.), International Critical Tables of Numerical Data: Physics, Chemistry and Technology, McGraw-Hill, New York (1927).
Factory Mutual System, Handbook of Industrial Loss Prevention, Factory Mutual Research Corporation, Norwood, MA (1968).
C.L. Yaws (ed.), Chemical Properties Handbook: Physical, Thermodynamic, Environmental, Transport, Safety and Health Related Properties for Organic and Inorganic Chemicals. McGraw Hill, New York (1999).
R.C. Weast (ed.), Handbook of Chemistry and Physics, 53rd ed., CRC, Cleveland, OH (1972/1973).
NFPA 30, Flammable and Combustible Liquids Code, National Fire Protection Association, Quincy, MA (2008).
P.F. Thorne, Fire Research Note 1022, Fire Research Station, Borehamwood, UK; P.F. Thorne, “Flashpoints of Mixtures of Flammable and Non-Flammable Liquids,” Fire and Materials, 1, pp. 134–140 (1976).
V. Babrauskas, Ignition Handbook, Fire Science Publishers, Issaquah, WA (2003).
B.P. Mullins and S.S. Penner, Explosions, Detonations, Flammability and Ignition, Pergamon Press, London, UK (1959).
D. Drysdale, Introduction to Fire Dynamics, 3rd ed., John Wiley and Sons, New York (2011).
NFPA, Fire Protection Handbook, 20th ed., National Fire Protection Association, Quincy, MA, pp. 21-135–21-149 (2008).
American Society for Testing and Materials, “Standard Test Method for Flashpoint by the Pensky-Martens Closed Tester,” ASTM D-93-94, ASTM, West Conshohocken, PA (1994).
American Society for Testing and Materials, “Standard Test Method for Flashpoint by the Tag Closed Tester,” ASTM D-56-87, ASTM, West Conshohocken, PA (1987).
American Society for Testing and Materials, “Standard Test Method for Flashpoint and Firepoint by the Cleveland Open Cup,” ASTM D-92-90, ASTM, West Conshohocken, PA (1990).
American Society for Testing and Materials, “Standard Test Method for Flashpoint and Firepoint of Liquids by the Tag Open Cup Apparatus,” ASTM D-1310-86, ASTM, West Conshohocken, PA (1986).
M.G. Zabetakis, “Flammability Characteristics of Combustible Gases and Vapours,” Bulletin 627, U.S. Bureau of Mines, Washington, DC (1961).
J.J. James, “A Method for Flammability Testing of Low Flammability Liquids,” MSc Thesis, University of Manchester, Manchester, UK (1991).
B.J. Tyler, personal communication.
K. Hasegawa and K. Kashuki, “A Method for Measuring the Upper Flashpoint—Practical Method Using the Setaflash Closed Cup Apparatus,” Report of the Fire Research Institute No. 71, Fire Research Institute, Tokyo, Japan (1991).
H. Mundwiler, “Brand im Hotel International Zürich, 16. Februar 1988”/“Incendie à l’Hôtel International, Zurich, 16 février 1988”; Schweizerische Feuerwehrzeitung/Journal des Sapeurs-Pompiers Suisses/Giornale dei Pompieri Svizzeri, 116, 3, pp. 144–162 (Mar. 1990).
I. Glassman and F.L. Dryer, “Flame Spreading Across Liquid Fuels,” Fire Safety Journal, 3, pp. 123–138 (1980/1981).
A.F. Roberts and B.W. Quince, “A Limiting Condition for the Burning of Flammable Liquids,” Combustion and Flame, 20, pp. 245–251 (1973).
D.J. Rasbash, “Relevance of Firepoint Theory to the Assessment of Fire Behavior of Combustible Materials,” International Symposium on Combustible Materials, Edinburgh University, Edinburgh, Scotland (1974).
C.L. Beyler, “A Unified Model of Fire Suppression,” Journal of Fire Protection Engineering, 4, pp. 5–16 (1992).
D.B. Spalding, “Some Fundamentals of Combustion,” Butterworths, London, UK (1957).
Highly Flammable Liquids and Liquefied Petroleum Gases Regulations, Her Majesty’s Stationery Office, London, UK (1972).
Chemicals (Hazard Information and Packaging for Supply): Regulations 2002, HSE Booklet L131, Health and Safety Executive, London, UK (2002).
Globally Harmonised System of Classification and Labelling of Chemicals (GHS), 1st rev. ed., UN Publication ST/SG/AC. 10/30/Rev.1, United Nations, Geneva, Switzerland (2005).
W.A. Sirignano and I. Glassman, “Flame Spreading Above Liquid Fuels: Surface Temperature Driven Flows,” Combustion Science and Technology, 1, pp. 307–312 (1970).
J.H. Burgoyne and A.F. Roberts, “Spread of Flame Across a Liquid Surface Part 2,” Proceedings of the Royal Society, London, A308, pp. 55–68 (1968).
S.K. Aggarwal, “A Review of Spray Ignition Phenomena: Present Status and Future Research,” Progress in Energy and Combustion Science, 24, pp. 565–600 (1998).
American Society for Testing and Materials, “Standard Test Method for Autoignition Temperature of Liquid Chemicals,” ASTME, 659–78 (Reapproved 2000) (1978).
N.P. Setchkin, “Self-Ignition Temperatures of Combustible Liquids,” Journal of Research National Bureau of Standards, 53, pp. 49–66 (1954).
J.D. Colwell and A. Reza,. “Hot surface ignition of automotive and aviation fluids” Fire Technology 41 105–123. (2005).
A Shaw and E Weckman, “Evaluation of the ignition of diesel fuels on hot surfaces” Fire Technology 46 407– (2010).
S. Davis, S Kelly and V Somandepalli, “Hot surface ignition of performance fuels” Fire Technology 46 363–374 (2010).
P.C. Bowes, “Self-Heating: Evaluating and Controlling the Hazards,” HMSO, London (1984).
W. McD Mackey, Journal of the Society of Chemical Industry, London, 14, p. 940 (1895); 15, p. 90 (1896).
National Fire Protection Association, Fire Protection Handbook, 20th ed., Table 6.17.11 “Materials Subject to Spontaneous Heating,” National Fire Protection Association, Quincy, MA (2008).
P.C. Bowes, “Fires in Oil-Soaked Lagging,” BRE Current Paper, CP 35/74, Fire Research Station, Borehamwood, UK (1974).
H. Lindner and H. Seibring, “Self-Ignition of Organic Substances in Lagging Material,” Chemie Ingenieur Technik, 39, p. 667 (1967).
L.G. Britton, “Spontaneous Insulation Fires,” AIChE Loss Prevention Symposium, San Diego, CA (August 19–22, 1990).
A.C. McIntosh, M. Bains, W. Crocombe, and J.F. Griffiths, “Autoignition of Combustible Fluids in Porous Insulating Materials,” Combustion and Flame, 99, pp. 541–550 (1994).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Society of Fire Protection Engineers
About this chapter
Cite this chapter
Drysdale, D.D. (2016). Ignition of Liquids. In: Hurley, M.J., et al. SFPE Handbook of Fire Protection Engineering. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2565-0_18
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2565-0_18
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2564-3
Online ISBN: 978-1-4939-2565-0
eBook Packages: EngineeringEngineering (R0)