Abstract
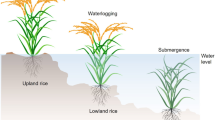
Flooding is a crucial factor affecting crop growth and yield in low-lying rainfed areas. Systematic investigation of flooding survival mechanisms in tolerant species has deciphered molecular, physiological, and developmental basis of soil flooding (waterlogging) and submergence survival. Flood escape and quiescence strategies of deepwater and submergence-tolerant rice (Oryza sativa) plants are regulated by ethylene-responsive factor (ERF) transcriptional activators. Ethylene induces genes of enzymes associated with aerenchyma formation, glycolysis, and fermentation pathway. Nonsymbiotic hemoglobin (NSHb) and nitric oxide (NO) have also been suggested as an alternative to fermentation to maintain lower redox potential (low NADH/NAD ratio). In rice (Oryza sativa L.), a calcineurin B-like interacting binding kinase (CIPK; OsCIPK15) is also involved in hypoxia tolerance. Detailed investigation revealed that ERFs are targets of a highly conserved O2-sensing protein turnover mechanism in Arabidopsis thaliana. Transcriptome and metabolome profiling of waterlogging-tolerant plant species reveals survival strategies that may be utilized through crop molecular breeding to develop tolerant cultivars.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ethylene
- Fermentation
- Flooding
- Nitric oxide
- Waterlogging
- Nonsymbiotic hemoglobin
- Calcineurin B-like interacting protein kinase
1 Introduction
Excess of water in the form of waterlogging (soil flooding) or complete submergence is lethal to majority of the terrestrial plants. Flooding events represent huge variation in duration and extent of inundation resulting in suboptimal levels of oxygen (hypoxia) or complete absence of oxygen (anoxia) affecting plant survival. Hampered availability of O2, CO2, and light hinders photosynthesis and aerobic respiration, leading to energy and carbohydrate shortage and thus limit growth and alter development (Voesenek and Bailey-Serres 2013). Further, reoxygenation of tissues and organs once floods subside often leads to oxidative stress (Fukao et al. 2011). Plants tolerate low oxygen stress either by altering its metabolism or by modifying its morphology and anatomy. Low oxygen sensing by group VII ERFs has been reported to regulate metabolic adaptation to flooding (Gibbs et al. 2011; Licausi et al. 2011). Use of carbohydrates and ATP is restricted only for processes considered necessary for survival under flooding. Developmental adaptation includes alterations in cellular and organ structure for enhancing the availability of oxygen (Bailey-Serres and Voesenek 2008).
2 Physiological and Anatomical Strategies for Flooding Tolerance
Plants face a sudden energy crisis under flooding due to nonavailability of oxygen for sustenance of aerobic respiration. Plants shift to anaerobic, glycolytic, and fermentative metabolism to sustain cell viability. Glycolytic pathway is energetically less efficient, yielding 2-ATP molecules in comparison to 36 molecules of ATP per hexose molecule produced by oxidative phosphorylation. Even glycolytic pathway is limited by the rate of NADH oxidation, as availability of oxidized NAD has a regulatory influence on the continuation of the glycolysis. Lactic acid fermentation involving lactate dehydrogenase (LDH) and ethanolic fermentation involving alcohol dehydrogenase (ADH) are the major mechanisms of NADH oxidation operating in the anaerobic tissues. However, there are certain lacunae with both the systems, while LDH may cause cytoplasmic acidosis, continuous ADH activity may result in toxic levels of ethanol and consequent injury to root cells (Bailey-Serres and Voesenek 2008). Energy-intensive processes like DNA replication, transcription, and cell division are curtailed during anoxia. Protein synthesis is finely regulated by selectively allowing translation of mRNAs encoding proteins involved in anaerobic and reactive oxygen species (ROS) metabolism. Syntheses of anaerobic proteins (ANP) like sucrose synthase, pyruvate decarboxylase, lactate dehydrogenase, and alcohol dehydrogenase are characteristic feature of anaerobic plant roots (Gibbs and Greenway 2003). Cellular carbohydrate reserves like soluble sugars and starch are mobilized during anoxia to support anaerobic metabolism. Anaerobic germination of seeds of rice and some other Poaceae members require slow mobilization of starch reserves (Guglielminetti et al. 1995). Sucrose is converted to UDP glucose and fructose by an enzyme called sucrose synthase (SS). SS was found to be the major enzyme catalyzing sucrose breakdown in anoxic rice seeds (Guglielminetti et al. 1995). Role of SS in anoxia tolerance is demonstrated in crops like maize, rice, pigeon pea, and mung bean (Bailey-Serres and Voesenek 2008; Kumutha et al. 2008a; Sairam et al. 2009a). Root carbohydrate status has often been correlated with flooding tolerance in crop plants (Kumutha et al. 2009). Studies on mung bean and pigeon pea suggest that tolerant genotypes were able to maintain higher root carbohydrate levels under waterlogging (Kumutha et al. 2008a, b). Waterlogged Arabidopsis plants accumulated higher levels of soluble sugars and amino acids as a result of increased starch and fatty acid catabolism (Hsu et al. 2011). Expression and activity of carbohydrate transporters were upregulated in shoots resulting in transport of carbohydrates from shoot to roots. Better phloem loading and soluble carbohydrate partitioning seem to be the basis for waterlogging tolerance of poplar and flooding tolerant oak (Quercus robur) as revealed by metabolite profiling (Ferner et al. 2012).
Some recent studies have suggested involvement of nonsymbiotic hemoglobin (NSHb) and nitric oxide (NO) in maintenance of NADH–NAD ratio, and thus in providing anaerobiosis tolerance (Hill 2012; Sairam et al. 2012). Expression and activity of NADPH oxidase increased under waterlogging in mung bean genotypes (Sairam et al. 2011a, b). Anaerobic roots apparently do not have a direct source of NADPH, i.e., photosynthetic light reaction and oxidative pentose phosphate pathway. Alternatively, a NADH kinase might be presumed to be involved in phosphorylation of NADH to NADPH. Consequently, NADH kinase, NADPH oxidase, and NADP phosphatase may provide another alternative route for NADH oxidation and thus continuation of glycolytic pathway (Kumutha et al. 2009; Sairam et al. 2009b).
Lower diffusion of ethylene in water leads to accumulation of ethylene in waterlogged and/or flooded plants and soil. Ethylene regulates physiological and morphological adaptive responses to flooding in plants. One such mechanism is development of soft tissues with large intercellular spaces called aerenchyma. Aerenchyma facilitates diffusion of photosynthetic and atmospheric oxygen from aerobic shoot to waterlogged roots. Primary aerenchyma arising from root cortex has been reported in cereal crops like rice, maize, wheat, and barley (Sauter 2013). Secondary, phellum-derived aerenchyma is observed in flooded roots of legumes like soybean and sesbania (Sauter 2013). In maize, a short span of 24 h of waterlogging is sufficient for production of cortical aerenchyma. Aerenchymas are produced by a programmed cell death process, signaled by ethylene, Ca2+, and ROS (Steffens et al. 2012). Transcriptome profiling of different cell layers of waterlogged maize roots reconfirmed that ethylene, Ca2+, and ROS signaling at root cortex layer induces production of aerenchymas (Rajhi et al. 2011). In rice and waterlogging-tolerant teosinte (Zea nicaraguensis), aerenchymas are constitutively formed in roots (Abiko et al. 2012; Steffens et al. 2012).
Specialized roots with poorly developed endodermis, emerging from submerged parts of stems, are called adventitious roots. Flooding-tolerant species produce adventitious roots as an adaptive response, to replace functions of flooded, anaerobic sedimentary root system. Adventitious roots are borne from shoots and without endodermis, hence distance and resistance to oxygen diffusion is less (Sauter 2013). Development of adventitious roots in deepwater rice, tomato, and Rumex palustris is an ethylene-dependent process (Sauter 2013). Flooding-adapted Oryza sp. has constitutively developed adventitious root primordia buried under nodal tissues (Coudert et al. 2010). Auxin–ethylene interaction leads to emergence of adventitious roots in rice. CRL1, an LBD (Lateral Organ Boundaries Domain) transcription factor acting upstream of auxin signaling pathway, regulates adventitious root initiation in rice (Inukai et al. 2005). Zhao et al. (2009) reported that WUSCHEL-related homeobox gene WOX11 regulates early development and emergence of adventitious roots in rice. Cell wall and cuticle layers of nodal tissue create barrier for emergence of adventitious roots in waterlogged plants. Epidermal cell death coordinated by ethylene and mechanical signals generated by root primordial facilitate adventitious roots emergence in rice (Steffens et al. 2012). Coordinated regulation of gibberellin and abscisic acid pathways by ethylene directs adventitious root elongation process in rice (Steffens et al. 2006).
Upward or hyponastic growth of leaves and petiole elongation of submerged leaves of semi-aquatic species Rumex palustris increases anoxia tolerance by facilitating the leaves to rise above water level. Accumulation of ethylene in submerged leaves leads to ABA insensitivity thereby increasing sensitivity to gibberellic acid (GA)-regulated cell expansion (Pierik et al. 2011). Leaf hyponasty was observed in Arabidopsis, following submergence in darkness (Colmer and Voesenek 2009). Flooding-intolerant species like tomato also exhibits leaf hyponasty post submergence, most likely mediated by an ethylene-dependent mechanism (Negi et al. 2010).
3 Low Oxygen Sensing and Protein Stability
Mechanism of low oxygen sensing was a mystery until 2011, when two independent groups demonstrated the involvement of N-end rule pathway of targeted proteolysis (NERP) in hypoxia signaling of Arabidopsis thaliana (Gibbs et al. 2011; Licausi et al. 2011). Discovery of flooding survival strategies in rice mediated by group VII ethylene response factors (ERF), SUBMERGENCE1A (SUB1A), and SNORKEL 1 (SK1) and SNORKEL 2 (SK2) paved way for identification of homologous genes in Arabidopsis (Xu et al. 2006; Hattori et al. 2009). Screening of flood-tolerant landraces of rice revealed the existence of multiple flooding survival strategies in rice. Deep-water rice follows a low oxygen escape strategy (LOES) mediated by ethylene-induced shoot elongation, regulated by ERFs, SK1, and SK2. SK1 and SK2 promote GA-induced stem elongation in rice. Flooding-tolerant rice landrace FR13A follows a quiescence mechanism, wherein GA induced growth; metabolism and other phenological processes are arrested to conserve energy for survival under anaerobiosis. Quiescence mechanism involves downregulation of growth and metabolism mediated by Sub1 locus containing either two or three genes (SUB1A, SUB1B, and SUB1C) belonging to group VII ERFs. Flooding-induced higher expression of SUB1A-1 restricts shoot elongation by regulating GA-signaling repressor SLENDER RICE-1 (SLR1) and the related SLR LIKE-1 (SLRL1) proteins. SUB1A-1 also reduces ethylene synthesis, gene expression of wall loosening enzyme (expansin) mRNAs, and carbohydrate depletion (Xu et al. 2006; Fukao and Bailey-Serres 2008). Five members of group VII ERFs are identified in Arabidopsis. Constitutively induced ERFs (RAP2.2, RAP2.12, and RAP2.13) and hypoxia-responsive ERFs (HRE1 and HRE2) regulate hypoxia tolerance in Arabidopsis. Constitutive overexpression of RAP2.12 in transgenic Arabidopsis plants resulted in increased postsubmergence survival (Licausi et al. 2011). Manipulation of N-terminal amino acids of RAP2.12 by deletion or addition of peptide tags negatively affected plant growth under normal and hypoxic conditions. N-terminal modification of RAP2.12 downregulated the oxygen-dependent expression of hypoxia marker genes in Arabidopsis (Licausi et al. 2011). Arabidopsis mutants defective in NERP, lacking either Arginine tRNA protein transferase (ate1, ate 2) or E3 ubiquitin ligase (proteolysis 6) demonstrated constitutive overexpression of hypoxia marker genes. N-terminal amino acids of most of the group VII ERFs are conserved, having cysteine (cys2) as the second amino acid. Oxidation of cys2 under normoxic conditions qualify group VII ERFs to be targeted to NERP-mediated proteolysis. Post-translational modification of cys2 is oxygen dependent and hence under anoxia, ERFs like RAP2.12 and HRE2 remain stable. Under normoxia RAP2.12 is plasma membrane localized, which following anoxia gets rapidly relocalized to nucleus for further signaling. Constitutively, active group VII ERFs like RAP2.12 are putative oxygen sensors in plants, and provide a rapid mechanism of flood adaptation. Group VII ERFs are regulated either by low oxygen, ethylene, or by both. NERP-insensitive ERFs regulate ethylene-mediated adaptive responses like production of aerenchyma, adventitious root formation, stem elongation, and hyponastic growth. However, metabolic adaptation of hypoxia tolerance is triggered by oxygen sensing property of NERP sensitive ERFs (Fig. 8.1).
Hypoxia signaling in plants. Flooding rapidly decreases availability of oxygen (O2) and energy status of plants and induces production of ethylene (C2H4) and nitric oxide (NO). These signals turn on downstream signal transduction pathways, which regulates developmental and metabolic adaptation for flooding tolerance
4 Nonsymbiotic Hemoglobins and Nitric Oxide Interaction Under Anoxia
Plants contain different classes of hemoglobins (Hb), and the first plant hemoglobin was discovered few decades back (Appleby 1992). Plant Hbs are classified as nonsymbiotic (NSHb) or symbiotic depending upon the plant tissue where they are found (Bogusz et al. 1988). Symbiotic hemoglobins are found exclusively in root nodules, where these functions in controlled transport of oxygen into bacteroids of symbiotic nitrogen-fixing bacteria (Appleby 1992). NSHb are found ubiquitously in plant kingdom and are expressed in seeds, root, shoot, and stem tissues of plants (Hill 2012). There are two classes of NSHb, class 1 NSHbs are induced by low cellular oxygen levels and nutrient toxicity; class 2 NSHbs are induced under cold stress and by cytokinins (Hunt et al. 2001). Taylor et al. (1994) isolated a class 1 nonsymbiotic hemoglobin, from barley. Overexpression of barley NSHb 1 in alfalfa root leads to increase in ascorbate content and higher activities of antioxidant enzymes in control as well as hypoxic roots (Dordas 2009). Expression of NSHb has been reported to be upregulated in response to hypoxia in barley (Taylor et al. 1994), Arabidopsis (Hunt et al. 2002), oak (Parent et al. 2008), and rice (Lira-Ruan et al. 2002). Respiratory inhibitors, which limit ATP production, are also as effective as hypoxia in inducing NSHb expression. Rapidly growing tissues like root tips also confront oxygen deficiency and show the presence of NSHb. The exact mechanism by which NSHb renders hypoxia tolerance is being unraveled. Low concentration of NSHb, low dissociation coefficient of oxyhemoglobin complex, and the induction of NSHb expression by low cellular energy levels indicates role of NSHb in stress signaling. Previous works clearly indicate involvement of NSHb in reactive oxygen and nitric oxide (NO) metabolism (Igamberdiev and Hill 2004). Experimental evidences have proved involvement of plant Hbs in catalyzing the conversion of NO to nitrate (Dordas 2009). Nitric oxide is a bioactive signal molecule involved in hormonal and stress signal transduction. NO involved in ROS scavenging, programmed cell death, and aerenchyma formation in plants. Nitric oxide is produced by hypoxia-induced activity of nitrate reductase. Excess of NO is scavenged by oxyhemoglobin form of NSHb in conjunction with NO dioxygenases, converting NO back to nitrate. NSHb is coupled with nitrate reductase, forming the Hb/NO cycle, in which excess NAD(P)H is oxidized (Igamberdiev and Hill 2004). This pathway plays a major role as an alternative of fermentation pathway (Fig. 8.1) in regeneration of NADH in waterlogging-affected mung bean plants (Sairam et al. 2012). Seed and embryo development in plants are also typical examples of hypoxic environment, with young embryos facing low energy levels (Rolletschek et al. 2002). Hypoxia-induced NO production in seeds (Borisjuk et al. 2007) leads to decrease in metabolism, while under normoxia, NO levels decrease and normal metabolism resumes. Manipulation of seed oxygen levels by seed-specific expression of NSHb (Thiel et al. 2011) in Arabidopsis led to increased seed metabolic activity.
5 Waterlogging, ROS Production, and Antioxidant Mechanism
Accelerated production of ROS is a ubiquitous phenomenon under stress conditions. Abiotic stresses like soil flooding and submergence lead to perturbation of the fine balance between oxidative and antioxidative capacity of plants. Hypoxia-induced increase in redox potential of both plant roots and surrounding soil is ideal for production of ROS. These ROS are necessary for inter- and intracellular signaling, but at high concentrations, they seriously disrupt normal metabolism of plants through oxidation of pigments, membrane lipids, proteins, and nucleic acids (Sairam et al. 2008). Short-term flooding for few hours enhances production of superoxide radicals in soybean roots (Van Toai and Bolles 1991). Accumulation of hydrogen peroxide (H2O2) was induced in hypoxia-stressed barley and wheat seedlings (Biemelt et al. 2000; Kalashnikov et al. 1994). Significant increase in lipid peroxidation, superoxide radical production, and membrane injury was observed in waterlogging-stressed, maize, pigeon pea, and mung bean genotypes (Yan et al. 1996; Kumutha et al. 2009; Sairam et al. 2011a). ROS production under soil flooding is owing to the induction of membrane bound NADPH oxidase, as indicated by inhibitor and gene expression studies in pigeon pea (Kumutha et al. 2009).
Detoxification of injurious levels of ROS is mediated by enzymatic antioxidants such as superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR), catalase (CAT), mono-dehydroascorbatereductase (MDHAR), dehydroascorbatereductase (DHAR), glutathione S transferase (GST), and nonenzymatic antioxidants viz., ascorbic acid, glutathione, α-tocopherol, and carotenoids. Upregulation of antioxidant defense system is often correlated with abiotic stress tolerance in crop plants. In Iris sp., a 14-fold increase in SOD activity was observed, following hypoxia (Monk et al. 1987). Similarly, in wheat seedlings increased activity of GR and higher contents of glutathione could mitigate post hypoxia oxidative stress (Ushimaru et al. 1997). A recent study comparing transcriptome changes in waterlogging-tolerant and -susceptible maize genotypes, showed upregulation of antioxidant defense and fermentation pathway genes as the basis of waterlogging tolerance (Thirunavukkarasu et al. 2013). There are reports of waterlogging stress-induced increase as well as decrease in antioxidant potential in crop plants (Biemelt et al. 2000; Kumutha et al. 2009). Waterlogging-tolerant pigeon pea genotypes displayed continuous increase in antioxidant enzyme activity over a period of six days of waterlogging, however in susceptible genotypes antioxidant enzyme activities declined after two days of submergence (Kumutha et al. 2009). In anoxia-stressed wheat seedlings, there was either no change or decrease in activities of MDHAR, DHAR, and GR enzymes (Biemelt et al. 2000). ROS production has been implicated in signal transduction for low oxygen stress. Screening Arabidopsis seedlings carrying a gene-trap transposon (DsGus) led to the identification of mutants with increased ADH-specific activity in response to hypoxia. Mutant phenotype was a result of insertion of DsGus in the first exon of a gene that encodes Rop (RHO-like small G protein of plants) guanosine triphosphatase (GTPase) activating protein 4 (ROPGAP4). Rop signaling is implicated in Ca2+ and H2O2-mediated signaling for abiotic and biotic stress tolerance of plants. Activation of ROP by GTP under low O2 induces production of H2O2 through a calcium-dependent NADPH oxidase. Accumulation of hydrogen peroxide is crucial for induction of ADH and RopGAP4 expression. RopGAP4 is needed for negative feedback regulation of ROP level; hence, ropgap4-1 seedlings succumb to oxidative stress due to excessive accumulation of H2O2 (Baxter-Burrell et al. 2002).
Heat shock transcription factors (HSF) have also been proposed to be H2O2 sensors in plants. Arabidopsis transgenic plants overexpressing HSFA2 are tolerant to hypoxia in comparison to wild type (Banti et al. 2010). Stress-induced induction of HSFs leads to transcription of genes encoding high-molecular-weight and low-molecular-weight heat shock proteins (HSPs) in plants (Banti et al. 2010). Increase in HSP transcripts in response to low oxygen stress is conserved across various plant species (Mustroph et al. 2010). Pucciariello et al. (2012) proposed that NADPH oxidase-dependent ROS pathway regulates HSFs and other ROS-regulated transcription factors in response to flooding stress. HSFs and other ROS-regulated transcription factors are not targets of NERP-dependent proteolysis, hence ROS signaling (Fig. 8.1) is an independent mechanism regulating flooding tolerance in plants (Banti et al. 2010).
6 Functional Genomics of Flooding Stress in Plants
The accessibility to sequence information has revolutionized research far ahead of transcriptomics and functional genomics. Transcriptome and proteome analysis of model and crop plants in response to flooding stress has widely been attempted. These studies especially those which compare responses of contrasting genotypes have thrown light on some evolutionarily conserved mechanism of flooding tolerance. Low oxygen-dependent changes in transcriptome (pool of total cellular mRNA) have been analyzed in Arabidopsis (Branco-Price et al. 2005; Liu et al. 2005), rice (Lasanthi-Kudahettige et al. 2007), and many more plant species. (Christianson et al. 2010; Voesenek and Bailey-Serres 2013) exposed to hypoxia have confirmed that low oxygen stress causes radical changes in gene expression. Apart from a specific set of hypoxia-induced genes, global gene transcription is downregulated under hypoxia. Genes encoding anaerobic proteins (ANPs) involved in sugar metabolism are preferentially expressed. Signal transduction components that activate RopGAP4, mitochondrial alternative oxidase (AOX), calmodulin, and CAP (calmodulin-associated peptide) were upregulated by hypoxia (Bailey-Serres and Chang 2005). Thirunavukkarasu et al. (2013) compared the whole transcriptome of contrasting subtropical maize genotypes at three stages of waterlogging stress. Genes responsible for programmed cell death that precedes aerenchyma formation was selectively upregulated in HKI 1105 (tolerant) exposed to waterlogging. Calmodulin, a Ca2+-binding protein that was highly expressed only in HKI 1105 interacts with glutamate decarboxylase and helps to maintain cytosolic pH under anoxia. A member of a flooding-specific gene family, XET A was found upregulated in HKI 1105 during both moderate (253-fold) and severe (16-fold) stresses, but downregulated in V 372 (sensitive). Ethylene-responsive factor-like protein 1, BBM2, AIL5-like, and WRI1 were upregulated in HKI 1105. It was also observed that in the tolerant genotype, auxin receptor genes such as IAA3, IAA14, and IAA16 were upregulated. Cross talk between ethylene and auxin signaling pathways probably enhances the formation of lateral and adventitious roots in waterlogging-tolerant genotypes of maize. Genes belonging to plant hormone biosynthesis and signal transduction were differentially regulated under waterlogging stress, including increases in ethylene, abscisic acid (ABA), gibberellic acid (GA), and auxin (IAA) and a reduction in cytokinin (CK) (Zou et al. 2013). Rice transcription factors Snorkeland Submergence-1A, belonging to group VII ERF (ethylene response factor) have been cloned by map-based cloning (discussed in section3). Apart from rice, extensive studies on quiescence and escape strategies were done in wild species Rumex acetosa and Rumex palustris, respectively (Hans et al. 2013). R. palustris escapes submergence by orientating its leaves in vertical position (hyponastic growth), in an ethylene-dependent manner followed by enhanced elongation rate of young petioles (Cox et al. 2006). In R. acetosa, submergence driven accumulation of ethylene suppresses petiole elongation and predisposes the plants towards metabolic rearrangement to minimize carbon use (Pierik et al. 2009). Hans et al. (2013) employed RNA sequencing (RNA-Seq) technology to investigate the molecular basis of adaptive traits of these two species. Upon submergence, there was enhanced expression of amino cyclopropane carboxylate (ACC) oxidase, enzyme catalyzing ethylene biosynthesis in both the species. In R. palustris, expression of an EIN3 BINDING F-BOX (EBF) was specifically upregulated. The putative rice ortholog regulates ethylene-induced growth stimulation through preventing negative regulation of GA biosynthesis by ethylene (Kim et al. 2012). The increase in EBF expression is consistent with elongation growth in R. palustris. Transcripts encoding ABA biosynthetic enzyme 9-cis-epoxycarotenoid dioxygenase was downregulated in R. palustris resulting in lower ABA levels in R. palustris. Transcript that encodes an ABA breakdown enzyme ABA-8-hydroxylase was induced in both the species. Transcripts encoding orthologs of two downstream components of ABA signaling (ABA-responsive element binding factor 2 and HOMEOBOX PROTEIN33) were exclusively induced in R. acetosa. Maintenance of ABA levels coupled with enhancement in ABA signaling induces metabolic reprogramming of R. acetosa. Transcripts regulating auxin transport like transcripts encoding orthologs of a PINOID like (WAG1) that are kinases regulating auxin transport properties of PIN family were upregulated in R. palustris. Transcripts of AUXIN (INDOLE-3-ACETIC ACID) 2-11 (AUXIAA2-11) auxin-responsive protein regulating auxin-mediated transcriptional responses were regulated only in R. palustris. In R. palustris auxin induces cell wall acidification by activating a plasma-membrane proton pump and thereby activates pH-sensitive cell wall-modifying enzymes expansins.
Comparison of whole transcriptome with translatome (mRNAs targeted to translation) revealed highly selective hypoxia-specific protein synthesis (Branco-Price et al. 2008) in hypoxia-stressed Arabidopsis plants. Hypoxia-induced translatome consisted of proteins belonging to anaerobic metabolism and ethylene biosynthesis and responses. Approximately half of the translated proteins were of no known functions and were designated as hypoxia-responsive unknown protein (HUP). Recent developments in low oxygen sensing paved way for the discovery of involvement of group VII ERFs in cellular level low oxygen sensing in Arabidopsis. However, there were earlier reports of involvement of various signaling molecules in anaerobic stress signaling in plants. Hypoxia signaling possibly senses changes in levels of cellular energy status, respirable substrates, transient elevations of Ca2+, ROS, and NO (Voesenek and Bailey-Serres 2013). Elevations of cytoplasmic calcium levels following anoxic stress have been observed in maize, rice, wheat, and cucumber plants (Yemelyanov et al. 2011; Subbaiah et al. 1998; He et al. 2012). Proteome analysis of calcium-treated hypoxia-stressed cucumber plants revealed calcium-dependent enhancement in levels of enzymes of primary metabolism and ROS scavenging (He et al. 2012). KIN10 and KIN11 are energy-sensing protein kinases belonging to SnRK1 clade of Arabidopsis and regulate carbon utilization under hypoxia (Baena-González et al. 2007). KIN10 positively regulates genes encoding enzymes catalyzing carbohydrate and amino acid catabolism in Arabidopsis (Baena-González et al. 2007; Cho et al. 2012). One of the KIN10/11-regulated genes is EXORDIUM-LIKE1, an HUP that is essential for carbon management under low oxygen conditions (Schröder et al. 2011). Calcium signals are transduced via SnRK1 group kinase and calcineurin B-like interacting binding kinase, CIPK15 regulates breakdown of starch, essential for anoxic germination of rice seeds (Lee et al. 2009). Another remarkable molecule regulating hypoxia signaling is nitric oxide (Hill 2012, discussed in section 4). NO homeostasis in hypoxic cells is largely dependent on nonsymbiotic hemoglobins, which are positively regulated by group VII ERF RAP2.12 (Mustroph et al. 2010). NO is a requisite for N-terminal Cys-oxidation and tagging of proteins for turnover in mammals (Hsu et al. 2011). It can be speculated that NO homeostasis under hypoxia may contribute to NERP dependent turnover of the ERFs. Anoxic stress and post-anoxic reoxygenation promotes mitochondrial generation of ROS at complex III (Discussed in Sect. 8.5). Elevated levels of cellular ROS levels ephemerally activate mitogen-activated protein kinases (MAPKs) (Chang et al. 2012). However, MAPK signaling maintains mRNAs selectively excluded from translation during anoxia, but might be essential for survival during post-anoxic reoxygenation.
Regulatory role of non-coding RNAs (nc RNAs) has been elucidated recently in model plants. Sequencing of small RNA libraries of hypoxic and control root tissues of Arabidopsis identified 65 unique microRNA (miRNA) sequences and 14 trans-acting small interfering RNA (tasiRNA). Putative targets for these hypoxia-responsive miRNA are transcription factors mainly from the MYB, NAC, Homeobox, SPL, ARF, AP2, MADS, and CCAAT-HAP2 families having important roles in plant growth and floral development (Moldovan et al. 2009). Wu et al. (2012) identified long non-coding RNAs (lnc RNA) responsive to hypoxia stress in Arabidopsis. Abundance of lnc RNA AtR8 was decreased by hypoxic treatments and recovered upon reoxygenation. AtR8 was preferentially localized to cytoplasm of root tissues. It is possible that the lnc RNA negatively regulates translation or ANPs and decrease in abundance of these RNAs upregulate translation of ANPs (Wu et al. 2012).
7 Conclusions and Future Perspectives
As flooding events depict huge variation in duration and extent of inundation ranging from waterlogging at root level to complete submergence and from few hours to few weeks duration, plant species show large variation in flooding tolerance. Identification of Sub1A locus from flooding-tolerant Indian rice landrace FR13A paved way for marker-assisted breeding of this locus into cultivated rice varieties. Current studies shows that under Indian conditions, Swarna-Sub1 can contribute up to 45 % increase in yields compared to current popular varieties under a 10-day period of submergence (Dar et al. 2013). Screening of 86 accessions of Arabidopsis and 100 accessions of Lolium perenne presents species level variation in flooding survival strategies in plants (Vashisht et al. 2011; Yu et al. 2012). Detailed analysis of the contrasting genotypes aided with transcriptome and proteome profiling and functional validation of candidate genes are required for reaching valuable conclusions. Some of the well characterized genes/proteins may be targeted for improving flooding tolerance by either transgenic manipulation of gene expression or for screening of the germplasm in a need-based manner (Table 8.1). Transcriptome comparison between submergence-tolerant wild Rorippa species with Arabidopsis revealed that genes of pyrophosphate-dependent pathway of phosphorylation are the candidate genes behind tolerance (Sasidharan et al. 2013). Similarly, transcriptome profiling of Rumex palustris and Rumex acetosa revealed two distinct mechanisms of survival in this related species. Similar to deep-water rice, Rumex palustris utilizes ethylene-mediated growth modification to avoid submergence. Rumex acetosa undergoes complete metabolic reprogramming to tolerate flood prone environments (Hans et al. 2013). Plant species belonging to flood prone ecosystems may serve as valuable models to understand flooding survival strategies useful in crop breeding.
References
Abiko T, Kotula L, Shiono K, Malik AI, Colmer TD, Nakazono M (2012) Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize (Zea mays ssp. mays). Plant Cell Environ 35:1618–1630
Appleby CA (1992) The origin and functions of haemoglobin in plants. Sci Progress 76:365–398
Baena-González E, Rolland F, Thevelein JM, Sheen J (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448:938–942
Bailey-Serres J, Chang R (2005) Sensing and signalling in response to oxygen deprivation in plants and other organisms. Ann Bot (Lond) 96:507–518
Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59:313–339
Banti V, Mafessoni F, Loreti E, Alpi A, Perata P (2010) The heat-inducible transcription factor HsfA2 enhances anoxia tolerance in Arabidopsis. Plant Physiol 152:1471–1483
Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J (2002) RopGAP4-dependent Rop-GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296:2026–2028
Biemelt S, Keetman U, Mock HP, Grimm B (2000) Expression and activity of isoenzymes of superoxide dismutase in wheat roots in response to hypoxia and anoxia. Plant Cell Environ 23:135–144
Bogusz D, Appleby CA, Landsmann J, Dennis ES, Trinick MJ, Peacock WJ (1988) Functioning haemoglobin genes in nonnodulating plants. Nature 331:178–180
Borisjuk L, Macherel D, Benamar A, Wobus U, Rolletschek H (2007) Low oxygen sensing and balancing in plant seeds: a role for nitric oxide. New Phytol 176:813–823
Branco-Price C, Kaiser KA, Jang CJ, Larive CK, Bailey-Serres J (2008) Selective mRNA translation coordinates energetic and metabolic adjustments to cellular oxygen deprivation and reoxygenation in Arabidopsis thaliana. Plant J 56:743–755
Branco-Price C, Kawaguchi R, Ferreira RB, Bailey-Serres J (2005) Genome wide analysis of transcript abundance and translation in Arabidopsis seedlings subjected to oxygen deprivation. Ann Bot 96:647–660
Chang R, Jang CJ, Branco-Price C, Nghiem P, Bailey-Serres J (2012) Transient MPK6 activation in response to oxygen deprivation and reoxygenation is mediated by mitochondria and aids seedling survival in Arabidopsis. Plant Mol Biol 78:109–122
Cho YH, Hong JW, Kim EC, Yoo SD (2012) Regulatory functions of SnRK1 in stress-responsive gene expression and in plant growth and development. Plant Physiol 158:1955–1964
Christianson JA, Llewellyn DJ, Dennis ES, Wilson IW (2010) Global gene expression responses to waterlogging in roots and leaves of cotton (Gossypium hirsutum L.). Plant Cell Physiol 51:21–37
Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681
Coudert Y, Périn C, Courtois B, Khong NG, Gantet P (2010) Genetic control of root development in rice, the model cereal. Trends Plant Sci 15:219–226
Cox MCH, Peeters AJM, Voesenek LACJ (2006) The stimulating effects of ethylene and auxin on petiole elongation and on hyponastic curvature are independent processes in submerged Rumex palustris. Plant Cell Environ 29:282–290
Dar MH, de Janvry A, Emerick K, Raitzer D, Sadoulet E (2013) Flood-tolerant rice reduces yield variability and raises expected yield, differentially benefitting socially disadvantaged groups. Sci Rep 3:3315
Dordas C (2009) Nonsymbiotichemoglobins and stress tolerance in plants. Plant Sci 176:433–440
Ferner E, Rennenberg H, Kreuzwieser J (2012) Effect of flooding on C metabolism of flood-tolerant (Quercus robur) and non-tolerant (Fagus sylvatica) tree species. Tree Physiol 32:135–145
Fukao T, Bailey-Serres J (2008) Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proc Natl Acad Sci U S A 105:16814–16819
Fukao T, Yeung E, Bailey-Serres J (2011) The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell 23:412–427
Gibbs DJ, Lee SC, Isa NM, Gramuglia S, Fukao T, Bassel GW, Correia CS, Corbineau F, Theodoulou FL, Bailey-Serres J, Holdsworth MJ (2011) Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479:415–418
Gibbs J, Greenway H (2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol 30:1–47
Guglielminetti L, Yamaguchi J, Perata P, Alpi A (1995) Amylolytic activities in cereal seeds under aerobic and anaerobic conditions. Plant Physiol 109:1069–1076
Hattori Y, Nagai K, Furukawa S, Song XJ, Kawano R, Sakakibara H, Wu J, Matsumoto T, Yoshimura A, Kitano H, Matsuoka M, Mori H, Ashikari M (2009) The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460:1026–1030
He L, Lu X, Tian J, Yang Y, Li B, Li J, Guo S (2012) Proteomic analysis of the effects of exogenous calcium on hypoxic-responsive proteins in cucumber roots. Proteome Sci 10(1):42
Hill RD (2012) Non-symbiotic haemoglobins—what’s happening beyond nitric oxide scavenging? AoB Plants 2012. doi: 10.1093/aobpla/pls004
Hsu FC, Chou MY, Peng HP, Chou SJ, Shih MC (2011) Insights into hypoxic systemic responses based on analyses of transcriptional regulation in Arabidopsis. PLoS One 6(12):e28888
Hunt PW, Klok EJ, Trevaskis B, Watts RA, Ellis MH, Peacock WJ, Dennis ES (2002) Increased level of hemoglobin 1 enhances survival of hypoxic stress and promotes early growth in Arabidopsis thaliana. Proc Natl Acad Sci U S A 99:17197–17202
Hunt PW, Watts RA, Trevaskis B, Llewelyn DJ, Burnell J, Dennis ES, Peacock WJ (2001) Expression and evolution of functionally distinct haemoglobin genes in plants. Plant Mol Biol 47:677–692
Igamberdiev AU, Hill RD (2004) Nitrate, NO and haemoglobin in plant adaptation to hypoxia: an alternative to classic fermentation pathways. J Exp Bot 55:2473–2482
Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Shibata Y, Gomi K, Umemura I, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M (2005) Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 17:1387–1396
Lee KW, Chen PW, Lu CA, Chen S, Ho THD, Yu SM (2009) Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding. Sci Signaling 2(91):ra61–ra61
Kalashnikov YE, Balakhnina TI, Zakrzhevsky DA (1994) Effect of soil hypoxia on activation of oxygen and the system of protection from oxidative destruction in roots and leaves of Hordeum vulgare. Russ J Plant Physiol 41:583–588
Kim J, Wilson RL, Case JB, Binder BM (2012) A comparative study of ethylene growth response kinetics in eudicots and monocots reveals a role for gibberellin in growth inhibition and recovery. Plant Physiol 160:1567–1580
Kumutha D, Ezhilmathi K, Sairam RK, Srivastava GC, Deshmukh PS, Meena RC (2009) Waterlogging induced oxidative stress and antioxidant activity in pigeonpea genotypes. Biol Plant 53(1):75–84
Kumutha D, Sairam RK, Meena RC (2008a) Role of root carbohydrate reserves and their mobilization in imparting waterlogging tolerance in green gram (Vigna radiata (L.) Wilczek) genotypes. Ind J Plant Physiol 13(4):339–346
Kumutha D, Sairam RK, Ezhilmathi K, Chinnusamy V, Meena RC (2008b) Effect of waterlogging on carbohydrate metabolism in pigeon pea (Cajanus cajan L.): upregulation of sucrose synthase and alcohol dehydrogenase. Plant Sci 175(5):706–716
Lasanthi-Kudahettige R, Magneschi L, Loreti E, Gonzali S, Licausi F, Novi G, Beretta O, Vitulli F, Alpi A, Perata P (2007) Transcript profiling of the anoxic rice coleoptile. Plant Physiol 144:218–231
Lee KW, Chen PW, Lu CA, Chen S, Ho THD, Yu SM (2009) Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding. Sci Signal 2(91):ra61
Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LACJ, Perata P, van Dongen JT (2011) Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilisation. Nature 479:419–422
Lira-Ruan V, Ross EJH, Sarath G, Klucas RV, Arredondo-Peter R (2002) Mapping and analysis of a hemoglobin gene family from Oryza sativa. Plant Physiol Biochem 40:199–202
Liu F, Vantoai T, Moy LP, Bock G, Linford LD, Quackenbush J (2005) Global transcription profiling reveals comprehensive insights into hypoxic response in Arabidopsis. Plant Physiol 137:1115–1129
Moldovan D, Spriggs A, Yang J, Pogson BJ, Dennis ES, Wilson IW (2009) Hypoxia-responsive microRNAs and trans-acting small interfering RNAs in Arabidopsis. J Exp Bot 14:165–177
Monk LS, Fagerstedt KV, Crawford RMM (1987) Superoxide dismutase as an anaerobic polypeptide—a key factor in recovery from oxygen deprivation in Iris pseudacorus. Plant Physiol 85:1016–1020
Mustroph A, Lee SC, Oosumi T, Zanetti ME, Yang H, Ma K, Yaghoubi-Masihi A, Fukao T, Bailey-Serres J (2010) Cross kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol 152:1484–1500
Negi S, Sukumar P, Liu X, Cohen J, Muday G (2010) Genetic dissection of the role of ethylene in regulating auxin dependent lateral and adventitious root formation in tomato. Plant J 61:3–15
Parent C, Berger A, Folzer H, Dat J, Crevecoeur M, Badot PM, Capelli N (2008) A novel nonsymbiotic hemoglobin from oak: cellular and tissue specificity of gene expression. New Phytol 177:142–154
Pierik R, De Wit M, Voesenek LACJ (2011) Growth mediated stress escape: convergence of signal transduction pathways activated upon exposure to two different environmental stresses. New Phytol 189:122–134
Pierik R, van Aken J, Voesenek LACJ (2009) Is elongation-induced leaf emergence beneficial for submerged Rumex species? Ann Bot 103:353–357
Pucciariello C, Parlanti S, Banti V, Novi G, Perata P (2012) Reactive oxygen species-driven transcription in Arabidopsis under oxygen deprivation. Plant Physiol 159(1):184–196
Rajhi I, Yamauchi T, Takahashi H, Nishiuchi S, Shiono K, Watanabe R, Mliki A, Nagamura Y, Tsutsumi N, Nishizawa NK, Nakazono M (2011) Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytol 190:351–368
Rolletschek H, Borisjuk L, Koschorreck M, Wobus U, Weber H (2002) Legume embryos develop in a hypoxic environment. J Exp Bot 53:1099–1107
Sairam RK, Dharmar K, Chinnusamy V, Meena RC (2009a) Waterlogging-induced increase in sugar mobilization, fermentation, and related gene expression in the roots of mung bean (Vigna radiata). J Plant Physiol 166(6):602–616
Sairam RK, Dharmar K, Chinnusamy V, Lekshmy S, Joshi R, Bhattacharya P (2011a) NADPH oxidase as the source of ROS produced under waterlogging in roots of mung bean. Biol Plant 55(4):741–746
Sairam RK, Dharmar K, Chinnusamy V, Lekshmy S, Joshi R, Bhattacharya P (2012) The role of non-symbiotic haemoglobin and nitric oxide homeostasis in waterlogging tolerance in Vigna species. Biol Plant 56(3):528–536
Sairam RK, Dharmar K, Lekshmy S, Chinnusamy V (2011b) Expression of antioxidant defense genes in mung bean (Vigna radiata L.) roots under water-logging is associated with hypoxia tolerance. Acta Physiol Plant 33(3):735–744
Sairam RK, Kumutha D, Ezhilmathi K, Chinnusamy V, Meena RC (2009b) Waterlogging induced oxidative stress and antioxidant enzyme activities in pigeon pea. Biol Plant 53(3):493–504
Sairam RK, Kumutha D, Ezhilmathi K, Deshmukh PS, Srivastava GC (2008) Physiology and biochemistry of waterlogging tolerance in plants. Biol Plant 52(3):401–412
Sasidharan R, Mustroph A, Boonman A, Akman M, Ammerlaan AMH, Breit T, Schranz ME, Voesenek LACJ, van Tienderen PH (2013) Root transcript profiling of two Rorippa species reveals gene clusters associated with extreme submergence tolerance. Plant Physiol 163(3):1277–1292
Sauter M (2013) Root responses to flooding. Curr Opin Plant Biol 16:282–286
Schröder F, Lisso J, Müssig C (2011) EXORDIUM-LIKE1 promotes growth during low carbon availability in Arabidopsis. Plant Physiol 156:1620–1630
Steffens B, Kovalev A, Gorb SN, Sauter M (2012) Emerging roots alter epidermal cell fate through mechanical and reactive oxygen species signaling. Plant Cell 24:3296–3306
Steffens B, Wang J, Sauter M (2006) Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 223:604–612
Subbaiah CC, Bush DS, Sachs MM (1998) Mitochondrial contribution to the Anoxic Ca2+ signal in maize suspension-cultured cells. Plant Physiol 118:759–771
Taylor ER, Nie XZ, MacGregor AW, Hill RD (1994) A cereal haemoglobin gene is expressed in seed and root tissues under anaerobic conditions. Plant Mol Biol 24:853–862
Thiel J, Rolletschek H, Friedel S, Lunn JE, Nguyen TH, Feil R, Tschiersch H, Muller M, Borisjuk L (2011) Seed-specific elevation of non-symbiotic hemoglobin AtHb1: beneficial effects and underlying molecular networks in Arabidopsis thaliana. BMC Plant Biol 11:48
Thirunavukkarasu N, Hossain F, Mohan S, Shiriga K, Mittal S, Sharma R, Singh RK, Gupta HS (2013) Genome-wide expression of transcriptomes and their co-expression pattern in subtropical maize (Zea mays L.) under waterlogging stress. PLoS One 8(8):e70433
Ushimaru T, Maki Y, Sano S, Koshiba K, Asada K, Tsuji H (1997) Induction of enzymes involved in the ascorbate-dependent antioxidative system, namely, ascorbate peroxidase, monodehydroascorbate reductase and dehydroascorbate reductase, after exposure to air of rice (Oryza sativa) seedlings germinated under water. Plant Cell Physiol 38:541–549
Van Toai TT, Bolles CS (1991) Post anoxic injury in soybean (Glycine max) seedlings. Plant Physiol 97:588–592
Vashisht D, Hesselink A, Pierik R, Ammerlaan JM, Bailey-Serres J, Visser EJ, Pedersen O, van Zanten M, Vreugdenhil D, Jamar DCL, Voesenek LACJ, Sasidharan R (2011) Natural variation of submergence tolerance among Arabidopsis thaliana accessions. New Phytol 190:299–310
Hans VV, Mustroph A, Barding GA, Eijk MV, Welschen-Evertman RAM, Pedersen O, Eric JWV, Larive CK, Pierik R, Bailey-Serres J, Voesenek LACJ, Sasidharan R (2013) Two Rumex species from contrasting hydrological niches regulate flooding tolerance through distinct mechanisms. The Plant Cell Online http://dx.doi.org/10.1105/tpc.113.119016
Voesenek LACJ, Bailey-Serres J (2013) Flooding tolerance: O2 sensing and survival strategies. Curr Opin Plant Biol 16(5):647–653
Wu T, Okada T, Fukushima T, Tsudzuki M, Sugiura Y, Yukawa Y (2012) A novel hypoxic stress-responsive long non-coding RNA transcribed by RNA polymerase III in Arabidopsis. RNA Biol 9:302–313
Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ (2006) Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442:705–708
Yan B, Da Q, Liu X, Huang S, Wang Z (1996) Flooding induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant Soil 179:261–268
Yemelyanov V, Shishova M, Chirkova T, Lindberg S (2011) Anoxia-induced elevation of cytosolic Ca2+concentration depends on different Ca2+ sources in rice and wheat protoplasts. Planta 234:271–280
Yu X, Luo N, Yan J, Tang J, Liu S, Jiang Y (2012) Differential growth response and carbohydrate metabolism of global collection of perennial ryegrass accessions to submergence and recovery following de-submergence. J Plant Physiol 169:1040–1049
Zhao Y, Hu Y, Dai M, Huang L, Zhou DX (2009) The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell 21:736–748
Zou X, Tan X, Hu C, Zeng L, Lu G (2013) The transcriptome of Brassica napus L. roots under waterlogging at the seedling stage. Int J Mol Sci 14:2637–2651
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Lekshmy, S., Jha, S.K., Sairam, R.K. (2015). Physiological and Molecular Mechanisms of Flooding Tolerance in Plants. In: Pandey, G. (eds) Elucidation of Abiotic Stress Signaling in Plants. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2540-7_8
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2540-7_8
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2539-1
Online ISBN: 978-1-4939-2540-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)