Abstract
Introduction. Immunosuppressive and nonimmunosuppressive therapy alternative to corticosteroids that can target the different antigens involved in pathogenic mechanisms of Graves’ ophthalmopathy (GO) have been hypothesized. Some have already been employed in clinical studies and showed interesting results, although the lack of randomized and controlled trials suggests caution for their use in clinical practice.
Evidence. Potential targets for therapy in GO are the TSH receptor and the IGF-1 receptor on the fibroblasts, inflammatory cytokines, and B and T cells. Most promising results are observed with small TSH-R molecules interacting the receptor on thyrocytes and fibroblasts, by blocking the PIK3/mTORC1 signaling cascades for adipogenesis and the anti-IGF-1R with the monoclonal antibody teprotumumab. A recent open study has shown that tocilizumab, an anti-sIL-6R antibody, inactivates GO. Consistent reports on the efficacy of rituximab need to be confirmed by randomized controlled trials, now just completed.
Conclusions. Clinical practice will greatly benefit from the use of disease modifying agents in GO, as compared to steroids, currently standard treatment for GO. Among these rituximab seems to be a better candidate, as preliminary results suggest better efficacy with a relative safe profile. Direct targeting of the orbital fibroblast via immunosuppression or nonimmunosuppressive drugs is emerging as a promising alternative.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The therapy of Graves’ disease (GD), when associated with orbitopathy (GO), aims at achieving restoration of euthyroidism as rapidly as possible. Available treatments for Graves’ hyperthyroidism include antithyroid drugs as the initial approach and subsequent definitive ablation of thyroid tissue by surgery or radioiodine therapy, as a causative treatment in GD is not available, due to the unknown pathogenic mechanisms at the basis of disease. The choice among the different therapeutic approaches for GD is based on several considerations such as the patient’s age, the thyroid volume, the presence of GO, and its degree of activity and severity. Restoration of permanent euthyroidism is frequently obtained only after definitive treatment with radioiodine or surgery. Based on the recent evidence, low-dose steroid prophylaxis should be encouraged when treating radioiodine patients with GD, with or without pre-existing GO, as long as risk factors for developing GO are identified [1–3]. On the other hand, no conclusive data are available so far in the literature on the possible risk of newly occurring GO or reactivation of previous GO after thyroidectomy.
Stable euthyroidism may induce spontaneous amelioration of milder degrees of GO [4, 5] and may contribute to the optimization of the potential responsiveness to immunosuppressive treatments in moderate-severe disease, when indicated. Euthyroidism also represents the essential condition in which patients may undergo rehabilitative surgical procedures in the burnt-out phase of GO.
Glucocorticoids
The treatment of moderate-severe GO is based on immunosuppressive therapy during the active phase of the disease. To date, glucocorticoids represent the mainstay of immunosuppression. However, treatment effectiveness much relies on great interindividual variability that may lead to either treatment failure or drug-induced toxicity. Significant response to therapy has been reported in as many as 60 % of patients with oral glucocorticoids (prednisone) [6, 7]. However, oral administration is more often associated with long-term side effects, including hepatotoxicity, Cushing’ syndrome, osteoporosis, glaucoma, and diabetes mellitus. More recent studies have shown that pulsed intravenous methylprednisolone (ivMP) is more effective (70–80 % of patients) [8] and has a better safety profile compared with oral prednisone. Nevertheless, this modality of treatment is also burdened by the occurrence of serious cardiovascular and hepatic morbidity that has led to reduction of methylprednisolone pulse doses in more recent years, down to a cumulative dose not larger than 8 g [9]. This dose is considered safe, but strict monitoring of liver function tests, hepatitis virus markers, serum glycemia, and blood pressure is warranted. A recent multicenter clinical trial of EUGOGO [10] has shown that an effective treatment schedule and dose of ivMP is 830 mg methylprednisolone administered weekly for 6 weeks followed by 415 mg for another 6 weeks, up to a cumulative dose of 7.47 g. This treatment schedule results in a short-term and transient advantage over lower doses, although it is associated with a slightly greater toxicity. Therefore it has been suggested that this dose regimen may be used in more severe cases of GO while an intermediate dose regimen may be used in most cases with moderate disease. The limitation of such treatment is that 20–30 % of patients are poorly responsive or unresponsive at all to ivMP and approximately 10–20 % of patients present with disease reactivation after drug withdrawal [9].
Targeted Therapy
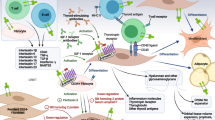
Over the past decade, innovative treatments have been sought based on better understanding of the mechanisms involved in GO pathogenesis. Some authors have recently proposed to directly target orbital tissue remodeling that results in tissue expansion in GO, without interfering with the immune reactions occurring in the orbit. Immunosuppression, on the other hand, would target the main players involved in the active, inflammatory phase of GO. These may be the antigens expressed on the target organ of inflammation, namely the TSH receptor (TSH-R) and the IGF-1 receptor (IGF-1R) on the fibroblasts, the cytokines and other humoral factors involved in the various stages of disease progression, and the immune effector cells, B and T cells (Figs. 23.1, 23.2, and 23.3).
Targets for Nonimmunosuppressive Therapy
It is well known that in GO adipogenesis and hyaluronic acid overproduction are the two central mechanisms leading to orbital tissue expansion that are driven by signal transduction through the TSH-R and IGF1-R [11, 12] (Fig. 23.1). Recently, Zhang and coworkers employed in vitro orbital fibroblasts and orbital fat tissue models to mimic GO pathology and were successful in inhibiting both adipogenesis and hyaluronic acid overproduction by blocking PIK3/mTORC1 signaling cascades [13]. In particular, by using inhibitors of PIK31A, PIK31B, and mTORC1, these authors demonstrated that mTORC1 is a critical player of adipogenesis, while PIK31A is more responsible for HA production. The PI3K/mTOR signaling pathway regulates many basic biological processes such as cell proliferation, survival, migration, glucose metabolism, and nutriment sensors [14, 15]. Targeting of this pathway to improve cancer control has been an intense and promising research field in the last decade. However, first-generation inhibitors, such as wortmannin, LY294002, or rapamycin, and its derivatives have shown undesirable side effects and low specificity in some experiments [16–18]. For these reasons, second-generation inhibitors with improved specificity and pharmacological properties have been developed and are currently used in clinical trials in patients with refractory cancers [19–21]. Another possible novel target for therapy might be the platelet-derived growth factor (PDGF) receptor, particularly the PDGF-BB isoform which has recently been found expressed and increased in GO orbital tissue [22]. PDGF-BB has been shown to markedly activate proliferation and production of pro-inflammatory cytokines such as CCL2, IL-6, IL-8, as well as hyaluronic acid by orbital fibroblasts [22], thus representing an attractive therapeutic target in GO treatment. Two small molecule-tyrosine kinase inhibitors, imatinib mesylate and nilotinib, have recently been demonstrated to prevent PDGF-induced PDGF-R autophosphorylation and signaling on orbital fibroblast of patients with GO [22] leading to block the PDGF-BB and PDGF-AB induced proliferation, hyaluronic and cytokine production by orbital fibroblasts [22–24]. Since the use of these two compounds has been associated with serious side effects [25], a structurally different tyrosine kinase inhibitor, dasatinib, has been developed and currently approved as a second-line therapy for treatment of chronic myeloid leukemia. Dasatinib has been shown to reduce the production of the extracellular matrix components fibronectin and collagen by skin fibroblasts in a systemic sclerosis patient [26], and very recently it has been found to suppress hyaluronic synthetase 2 (HAS-2), CCL2, IL-6, and IL-8 mRNA levels in orbital tissue from active GO, thus confirming that this or similar compounds may represent a promising therapeutic approach [27].
Targeting the TSH Receptor and the IGF-1 Receptor
Over the past few years some TSH analogs, called small TSH molecules, have been chemically synthesized. These low molecular weight compounds can be produced in large quantities and can be administered orally, since they are absorbed by the gastrointestinal tract. These molecules can be used as probes of TSH-R biology or for the treatment of both GD and GO, as they have been tested for their effects on both thyrocytes and orbital fibroblasts (Fig. 23.1). Three classes of small molecules have been developed: (1) TSH-R agonists (ligands that activate receptors), (2) neutral antagonists (ligands that inhibit receptor activation by agonists), and (3) inverse agonists (ligands that inhibit receptor activation by agonists and also basal or constitutive activity) [28].
In primary cultures of human thyrocytes, TSH-R agonists increase mRNA levels of thyroglobulin (Tg), thyroperoxidase (TPO), sodium-iodide symporter (NIS), and deiodinase type 2; more importantly, they increase serum thyroxine and radioiodine uptake by the mouse thyroid gland, after absorption from the gastrointestinal tract following administration by esophageal gavage [29]. The antagonists of TSH-R bind to the transmembrane region of the receptor and act in an allosteric manner by preventing the conformational changes necessary for receptor activation, without interfering with TSH or TSAb binding [30]. The inverse TSH-R agonists decrease the expression of the mRNAs of TPO, TSH-R, Tg, and NIS in the absence of any agonist in primary cultures of human thyrocytes [31], supporting the hypothesis that they could be used to suppress TSH-independent signaling in humans. TSH-R analogs, antagonists, and TSH-R inverse agonists represent an emerging novel class of therapeutic agents for both GD and GO.
By modifying the chemical structure of the TSH-R agonist NCGC00161870 [32], a series of TSH-R antagonists was developed. Among those, NCGC00242595 is a neutral antagonist while NCGC00161856 and the NCGC00229600 are inverse agonists. This latter compound was shown to inhibit the activation of TSH-R signaling stimulated by TSAb from GD patients’ sera in a model cell system and in primary human thyrocytes [33]. More recently, a new compound NCGC00242364 (ANTAG3) has been described that appears effective in inhibiting thyroid gland stimulation by inhibition of TSH-R activation in mice in vitro [34]. It is possible to speculate on the potential use of these molecules in GD patients who are most likely to achieve remission or in patients with thyroid storm, or in those in whom a rapid control of thyrotoxicosis is required [35].
TSH-R antagonists may also represent a novel approach for the therapy of GO, as they can inhibit the activation of TSH-R on Graves’ orbital fibroblasts. Recently, it has been demonstrated that the TSH-R autoantibody M22 is able to stimulate cAMP production by GO orbital fibroblasts and that this stimulation can be inhibited by TSH-R small molecule antagonists [36]. NCGC00229600 and Org-274179-0 have been tested on both undifferentiated orbital fibroblasts and orbital fibroblasts differentiated into adipocytes. NCGC00229600 has been found to inhibit both constitutive and GD-IgG or TSH-stimulated cAMP production in model cells overexpressing human TSH-R (HEK-EM293) and in human thyroid cell cultures [33], and to also inhibit the production and accumulation of HA in the orbit [37]. Org-274179-0 dose dependently inhibited cAMP production induced by rhTSH in a hTSH-R-expressing CHO cell line [38] and inhibited cAMP production in differentiated human orbital fibroblasts. More recently, a novel compound ANTAG3 was proposed as a possible useful small TSH molecule because of its ability to inhibit TSH-R signaling in other tissues expressing TSH-R, including orbital fibroblasts/preadipocytes and adipocytes [34].
The IGF-1R has been shown to be co-expressed in orbital fibroblasts with the TSH-R in GO [39]; blocking IGF-1R appears to attenuate TSH-dependent signaling [40]. Teprotumumab (RV 001, R1507) is a specific fully human monoclonal antibody that binds to the extracellular-subunit domain of IGF-1R and has been developed as a therapeutic strategy for several types of solid tumors and lymphomas [41]. Very recently, Chen and coworkers have demonstrated that teprotumumab is able either to decrease the expression of TSH-R and IGF-1R on fibrocytes or to attenuate TSH-dependent IL-6 and IL-8 expression and Akt phosphorylation [42]. Teprotumumab is currently under investigation in patients with moderate-severe GO in a phase 2 multicenter placebo-controlled randomized clinical trial conducted in the USA and in Europe.
Cytokines
The immune reactions in orbital tissues appear to depend on resident immune cells or cells recruited from the bone marrow through the expression and release of cytokines. It has been shown that in the active phase of GO, there is a predominant production of pro-inflammatory and Th1-derived cytokines such as IL-6 and IL-1, and IFN-gamma-induced chemokines, such as CXCL10. Th2-derived cytokines, including IL-4, IL-5, and IL-10, are more likely associated with the inactive phase of GO (Fig. 23.2).
Tumor necrosis factor-α (TNF-α) is a naturally occurring cytokine that plays a pivotal role in inflammatory and immune responses. The progressive understanding of the pathogenic processes involved in autoimmune conditions has driven the development of biological compounds that target specific inflammatory mediators, with the first available class represented by TNF inhibitors (etanercept, infliximab, adalimumab, certolizumab). Subsequently, other molecules targeting different immune pathways have been approved, including the IL-6 receptor antagonist tocilizumab and the IL-1 inhibitor anakinra.
Etanercept is a recombinant dimeric fusion protein consisting of two molecules of the soluble, extracellular ligand-binding portion of the human 75 kDa TNF receptor linked to the Fc portion of human immunoglobulin G1. It binds TNF and lymphotoxin-alfa and blocks the interaction of TNF with receptors on the cell surface, thereby preventing TNF-mediated inflammatory cellular responses and modulating the effect of other TNF-induced or regulated molecules [43]. Based on the evidence for a role of TNF in the pathogenesis of GO [44], in 2005 Paridaens et al. [45] have treated in a pilot study ten patients with active moderate-severe GO and noted clinical improvement of the soft tissue signs in six of ten. The authors could not rule out that the improvement was due to the natural course of the disease and could not show an advantage over therapy with ivMP, in terms of efficacy and side effects.
Another important pathway in active GO is represented by the IL-6/sIL-6 receptor system. Elevated serum sIL-6R concentrations were in fact measured in patients with active GO [46]. Tocilizumab is a recombinant, humanized monoclonal antibody that acts as an interleukin (IL)-6 receptor antagonist and binds selectively and competitively to soluble and membrane-expressed IL-6 receptors, thereby blocking IL-6 signal transduction. Several studies demonstrated the efficacy of intravenous tocilizumab in improving disease activity, structural joint damage, and/or HR-QOL in patients with early or long-standing rheumatoid arthritis, including those with refractory to standard therapy. Promising results have been recently obtained in a study in patients with GO refractory to ivMP treatment [47]. After therapy with tocilizumab, CAS improved in 18/18 patients, proptosis decreased in 13/18, and ocular motility improved in 15/18. One patient with compressive optic neuropathy also improved and did not undergo orbital decompression. These positive preliminary results warrant further clinical trials.
In a study by Cawood et al. [48], a synergic effect on adipogenesis of cigarette smoke extract and IL-1 has been observed in an in vitro model of GO. This synergic effect may explain why smoking in the presence of local orbital inflammatory response driven by cytokines may result in the increased frequency and severity of GO in smokers. In this study, the authors used an IL-1 antagonist, employed in the treatment of rheumatoid arthritis, to block the IL-1 stimulatory effect on adipogenesis and prevent the synergic effect of the smoke extract. This observation may have important therapeutic implications for the treatment of GO, although this hypothesis has not been challenged further in a therapeutic trial.
B Cell Depletion
Rituximab
Rituximab (RTX) has been used off-label in various autoimmune disorders but is approved for clinical use only in rheumatoid arthritis (RA) and antineutrophil cytoplasmic antibody-associated (ANCA) vasculitis. While the success of B cell-depleting therapy has reinforced the value of this approach in several autoimmune disease, many questions on the disparate effect of RTX in specific disease states remain unanswered. In general, RTX depletes more than 95 % of mature B cells in blood and primary lymphoid organs after 2 days by a single treatment, but it is not clear if treatment efficacy is dependent upon full or only partial B cell depletion or on targeting of specific B cell subsets [49]. The entire wide spectrum of B cell functions can be affected by RTX depletion.
Effects on Antibody Production
Germinal centers are functional sites where classical reactions leading to high-affinity antibodies production occur, although T cell-dependent reactions may even occur in extrafollicular sites [50]. Following antigen-specific proliferation, B cells enter into the germinal center microenvironment, where they diversify their antigen receptors and generate pools of long-lived memory B cells [51] which give rise to long-lived plasma cells within the bone marrow that are responsible for producing and maintaining serum antibody levels [52] (Fig. 23.3). Recent work has shown that germinal centers are constituted of a distinct dark zone in which proliferation of B cells results from interaction of the autoantigen(s) and MHC class II molecules presented to follicular helper T cells (classical germinal center autommunity) and of a light zone in which B cell autoreactive clones after clonal expansion are activated by irrelevant selecting antigens bound to follicular dendritic cells (bystander germinal center autoimmunity) [53]. Therefore, B cells trafficking to germinal centers are continuously involved in cycles of proliferation and selection and, when in the light zone, B cells are in close contact with dendritic cells and present antigen to helper T cells [54]. Interactions of B and T cells in germinal centers depend on the availability of the antigen stimulating proliferation and selection of B cells, with potentially important implications for B cell-depleting therapies. RTX may not sufficiently deplete B cells in germinal centers or may affect only specific clones arising from the different pathways described within the germinal centers or even extrafollicular lymphoid structures and, therefore, may not impact on B and T cell interaction, with resulting little therapeutic impact on pathogenic specific autoantibodies [55]. Autoantibodies may be pathogenic through direct binding to specific receptors (e.g., the TSH receptor on the thyrocyte membrane in Graves’ disease) or through the formation of immune complexes in tissues that locally activate complement reactions and induce inflammation [56]. Although in experimental models there is no direct evidence that autoantibodies alone initiate autoimmune disease, it is well known that they are associated with the disease and change in relation to the disease course, suggesting their involvement in the mechanisms of disease pathogenesis.
Antigen Presentation and Cytokines Production
B cells are also important antigen-presenting cells in the initiation of immune responses [57, 58] and in the production of cytokines and chemokines, including GM-CSF, IL-10, IL-4, IL-6, lymphotoxin-α, TGF-β, and IFN-γ [59, 60]. Decrease of cytokines levels after RTX treatment might be an indirect effect of B cell depletion on CD4+ T cell differentiation into Th1 and Th2 cytokine subsets and may contribute to improvement of disease progression, as has been observed in primary Sjogren syndrome [61].
Effects on T Cells
Unexpected depletion of T cells, mainly CD4+ cells, has been recently observed in patients with RA treated with RTX [62] (Fig. 23.3). Peripheral depletion of T cell was delayed, as compared to that of B cell, but likewise persisted as long as 6 months. Interestingly, T cell depletion was shown to correlate with the patients’ clinical response better than B cell depletion and therefore it has been suggested that T cell count may help monitoring response to RTX therapy. The implications of this data are that RTX may be indirectly responsible also for the depletion of autoreactive T cells, as a consequence of the decrease of T cell promoting cytokines and chemokines released by B cells, depleted by RTX. In addition, RTX may target T cells expressing low level CD20, which have been reported to account for as many as 5 % peripheral blood CD3+ T cells in patients with RA [63]. These very recent findings disclose a new scenario in the mechanism(s) by which RTX may impact on the direct pathogenetic reactions of autoimmune disease.
Regulatory B Cells
The IL-10-producing subset of B cells, known as regulatory B cells (B regs) or B10 cells [64], plays an important role in the suppression of autoimmune and inflammatory disease. IL-10 induces suppression of both Th1 and Th2 cytokine polarization and inhibits antigen presentation and pro-inflammatory cytokine production by monocytes and macrophages [65]. As a consequence of B cell depletion, there might be disease exacerbation in some autoimmune conditions because IL-10-producing cells, that inhibit regulation on T cell-mediated inflammatory responses, are also eliminated. RTX has in fact been reported to exacerbate ulcerative colitis [66, 67] and trigger psoriasis [68], both conditions representing Th1-mediated autoimmune conditions.
Pharmacokinetics and Dosing
Pharmacokinetics and pharmacodynamics studies performed in patients with B cell lymphomas have shown that serum concentration of RTX usually correlates directly with response and inversely with tumor mass [69]. Reports of variable half lives (11–105 h) may be the result of the different tumor burden and of the changes of CD20 expression on B cells, consequent to repeated RTX administration [70]. In RA, RTX half life has been reported to be as long as 20 days after two doses of 1,000 mg, the dose being used in most autoimmune diseases [71]. Studies that have directly addressed the most appropriate dose/response relationship of RTX are lacking. A recent meta-analysis of randomized trials in RA has shown no significant differences in the primary clinical outcomes when 1,000 mg twice was compared to 500 mg twice, 2 weeks apart [72]. The incidence of first infusion reactions was also decreased with the low-dose RTX treatment. The use of lower RTX doses may ultimately lead to significant reduction of treatment costs for a chronic disease, making it affordable by more clinical centers [70].
Side Effects
Infusion-related reactions are the most frequently reported side effects of RTX [73]. These reactions may be present in about 10–30 % of patients at first infusion and can be severe, but reversible. Release of pro-inflammatory cytokines from macrophages, monocytes, lymphocytes, and NK cells is the underlying mechanism. Activation of complement cascade may be responsible for fever, chill, and skin rashes [74]. Interestingly, during complement activation small fragments (C3a and C5a), which function as anaphylatoxins, help recruit effector cells to the site of inflammation and bind on locally infiltrating macrophages and enhance ADCC activity [75].
Among major side effects, infections have been attributed to the decrease of immunoglobulin levels after RTX repeated doses. Recently, a large retrospective study on 191 patients affected with multisystem autoimmune disease has looked at the incidence, severity, and complications of hypogammaglobulinemia as a consequence of combined immunosuppression with steroids, cyclophosphamide, and RTX [76]. The study has shown that although RTX therapy induced relative low immunoglobulin G levels, severe infections observed in the patients were associated with higher exposure to steroids but not to hypogammaglobulinemia. Another recent review of over 3,000 patients with rheumatoid arthritis showed comparable serious infection rates in those treated with RTX versus placebo plus methotrexate [77]. Data from these large studies are consistent with RTX being considered a safe therapy at least in the population affected with RA, but probably in many autoimmune disease in which lower therapeutic doses may be adopted.
Progressive multifocal leukoencephalopathy (PML) has rarely been reported in patients receiving RTX, especially those with systemic lupus erythematosus (SLE). It is again important to point out that all these patients had previously been treated with other immunosuppressive therapies including cyclophosphamide, azathioprine and even steroids, oral prednisone, or intravenous steroids [78]. Of note, more than 40 % of cases of PML have been reported in patients with SLE who were only minimally immunosuppressed, as if SLE itself may predispose for PML [79].
Other Monoclonal Antibodies Indirectly Targeting B Cells
Atacicept is a fusion protein of the human IgG Fc protein and the BAFF/APRIL receptor TACI. Targeting BAFF or APRIL leads to depletion of B cell progenitors (Fig. 23.3), but not memory B cells; therefore humoral immunity and memory responses to pathogens remain intact. Belimumab, an mAb against BAFF, has also been tried in patients with RA and SLE and has shown clear biologic effects on B cells and Ig levels with moderate clinical benefits [80, 81] in SLE, but only modest clinical benefits compared with placebo [82] in RA. Increased serum BAFF concentrations have been detected in patients with Hashimoto’ thyroiditis [83] and more recently in those with Graves’ disease with and without GO [84], suggesting that anti-BAFF therapy may be an option in the management of Graves’ disease.
B Cell Depletion Therapy in Graves’ Disease and Orbitopathy
Effects of RTX in Graves’ Disease
One controlled [85] and two non-controlled clinical trials have studied the effect of RTX on the hyperthyroidism of GD [86, 87], but included a rather limited number of patients with inconsistence of the clinical parameters considered. In addition, these preliminary studies have been conducted employing variable schedule and dosing of RTX, from 375 mg/m2 for four cycles to 1,000 mg twice, 2 weeks apart. El Fassi et al. [85] treated ten patients with newly diagnosed hyperthyroidism with methimazole (MMI) and RTX and ten with only MMI until they became euthyroid. Within 1 year of follow-up all patients treated with MMI alone, but only six of ten treated with MMI and RTX had relapse of hyperthyroidism. Those who did not relapse had persistently low values of serum TRAb levels, considered predictive of sustained remission after RTX. Peripheral B cells were not measured and therefore changes in serum TRAb could not be related to either B cell depletion after RTX or B cell return in the peripheral blood [84]. In a follow-up study, El Fassi et al. [88] proposed that RTX treatment in GD patients may favorably affect disease remission by distinctively acting on the TSAb subpopulation with TRAb, as a result of the effect of RTX on autoreactive short-lived TSAb-producing plasma cells [89].
Heemstra et al. [87] treated 13 patients with relapsing GD, of whom three with mild GO (23 %). Ten patients were also treated with MMI, when hyperthyroid. In four of 13 patients hyperthyroidism relapsed 26 weeks after RTX treatment, while in nine stable euthyroidism was observed for a median of 18 months. Similarly to what reported by El Fassi et al. [85], GD patients who remained euthyroid had relatively low serum TRAb level before RTX therapy. It is unclear why RTX treatment would have no effect on GD patients who were more hyperthyroid and had higher serum TRAb levels, who eventually needed radioiodine therapy. In the study of Salvi et al. [86] none of nine patients treated had improvement of thyroid function after RTX and required MMI to maintain euthyroidism. Of note, one patient, upon MMI withdrawal (for 7–8 days), had a very rapid relapse of hyperthyroidism associated with a surge of serum TRAb, while still being totally B cell-depleted in the peripheral blood after RTX [85]. More recently, Vannucchi et al. [90] were not able to observe a specific effect of RTX on serum TSAb autoantibodies, which did not change after therapy but fluctuated with an identical pattern to serum TRAb in both hyperthyroid and euthyroid GD patients. More studies are needed to address the effect of RTX on TSH receptor autoantibodies and a more conclusive interpretation on the potential role of RTX on the remission of GD hyperthyroidism can only be obtained from larger prospective, controlled studies.
RTX in GO: Dosing and Efficacy
Since the first report on successful treatment of one patient with moderate-severe GO [91], several non-controlled studies on the effects of RTX in GO have appeared in the literature, reporting data on as many as 43 patients. In addition, one randomized controlled trial comparing RTX to placebo [92] and one comparing RTX to steroids [93] in moderate-severe GO have just been completed. Only the preliminary results of these studies have so far been presented and definitive results are awaited.
Dosing schedules of RTX employed in the treatment of GO have been quite different and the lack of randomized trials and dose finding studies leaves us with the question of the appropriate dose for therapy of active GO unanswered. Some investigators have underscored that RTX may be effective in patients with GO even at lower doses than currently suggested in autoimmune rheumatic disease, although the dose of the drug has never been addressed as a study outcome. The infusion of 1,000 mg twice, with a 2-week interval, standardized for the treatment of RA and other autoimmune diseases has been reported to be effective in most open studies [84, 94, 95] and in case reports [91, 96]. The standard RTX dose used in lymphomas and neoplasia of 375 mg/sq meter has also been used successfully in a randomized controlled trial in GD patients [85], described above. Total peripheral B cell depletion following very low-dose RTX was shown in a study by Salvi et al. [97] in two patients in whom RTX was discontinued because of the development of a transient cytokine release reaction, after receiving only 100 mg of the drug. Interestingly, after spontaneous resolution of the side effect, clinical improvement with GO inactivation occurred within a few weeks, despite the administration of a dose about 20 times less than the standard dose used in systemic autoimmune disease. More recently, Mitchell et al. [95] have treated nine patients with steroid-refractory GO, of whom five (55 %) had signs suggestive of DON. While two patients received RTX at the full dose of 1,000 mg twice, six received only 500 mg twice and one patient three times, based on the attainment of peripheral B cell depletion. Monitoring of peripheral B cell depletion in order to titrate the RTX dose has in fact been suggested by some authors in autoimmune renal disease [98]. GO improved in all active patients, with four of nine patients (44.4 %) experiencing minor side effects at the first infusion. Patients with signs of DON also improved their NOSPECS score.
In earlier reports [85, 91] RTX was mainly used in patients with active GO who were unresponsive to standard ivMP therapy. They had a significant decrease of the CAS (<3) and improvement of ocular motility, as early as 4–6 weeks after RTX, that persisted without any additional therapy. Subsequently, RTX therapy has been used as a first-line treatment in patients not treated previously with steroids. In the open study of Salvi et al. [86], the mean CAS values of nine patients with active GO significantly decreased from 4.7 to 1.8 at the end of follow-up and also proptosis, eye muscle motility, and signs of soft tissue inflammation improved significantly in response to RTX. Reactivation of GO was never observed after RTX, but it is known to occur in 10–20 % of patients treated with steroids [10]. In the study of Khanna et al. [94] six patients with active and severe GO, unresponsive to glucocorticoid therapy, were treated concomitantly with steroid therapy. RTX (1,000 mg, twice) had a rapid and sustained therapeutic effect on both activity and severity. While the CAS decreased significantly at 8 weeks and remained low at 6 months, no patients improved in extraocular motility or proptosis. Four of these patients, who had optic neuropathy, showed improvement of visual acuity within 4 weeks, with return to premorbid values at 8 weeks from treatment. When glucocorticoids were tapered off, there was no disease reactivation. Silkiss et al. [99] treated with RTX 12 patients with active GO administered at the dose of 1 g, 2 weeks apart. Disease inactivation was shown by a decrease of the mean CAS at 16 weeks as well as by a decrease of the mean Thyroid Associated Ophthalmopathy Scale (TAOS), as modified by Dolman and Rootman (VISA Classification) [100]. Patients were studied up to 52 weeks of follow-up, with no evidence of disease reactivation and side effects.
In contrast to all these reports, failure of RTX has been reported in one patient whose GO did not respond to therapy and subsequently progressed to acute DON [101]. Two similar cases have been observed among the series of patients treated by Stan et al. [92], in the preliminary results of their randomized controlled study. It is possible that in these patients subclinical DON was already present at the time of therapy and that the orbital edema caused by cytokine release after the administration of RTX may have increased intraorbital tissue congestion and optic nerve compression. On the other hand, RTX has also been employed successfully in another ten patients with DON [94, 95, 100], resulting in improvement of visual sight.
This data need to be confirmed in larger studies and until then we suggest caution in administering RTX in severe disease, particularly when patients have GO of long duration or subclinical DON.
The preliminary results of two randomized clinical trials employing RTX in GO have just been completed and preliminary results recently presented. Salvi et al. [93] have compared RTX with ivMP in patients with active moderate-severe GO and studied the decrease of the CAS as a primary end point. The CAS decreased more significantly after RTX, whether patients had received 1,000 mg twice or a single dose of 500 mg and, at 24 weeks, 100 % of patients after RTX improved compared to 69 % after ivMP (P < 0.001). Disease reactivation was never observed in patients treated with RTX, but in five after ivMP. Data on secondary end points (total eye score, motility and quality of life) will allow to assess whether RTX acts as a disease modifying therapy, compared to steroids. Stan and colleagues [92] did not find RTX effective in treating active GO, when compared to placebo. The study was conducted on 21 patients, of whom two, after RTX, had disease progression and developed optic neuropathy. The publication of their final data will probably allow comparing the differences in the criteria of recruitment of the patients, i.e., disease duration, degree of activity, and others that might account for these discrepant results. We envisage that further and larger randomized controlled trials will be needed for definitive data on the potential disease modifying role of RTX in GO and its superiority over standard treatment with steroids.
References
Träisk F, Tallstedt L, Abraham-Nordling M, Andersson T, Berg G, Calissendorff J, Hallengren B, Hedner P, Lantz M, Nyström E, Ponjavic V, Taube A, Törring O, Wallin G, Asman P, Lundell G. Thyroid Study Group of TT 96. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94(10):3700–7. doi:10.1210/jc.2009-0747.
Lai A, Sassi L, Compri E, Marino F, Sivelli P, Piantanida E, Tanda ML, Bartalena L. Lower dose prednisone prevents radioiodine-associated exacerbation of initially mild or absent graves’ orbitopathy: a retrospective cohort study. J Clin Endocrinol Metab. 2010;95(3):1333–7. doi:10.1210/jc.2009-2130.
Vannucchi G, Campi I, Covelli D, Dazzi D, Currò N, Simonetta S, Ratiglia R, Beck-Peccoz P, Salvi M. Graves’ orbitopathy activation after radioactive iodine therapy with and without steroid prophylaxis. J Clin Endocrinol Metab. 2009;94(9):3381–6. doi:10.1210/jc.2009-0506.
Perros P, Crombie AL, Kendall-Taylor P. Natural history of thyroid associated ophthalmopathy. Clin Endocrinol (Oxf). 1995;42(1):45–50.
Tanda ML, Piantanida E, Liparulo L, Veronesi G, Lai A, Sassi L, Pariani N, Gallo D, Azzolini C, Ferrario M, Bartalena L. Prevalence and natural history of Graves’ orbitopathy in a large series of patients with newly diagnosed graves’ hyperthyroidism seen at a single center. J Clin Endocrinol Metab. 2013;98(4):1443–9. doi:10.1210/jc.2012-3873.
Marcocci C, Bartalena L, Tanda ML, Manetti L, Dell’Unto E, Rocchi R, Barbesino G, Mazzi B, Bartolomei MP, Lepri P, Cartei F, Nardi M, Pinchera A. Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves’ ophthalmopathy: results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab. 2001;86:3562–7.
Kahaly GJ, Pitz S, Hommel G, Dittmar M. Randomized, single-blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab. 2005;90:5234–40.
Kahaly GJ, Shimony O, Gellman YN, Lytton SD, Eshkar-Sebban L, Rosenblum N, Refaeli E, Kassem S, Ilany J, Naor D. Regulatory T-cells in Graves’ orbitopathy: baseline findings and immunomodulation by anti-T lymphocyte globulin. J Clin Endocrinol Metab. 2011;96:422–9.
Zang S, Ponto KA, Kahaly GJ. Clinical review: Intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab. 2011;96(2):320–32. doi:10.1210/jc.2010-1962.
Bartalena L, Krassas GE, Wiersinga W, Marcocci C, Salvi M, Daumerie C, Bournaud C, Stahl M, Sassi L, Veronesi G, Azzolini C, Boboridis KG, Mourits MP, Soeters MR, Baldeschi L, Nardi M, Currò N, Boschi A, Bernard M, von Arx G, European Group on Graves’ Orbitopathy. European Group on Graves’ Orbitopathy Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97:4454–63.
Kumar S, Nadeem S, Stan MN, Coenen M, Bahn RS. A stimulatory TSH receptor antibody enhances adipogenesis via phosphoinositide 3-kinase activation in orbital preadipocytes from patients with Graves’ ophthalmopathy. J Mol Endocrinol. 2011;46(3):155–63. doi:10.1530/JME-11-0006.
Kumar S, Iyer S, Bauer H, Coenen M, Bahn RS. A stimulatory thyrotropin receptor antibody enhances hyaluronic acid synthesis in graves’ orbital fibroblasts: inhibition by an IGF-I receptor blocking antibody. J Clin Endocrinol Metab. 2012;97(5):1681–7. doi:10.1210/jc.2011-2890.
Zhang L, Grennan-Jones F, Draman MS, Lane C, Morris D, Dayan CM, Tee AR, Ludgate M. Possible targets for nonimmunosuppressive therapy of graves’ orbitopathy. J Clin Endocrinol Metab. 2014;99(7):E1183–90. doi:10.1210/jc.2013-4182.
Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signaling. Nat Rev Mol Cell Biol. 2010;11:329–41.
Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84.
Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR pathway–beyond rapalogs. Oncotarget. 2010;1(7):530–43.
Prevo R, Deutsch E, Sampson O, Diplexcito J, Cengel K, Harper J, O’Neill P, McKenna WG, Patel S, Bernhard EJ. Class I PI3 kinase inhibition by the pyridinylfuranopyrimidine inhibitor PI-103 enhances tumor radiosensitivity. Cancer Res. 2008;68(14):5915–23.
Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2006;26(13):1932–40.
Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9(5):341–9.
Kurtz J-E, Ray-Coquard I. PI3 kinase inhibitors in the clinic: an update. Anticancer Res. 2012;32(7):2463–70.
Potiron VA, Abderrahmani R, Giang E, Chiavassa S, Di Tomaso E, Maira SM, Paris F, Supiot S. Radiosensitization of prostate cancer cells by the dual PI3K/mTOR inhibitor BEZ235 under normoxic and hypoxic conditions. Radiother Oncol. 2013;106(1):138–46.
van Steensel L, Paridaens D, Schrijver B, Dingjan GM, van Daele PL, van Hagen PM, van den Bosch WA, Drexhage HA, Hooijkaas H, Dik WA. Imatinib mesylate and AMN107 inhibit PDGF-signaling in orbital fibroblasts: a potential treatment for Graves’ ophthalmopathy. Invest Ophthalmol Vis Sci. 2009;50(7):3091–8. doi:10.1167/iovs. 08-2443.
van Steensel L, Paridaens D, Dingjan GM, van Daele PL, van Hagen PM, Kuijpers RW, van den Bosch WA, Drexhage HA, Hooijkaas H, Dik WA. Platelet-derived growth factor-BB: a stimulus for cytokine production by orbital fibroblasts in Graves’ ophthalmopathy. Invest Ophthalmol Vis Sci. 2010;51(2):1002–7. doi:10.1167/iovs. 09-4338.
van Steensel L, Paridaens D, van Meurs M, van Hagen PM, van den Bosch WA, Kuijpers RW, Drexhage HA, Hooijkaas H, Dik WA. Orbit-infiltrating mast cells, monocytes, and macrophages produce PDGF isoforms that orchestrate orbital fibroblast activation in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2012;97(3):E400–8. doi:10.1210/jc.2011-2697.
Kim TD, Rea D, Schwarz M, Grille P, Nicolini FE, Rosti G, Levato L, Giles FJ, Dombret H, Mirault T, Labussière H, Lindhorst R, Haverkamp W, Buschmann I, Dörken B, le Coutre PD. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia. 2013;27(6):1316–21. doi:10.1038/leu.2013.70.
Akhmetshina A, Dees C, Pileckyte M, Maurer B, Axmann R, Jüngel A, Zwerina J, Gay S, Schett G, Distler O, Distler JH. Dual inhibition of c-abl and PDGF receptor signaling by dasatinib and nilotinib for the treatment of dermal fibrosis. FASEB J. 2008;22(7):2214–22. doi:10.1096/fj.07-105627.
Virakul S, Dalm VA, Paridaens D, van den Bosch WA, Hirankarn N, van Hagen PM, Dik WA. The tyrosine kinase inhibitor dasatinib effectively blocks PDGF-induced orbital fibroblast activation. Graefes Arch Clin Exp Ophthalmol. 2014;252(7):1101–9. doi:10.1007/s00417-014-2674-7.
Gershengorn MC, Neumann S. Update in TSH receptor agonists and antagonists. J Clin Endocrinol Metab. 2012;97(12):4287–92. doi:10.1210/jc.2012-3080.
Neumann S, Huang W, Titus S, Krause G, Kleinau G, Alberobello AT, Zheng W, Southall NT, Inglese J, Austin CP, Celi FS, Gavrilova O, Thomas CJ, Raaka BM, Gershengorn MC. Small-molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice. Proc Natl Acad Sci U S A. 2009;106(30):12471–6. doi:10.1073/pnas.0904506106.
Neumann S, Kleinau G, Costanzi S, Moore S, Jiang JK, Raaka BM, Thomas CJ, Krause G, Gershengorn MC. A low-molecular-weight antagonist for the human thyrotropin receptor with therapeutic potential for hyperthyroidism. Endocrinology. 2008;149(12):5945–50. doi:10.1210/en.2008-0836.
Neumann S, Huang W, Eliseeva E, Titus S, Thomas CJ, Gershengorn MC. A small molecule inverse agonist for the human thyroid-stimulating hormone receptor. Endocrinology. 2010;151(7):3454–9. doi:10.1210/en.2010-0199.
Kleinau G, Haas AK, Neumann S, Worth CL, Hoyer I, Furkert J, Rutz C, Gershengorn MC, Schülein R, Krause G. Signaling-sensitive amino acids surround the allosteric ligand binding site of the thyrotropin receptor. FASEB J. 2010;24(7):2347–54. doi:10.1096/fj.09-149146.
Neumann S, Eliseeva E, McCoy JG, Napolitano G, Giuliani C, Monaco F, Huang W, Gershengorn MC. A new small-molecule antagonist inhibits Graves’ disease antibody activation of the TSH receptor. J Clin Endocrinol Metab. 2011;96(2):548–54. doi:10.1210/jc.2010-1935.
Neumann S, Nir EA, Eliseeva E, Huang W, Marugan J, Xiao J, Dulcey AE, Gershengorn MC. A selective TSH receptor antagonist inhibits stimulation of thyroid function in female mice. Endocrinology. 2014;155(1):310–4. doi:10.1210/en.2013-1835.
Emerson CH. When will thyrotropin receptor antagonists and inverse thyrotropin receptor agonists become available for clinical use? Thyroid. 2011;21(8):817–9. doi:10.1089/thy.2011.2108.ed.
Turcu AF, Kumar S, Neumann S, Coenen M, Iyer S, Chiriboga P, Gershengorn MC, Bahn RS. A small molecule antagonist inhibits thyrotropin receptor antibody-induced orbital fibroblast functions involved in the pathogenesis of Graves ophthalmopathy. J Clin Endocrinol Metab. 2013;98(5):2153–9. doi:10.1210/jc.2013-1149.
Neumann S, Pope A, Geras-Raaka E, Raaka BM, Bahn RS, Gershengorn MC. A drug-like antagonist inhibits thyrotropin receptor-mediated stimulation of cAMP production in Graves’ orbital fibroblasts. Thyroid. 2012;22(8):839–43. doi:10.1089/thy.2011.0520.
van Zeijl CJ, van Koppen CJ, Surovtseva OV, de Gooyer ME, Plate R, Conti P, Karstens WJ, Timmers M, Saeed P, Wiersinga WM, Miltenburg AM, Fliers E, Boelen A. Complete inhibition of rhTSH-, Graves’ disease IgG-, and M22-induced cAMP production in differentiated orbital fibroblasts by a low-molecular-weight TSHR antagonist. J Clin Endocrinol Metab. 2012;97(5):E781–5. doi:10.1210/jc.2011-2931.
Smith TJ. Is IGF-I receptor a target for autoantibody generation in Graves’ disease? J Clin Endocrinol Metab. 2013;98(2):515–8. doi:10.1210/jc.2013-1004.
Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A, Gianoukakis AG, Douglas RS, Smith TJ. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol. 2008;181(6):4397–405.
Huang HJ, Angelo LS, Rodon J, Sun M, Kuenkele KP, Parsons HA, Trent JC, Kurzrock R. R1507, an anti-insulin-like growth factor-1 receptor (IGF-1R) antibody, and EWS/FLI-1 siRNA in Ewing’s sarcoma: convergence at the IGF/IGFR/Akt axis. PLoS One. 2011;6(10):e26060. doi:10.1371/journal.pone.0026060.
Chen H, Mester T, Raychaudhuri N, Kauh CY, Gupta S, Smith TJ, Douglas RS. Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. J Clin Endocrinol Metab. 2014;99(9):E1635–40. jc20141580.
Scott LJ. Etanercept: a review of its use in autoimmune inflammatory diseases. Drugs. 2014;18.
Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362(8):726–38. doi:10.1056/NEJMra0905750.
Paridaens D, van den Bosch WA, van der Loos TL, Krenning EP, van Hagen PM. The effect of etanercept on Graves’ ophthalmopathy: a pilot study. Eye (Lond). 2005;19(12):1286–9.
Salvi M, Girasole G, Pedrazzoni M, Passeri M, Giuliani N, Minelli R, Braverman LE, Roti E. Increased serum concentrations of interleukin-6 (IL-6) and soluble IL-6 receptor in patients with Graves’ disease. J Clin Endocrinol Metab. 1996;81(8):2976–9.
Pérez-Moreiras JV, Alvarez-López A, Gómez EC. Treatment of active corticosteroid-resistant graves’ orbitopathy. Ophthal Plast Reconstr Surg. 2014;30(2):162–7. doi:10.1097/IOP.0000000000000037.
Cawood TJ, Moriarty P, O’Farrelly C, O’Shea D. Smoking and thyroid-associated ophthalmopathy: a novel explanation of the biological link. J Clin Endocrinol Metab. 2007;92(1):59–64.
Dörner T, Lipsky PE. B cells: depletion or functional modulation in rheumatic diseases. Curr Opin Rheumatol. 2014;26(2):228–36.
Daridon C, Loddenkemper C, Spieckermann S, Kühl AA, Salama A, Burmester GR, Lipsky PE, Dörner T. Splenic proliferative lymphoid nodules distinct from germinal centers are sites of autoantigen stimulation in immune thrombocytopenia. Blood. 2012;120(25):5021–31.
Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J Exp Med. 1998;187:885–95.
DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, Kelsoe G, Tedder TF. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180:361–71.
Dörner T, Giesecke C, Lipsky PE. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther. 2011;13(5):243.
Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–57.
Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, Wu Y, Meng YG, Gribling P, Lin Z, Nguyen K, Tran T, Zhang Y, Rosen H, Martin F, Chan AC. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174(2):817–26.
Abbas AK, Lichtman AH, Pober JS. (eds) B cell activation and antibody production. In: Cellular and Molecular Immunology, W.B. Saunders Co.:Philadelphia, 1991; pp. 186–203.
Kurt-Jones EA, Liano D, HayGlass KA, Benacerraf B, Sy MS, Abbas AK. The role of antigen-presenting B cells in T cell priming in vivo. Studies of B cell-deficient mice. J Immunol. 1988;140:3773–8.
Constant S, Schweitzer N, West J, Ranney P, Bottomly K. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J Immunol. 1995;155:734–3741.
Pistoia V. Production of cytokines by human B cells in health and disease. Immunol Today. 1997;18:343–50.
Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1(6):475–82.
Meijer JM, Meiners PM, Vissink A, Spijkervet FK, Abdulahad W, Kamminga N, Brouwer E, Kallenberg CG, Bootsma H. Effectiveness of rituximab treatment in primary Sjögren’s syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62(4):960–8.
Mélet J, Mulleman D, Goupille P, Ribourtout B, Watier H, Thibault G. Rituximab-induced T cell depletion in patients with rheumatoid arthritis: association with clinical response. Arthritis Rheum. 2013;65(11):2783–90.
Eggleton P, Bremer E. Direct and indirect rituximab-induced T-cell depletion: Comment on the article by Mélet et al. Arthritis Rheum. 2014. doi:10.1002/art.38347.
Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–50.
Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy-review of a new approach. Pharmacol Rev. 2003;55:241–69.
Goetz M, Atreya R, Ghalibafian M, Galle PR, Neurath MF. Exacerbation of ulcerative colitis after rituximab salvage therapy. Inflamm Bowel Dis. 2007;13:1365–8.
El Fassi D, Nielsen CH, Kjeldsen J, Clemmensen O, Hegedüs L. Ulcerative colitis following B lymphocyte depletion with rituximab in a patient with Graves’ disease. Gut. 2008;57:714–5.
Dass S, Vital EM, Emery P. Development of psoriasis after B cell depletion with rituximab. Arthritis Rheum. 2007;56:2715–8.
Cartron G, Trappe RU, Solal-Céligny P, Hallek M. Interindividual variability of response to rituximab : from biological origins to individualized therapies. Clin Cancer Res. 2011;17:19–30.
Pescovitz MD. Rituximab, an anti-CD20 monoclonal antibody: history and mechanism of action. Am J Transplant. 2006;6:859–66.
Edwards JC, Leandro MJ, Cambridge G. B lymphocyte depletion therapy with rituximab in rheumatoid arthritis. Rheum Dis Clin North Am. 2004;30:393–403.
Bredemeier M, de Oliveira FK, Rocha CM. Low- versus high dose rituximab for rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2014;66(2):228–35.
Descotes J. Immunotoxicity of monoclonal antibodies. MAbs. 2009;1:104–11.
van der Kolk LE, Grillo-López AJ, Baars JW, Hack CE, van Oers MH. Complement activation plays a key role in the side-effects of rituximab treatment. Br J Haematol. 2001;115:807–11.
Boross P, Jansen JH, de Haij S, Beurskens FJ, van der Poel CE, Bevaart L, Nederend M, Golay J, van de Winkel JG, Parren PW, Leusen JH. The in vivo mechanism of action of CD20 monoclonal antibodies depends on local tumor burden. Haematologica. 2011;96:1822–30.
Marco H, Smith RM, Jones RB, Guerry MJ, Catapano F, Burns S, Chaudhry AN, Smith KG, Jayne DR. The effect of rituximab therapy on immunoglobulin levels in patients with multisystem autoimmune disease. BMC Musculoskelet Disord. 2014;15:178.
van Vollenhoven RF, Emery P, Bingham 3rd CO, Keystone EC, Fleischmann RM, Furst DE, Tyson N, Collinson N, Lehane PB. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann Rheum Dis. 2013;72:1496–502.
Food and Drug Administration (2006) FDA Public Health Advisory: life-threatening brain infection in patients with systemic lupus erythematosus after Rituxan (rituximab) treatment. http://www.fda.gov/cder/drug/
Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy in patients with rheumatic diseases: are patients with systemic lupus erythematosus at particular risk? Autoimmun Rev. 2008;8:144–6.
Wallace DJ, Lisse J, Stohl W, McKay J, Boling E, Merrill JT. Belimumab (BmAb) reduces SLE disease activity and demonstrates durable bioactivity at 76 weeks. Arthritis Rheum. 2006;54 Suppl 9:S790.
Furie R, Stohl W, Ginzler EM, Becker M, Mishra N, Chatham W, Merrill JT, Weinstein A, McCune WJ, Zhong J, Cai W, Freimuth W. Belimumab Study Group. Biologic activity and safety of belimumab, a neutralizing anti-B-lymphocyte stimulator (BLyS) monoclonal antibody: a phase I trial in patients with systemic lupus erythematosus. Arthritis Res Ther. 2008;10:R109.
Mackay F, Groom JR, Tangye SG. An important role for B-cell activation factor and B cells in the pathogenesis of Sjogren’s syndrome. Curr Opin Rheumatol. 2007;19:406–13.
Fabris M, Grimaldi F, Villalta D, Picierno A, Fabro C, Bolzan M, De Vita S, Tonutti E. BLyS and April serum levels in patients with autoimmune thyroid diseases. Autoimmun Rev. 2010;9(3):165–9.
Vannucchi G, Covelli D, Currò N, Dazzi D, Maffini A, Campi I, Bonara P, Guastella C, Pignataro L, Ratiglia R, Beck-Peccoz P, Salvi M. Serum BAFF concentrations in patients with Graves’ disease and orbitopathy before and after immunosuppressive therapy. J Clin Endocrinol Metab. 2012;97(5):E755–9.
El Fassi D, Nielsen CH, Bonnema SJ, Hasselbalch HC, Hegedüs L. B lymphocyte depletion with the monoclonal antibody Rituximab in Graves’ disease. A controlled pilot study. J Clin Endocrinol Metab. 2007;92:1769–72.
Salvi M, Vannucchi G, Campi I, Currò N, Dazzi D, Simonetta S, Bonara P, Rossi S, Sina C, Guastella C, Ratiglia R, Beck-Peccoz P. Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol. 2007;156:33–40.
Heemstra KA, Toes RE, Sepers J, Pereira AM, Corssmit EP, Huizinga TW, Romijn JA, Smit JW. Rituximab in relapsing Graves’ disease, a phase II study. Eur J Endocrinol. 2008;159:609–15.
El Fassi D, Banga JP, Gilbert JA, Padoa C, Hegedüs L, Nielsen CH. Treatment of Graves’ disease with rituximab specifically reduces the production of thyroid stimulating autoantibodies. Clin Immunol. 2009;130:252–8.
Huang H, Benoist C, Mathis D. Rituximab specifically depletes short-lived autoreactive plasma cells in a mouse model of inflammatory arthritis. Proc Natl Acad Sci U S A. 2010;107:4658–63.
Vannucchi G, Campi I, Bonomi M, Covelli D, Dazzi D, Currò N, Simonetta S, Bonara P, Persani L, Guastella C, Wall J, Beck-Peccoz P, Salvi M. Rituximab treatment in patients with active Graves’ orbitopathy: effects on proinflammatory and humoral immune reactions. Clin Exp Immunol. 2010;31.
Salvi M, Vannucchi G, Campi I, Rossi S, Bonara P, Sbrozzi F, Guastella C, Avignone S, Pirola G, Ratiglia R, Beck-Peccoz P. Efficacy of rituximab treatment for thyroid-associated ophthalmopathy as a result of intraorbital B-cell depletion in one patient unresponsive to steroid immunosuppression. Eur J Endocrinol. 2006;154:511–7.
Stan MN, Garrity JA, Bradley EA, Woog JJ, Bahn MM, Brennan MD, Bryant SC, Achenbach SJ, Bahn RS. Randomized double-blind placebo-controlled trial of rituximab for treatment of Graves’ ophthalmopathy. 83rd Annual Meeting of the American Thyroid Association, San Juan, Puerto Rico, October 16–20th, 2013 (Abs #3)
Salvi M, Vannucchi G, Campi I, Covelli D, Currò N, Dazzi D, Avignone S, Sina C, Beck-Peccoz P. Double blind randomized controlled study of rituximab and intravenous steroid treatment in Graves’ orbitopathy (GO): analysis of the primary endpoint at 24 weeks. 37th Annual meeting of the European Thyroid Association, Leiden, The Netherlands, September 7-11th, 2013 (Abs).
Khanna D, Chong KK, Afifiyan NF, Hwang CJ, Lee DK, Garneau HC, Goldberg RA, Darwin CH, Smith TJ, Douglas RS. Rituximab treatment of patients with severe, corticosteroid-resistant thyroid-associated ophthalmopathy. Ophthalmology. 2010;117:133–9.
Mitchell AL, Gan EH, Morris M, Johnson K, Neoh C, Dickinson AJ, Perros P, Pearce SH. The effect of B cell depletion therapy on anti-TSH receptor antibodies and clinical outcome in glucocorticoid refractory Graves’ orbitopathy. Clin Endocrinol (Oxf). 2013;79:437–42.
Madaschi S, Rossini A, Formenti I, Lampasona V, Bianchi Marzoli S, Cammarata G, Politi L, Martinelli V, Bazzigaluppi E, Scavini M, Bosi E, Lanzi R. Treatment of thyroid-associated orbitopathy with rituximab – a novel therapy for an old disease: case report and literature review. Endocr Pract. 2010;16:677–85.
Salvi M, Vannucchi G, Currò N, Introna M, Rossi S, Bonara P, Covelli D, Dazzi D, Guastella C, Pignataro L, Ratiglia R, Golay J, Beck-Peccoz P. A small dose of rituximab may be sufficient to treat Graves’ orbitopathy: new insights into the mechanism of action. Arch Ophthalmol. 2012;130:122–4.
Cravedi P, Ruggenenti P, Sghirlanzoni MC, Remuzzi G. Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2007;2:932–7.
Silkiss RZ, Reier A, Coleman M, Lauer S. Rituximab for thyroid eye disease. Ophthal Plast Reconstr Surg. 2010;26:310–4.
Salvi M, Vannucchi G, Campi I, Currò N, Simonetta S, Covelli D, Pignataro L, Guastella C, Rossi S, Bonara P, Dazzi D, Ratiglia R, Beck-Peccoz P. Rituximab treatment in a patient with severe thyroid-associated ophthalmopathy: effects on orbital lymphocytic infiltrates. Clin Immunol. 2009;131:360–5.
Krassas GE, Stafilidou A, Boboridis KG. Failure of rituximab treatment in a case of severe thyroid ophthalmopathy unresponsive to steroids. Clin Endocrinol. 2010;72:853–5.
Acknowledgements
This work was supported in part by MIUR, Roma, and by Fondazione Cà Granda, IRCCS, Milano, Italy.
The authors have nothing to disclose. No conflicting relationship exists for the author.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Salvi, M., Vannucchi, G. (2015). Future Therapy for Graves’ Disease and Ophthalmopathy. In: Bahn, R. (eds) Graves' Disease. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2534-6_23
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2534-6_23
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2533-9
Online ISBN: 978-1-4939-2534-6
eBook Packages: MedicineMedicine (R0)