Abstract
The betalamic acid derivatives of betalain pigments (purple-red betacyanins and yellow betaxanthins) are dietary compounds occurring in a few plant foods including beets (Beta vulgaris) and cactus pears (Opuntia spp.). Belonging to betaxanthins, indicaxanthin is rich in yellow cactus pear (Opuntia ficus indica L., Mill). High dietary bioavailability of indicaxanthin in humans, as well as its physicochemical properties, radical-scavenging and antioxidant activities in various experimental models suggest this molecule as a promising nutraceutical agent and open perspectives for its applications. Life-long modulatory activity at the epigenetic level now appears as the new frontier to shed light on the beneficial effects from a chronic exposure at dietary concentrations of redox-active phytochemicals. Data obtained from studies on human intestinal carcinoma cell cultures show that indicaxanthin has the potential to affect global DNA methylation, revert onco-suppressor gene silencing, and induce arrest of cell growth. Elucidation of cell pathways and molecular mechanisms underlying epigenetic activities in various cells and conditions is needed to understand these benefits and limitations of dietary indicaxanthin.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Betalains
- Hormesis
- Xenohormesis
- Redox homeostasis
- Human bioavailability
- Intestinal absorption
- Safety
- Antioxidant protection
- Membrane activity
- Epigenetics
Introduction
In the recent 25 years, enormous progresses have been made in understanding the redox biology of cells and tissues. Epidemiological observations have revealed that fruit and vegetable diets have health benefits and protective functions against oxidative stress-based chronic disorders, such as cardiovascular disease and cancer, which lead to broad research on redox-active phytochemicals [1–3]. At first, these compounds were believed to act mostly by providing direct antioxidant protection by trapping radicals and/or chelating redox-active metals. A significant body of evidence, however, now supports the participation of these molecules in the regulation of a complex web of cell signaling as the means by which they affect cell activities and functions [4–6]. The physicochemical characteristics of phytochemicals can play important roles in cell signaling, including the regulation of the cell redox state by interfering with oxidant production and/or antioxidant defenses, interactions with signaling proteins, and the regulation of membrane-associated cell signaling. As a final target of these activities, the epigenetic modulation of gene expression has recently emerged as a finely tuned means to explain the effects of these compounds.
A so-called xenohormesis theory [7] has been put forward to explain why the plant kingdom is such a rich source of redox-active beneficial compounds (Fig. 7.1).
Redox-regulated cell functions in the context of hormesis and xenohormesis . In response to adverse environmental conditions such as dryness, light, heat, or exogenous stressors including wounding and oxidative stress [8–10] plants synthesize their own molecular defense, i.e., phytochemicals, that in turn modulate redox-regulated cellular adaptative responses, a process known as hormesis [11, 12]. Xenohormesis postulates that heterotrophs (animals and fungi) have evolved the ability to sense/interact with these compounds and use them as chemical cues finally inducing cell responses [13, 14]
Indicaxanthin
Chemical and Physicochemical Characteristics
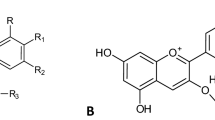
Indicaxanthin is a yellow pigment in the betalain class [15]. These nitrogenous compounds are restricted to 13 plant families of the Cariophyllales order and a few superior fungi in the genus Amanita of the Basidiomycetes [16], in which anthocyanins are absent and replaced [17]. Yellow and red beet (Beta vulgaris) and cactus pear (Opuntia genus) are the main food sources of these phytochemicals [18]. The edible berries of Rivina humilis L. were recently identified to be new sources [19]. The main common structure of all betalains is the dihydropyridine moiety from betalamic acid (Fig. 7.2a). This is conjugated with amino acids or the corresponding amines (including dopamine) to generate the yellow betaxanthins (λ m = 480 nm). Indicaxanthin, in particular, is the immonium derivative of proline with betalamic acid (Fig. 7.2b) [20]. The violet-red pigmented betacyanins (λ m = 535 nm) are derivatives of betanidin , the conjugate of betalamic acid with cyclo-DOPA (Fig. 7.2a). The fruit of Opuntia ficus indica (yellow variety) is by far the best source of indicaxanthin, with an amount from 8.5 mg/100 g fresh fruit pulp (Sicilian cultivated fruit) [21] to 15 mg/100 g fresh fruit pulp (Sicilian wild fruit, unpublished data from the authors’ laboratory). It also contains betanin , around one tenth of indicaxanthin [21]. As a comparison, the Rivina humilis fresh berries reportedly contain a minor amount of indicaxanthin, but up to 180 mg/100 g eight betaxanthins [19]. In accordance with the hormetic theory [11], it is not surprising that wild Sicilian cactus pear fruits possess higher amounts of betalains than cultivated fruits.
Betacyanins have been known for many years as additives in the food industry, due to their colorant properties, high solubility in water, pH stability in the range 3–7 [22], and the absence of toxicity [22, 23]. Renewed interest in betalains has come in the recent decade to characterize the redox chemistry and radical-scavenging and antioxidant activities of these molecules [21, 24–26], which has then stimulated a number of studies on their potential activities in biological environments and cell contexts.
The redox potential of indicaxanthin has been measured by cyclic voltammetry [21]. The differential pulse voltammogram showed two anodic waves, with calculated peak potentials of 611 and 895 mV indicating that indicaxanthin is able to donate its electrons to organic radicals commonly formed in cell environments, including alkyl-peroxyl and lipoperoxyl radicals [27], which has been shown [28].
Indicaxanthin is a cationized molecule, with a positive charge localized in proximity of the N1 nitrogen (Fig. 7.2b) and possesses a number of ionizable carboxyl groups. A recent computational analysis predicted the solubility parameters and the polar and nonpolar surface area (NPSA) of indicaxanthin, as well as the dissociation constants of the carboxyl groups [29]. According to these calculations, indicaxanthin mainly exists as a bis-anion within a large range of pH, including physiological pH, and the pH gradient relevant to the intestinal environment during digestion (pH 6.0–7.4) [30, 31], a condition used to investigate the mechanisms of the intestinal absorption [29]. According to the octanol/water partition coefficients ClogP and ClogD (pH 6.0), indicaxanthin has a moderately nonpolar character [29]. However, the calculated NPSA suggested that it has a quite large nonpolar surface, accounting for more than 50 % of the surface area, which appears to substantiate observations on its ability to interact with membranes [25, 32–34] and low-density lipoproteins (LDLs) [28, 35, 36]. Table 7.1 summarizes the chemical and physicochemical properties of indicaxanthin.
Interactions of Indicaxanthin with Membranes
Because it possesses ionizable groups (the charged portions) and lipophilic moieties, indicaxanthin may act as an amphiphilic-like compound at a physiological pH. These chemical properties are critical to drive interaction with cell surface, where bioactive compounds binds or interacts with lipids or/and membrane effectors to initiate cell signaling transduction or transports through the cell membrane to reach inner sites, including nuclear ones, possibly affecting regulation of gene expression.
The location of the pigment in vesicles of either L-R-dipalmitoyl-phosphatidylcholine (DPPC), or l-R-dimyristoyl-phosphatidylcholine (DMPC) has recently been investigated by monitoring the characteristic absorbance spectrum of indicaxanthin in the visible light (Fig. 7.2b) and its variation as a function of temperature and phospholipid concentration [33]. While ruling out that the pigment partitioned at the hydrophobic interior, that study did not unequivocally conclude on the location of indicaxanthin in these vesicles. The partition of indicaxanthin between water and vesicular pseudo phases was investigated with a Gepasi modeling approach [34]. The rate constants of the reaction of indicaxanthin with the water-soluble azo-initiator 2,2′-azobis [2-methylpropionamidine] dihydrochloride (AAPH) in aqueous solution were calculated and compared with the constants from analogous reaction in the presence of DPPC or DMPC bilayers, at varying phospholipid concentration and temperature. Analysis of the data established that indicaxanthin can solubilize in the bilayer between the polar head groups and hydrophobic region, the so-called palisade domain. This location may allow indicaxanthin to scavenge radicals from the water phase, as well as propagating lipoperoxyl radicals floating to the bilayer polar interface ([37]; Fig. 7.3). All this appears consistent with the antioxidant activity observed in simple chemical systems such as soybean phosphatidylcholine (PC) bilayers [28]. In addition, interactions of indicaxanthin with membrane lipids and possibly other components, could elicit cell responses, and account for cell effects of indicaxanthin, including anti-inflammatory and anti-apoptotic effects [38–40].
Proposed model for the biophysical properties of Indicaxanthin in which the phytochemical can partition in the interface between hydrophobic core and hydrophilic head groups of lipid bilayers. At this level Indicaxanthin can react with radicals from the water phase as well as chain propagating lipoperoxyl radicals floating to the bilayer polar interface. Only one leaflet of the bilayer is represented
Bioavailability
Bioavailability is considered a critical factor for dietary compounds treatment . Bioactivity and potential effects of dietary phytochemicals on human health can be assessed only on absorbed active molecules and transformed digests. Bioavailability is affected by a number of exogenous and endogenous variables and factors, such as structure of food matrix, processing of food, chemistry and stability of ingested compounds, dietary amount, interactions with other food components, bacterial metabolism, and individual differences. All of these could affect intestinal absorption and/or circulation in plasma and excretion, and ultimately the actual concentration of phytochemicals available to target cells.
Betacyanins appeared to be absorbed, which were detected in the urine of subjects who consumed red beet juice [25, 30], beetroot [41], or cactus pear fruit [36]. Early human studies in the authors’ laboratory [36], provided evidence that bioavailability of dietary indicaxanthin was higher than a majority of dietary polyphenol phytochemicals [42], and even than other betalain pigments such as betanin [25, 30, 36]. Data from plasma kinetics and urinary excretion indeed showed that indicaxanthin reached a maximum plasma concentration of 7 μmol/L, 3 h after a cactus pear fruit meal with 28 mg of the pigment, and disappeared from plasma 12 h after the ingestion. The urinary excretion over 12 h represented more than 70 % of the amount ingested [36]. Two major points emerged from these studies are: indicaxanthin is absorbed in a quite high amount, and enters circulation in its native form. The evidence that dietary indicaxanthin did not undergo metabolism in enterocytes or hepatocytes allowed a number of studies to investigate effects and mechanistic aspects of indicaxanthin activity in cell models, utilizing the phytochemical concentrations reflecting dietary conditions and post-intestinal blood levels [32, 40, 43]. Data from these studies suggest that dietary indicaxanthin is a potential nutraceutical .
In vitro studies under simulated gastrointestinal conditions shed light on factors that would account for the pigment bioavailability observed in humans. Digestive stability and bioaccessibility of indicaxanthin, that is, the amount of the compound soluble in a post-intestinal digest [44] have been evaluated after a simulated oral, gastric and small intestinal digestion of cactus pear fruit preparations and compared with the stability and bioaccessibility of purified pigment [45]. A minor loss of indicaxanthin was observed at the gastric-like environment only, which was not affected by food matrix, whereas the molecule appeared wholly soluble in the aqueous fraction of post-intestinal digest. Interestingly, the bioaccessibility of indicaxanthin, expressed as percent of the food content, was 77 % of the amount in the cactus pear fruit, a finding quite in accordance with the bioavailability in humans [36]. That indicaxanthin bioavailability is mainly determined by stability of the molecule to the digestive environment has also been confirmed by studies on the intestinal trans-epithelial transport of the pigment [29] .
Like for xenobiotics, the intestinal absorption of phytochemicals may occur passively, through trans-cellular permeation or paracellular route in accordance with molecular mass and physicochemical characteristics, and could involve either influx or efflux membrane transporters. Generally, unless utilizing transporters in the epithelial membrane, charged solutes of a suitable molecular mass should diffuse through the paracellular route and be transported passively by solvent drag [46–49]. The intestinal permeation of indicaxanthin was investigated using Caco-2 cell monolayers grown on Transwell® insert, an established model of the intestinal barrier. Originating from a human colorectal carcinoma, these cells spontaneously differentiate into polarized monolayers that exhibit morphological and functional characteristics of the intestinal absorptive epithelium [50, 51] . The trans-epithelial transport of dietary-consistent amounts of indicaxanthin was measured in apical-to-basolateral and basolateral-to-apical direction under an inwardly directed pH gradient (pH 6.0–7.4), mimicking luminal and serosal sides of human intestinal cells [29]. According to the apparent bidirectional permeability coefficient (P app), transport kinetics as a function of time and concentration, absence of interference by inhibitors of membrane transporters, and remarkable increase of permeation after ethylene diamine tetra-acetic acid (EDTA) treatment, it was concluded that, quite consistent with its physicochemical features, indicaxanthin crosses the intestinal barrier through paracellular junctions reaching unaltered the basolateral compartment. Moreover, the magnitude of P app ((4.4 ± 0.4) × 10−6 cm s−1), either pure or food-derived compound, suggests that the trans-epithelial gradient of dietary indicaxanthin at the intestinal lumen, with the continuous removal by the bloodstream at the serosal side, would allow a significant intestinal transport and absorption in vivo. According to these findings, the bioavailability of dietary indicaxanthin in humans [36] appears as a result of (i) relatively high stability of the molecule to the digestive process [45], (ii) favorable intestinal absorption through paracellular route by solvent drag, and (iii) easy release from food [29]. It is peculiar that indicaxanthin does not undergo metabolic transformations such as glucuronidation, sulfation, or methylation to be released in plasma and circulates as unconjugated molecule. This may be advantageous for the interaction of this amphypathic molecule with membranes and/or membrane effectors leading to cell signaling and response .
Radical-Scavenging and Antioxidant Activity of Indicaxanthin
Structural Implications in the Free Radical-Scavenging Activity of Betalains
Several studies in the latest decade investigated the antiradical activity of betalains [21, 24–26, 52–54] . The cyclic amine of betalamic acid, the building block of the betalain pigments, may be envisaged as the reactive group acting similarly to the amine group of ethoxyquin, a potent antioxidant in lipid systems [55, 56] and in vivo [57]. A recent systematic analysis [52, 53] explored the relations between structures, spectroscopic properties and antiradical activity of various betalains, measured by the assay of decolorization of the 2,2′-azino-bis[3-ethylbenzthiazoline-6-sulfonic acid (ABTS) radical cation [58]. The studies considering both natural and synthetic compounds, with or without hydroxyl groups or aromaticity in the pigment structure, ascertained that in betaxanthins there is an “intrinsic activity” not linked to the presence of hydroxyl groups or aromaticity, which might be associated with the common electronic resonance system supported between the two nitrogen atoms, and be general to all betalains that contain a similar resonance system. The presence of phenolic hydroxy group, however, enhances significantly the radical-scavenging activity of betaxanthins [52]. In this context, the proline-derivative of betalamic acid (indicaxanthin) should act as a radical scavenger less effective than betacyanins, e.g., betanin , which has indeed been shown [21].
Chemical Models
The decolorization of the ABTS radical cation is an accurate assay universally used for screening radical-scavenging activity of either food extracts or natural pure compounds, as compared with the hydrophilic vitamin E-analogue Trolox [58]. The reducing activity of indicaxanthin has been evaluated by the reaction with the ABTS radical cation, generated by reacting ABTS with potassium persulfate [21]. When expressed as Trolox equivalents, the radical-scavenging activity of indicaxanthin was one order of magnitude lower than the betacyanin betanin [21], which was in accordance with the structural features [52, 53] and the redox potential of these two betalains [21]. The higher scavenging capacity of betanin can be explained by the ease with which it is possible to withdraw an electron from its phenolic hydroxyl group, and by the stability of the resulting delocalized radical [24]. In contrast, the electron abstracted from the betaxanthins could only be from the π-orbitals, this loss being hindered by the positive charge of the N-atom.
Lipoperoxyl radical-scavenging activity of indicaxanthin has been evaluated by the reaction with radicals generated in methyl linoleate methanol solution by 2,2′-azobis[2,4-dimethylvaleronitrile] (AMVN), and in aqueous soybean PC unilamellar liposomes by AAPH [28]. Indicaxanthin acts as a classical chain-terminating lipoperoxyl radical scavenger in solution, with calculated inhibition constant and stoichiometric factor quite comparable with those reported for the most effective natural lipoperoxyl radical scavenger, α-tocopherol [59], under comparable conditions. According to the data, after H-atom donation, the polyene system of indicaxanthin may allow the formation of a resonance-stabilised aminyl radical, whose reactivity will be affected by the environment. In methanol solution of peroxidizing methyl linoleate, the observed stoichiometry suggests reaction with a second lipoperoxyl radical (n = 1.98) [28].
The radical-scavenging capacity is not the only requisite for a compound to act as an antioxidant in a biological context. Kind and site of radicals generated and location of the compound (membrane or solution) may finally decide about the potential of a molecular antioxidant [59–61]. Though effective in preventing lipid oxidation, when incorporated in soybean PC liposomes submitted to aqueous radicals from AAPH, indicaxanthin did not exhibit an antioxidant activity consistent with the activity in solution [28]. The data suggested a mechanism more complex than a classical chain-breaking antioxidant, involving indicaxanthin regeneration and recycling. After reaction of indicaxanthin with a lipid peroxyl radical, reduction of a short-lived indicaxanthin radical intermediate would occur at the expenses of unsaturated lipids, that is a potentially prooxidant insult. Since a clear antioxidant effect is evident, however, the rate at which the indicaxanthin radical starts oxidation events must be lower than that of the lipid peroxyl radical (Fig. 7.4). In this context, the ratio indicaxanthin/unsaturated lipid should be critical in determining the recycling effectiveness, as well as the actual equilibrium between antioxidant and potential prooxidant activity of the molecule.
Proposed scheme of inhibition of peroxyl-induced lipid oxidation in aqueous soybean PC liposomes by Indicaxanthin and phytochemical regeneration. Reaction of indicaxanthin (Ind) with lipoperoxyl or aqueous peroxyl radicals (ROO●) generates a resonance stabilized intermediate cation radical (Ind●) and hydroperoxide (ROOH) (reaction 1). It is proposed that Ind● is reduced by reaction with polyunsatured fatty acid (LH) to regenerate Ind and a carbon centered radical (L●) (reaction 2) that in turn reacts with oxygen (O2) to form LOO● capable of initiating a new oxidation chain (reaction 3). Phytochemical regeneration does not result in a prooxidant event as the rate at which Ind starts oxidation events is much lower than that of the lipid peroxyl radical (k 2«k 3). This process competes with termination reactions, which results in consumption of the phytochemical (reaction 4)
Indicaxanthin and α-tocopherol, simultaneously incorporated in liposomes, exhibited cooperative antioxidant effects and reciprocal protection [28]. In accordance with the potential “prooxidant” character of indicaxanthin in this system, the extent of synergism decreased at the increase of the ratio (indicaxanthin)/(α -tocopherol).
Biological Models
Ex vivo spiking of human plasma with indicaxanthin, followed by isolation of LDL has provided evidence that the betalain can bind to LDL in a saturable fashion, with a maximum binding of 0.5 nmol/mg LDL protein. The indicaxanthin-enriched LDL appeared more resistant than the homologous native particles to the copper-induced oxidation, as assessed by the elongation of the period required to start lipoperoxide production [35]. Mechanistic aspects of the antioxidant activity of indicaxanthin in LDL appeared comparable with the observations in liposomes [28], including synergistic interactions with the LDL vitamin E and recycling of indicaxanthin at the expenses of unsaturated LDL lipids, with no evidence of prooxidant effects [35].
The oxidation status of the heme iron in proteins such as hemoglobin (Hb), myoglobin (Mb), or myeloperoxidase (MPO) must strictly be controlled in order that the proteins accomplish appropriately their functional roles, and do not become powerful oxidizing agents. Indeed, due to their peroxidase or peroxidase-like activity, in the presence of hydrogen peroxide or organic hydroperoxides these proteins undergo a two-electron oxidation, giving rise to high-valent iron radical species (X•[FeIV = O]) [62–64]. Unless rapidly reduced, the latter can oxidize cell components and damage the protein itself thus impairing its function.
The Hb heme-iron is extensively oxidized under pathological conditions, e.g., β-thalassemia [65], while continuous slow autoxidation of Hb also occurs in healthy individuals resulting in red cell oxidative stress [66, 67]. Kinetic studies of the reaction of indicaxanthin with perferryl-Hb, the two-electron oxidized intermediate in the oxidative degradation of Hb [62], showed that indicaxanthin reduces the hypervalent heme-iron at a rate one order of magnitude higher than that of reductants such as ascorbate and Trolox, on a molar basis [43].
Dietary phytochemicals may act in the gastrointestinal tract [68–71]. Scavenging of highly oxidizing hypervalent-iron myoglobin (perferryl-Mb) formed during meat digestion [68] may preserve oxidable lipids and avoid formation of potentially toxic lipid hydroperoxides. In this context, unpublished observations in the authors’ laboratory provided evidence that indicaxanthin concentrations consistent with a dietary intake reduced perferryl-Mb and prevented the lipid oxidation of heated red meat under a simulated gastric digestion.
Despite MPO plays key roles in the defense against invading pathogens by oxygen-dependent antimicrobial action [72, 73], the enzyme has an enormous potential to inflict damage to host tissues, through its ability to catalyze the production of a complex array of reactive oxidants including its product hypochlorous acid (HOCl), and nitrogen dioxide, organic-free radicals and drug metabolites [74–77]. In addition, the harmful potential of MPO in the onset and progression of atherogenetic processes is to be considered because the demonstrated capacity of the enzyme to oxidize LDL [78–81]. According to its halogenation and peroxidase cycles [82], MPO can catalyze both two- and one-electron oxidation reactions, leading to production of the cytotoxic HOCl and generating the hypervalent iron redox intermediates compound I (CI) and compound II (CII). Indicaxanthin has been shown to be a good electron donor for both CI and CII, at very low μmolar concentrations [83]. In addition, it has been reported that the betalain can scavenge HOCl [83].
Table 7.2 reports quantitative parameters of the reaction of indicaxanthin with various oxidants/radicals.
Effects of Indicaxanthin in Cell, Tissue, and Animal Models
The redox chemistry, physicochemical properties, bioactivity in solution and high bioavailability of indicaxanthin were a promising approach to investigate on the activity and eventual mechanism of action in the cells. A number of experimental setups with various either human or mouse cell cultures showed positive effects of indicaxanthin on the cell response to toxic stimuli modeling pathophysiological conditions, from serious oxidative stress to inflammation and apoptosis. In other studies, evidence has been provided that indicaxanthin can modulate the differentiation of T cells toward an immune-modulatory phenotype, and affect mouse ileal contractility. Anti-inflammatory activity of indicaxanthin was tested in a murine model.
Ex vivo spiking of blood from healthy human subjects with varied indicaxanthin concentrations resulted in a saturable incorporation of the betalain in the RBC, to the extent of approximately 1 nmol/mL packed cell, an amount very close to that measured in human RBCs after consumption of dietary indicaxanthin [32, 36]. Indicaxanthin-enriched erythrocytes resulted more resistant to the cumene hydroperoxide-indicaxanthin-induced oxidative haemolysis than the homologous nonenriched cells, with a significant correlation between the increase of resistance and the amount of the incorporated indicaxanthin [32]. High oxygen tension and large amounts of iron, a transition metal promoting formation of oxygen free radicals [84], make red blood cells (RBCs) highly susceptible to oxidation, leading to impairment of the cell function and premature aging [67]. In addition, the oxidative alterations of the RBC membrane can even cause injury to the endothelial cells [67]. Protective effects in blood of dietary indicaxanthin may be suggested.
An increased generation of reactive oxygen species, first caused by Hb auto-oxidation and precipitation, characterizes genetic hemolytic disorder such as β-thalassemia. The pathology is associated to depletion of the RBC antioxidant defense resulting in damage to cell components, impairment of morphology and function of cell membrane, and accelerated RBC destruction [65, 85–88]. Protective dose-dependent effects of indicaxanthin have been shown in RBC from β-thalassemia patients submitted to an in vitro oxidation by cumene hydroperoxide [43]. The betalain, that entered red blood cells, enhanced the resistance of thalassemia RBC to hemolysis, prevented lipid and Hb oxidation, and retarded vitamin E and GSH depletion. In the same work, spiking of blood from thalassemia patients with indicaxanthin resulted in its incorporation in the RBCs, indicating that the pathological alterations to the membrane do not affect a trans-bilayer movement of this phytochemical. Antioxidant vitamins and phytochemicals may be helpful to treat β-thalassemia [89–91]. The finding that indicaxanthin can be incorporated in the redox machinery of β -thalassemia RBCs, may offer novel opportunities of therapeutic interest in this pathology.
Atherosclerosis is a chronic inflammatory condition of the arterial intima. This complex process progresses slowly during a lifetime, with initial dysfunction of the vascular endothelium to a great extent mediated by oxidized LDL, immuno-competent cells, cytokines, growth factors, adhesion molecules, and compounds involved in redox-sensitive regulatory mechanisms underlying inflammatory processes [92–96]. Since atherosclerosis and related cardiovascular problems are a leading cause of death in the western world [97], research on dietary compounds capable of preventing or controlling pathological events is now very active. In this context, the activity of indicaxanthin has been explored in various model systems.
Vascular endothelial cells are a direct target of proinflammatory stimuli that remarkably affect a number of redox-mediated signaling pathways, leading to production of chemotactic factors, lipid mediators, and cytokines [98], as well as adhesion molecules such as the adhesion molecule-1 (ICAM-1) [99, 100]. With an in vitro model of inflammation consisting of umbilical vein endothelial cells (HUVEC) stimulated with the proinflammatory cytokine tumor necrosis factor-α (TNF-α), it has been shown that μmolar indicaxanthin concentrations can modulate the expression of ICAM-1 [38].
Apoptosis of macrophages triggered by oxidized cholesterol derivatives, especially 7-ketocholesterol (7-KC), is considered a key event in the development of human atheromas [101, 102]. A recent in vitro study showed that dietary consistent amounts of indicaxanthin exert protective effects and prevent the 7-KC-induced pro-apoptotic events in human monocyte/macrophage THP-1 cells. The effects of indicaxanthin have been related to inhibition of the basal activity and overexpression of NADPH oxidase-4 (NOX-4), prevention of the redox-sensitive nuclear factor-kB activation, maintaining of cell redox balance and inhibition of the increase of cytosolic calcium, which prevented mitochondrial damage and consequently apoptosis. About the molecular mechanism, though other explanations cannot be ruled out, interactions of indicaxanthin with 7-KC at the level of the THP-1 cell membrane could bring about some stabilizing effect leading to modulate NOX-4 enzyme activity and possibly prevent opening of calcium channels [102].
Immunomodulatory effects of indicaxanthin have been revealed in vitro with mouse splenocytes allowed to differentiate in Th1 and Th17 skewing conditions, either in the presence or in the absence of indicaxanthin [103]. Indicaxanthin inhibited both IFN-γ and IL-17 production in the Th17 conditions, accompanied by a marked increase of IL-6 and a significant reduction of IL-10 levels, indicating a modulation of T regulatory differentiation and/or function. The data also showed a reduction of percentage of IL-17- and a parallel increase of IFN-γ- producing cells in indicaxanthin-treated compared to control cells. Consistent with this, indicaxanthin almost doubled the number of IFN-γ + T cells in splenocytes differentiated in Th1 skewing conditions. The data suggest potential activity of indicaxanthin in Th1-mediated protective immunity against pathogens. In addition, positive effects could be hypothesized on Th17-driven autoimmune disorders, e.g., multiple sclerosis or rheumatoid arthritis.
An experimental organ bath technique to record the mechanical activity of the isolated mouse ileum longitudinal muscle was used to investigate the effects of indicaxanthin on the spontaneous and evoked contractility [104], and mechanism of action underlying [105]. Indicaxanthin showed a remarkable spasmolytic effect on the intestinal contractility, with its action ascribable to phosphodiesterase inhibition and elevation of cAMP levels. In view of the capacity of indicaxanthin to interact with and cross cell membranes [32–34, 43], these findings open interesting questions on how this action may be accomplished at a molecular level.
To date, there is only one in vivo report on pharmacological activity of indicaxanthin. Remarkable anti-inflammatory effects have been observed in a carrageenin-induced rat pleurisy model [39]. Orally given to the extent of 8 µmol/kg, which is consistent with a human dietary assumption from cactus pear fruits [36], prior to carrageenin injection in the pleural cavity, indicaxanthin caused a 72 % reduction of the inflammatory exudate volume, and 95 % reduction of total leukocyte number. Table 7.3 reports on biological actions of indicaxanthin in various systems.
Epigenetics
Occurrence and frequencies of epigenetic changes throughout the life are now acknowledged crucial for gene activation or silencing [106] . This finally affects the entire cell behavior allowing normal cell functions or, conversely, leading to pathophysiological states, including inflammation, cancer, and aging [107–109]. Differently from definite aberrations in the DNA sequences, epigenetic alterations are potentially reversible [106]. Numerous data pointing out to the influence of food components, especially redox-active phytochemicals, on epigenetic mechanisms emphasize the role of diet as an important environmental factor for maintaining a healthy status, and offer promising evidence for their eventual chemopreventive efficacy [110]. It may be worth noting that steady-state oxidation of cell proteins has been reported to be responsive to diet [111].
Longevity and lower incidence of chronic degenerative disorders and cardiovascular disease among Mediterranean populations have been associated with a peculiar dietary model rich of fruits and vegetables and low of animal fat. As a part of the Mediterranean dietary habit, the cactus pear fruit and its betalain phytochemicals, i.e., indicaxanthin and betanin , may have contributed to these beneficial effects, especially in the past when food was less globalized [112, 113]. Repetitive long-life exposure to physiological concentrations of betalains could play a role in maintaining cell functions through epigenetic machinery. The highly bioavailable and bioactive indicaxanthin may represent an important factor in this context, so our current research is going in this direction. Epigenetic activity of indicaxanthin has recently been shown [114]. Hypermethylation of onco-suppressor gene promoters is a well-established epigenetic modification [115]. Among others, the hypermethylation of the p16 gene, an onco-suppressor controlling the cell cycle through cycline inhibition, is associated with tumor development [116–118] . A number of data with a human intestinal cancer (Caco-2) cell line provided evidence that the amount of indicaxanthin consistent with that in the intestinal digests after a dietary consumption of cactus pear fruit pulp [36] could induce demethylation of the p16 gene promoter, reactivation of the p16 synthesis and finally cell apoptosis. Exploring indicaxanthin activity on other onco-suppressor genes in the same as well as other human cancer cell lines and, understanding pathways by which indicaxanthin can modulate epigenetic mechanisms is the near future challenge.
Molecular Toxicology and Biosafety
Betalains, especially red betacyanins , have long been considered important natural pigments for industry, from food to cosmetics and pharma products, then safety of these molecules has been checked long time ago [24, 119, 120]. Studies carried out to determine decomposition and stability [121, 122], mutagenicity [120, 123], and toxicological and toxicokinetic effects [124], showed that these pigments are not harmful. Some in vivo studies in rats indicated that a betalain extract from Garambullo, a cactacea fruit (Myrtillocactus geometrizans) did not have toxic effects at any of the doses tested, up to 5 g/kg body weight [124]. Nevertheless, in spite of the acute or subacute safety assessment in animals, current and emerging knowledge on (i) the redox regulation of signaling pathways involved in cell survival and death, (ii) the activity of phytochemicals as redox modulators with potential dual behavior of acting (antioxidant vs. prooxidant), and (iii) the cell epigenetic response to changes of its redox milieu, require that actions of redox active phytochemicals of our diet should be checked at a molecular level. In addition, their eventual involvement in the epigenome regulation should at least been established in cellular models under normal and pathophysiological states. Since these substances may have the potential to behave as nutraceutical/ pharmaceutical agents, it should be assured that they do not cause harmful variations of the cell redox homeostasis at recurring dietary-consistent amounts, or eventually at amounts of pharmacological interest. It should be kept in mind that a potential chemoprevention, through modulation of the cell redox state in turn controlling various signaling transduction mechanisms, may require very minute amounts of phytochemicals. Then, in normal cells, eventual prooxidant activity and dangerous effects by high concentrations may occur. Conversely, in tumor cells, where the redox milieu is disturbed [125], prooxidant activity of a given phytochemical could be required [126, 127], to restore homeostatic patterns and cause apoptosis (chemotherapy), which may be toxic for normal cells [14].
So far, with respect to the above mentioned tasks, potential adverse effects of high concentrations of indicaxanthin have been searched in a few experimental conditions in the authors’ laboratory, in contexts where low indicaxanthin concentrations exhibited antioxidant , or radical-scavenging effects [28, 35]. Prooxidant activity was not observed neither in the model of copper-stimulated oxidation of human LDL [35], nor in liposomal membranes under the prooxidant action of the azo-initiator AAPH [28], rather indicaxanthin dose-dependently inhibited lipid peroxidation in both systems, even when tested at very high micromolar concentrations (up to 1 mM) [35]. Similarly, no prooxidant activity was evident in human RBC [32]. With its peculiar location in lipid bilayers [33, 34], antioxidant activity of indicaxanthin at the cell membrane could be relevant not only to the integrity of membrane but also to maintain cell homeostasis. Redox-driven signaling pathways may be started by oxidized membrane lipid components, and their soluble derivatives such as reactive aldehydes, being the latter capable of starting oxidation pathways inside the cell [128], damaging proteins, DNA, and also affecting epigenetic mechanisms [129].
The membrane enzymes of the NADPH oxidase (NOX) family are involved in the production of reactive oxygen species (ROS) in all animal cells and their primary role in the control of cell redox homeostasis is widely acknowledged [130–132]. In this context, investigating the activity of phytochemicals at the level of these enzymes may be a strategy to investigate about potentially harmful mechanisms underlying their biological actions in various cells and under different conditions. A surprising pro-senescent activity of resveratrol in human endothelial cells, for instance, has been reported to be mediated by NOX-1 and NOX-4 [133]. Research with monocyte/macrophage THP-1 cells [40] showed that indicaxanthin, at nutritionally relevant amounts, can prevent the NOX-4 dependent ROS formation induced by the toxic 7-keto-cholesterol, however, does not modify the basal activity of the enzyme in the absence of the oxysterol, even at ten-times higher amounts. Then, while protecting THP-1 cells from an oxidant agent, indicaxanthin itself did not modify the basal redox environment of these cells under normal conditions.
Indicaxanthin toxicity has been checked on Caco-2 cells grown at confluence for 15 days, to reach characteristics of normal human colon epithelial cells [50, 51]. The assays were carried out by measuring LDH activity released from the cells exposed to high micromolar concentrations of the phytochemical, in the range 25–100 µM. No significant release of LDH activity was detected after a 24-h incubation, showing that cell integrity had been preserved.
Other studies with a panel of animal cells are necessary to provide a much more complete picture on potentially harmful actions of indicaxanthin and to test influence on epigenome targets. However, to date, adverse effects of the phytochemical have not been observed in any either solution or cell system, even in a range of concentrations widely exceeding the physiological blood levels obtained with food intake [36].
Conclusive Remarks and Perspectives
Dietary redox active phytochemicals are currently studied intensely for basic science and applied research. Understanding how these compounds exert physiological effects when ingested with food and exploiting their nutraceutical/ pharmacological potential are the key goals in the study of molecular nutrition [134]. This hard task requires interdisciplinary collaborations and clinical studies.
There is now a consensus that redox-active phytochemicals are utilized in animal cells as bio-signals to start pathways regulating protective and/or defense activities. How a cell interprets these signals depends on the cell type, the response machinery of that cell, and its history in terms of what signals that cell previously responded to and the proteomic setup the cell had at the time of the arrival of the new signal. In this picture, environment- induced epigenetic modifications, reversibly silencing or activating certain genes may play an important role in the great variability of cell responses to dietary phytochemicals (which is the field of nutrigenetics), but at the same time may represent molecular targets on which a phytochemical can intervene (which is the field of nutrigenomics) . While signal transduction mechanisms evoked by phytochemicals are mostly unknown, the modulation of epigenetic mechanisms in nonmalignant cells has been associated with the positive effects of such compounds (chemoprevention). Once molecular targets for selected dietary compounds are found, dietary strategies could be designed to maintain cell functions and possibly prevent or reduce risk of developing chronic/degenerative diseases.
Almost all fruits and vegetables on our table are now extensively cultivated, so they do not cope with, or receive much less, environmental stress. When considering hormetic production of defensive phytochemicals, this “easy and cozy” condition may possibly decrease the protective value of green food, which deserves to be tested. In this context, cactus, pear, and its betalains, may be an interesting exception. Even cultivated, the plant is not subjected to continuous reproductive cycles; fruits cannot long be stored and are available for some months in a year. In addition, many plants are still growing wild in Mediterranean areas, facing such tough environmental conditions as intense sunlight, dryness, heat.
Since dietary betalains are only a very limited number as compared with thousands phenol/polyphenol phytochemicals, their biological and health-promoting properties have been the object of less, but recently growing, interest and research [135]. However, among so many compounds indicaxanthin, a yellow betalain pigment, holds a special place and appears to be a promising dietary factor to maintain cell functions and positively affect human health. As a result of relatively high stability during the digestive process [45], favorable intestinal absorption through paracellular route without metabolic transformation, and easy release from food [29], dietary indicaxanthin possesses high bioavailability in humans [36]. Moreover, its redox chemistry and physicochemical characteristics [21, 29] allow the molecule to interact with membranes [34], penetrate cells [32] and scavenge both lipid radicals and water-soluble oxidants [28, 35]. So far, evidence of anti-apoptotic [40] and immune-modulator [103] activity has been provided, and capacity of indicaxanthin of reverting epigenetic changes associated with cancer development [111] shown in in vitro models, whereas only one study reported on anti-inflammatory effects in rat [39]. Future investigations in animal models, and characterization of indicaxanthin from a “nutri-epigenomic perspective,” will provide important information on the potential to act as a chemopreventive agent.
The edible fruits of the cactus Opuntia ficus indica, L., a plant spread in the South of Italy, are the only abundant source of indicaxanthin, which may somewhat account for why nutritional studies and basic science investigations, including biological actions, on this molecule have been carried out almost exclusively in the authors’ laboratory in Sicily. While feeling this as a great commitment, we are challenged to find out as many information as possible on the potentiality of this pigment and provide scientific and sound evidence of eventual beneficial effects from its chronic use as a nutraceutical agent. The road ahead is still very long, but really exciting.
References
Slavin JL, Lloyd B (2012) Health benefits of fruits and vegetables. Adv Nutr 3:506–516
Murakami A, Ohnishi K (2012) Target molecules of food phytochemicals: food science bound for the next dimension. Food Funct 3:462–476
Obrenovich ME, Li Y, Parvathaneni K, Yendluri BB, Palacios HH, Leszek J, Aliev G (2011) Antioxidants in health, disease and aging. CNS Neurol Disord Drug Targets 10:192–207
Halliwell B, Rafter J, Jenner A (2005) Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not? Am J Clin Nutr 81:268S–276S
Doré S (2005) Unique properties of polyphenol stilbenes in the brain: more than antioxidant actions; gene/protein regulatory activity. Neurosignals 14:61–70
Izzi V, Masuelli L, Tresoldi I, Sacchetti P, Modesti A, Galvano F, Bei R (2012) The effects of dietary flavonoids on the regulation of redox inflammatory networks. Front Biosci 17:2396–2418
Howitz KT, Sinclair DA (2008) Xenohormesis: sensing the chemical cues of other species. Cell 133:387–391
Sepúlveda-Jiménez GP, Rueda-Benítez P, Porta H, Rocha-Sosa M (2004) Betacyanin synthesis in red beet (Beta vulgaris) leaves induced by wounding and bacterial infiltration is preceded by an oxidative burst. Physiol Mol Plant Pathol 64:125–133 (Erratum in Physiological and Molecular Plant Pathology 66:75)
Suzuki N, Kousevitzky S, Mittler R, Miller G (2012) ROS and redox signaling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Suzuki N, Mittler R (2012) Reactive oxygen species-dependent wound responses in animals and plants. Free Radic Biol Med 53:2269–2276
Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG et al (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425:191–196
Calabrese V, Cornelius C, Dinkova-Kostova AT, Iavicoli I, Di Paola R, Koverech A et al (2012) Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim Biophys Acta 1822:753–783
Surh YJ (2011) Xenohormesis mechanisms underlying chemopreventive effects of some dietary phytochemicals. Ann N Y Acad Sci 1229:1–6
Nair S, Li W, Kong AT (2007) Natural dietary anti-cancer chemopreventive compounds: redox-mediated differentiatal signaling mechanisms in cytoprotection of normal cells versus cytotoxycity in tumor cells. Acta Pharmacol Sin 28:459–472
Piattelli M (1981) The betalains: structure, biosynthesis and chemical taxonomy. In: Conn EE (ed) The biochemistry of plants: a comprehensive treatise, vol 7. Academic, New York, pp 557–575
Strack D, Vogt T, Schliemann W (2003) Recent advances in betalain research. Phytochemistry 62:247–269
Stafford HA (1994) Anthocynins and betalains: evolution of the mutually exclusive pathways. Plant Sci 101:91–98
Stintzing FC, Schieber A, Carle R (2002) Identification of betalains from yellow beet (Beta vulgaris, L) and cactus pear (Opuntia ficus indica, L Mill) by high performance liquid chromatography-electrospray ionization mass spectrometry. J Agric Food Chem 50:2302–2307
Khan MI, Harsha PSC, Giridhar P, Ravishankar GA (2012) Pigment identification, nutritional composition, bioactivity, and in vitro cancer cell cytotoxicity of Rivina humilis L. berries, potential source of betalains. LWT Food Sci Technol 47:315–323
Piattelli M, Minale L, Prota G (1964) Isolation structure and absolute configuration of indicaxanthin. Tetrahedron 20:2325–2329
Butera D, Tesoriere L, Di Gaudio F, Bongiorno A, Allegra M, Pintaudi AM et al (2002) Antioxidant activities of sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: betanin and indicaxantin. J Agric Food Chem 50:6895–6901
Stintzing F, Carle R (2007) Betalains—emerging prospects for food scientists. Trends Food Sci Technol 18:514–525
Schwartz SJ, von Elbe JH, Pariza MW, Goldsworthy T, Pitot HC (1983) Inability of red beet betalain pigments to initiate or promote hepatocarcinogenesis. Food Chem Toxicol 21:531–535
Escribano J, Pedreno MA, Garcia-Carmona F, Munoz R (1998) Characterization of the antiradical activity of betalains from Beta vulgaris L. roots. Phytochem Anal 9:124–127
Kanner J, Harel S, Granit R (2001) Betalains—a new class of dietary cationized antioxidants. J Agric Food Chem 49:5178–5185
Pedreno MA, Escribano J (2000) Studying the oxidation and the antiradical activity of betalain from beetroot. J Biol Educ 35:49–51
Buettner GR (1993) The pecking order of free radicals and antioxidants: lipid peroxidation, a-tocopherol, and ascorbate. Arch Biochem Biophys 300:535–543
Tesoriere L, Allegra M, Butera D, Gentile C, Livrea MA (2007) Kinetics of the lipoperoxyl radical scavenging activity of indicaxanthin in solution and in unilamellar liposomes. Free Radical Res 41:226–233
Tesoriere L, Gentile C, Angileri F, Attanzio A, Tutone M, Allegra M, Livrea MA (2013) Trans-epithelial transport of the betalain pigments indicaxanthin and betanin across Caco-2 cell monolayers and influence of food matrix. Eur J Nutr 52:1077–1088
Frank T, Stintzing FC, Carle R, Bitsch I, Quaas D, Strass G et al (2005) Urinary pharmacokinetics of betalains following consumption of red beet juice in healthy humans. Pharmacol Res 52:290–297
Gliszczynska-Swiglo A, Szymusiak H, Malinowska P (2006) Betanin, the main pigment of red beet: molecular origin of its exceptionally high free radical-scavenging activity. Food Addit Contam 23:1079–1087
Tesoriere L, Butera D, Allegra M, Fazzari M, Livrea MA (2005) Distribution of betalain pigments in red blood cells after consumption of cactus pear fruits and increased resistance of the cells to ex vivo-induced oxidative hemolysis in humans. J Agr Food Chem 53:1266–1270
Turco Liveri ML, Sciascia L, Lombardo R, Tesoriere L, Passante E, Livrea MA (2007) Spectrophotometric evidence for the solubilization site of betalain pigments in membrane biomimetic systems. J Agric Food Chem 55:2836–2840
Turco Liveri ML, Sciascia L, Allegra M, Tesoriere L, Livrea MA (2009) Partition of indicaxanthin in membrane biomimetic systems. A kinetic and modeling approach. J Agric Food Chem 57:10959–10963
Tesoriere L, Butera D, D’Arpa D, Di Gaudio F, Allegra M, Gentile C, Livrea MA (2003) Increased resistance to oxidation of betalain-enriched human low density lipoproteins. Free Radic Res 37:689–696
Tesoriere L, Allegra M, Butera D, Livrea MA (2004) Absorption, excretion, and distribution in low density lipoproteins of dietary antioxidant betalains. Potential health effects of betalains in humans. Am J Clin Nutr 80:941–945
Barclay LRC (1993) Model biomembranes: quantitative studies of peroxidation, antioxidant action, partitioning, and oxidative stress. Can J Chem 71:1–16
Gentile C, Tesoriere L, Allegra M, Livrea MA, D’Alessio P (2004) Antioxidant betalains from cactus pear (Opuntia ficus-indica) inhibit endothelial ICAM-1 expression. Ann NY Acad Sci 1028:481–486
Allegra M, Ianaro A, Tersigni M, Tesoriere L, Livrea MA (2009) Indicaxanthin exerts anti-inflammatory effects on carrageenin-induced rat pleurisy. Free Radical Res 43 (Suppl 1):S47
Tesoriere L, Attanzio A, Allegra M, Gentile C, Livrea MA (2013) Phytochemical indicaxanthin suppresses 7-ketocholesterol-induced THP-1 cell apoptosis by preventing cytosolic Ca2 + increase and oxidative stress. Br J Nutr 1–11 (e-pub), 110:230–240
Watts AR, Lennard ML, Tucker GT, Woods HF (1993) Beeturia and the biological fate of beetroot pigments. Pharmacogenetics 3:302–311
Manach C, Williamson G, Morand C, Scalbert A, Rémésy C (2005) Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 81:230S–242S
Tesoriere L, Allegra M, Butera D, Gentile C, Livrea MA (2006) Cytoprotective effects of the antioxidant phytochemical indicaxanthin in beta-thalassemia red blood cells. Free Radic Res 40:753–761
McDougall GJ, Dobson P, Smith P, Blake A, Stewart D (2005) Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J Agric Food Chem 53:5896–5904
Tesoriere L, Fazzari M, Angileri F, Gentile C, Livrea MA (2008) In vitro digestion of betalainic foods. Stability and bioaccessibility of betaxanthins and betacyanins and antioxidative potential of food digesta. J Agric Food Chem 56:10487–10492
Adson A, Raub TJ, Burton PS, Barshun CL, Hilgers AR, Audus KL, Ho NF (1994) Quantitative approach to delineate paracellular diffusion in cultured epithelial cell monolyer. J Pharm Sci 83:1529–1536
Karlsson J, Ungell AL, Artursson P (1994) Effect of an oral rehydration solution on paracellular drug transport in intestinal epithelial cells and tissues: assessment of charge and tissue selectivity. Pharm Res 11:S248
Pade V, Stavchansky S (1997) Estimation of the relative contribution of the transcellular and paracellular pathway to the transport of passively absorbed drugs in the Caco-2 cell culture model. Pharm Res 14:1210–1215
Knipp GT, Ho NF, Barsuhn CL, Borchardt R (1997) Paracellular diffusion in Caco-2 cell monolayers: effect of perturbation on the transport of hydrophilic compounds that vary in charge and size. J Pharm Sci 86:1105–1110
Galijatovic A, Otake Y, Walle UK, Walle T (2001) Induction of UDP-glucuronyltransferase UGT1A1 by the flavonoid chrisin in Caco2 cells- potential role in carcinogen bioactivation. Pharmaceutical Res 18:374–379
Sun DX, Lennernas H, Welage LS, Barnett JL, Landowski CP, Foster D et al (2002) Comparison of human duodenum and Caco2 gene expression profiles for 12,000 gene sequence tags and correlation with permeabililty of 26 drugs. Pharm Res 19:1400–1416
Gandía-Herrero F, Escribano J, García-Carmona F (2009) The role of phenolic hydroxy groups in the free radical scavenging activity of betalains. J Nat Prod 72:1142–1146
Gandía-Herrero F, Escribano J, García-Carmona F (2010) Structural implications on color, fluorescence, and antiradical activity in betalains. Planta 232:449–460
Gandía-Herrero F, Escribano J, García-Carmona F (2012) Purification and antiradical properties of the structural unit of betalains. J Nat Prod 75:1030–1036
Belitz HD, Grosch W (1987) Food chemistry. Springer-Verlag, Berlin, pp 177
Lin JC, Olcott HS (1995) Ethoxyquin nitroxide. J Agric Food Chem 23:798–800
Lockart B, Bonhomme N, Roger A, Dorey G, Casara P, Lestage P (2001) Protective effect of the antioxidant 6-ethoxy-2,2-pentamethylen-1,2-dihydroquinoline (S 33113) in models of cerebral neurodegeneration. Eur J Pharmacol 416:59–68
Pellegrini N, Re R, Yang M, Rice-Evans C (1999) Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2†²-azinobis(3-ethylenebenzothiazoline-6-sulfonic cid) radical cation decolorization assay. Methods Enzymol 299:379–389
Niki E, Saito T, Kawakami A, Kamiya Y (1984) Inhibition of oxidation of methyl linoleate in solution by vitamin E and vitamin C. J Biol Chem 259:4177–4182
Burton GW, Ingold KU (1981) Autoxidation of biological molecules. 1. The antioxidant activity of vitamin E and related chainbreaking phenolic antioxidants in vitro. J Am Chem Soc 103:6472–6477
Barclay LRC, Ingold KU (1981) Autoxidation of biological molecules. 2. Autoxidation of a model membrane. Comparison of the autoxidation of egg lecithin phosphatidylcholine in water and in chlorobenzene. J Am Chem Soc 103:6478–6485
Everse J, Hsia N (1997) The toxicities of native and modified hemoglobins. Free Radic Biol Med 22:1075–1099
Everse J (1998) The structure of heme proteins compounds I and II: some misconceptions. Free Radic Biol Med 24:1338–1346
Furtmuller PG, Obinger C, Hsuanyu Y, Dunford HB (2000) Mechanism of reaction of myeloperoxidase with hydrogen peroxide and chloride ion. Eur J Biochem 267:5858–5864
Rund D, Rachmilewitz E (2005) Beta-thalassemia. New Engl J Med 353:1135–1146
Vollaard NB, Reeder BJ, Shearman JP, Menu P, Wilson MT, Cooper CE (2005) A new sensitive assay reveals that hemoglobin is oxidatively modified in vivo. Free Radic Biol Med 39:1216–1228
Rifkind JM, Nagababu E (2012) Hemoglobin redox reactions and red blood cell aging. Antiox Redox Sign. doi:10.1089/ars.2012.4867
Halliwell B, Zhao K, Whiteman M (2001) The gastrointestinal tract: a major site of antioxidant action? Free Radic Res 33:819–880
Kanner J, Lapidot T (2001) The stomach as a bioreactor: dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radic Bio Med 31:1388–1395
Scalbert A, Morand C, Manach C, Remesy C (2002) Absorption and metabolism of polyphenols in the gut and impact on health. Biomed Pharmacother 56:276–282
Ursini F, Sevanian A (2002) Wine polyphenols and optimal nutrition. Ann NY Acad Sci 957:200–209
Klebanoff SJ (1980) Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med 93:480–489
Klebanoff SJ (1975) Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Seminar Haematol 12:117–142
Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R (1996) Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest 97:1535–1544
Heinecke JW (1998) Oxidants and antioxidants in the pathogenesis of atherosclerosis: implications for the oxidized low density lipoprotein hypothesis. Atherosclerosis 141:1–15
Klebanoff SJ (2005) Myeloperoxidase: friend and foe. J Leukoc Biol 77:598–525
Leeuwenburgh C, Hardy MM, Hazen SL, Wagner P, Oh-ishi S, Steinbrecher UP, Heinecke JW (1997) Reactive nitrogen intermediates promote low density lipoprotein oxidation in human atherosclerotic intima. J Biol Chem 272:1433–1436
Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R (1996) Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest 97:1535–1544
Hazell LJ, Stocker R (1993) Oxidation of low-density lipoprotein with hypochlorite causes transformation of the lipoprotein into a high-uptake form for macrophages. Biochem J 290:165–172
Hazen SL, Heinecke JW (1997) 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest 99:2075–2081
Kostyuk VA, Kraemer T, Sies H, Schewe T (2003) Myeloperoxidase/nitrite-mediated lipid peroxidation of low-density lipoprotein as modulated by flavonoids. FEBS Lett 537:146–150
Dunford HB (ed) (1999) Heme peroxidases. Wiley-VCH: New York and Toronto
Allegra M, Furtmüller PG, Jantschko W, Zederbauer M, Tesoriere L, Livrea MA, Obinger C (2005) Mechanism of interaction of betanin and indicaxanthin with human myeloperoxidase and hypochlorous acid. Biochem Biophys Res Commun 332:837–844
Halliwell B, Gutteridge JMC (1999) Free radical in biology and medicine, 3rd edn. Oxford University Press: New York
Chiu DT, van den Berg J, Kuypers FA, Hung IJ, Wie JS, Liu TZ (1996) Correlation of membrane lipid peroxidation with oxidation of hemoglobin variants: possibly related to the rates of hemin release. Free Radic Biol Med 21:89–95
Grinberg LN, Rachmilewitz EA, Kitrossky N, Chevion M (1995) Hydroxyl radical generation in beta-thalassemic red blood cells. Free Radic Biol Med 18:611–615
Scott MD, van den Berg JJ, Repka T, Rouyer-Fessard P, Hebbel RP, Beuzard Y, Lubin BH (1993) Effect of excess alpha-hemoglobin chains on cellular and membrane oxidation in model beta-thalassemic erythrocytes. J Clin Invest 91:1706–1712
Van Dyke B, Saltman P (1996) Hemoglobin: a mechanism for the generation of hydroxyl radicals. Free Radic Biol Med 20:985–989
Das N, Chowdhury TD, Chattopadhyay A, Datta AG (2004) Attenuation of oxidative stress-induced changes in thalassemic erythrocytes by vitamin E. Polish J Pharmacol 56:85–96
Grinberg LN, Rachmilewitz EA, Newmark H (1994) Protective effects of rutine against hemoglobin oxidation. Biochem Pharmacol 48:643–49
Tesoriere L, D’Arpa D, Butera D, Allegra M, Renda D, Maggio A, Bongiorno A, Livrea MA (2001) Oral supplements of vitamin E improve measures of oxidative stress in plasma and reduce oxidative damage to LDL and erythrocytes in beta-thalassemia intermedia patients. Free Radic Res 34:529–540
Stocker R, Keany J Jr (2004) Role of the oxidative modifications in atherosclerosis. Physiol Rev 84:1381–1478
Lusis AJ (2000) Atherosclerosis. Nature 407:233–241
Moore KJ, Tabas I (2011) Macrophages in the pathogenesis of atherosclerosis. Cell 145:341–355
Lonn ME, Dennis JM, Stocker R (2012) Actions of “antioxidants” in the protection against atherosclerosis. Free Radic Biol Med 53:863–884
Badimon L, Martinez-Gonzalez J, Llorente-Cortes V, Rodriguez C, Padro T (2006) Cell biology and lipoproteins in atherosclerosis. Curr Mol Med 5:439–456
D’Alessio P (2004) Aging and the endothelium. Exp Gerontol 36:165–171
Loyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C (2010) American Heart Association Statistics Committee and Stroke Statistics Subcommittee. 2010. Update. Circulation 121:948–954
D’Alessio P (2002) Endothelium as a pharmacological target. Curr Op Invest Drugs 2:1720–1724
Carman CV, Jun CD, Salas A, Springer TA (2003) Endothelial cells proactively form microvilli-like membrane projections upon intracellular adhesion molecule-1 engagement of leukocyte LFA-1. J Immunol 171:6135–6144
Brown AJ, Jessup W (1999) Oxysterols and atherosclerosis. Atherosclerosis 142:1–28
Larsson DA, Baird S, Nyhalah JD, Yuan XM, Li W (2006) Oxysterol mixtures, in atheroma-relevant proportions, display synergistic and proapoptotic effects. Free Radic Biol Med 41:902–910
Allegra M, Rattazzi A, Attanzio A, Tesoriere L, Livrea MA, D’Acquisto F (2012) Modulation of TH1/TH17 equilibrium in vitro by indicaxanthin from Opuntia ficus indica (L. Mill). Free Radic Biol Med 53 (Suppl 1):S47
Baldassano S, Tesoriere L, Rotondo A, Serio R, Livrea MA, Mulè F (2010) Inhibition of the mechanical activity of mouse ileum by cactus pear (Opuntia Ficus Indica, L, Mill.) fruit extract and its pigment indicaxanthin. J Agric Food Chem 58:7565–7571
Baldassano S, Rotondo A, Serio R, Livrea MA, Tesoriere L, Mulè F (2011) Inhibitory effects of indicaxanthin on mouse ileal contractility: analysis of the mechanism of action. Eur J Pharmacol 658:200–205
Lin HJ, Zuo T, Chao JR, Peng Z, Asamoto LK, Yamashita SS, Huang TH (2009) Seed in soil, with an epigenetic view. Biochim Biophys Acta 1790:920–924
Dolinoy DC Weidman JR, Jirtle RL (2007) Epigenetic gene regulation: linking early developmental environment to adult disease. Reprod Toxicol 23:297–307
Esteller M (2008) Epigenetics in cancer. N Engl J Med 358:1148–1159
Huang J, Plass C, Gerhauser C (2011) Cancer chemoprevention by targeting epigenome. Curr Drug Targets 12:1925–1956
Malireddy S, Kotha SR, Secor JD, Gurney TO, Abbott JL, Maulik G et al (2012) Phytochemical antioxidants modulate mammalian cellular epigenome:implications in health and disease. Antiox Redox Signal 17:327–339
Moriarty-Craige SE, Jones DP (2004) Extracellular thiols and tiol/disulfide redox in metabolism. Annu Rev Nutr 24:481–509
Martínez-González MA, García-López M, Bes-Rastrollo M, Toledo E, Martínez-Lapiscina EH, Delgado-Rodriguez M, Vazquez Z, Benito S, Beunza JJ (2011) Mediterranean diet and the incidence of cardiovascular disease: a Spanish cohort. Nutr Metab Cardiovasc Dis 21:237–244
Sofi F, Cesari F, Abbate R, Gensini GF, Casini A (2008) Adherence to Mediterranean diet and health status: meta-analysis. BMJ 337:a1344
Naselli F, Modica M, Attanzio A, Caradonna F, Gentile C, Tesoriere L, Livrea MA (2012) Pro-apoptotic activity of the phytochemical Indicaxanthin on colorectal carcinoma cells (Caco-2) and epigenetic CpG demethylation of the promoter and reactivation of the expression of p16. 56th National Meeting of the Italian Society of Biochemistry and Molecular Biology, Chieti
Draht MX, Riedl RR, Niessen H, Carvalho B, Meijer GA, Herman JG, van Engeland M, Melotte V, Smits KM (2012) Promoter CpG island methylation markers in colorectal cancer: the road ahead. Epigenomics 4:179–194
Matsuda Y (2008) Molecular mechanism underlying the functional loss of cyclin-dependent kinase inhibitors p16 and p27 in hepatocellular carcinoma. World J Gastroenterol 14:1734–1740
Zang JJ, Xie F, Xu JF, Qin YY, Shen RX, Yang JM, He J (2011) P16 gene hypermethylation and hepatocellular carcinoma: a systematic review and meta-analysis. World J Gastroenterol 17:3043–3048
Shima K, Nosho K, Baba Y, Cantor M, Meyerhardt JA, Giovannucci EL, Fuchs CS, Ogino S (2011) Prognostic significance of CDKN2A (p16) promoter methylation and loss of expression in 902 colorectal cancers: cohort study and literature review. Int J Cancer 128:1080–1094
von Elbe JH, Klement JT, Amundson CH, Cassens RG, Lindsay RC (1974) Evaluation of betalain pigments as sausage colorants. J Food Sci 39:128–132
von Elbe JT, Schwartz SJ (1981) Absence of mutagenic activity and a short term toxicity study of beet pigments as food colorants. Arch Toxicol 49:93–98
Patkai G, Barta J (1997) Decomposition of anticarcinogen factors of the beetroot during juice and nectar productions. Canc Lett 114:105–106
Reynoso R, Garcia FA, Morales D, de Mejia Gonzales E (1997) Stability of betalain pigments from a Cactacea fruit. J Agric Food Chem 45:2884–2889
Haveland-Smith RB (1981) Evaluation of the genotoxicity of some natural food colours using bacterial assays. Mutat Res 91:285–289
Reynoso RC, Giner TV, de Mejia Gonzales E (1999) Safety of a filtrate of fermented Garambullo fruit: biotransformation and toxicity studies. Food Chem Toxicol 37:825–830
Trachootham D, Lu W, Ogasawara MA, Rivera-Del Valle N, Huang P (2008) Redox regulation of cell survival. Antiox Redox Signal 1:1343–1347
Fruehauf JP, Meyskens FL Jr (2007) Reactive oxygen species: a breath of life or death? Clin Cancer Res 13:789–794
Lambert JD, Elias RJ (2010) The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys 501:65–72
Dianzani U (2003) 4-Hydroxynonenal from pathology to physiology. Mol Aspects Med 24:213–218
Higdon A, Diers AR, Oh JY, Landar A, Darley-Usmar VM (2012) Cell signaling by reactive lipid species: new concepts and molecular mechanisms. Biochem J 442:453–464
Jiang F, Zhang Y, Dusting GJ (2011) NADPH-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev 63:218–242
Bedard K, Krause K-H (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313
Leto TL, Morand S, Hurt D, Ueyama T (2009) Targeting and regulationof reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal 11:2607–2619
Schilder Y, Heiss EH, Schachner D, Ziegler J, Reznicek G, Sorescu D, Dirsch VM (2009) NADPH oxidases 1 and 4 mediate cellular senescence induced by resveratrol in human endothelial cells. Free Radic Biol Med 46:1598–1606
Konishi T (2011) From herb to kitchen and bedside: food factors are pharmacological molecules with antioxidant activity. Free Radic Res 45:863–864
Moreno DA, Garcia-Viguera C, Gil JI, Gil-Izquierdo A (2008) Betalains in the era of global agri-food science, technology and nutritional health. Phytochem Rev 7:261–280
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Livrea, M., Tesoriere, L. (2015). Indicaxanthin Dietetics: Past, Present, and Future. In: Chen, C. (eds) Pigments in Fruits and Vegetables. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2356-4_7
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2356-4_7
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2355-7
Online ISBN: 978-1-4939-2356-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)