Abstract
Hypertension is widespread and causes incalculable misery. Much of the population at risk of hypertension is not in regular contact with healthcare providers. The idea of a vaccine for hypertension that could ameliorate or cure the disease with a single procedure is innately appealing. The discovery that renin increased blood pressure raised the possibility that renin could be the key to the etiology of hypertension. Administering renin from one species to another resulted in the recipient developing anti-renin antibodies sparking interest in a vaccine against renin. Some of these renin vaccines were successful at lowering blood pressure but resulted in severe vasculitis and kidney damage. This turned the scientific focus downstream in the renin–angiotensin–aldosterone system, angiotensin I, angiotensin II, and the angiotensin II receptor all have tested as vaccine targets. Many of these peptides are small and unable to initiate an immune response by themselves. This stimulated research into possible adjuvants. Viral-like particle (VLP) showed strong immune response and has a good safety profile. The CYT006-AngQb vaccine combines angiotensin II and VLP. This is the only hypertension vaccine shown to significantly reduce blood pressure in hypertensive humans (mean − 9/− 4 mmHg). This was considered an ineffective response and has not been improved by larger or more frequent doses. Angiotensin II receptor vaccines are now undergoing active research. The chapter will discuss each vaccine and the future outlook for the field.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Background
Current pharmacologic therapy for hypertension is focused on managing it. A better treatment would be to employ the immune system to modulating hypertension mediators and targets in order to control the blood pressure.
Vaccines are therapies that stimulate the immune system to provide protection against disease. Usually, this is via antibodies against infectious diseases; however, vaccines can be used in other chronic disease, e.g., cancer, asthma, smoking cessation, and hypertension [1–3].
Vaccines need a target. In infectious disease, vaccines target bacterial or viral outer surface proteins. What is the appropriate target for hypertension? The causes of hypertension are complex and it is unlikely that there is a single pinpoint cause, however, the renin–angiotensin–aldosterone system (RAAS) is a central actor and makes a logical target for vaccine development . See Chap. 6 for a full discussion of the RAAS. The remainder of this chapter will focus on the research and achievements of vaccines targeting elements of the RAAS.
Renin Vaccine
Renin was discovered in 1898 by Tigerstedt and Bergman . They extracted renin from the kidney of a hypertensive dog and found that it could induce hypertension in another nephrectomized dog [4].
Johnson and Wakerlin first demonstrated that parenteral administration of renin from one species to another species results in the production of anti-renin antibodies [5]. Goldblatt conducted the first human study of a renin vaccine when he injected human subjects with porcine renin [6]. The subjects, who all had primary hypertension , developed antibodies to the foreign renin, but the antibodies did not lower blood pressure. The investigators theorized that there must have been a lack of cross-reactivity between the antibodies developed against porcine renin and native human renin .
There were several studies on renin vaccine from 1950 to 1980 with both active and passive immunization [7]. Most of the studies were done using cross-species renin infusions to develop the antibodies. The procedure was most successful at reducing renal vascular hypertension (the experimental model for the hypertension usually involved nephrectomy and partial renal artery obstruction). Some studies found blood pressure reductions as high as 20–50 mmHg. The procedure appeared safe with no immune complex depositions discovered in renal biopsies
The early promising results prompt Michael et al. to test a renin vaccine with adjuvant [8]. The use of an adjuvant was in order to generate a more sustained clinical response. He combined purified human renin with Freund’s adjuvant, water-in-oil emulsion and dead mycobacterium. When tested in marmosets, this vaccine resulted in high titers of anti-renin antibodies and a significant reduction in blood pressure. However, 1–4 months after immunization, the animals became sick and died. Autopsies showed immunoglobulin deposition in the afferent arterioles. There was also evidence of cellular inflammation around the renal arterioles and interstitial nephritis. This setback closed this avenue of research and no further work being done on renin vaccines .
Angiotensin I Vaccine
Angiotensin I and angiotensin II are small peptides, 10 and 8 amino acids respectively. Because of their small size, investigators theorized that anti-angiotensin antibodies should pose less of an autoimmune threat. Angiotensin I molecules need to be prepared with an adjuvant to make them immunogenic .
Reade et al. immunized spontaneously hypertensive rats (SHR) with angiotensin I coupled to Limulus polyphemus hemocyanin. Despite the successful induction of high titers of angiotensin I antibodies, there was no reduction in blood pressure [9].
Gardiner et al. immunized normotensive rats with angiotensin I coupled to a tetanus toxoid (TT) carrier protein adjuvanted with aluminum hydroxide (ALOH). The vaccine was injected on days 0, 21, and 42. Vaccinated rats had blunted responses to exogenous angiotensin I on day 63 and had no response to angiotensin II administration. The anti-angiotensin antibody titer increased 32, 100 fold [10].
Downham et al. compared angiotensin I vaccines made with two different carriers, TT and keyhole limpet hemocyanin (KLH) vaccine (PMD−3117) in humans and rats [11]. The researchers thought that since tetanus toxoid is a common antigen that most of adults have been exposed to, it may reduce the effectiveness of the vaccine [12]. In rats, both vaccines induced similar immune responses and similar protection from the presser effects of exogenous angiotensin I . In humans, however, the antibodies were unable to blunt the hypertensive effect of either angiotensin I or angiotensin II challenges.
Brown et al. tested an angiotensin I vaccine on patients with primary hypertension [13]. Patients, who had already been shown to be responsive to an ACEi or ARB, were randomly assigned to receive PMD3117 or placebo over a 6-week period. Patients stopped ACEi/ARB therapy 2 weeks before starting the study drug and resumed it 6 weeks later. The vaccine increased anti-angiotensin antibody titer after the second injection and titers peaked on day 64. Median half-life was 85 (95 % CI, 44 and 153) days. The vaccine did not influence blood pressure. Biochemical assessment showed patients randomized to vaccine had higher levels of renin (P = 0.033) and lower levels of aldosterone (6 % of values seen in patients receiving placebo, P = 0.012). The author concluded that the vaccine had biochemical but not clinical suppression of the RAAS.
Turki et al. created an angiotensin I vaccine using a novel adjuvant, CoVaccine HT. This is an adjuvant made of synthetic sucrose fatty acid sulfate esters immobilized on the droplets on a submicron emulsion of squalane in water. Mild to moderate hypertension patients (n = 124) were randomly assigned to receive vaccine every 3 weeks for or placebo for a 9-week period. The study had to be terminated due to adverse effects [NCT00702221].
Angiotensin II Vaccine
Johnston et al. theorized that, although immunization against angiotensin II greatly reduced the hypertensive response to exogenous angiotensin II, it played no direct role in the production or maintenance of experimental renal hypertension [14] . He also found that angiotensin II antibodies have a half-life of only 11 h. Similar findings, discrediting angiotensin II as having a key role in hypertension were provided by MacDonald et al. [15].
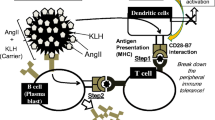
In 2007, Cytos Biotechnology AG (Switzerland) developed an angiotensin II-specific vaccine called CYT006-AngQb. CYT006-AngQb uses a modified angiotensin II peptide, with a N-terminal CysGlyGly extension. This antigen is covalently bound to virus-like particle (VLP) derived from protein coat of the bacteriophage Qβ (Fig. 9.1).
VLPs have supramolecular structures with rods or icosahedrons with diameters in the range of 25–100 nm. They are composed of multiple copies of one or more recombinant, expressed, viral, structural proteins which spontaneously assemble into particles. In addition to being virus-like in structure, they are often antigenically indistinguishable from the virus from which they were derived [16].
VLPs stimulate a strong B-cell response against self-antigens and this new technology helps overcome the self-tolerance limitations of immunization against angiotensin II [17, 18].
Tissot et al. did a multi-center, double-blind, randomized, placebo-controlled phase II trial. They randomized 72 patients with mild-to-moderate hypertension to receive subcutaneous injections of either 100 μg CYT006-AngQb, 300 μg CYT006-AngQb, or placebo, at weeks 0, 4, and 12 [19]. Twenty-four-hour ambulatory blood pressures were recorded before treatment and at week 14 . After a single injection, all patients receiving the vaccine responded with high anti-angiotensin II IgG titers. The antibody response was boosted after the second injection, and reached peak levels of response about 2 weeks after the third injection. The anti-angiotensin II IgG response was dose dependent, higher titers in patients randomized to 300 mcg than those randomized to 100-mcg dose. The half-life after the third injection was only 17 weeks. In the 300-mcg group, the ambulatory blood pressures fell 9.0/4.0 mmHg from baseline, with very dramatic decreases in early-morning blood pressures (25.0/13.0 mmHg).
The investigators could not document any change in the concentration of C1, C3, or factor C3a, which suggests that there was little to no immune complex deposition. The plasma renin levels increased in the vaccine group, likely due to a reduction in blood pressure. This trial is the first to show that vaccination against a vasoactive endogenous substance can reduce blood pressure in human beings .
Further work by the same team investigated more frequent dosing (at weeks 0, 2, 4, 6, and 10). This modification showed a fivefold increase in antibody titer but only − 2.3/− 0.4 mmHg improvement in blood pressure. Antibody affinities for angiotensin II were significantly lower in the second study than in the first (P < 0.001). The authors concluded that both the quantity and the quality of the antibody is important for blood pressure reduction. Future studies on CYT006-AngQb are on hold due to financial reasons .
Angiotensin II Receptor Type 1 Vaccine
The newest vaccine approach does not target renin, angiotensin I or angiotensin II, rather it creates antibodies to block angiotensin II receptor type 1 (ATR; Fig. 9.2) .
Schematic representation of the classic renin–angiotensin system with oral medication and vaccines blocking at their specific targets. The inhibitory actions are shown in dashed lines with arrows. ACE angiotensin converting enzyme, ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker, AT 1 R Ang II-type 1 receptors
Zhu et al. immunized spontaneous hypertensive rat with a peptide-based vaccine made of a seven-amino-acid sequence (AFHYESR) from the second extracellular loop of rat AT−1 A receptor (ATR12181; [20, 21]). The carrier protein is a TT complex in combination with Freund’s adjuvant. The vaccine induced anti-ATR12181 antibodies and a 17-mmHg reduction in systolic blood pressure. They also noted decreased cardiac hypertrophy and decreased kidney injuries. No signs of autoimmune disease were found after sacrificing the rats.
The same group changed the adjuvant to VLP, which have a better safety profile than Freund’s adjuvant [22]. The vaccine significantly decreased the blood pressure of angiotensin II-induced hypertensive mice up to 35 mmHg and that of spontaneously hypertensive rats up to 19 mmHg and prevented remodeling of hypertensive-vulnerable target organs. The half-life of the antibody was 14.4 days. The antibody specifically bound to angiotensin II receptor type 1 and inhibited angiotensin II- induced calcium-dependent signal transduction events, including protein kinase C-α translocation, extracellular signal-regulated kinase 1/2 phosphorylation (72 % decrease; P = 0.013). They also saw a 68 % decrease in intracellular Calcium (P = 0.017). The antibody did not inhibit angiotensin II binding to the receptor but rather diminished the pressure response and signal transduction initiated by angiotensin II .
Road Block and Future Direction
A vaccine for hypertension has been investigated for over 100 years. Though an effective therapy has not emerged, the journey has resulted in significant scientific breakthroughs. Summary of the clinical trial of hypertension vaccine is given in Table 9.1. The earliest efforts used renin vaccines. This was a proof of concept. By transferring renin across species, renin could be made immunogenic and the anti-renin antibodies effectively lowered blood pressure in animals. Unfortunately, the effectiveness came with devastating autoimmune consequences forcing the abandonment of this target molecule. The autoimmune complications were at least partly due to the large size of the antigen [23]. Renin is a 406-amino acid peptide while angiotensin I , angiotensin II, and the angiotensin receptor target-region are 10, 8, and 7 amino acid peptides respectively. The smaller molecular target decrease the likelihood of simultaneous binding of two antibodies to a single antigen resulting in less cross-linking and decreased immune-complex formation.
Second-generation antihypertensive vaccines targeted angiotensin I were able to successfully generate anti-angiotensin I antibodies but failed to show any blood pressure reduction in both animal and human studies. This not only showed limitations in the therapeutic strategy of an angiotensin I vaccine but also helped researchers better understand the pathophysiology and determinants of primary hypertension .
The adjuvant is critical in boosting the immune response to a vaccine. Freund’s adjuvant induces strong immune response in an animal but can be toxic in humans. Aluminum gels and salts are the most commonly used adjuvants in human vaccines. VLP is a newer technology that is more efficient than aluminum [16]. VLP has a good safety profile and is used in commercial vaccines, Gardisil® and Cervavix TM. The hypertension vaccines that use VLP as an adjuvant have not shown any autoimmune side effects.
The third-generation antihypertensive vaccines targeted angiotensin II with a VLP adjuvant. This vaccine was tested in humans and is the only hypertension vaccine ever to be safe and effective in humans. Unfortunately, the efficacy was inconsistent and below the expectations, − 9/− 4 mmHg and − 2.3/− 0.4 mmHg from two studies. The vaccines are no longer under active investigation and were never approved for clinical use. The vaccines’ effectiveness was limited by an inability to generate sufficient titers with significant longevity. The chief appeal of a vaccine is the ability to treat once and provide a durable therapy.
The fourth-generation hypertension vaccine research changed strategies in order to decrease the need for such high titers. Angiotensin receptor (ATR) vaccines effectively reduce blood pressure and prevent target organ damage in animal models. Uniquely, the ATR vaccine does not sequestrate peptides through antigen–antibody complexes, but desensitizes the angiotensin II receptor. The strategy of changing the receptor will hopefully lower the antibody titers needed to be clinically effective and allow less frequent vaccine injections. This ATR vaccine is the only hypertension vaccine that is currently being investigated.
In summary, the current problem of hypertension vaccine is not a safety issue but one of efficacy. In order to lower blood pressure, individuals must have brisk antibody responses to the vaccine. This can be unpredictable. Additionally, not every antibody is created equal, with some having greater efficacy than others. Continued research and creativity may ultimately produce an effective vaccine for hypertension but for now that seems to be a distant and cloudy future.
Reference
Hoogsteder PH, Kotz D, van Spiegel PI, Viechtbauer W, Brauer R, Kessler PD, Kalnik MW, Fahim RE, van Schayck OC. The efficacy and safety of a nicotine conjugate vaccine (NicVAX®) or placebo co-administered with varenicline (Champix®) for smoking cessation: study protocol of a phase IIb, double blind, randomized, placebo controlled trial. BMC Public Health. 2012;12:1052.
Bachmann MF, Jennings GT. Therapeutic vaccines for chronic diseases: successes and technical challenges. Philos Trans R Soc Lond B Biol Sci. 2011;366(1579):2815–22.
Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341(6151):1192–8.
Solandt DJ, Massin R, Cowan CR. Hypertensive effect of blood from hypertensive dogs. Lancet. 1940;1:873–4.
Johnson CA, Wakerlin GE. Antiserum for renin. Proc Soc Exp Biol Med. 1940;44:277.
Goldblatt H, Haas E, Lamfrom H. Antirenin in man and animals. Trans Assoc Am Physicians. 1951;64:122.
Michel JB, Galen FX, Guettier C, Sayah S, Hinglais N, Fehrentz JA, le N’guyen D, Carelli C, Castro B, Corvol P. Immunological approach to blockade of the renin-substrate reaction. J Hypertens Suppl. 1989;7(2):S63–70 (review).
Michel JB, Guettier C, Philippe M, Galen FX, Corvol P, Menard J. Active immunization against renin in normotensive marmoset. Proc Natl Acad Sci U S A. 1987;84:4346–50.
Reade R, Michel JB, Carelli C, et al. Immunization of spontaneously hypertensive rats against Angiotensin1. Arch Mal Coeur Vaiss. 1989;82:1323–8.
Gardiner SM, Auton TR, Downham MR, Sharp HL, Kemp PA, March JE, Martin H, Morgan PJ, Rushton A, Bennet T, Glover JF. Active immunization with angiotensin I peptide analogue vaccines selectively reduces the pressor effects of exogenous angiotensin I in conscious rats. Br J Pharmacol. 2000;129(6):1178–82.
Downham MR, Auton TR, Rosul A, Sharp HL, Sjöström L, Rushton A, Richards JP, Mant TGK, Gardiner SM, Bennett T, Glover JF. Evaluation of two carrier protein-angiotensin I conjugate vaccines to assess their future potential to control high blood pressure (hypertension) in man. Br J Clin Pharmacol. 2003;56(5):505–12.
LeClerc C, Schutze M-P, Deriaud E, Przewlocki G. The in vivo elimination of CD4 + cells prevents the induction but not the expression of carrier-induced epitopic suppression. J Immunol. 1990;145:1343–9.
Brown MJ, Coltart J, Gunewardena K, Ritter JM, Auton TR, Glover JF. Randomized double-blind placebo-controlled study of an angiotensin immunotherapeutic vaccine (PMD3117) in hypertensive subjects. Clin Sci (London). 2004;107(2):167–73.
Johnston CI, Hutchinson JS, Mendelsohn FA. Biological significance of renin-angiotensin immunization. Circ Res. 1970;26(suppl II):II-215–22.
Macdonald GJ, Louis WJ, Renzini V, Boyd GW, Peart WS. Renal-clip hypertension in rabbits immunized against angiotensin II. Circ Res. 1970;27:197–211.
Spohn, G, Bachmann, MF. Exploiting viral properties for the rational design of modern vaccines. Expert Rev Vaccines. 2008;7(1):43–54.
Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262(5138):1448–51.
Jegerlehner A, Tissot A, Lechner F, Sebbel P, Erdmann I, Kundig T, Bachi T, Storni T, Jennings G, Pumpens P, Renner WA, Bachmann MF. A molecular assembly system that renders antigens of choice highly repetitive for induction of protective B cell responses. Vaccine. 2002;20(25–26):3104–12.
Tissot AC, Maurer P, Nussberger J, Sabat R, Pfister T, Ignatenko S, Volk HD, Stocker H, Müller P, Jennings GT, Wagner F, Bachmann MF. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind. Lancet. 2008;371(9615):821–7.
Zhu F, Liao YH, Li LD, et al. Target organ protection from a novel angiotensin II receptor (AT1) vaccine ATR 12181 in spontaneously hypertensive rats. Cell Mol Immunol. 2006;3:107–14.
Zhu F, Liao Y-H, Li L, et al. Abstract 2753: observation of long-term efficacy and safety of an ATR12181 vaccine against hypertension in SHR. Circulation. 2006;114:II_575.
Chen X, Qiu Z, Yang S, Ding D, Chen F, Zhou Y, Wang M, Lin J, Yu X, Zhou Z, Liao Y. Effectiveness and safety of a therapeutic vaccine against angiotensin II receptor type 1 in hypertensive animals. Hypertension. 2013;61(2):408–16.
Janin J, Cothia C. The structure of protein-protein recognition sites. J Biol Chem. 1990;265:16027–30.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Phisitkul, S., Topf, J. (2015). Blood Pressure Vaccines. In: Weir, M., Lerma, E. (eds) Chronic Kidney Disease and Hypertension. Clinical Hypertension and Vascular Diseases. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-1982-6_9
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1982-6_9
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-1981-9
Online ISBN: 978-1-4939-1982-6
eBook Packages: MedicineMedicine (R0)