Abstract
Inhibition of the renin-angiotensin system (RAS) by administration of either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) or a direct renin inhibitor (DRI) similarly reduces blood pressure (BP) when each is used as monotherapy in patients with hypertension. Both ACE inhibitors and ARBs also slow down the progressive decline in renal function, which marks renal injury, particularly in patients with diabetic nephropathy with the renoprotective effects of these drugs, in part, relating to their capacity to reduce protein excretion. Both ACE inhibitor and ARB therapy also decrease the high cardiovascular (CV) event rate common to high-risk cardiac patients. Moreover, ACE inhibitors and ARBs are both of proven benefit in forms of heart failure (HF) characterized by a reduced ejection fraction (EF).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Renin-angiotensin system (RAS)
- Angiotensin-converting enzyme (ACE)
- Angiotensin receptor blocker (ARB)
- Direct renin inhibitor (DRI)
Introduction
Inhibition of the renin-angiotensin system (RAS) by administration of either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) or a direct renin inhibitor (DRI) similarly reduces blood pressure (BP), when each is used as monotherapy in patients with hypertension [1, 2]. Both ACE inhibitors and ARBs also slow down the progressive decline in renal function, which marks renal injury, particularly in patients with diabetic nephropathy [3–5] with the renoprotective effects of these drugs, in part, relating to their capacity to reduce protein excretion [6]. Both ACE inhibitor and ARB therapy also decrease the high cardiovascular (CV) event rate common to high-risk cardiac patients [7–10]. Moreover, ACE inhibitors and ARBs are both of proven benefit in forms of heart failure (HF) characterized by a reduced ejection fraction (EF) [11, 12].
Experimental Basis for Combining an Angiotensin-Converting Enzyme Inhibitor and an Angiotensin-Receptor Blocker or a Direct Renin Inhibitor and/or an Aldosterone Receptor Antagonist
The pharmacologic actions of ACE inhibitors and ARBs and/or a DRI have been well characterized. BP reduction and/or tissue-based protection, achieved through interruption of the RAS , relates specifically to the distinctive pharmacodynamic properties of either an ACE inhibitor , a DRI, or an ARB [13, 14]. Factors that have some bearing on the final response to these drug classes include drug pharmacokinetic and pharmacodynamic half-life, the phenomenon of “angiotensin-II”, or “aldosterone escape” and/or interruption of the short feedback loop, which increases upstream components of the RAS—so-called reactive hyperreninemia [14, 15].
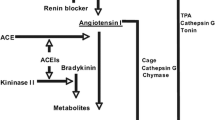
At the outset of therapy with an ACE inhibitor, both circulating and tissue concentrations of angiotensin-II (ang-II) drop. This fall in ang-II concentrations is to be expected given that ACE inhibition per se dose dependently lessens the enzymatic conversion of angiotensin-I (ang-I) to ang-II. Alternatively, with more long-term ACE inhibitor use, there is a gradual return of circulating and tissue ang-II concentrations to pretreatment levels, a process termed “angiotensin-II escape” [16]. One suggested explanation for ang-II escape focuses on the ability of tissue-based enzymes, such as chymase, cathepsin G, and CAGE (chymostatin-sensitive angiotensin-generating enzyme), to alternatively generate ang-II from a number of peptide substrates [17].
Since an ARB works by blocking the AT1-receptor, it was initially presumed that this mechanism of action, in addition to possibly AT2-receptor stimulation, would be additive to an ACE inhibitor effect by lessening the opposing BP effects that could in theory result from “angiotensin II escape.” The relevance of ang-II escape however remains unclear. In the treatment of hypertension and HF, there is scant evidence to support a role for ang-II escape in disabling the response to an ACE inhibitor [18]. If ang-II escape with ACE inhibitor use is ever to be clinically relevant, it will be on the basis of “suboptimal tissue protection,” a process that is not readily quantified. DRIs were originally held to offer incremental benefit for BP reduction in addition to what might be seen with an ACE inhibitor or an ARB, as they provide a more complete blockade of the RAS . It was posited that a DRI would suppress residual ang-II production and the counter-regulatory increase in plasma renin activity (PRA) observed in patients receiving ACE inhibitor and ARB monotherapy, and/or by blocking “aldosterone escape” that is seen with an ACE inhibitor or an ARB [19] .
ARAs can further reduce BP when given together with an ACE inhibitor, ARB, or a DRI . Fundamentally, nullifying the effect of aldosterone effect on BP with an ARA would be expected to further reduce BP beyond what would be seen with any of these classes given alone or together. Such BP reduction relates to an ARA effect on aldosterone/volume, which would not be mechanistically redundant as is the case when an ACE inhibitor is added to an ARB or a DRI [20–22].
Interpretive Considerations in Combining Angiotensin-Converting Enzyme Inhibitors and Angiotensin-Receptor Blockers and/or a Direct Renin Inhibitor or an Aldosterone Receptor Antagonist
The basis for combining an ACE inhibitor with an ARB or a DRI is to achieve a therapeutic outcome better than that seen with either drug given as monotherapy. Giving two RAS inhibitors together is not merely “giving two drugs” in that there are various pharmacologic considerations that influence the interpretation of the observed response. To accurately interpret the response to the combination of an ACE inhibitor and an ARB and/or a DRI requires that consideration be given to the pharmacologic profile of individual class members, time-of-day of dosing of each compound, the sequence with which the ARB or ACE inhibitor is added, and continuing dual therapy for a long-enough period of time to ensure that a long-term response has been identified [23].
There are more than 20 ACE inhibitors and ARBs, and one DRI marketed worldwide. There was one fixed dose combination of a DRI/ARB (aliskiren/valsartan [Valturna®]); however, it is off the market since 2012. Drugs within each of these classes have divergent durations of action; thus, the combination of the short-acting ACE inhibitor, captopril, with the long-acting ARB, candesartan, can produce a greater end-of-day response than if captopril were to be given with the more short-acting ARB, losartan. This can be mistakenly viewed as an additive response when it may simply reflect a more extended effect from the longer half-life compound. This is particularly the case with the long-acting compound, aliskiren [24]. When an ACE inhibitor and ARB are both long acting, meaningful additivity in BP reduction does not occur [25]. The timing of drug administration should also be accounted for in assessing a response to combination therapy in that, giving an ACE inhibitor and an ARB separated by several hours may conceivably prove more effective than if both medications were given simultaneously. Finally, the sequence in which these medications are given, such as whether an ACE inhibitor or an ARB is first given and when the alternative drug is added, may influence the final BP reduction and/or an end-organ effect such as a drop in urinary protein excretion [26].
Clinical Trial Considerations of Dual Renin-Angiotensin System Blockade
The concept of dual blockade of the RAS being inherently better than a single agent-modifying activity in this class seemed quite logical with the early experimental evidence from Menard et al. and therein rapidly emerged as a therapeutically attractive option [27]. Much of the early enthusiasm for dual blockade of the RAS system, however, derived from beneficial changes in surrogate end points such as BP, proteinuria, and/or endothelial dysfunction; however, a not insignificant amount of this unbridled excitement about combined RAS inhibition proved to be unjustified as the results from various trials became available with studies such as the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), the Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE), and the Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) [28–30].
Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial
ONTARGET was a double-blind, randomized, parallel-group study involving 25,620 patients in 40 countries. Patients were 55 years of age or older with either a history of coronary artery disease, stroke, peripheral vascular disease, or diabetes with end-organ damage. Subjects were randomized to telmisartan 80 mg/day, ramipril 10 mg/day, or telmisartan 80 mg/day plus ramipril 10 mg/day. The primary end points were CV mortality, nonfatal stroke, acute myocardial infarction (MI), and HF hospitalization. The secondary end points were newly diagnosed HF, diabetes mellitus, or atrial fibrillation; revascularization procedures, development of dementia/cognitive decline, and nephropathy. Study results showed that mean BP was lower in the telmisartan (0.9/0.6 mmHg greater reduction) and the combination therapy groups (2.4/1.4 mmHg greater reduction) than in the ramipril group. At study’s end, the primary end point had occurred in a similar number of patients in all three patient groups. Patients receiving combination treatment had higher rates of hypotensive symptoms, syncope, renal dysfunction, and hyperkalemia, with a trend toward an increased risk of progressing to a need for dialysis. At its conclusion, ONTARGET provided the largest evidence base available to determine, if the combination of an ACE inhibitor/ARB could reduce CV disease-related events and mortality in high-risk patients, including those with diabetes [28].
Assessment
The ONTARGET results strongly suggested that combination therapy with the ACE inhibitor, ramipril, and the ARB, telmisartan, was not to be recommended in high-risk patients with vascular disease or diabetes in the absence of HF. Shortly after the publication of the ONTARGET trial results, Messerli published in early 2009 a viewpoint advising physicians to avoid using dual RAS blockade because of the greater risk of side-effects [31]. Also, in early 2009 about the same time, the Canadian Hypertension Education Program urged physicians to no longer use these two drug classes together and the Canadian Heart and Stroke Foundation offered a similar guideline for patients with hypertension [32]. The bar for subsequent event trials with dual RAS inhibitor therapy would already appear to have been set high in early 2009 based on the academic perception of the ONTARGET results.
Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints
In the ALTITUDE study, the utility of the renin inhibitor, aliskiren, was tested in 8561 high-risk type 2 diabetic patients, the majority of whom had albuminuria, who were adjunctively given aliskiren 300 mg/day or placebo in addition to treatment with an ACE inhibitor or an ARB. A composite CV and renal end point was selected. The trial was discontinued prematurely after 18.3 % of the aliskiren group had reached the primary end point compared to 17.1 % in the placebo group. About 41 % of patients had a baseline systolic BP > 140 mmHg, and 12 % had a diastolic BP > 85 mmHg. Oddly, BP actually increased about 3 mmHg in both groups, although this increase was less in the group given aliskiren. Glomerular filtration rate (GFR) decreased 5 ml/min over the 42 months of observation in both groups. Potassium increased in both groups but more so in the aliskiren group. Hyperkalemia (> 5.5 mmol/l) was both the most common adverse event reported by investigators and the lead cause of study drug discontinuation. The Data and Safety Monitoring Board terminated this study early citing issues of therapeutic futility as well as an increased incidence of nonfatal stroke, renal complications, hyperkalemia, and hypotension over the 18–24 months of follow-up [29].
Assessment
The investigators in ALTITUDE quite appropriately underscored the need to go beyond surrogate biomarkers and obtained risk-benefit data from a clinical end point trial to better inform clinical decisions with aliskiren use in combination. There were several study design issues in ALTITUDE including most importantly the fact that patients in this study did not have any dose reduction or drug withdrawal when aliskiren was added making low BP occurrence more likely (median systolic BP at baseline was 135 mmHg systolic). At the completion of the ALTITUDE, the physician community awaited results from VA NEPHRON-D to make a final decision on the therapeutic positioning of dual RAS inhibitor therapy. Physician opinion was such that the premature termination of the ALTITUDE trial did not bode well for NEPHRON-D [29, 30].
Veterans Affairs Nephropathy in Diabetes
The VA NEPHRON-D trial studied the effect on CKD progression of 100 mg of the ARB losartan with or without the ACE inhibitor lisinopril (10–40 mg/day) in 1448 mainly male veteran patients with type 2 diabetes and overt nephropathy (GFR 54 mL/min). The primary end point was a composite of a 50 % decline in eGFR, end-stage renal disease (ESRD) requiring dialysis, or death. Safety end points included mortality, hyperkalemia (serum potassium > 6.0 mmol/L or that required an Emergency Department visit, hospitalization, or dialysis), and acute kidney injury adverse events, which were episodes occurring during or requiring hospitalization. BP values were similar in the two groups at enrollment, during adjustment of the losartan dose, and at randomization. The combination group had slightly lower BP readings on treatment by 2 mmHg. A total of 152 primary end point events occurred in the monotherapy group and 132 in the combination therapy group, a nonsignificant difference (hazard ratio (HR) with combination therapy, 0.88; 95 % confidence interval [CI], 0.70–1.12; P = 0.30). A trend toward a benefit from combination therapy with respect to the secondary end point (HR, 0.78; 95 % CI, 0.58–1.05; P = 0.10) decreased with time (P = 0.02 for nonproportionality). There was no benefit with respect to mortality (HR for death, 1.04; 95 % CI, 0.73–1.49; P = 0.75) or CVR events. Total mortality or CV end points were not different between treatments. This study was prematurely terminated for safety concerns. Combination therapy increased the risk of hyperkalemia (6.3 events per 100 person-years, vs. 2.6 events per 100 person-years with monotherapy; P < 0.001) and acute kidney injury (12.2 vs. 6.7 events per 100 person-years, P < 0.001) [30].
Assessment
Similar to the ALTITUDE study, this was an outcome trial with combination RAS therapy that was prematurely terminated based on safety consideration with modest, if any, outcome benefits. As in ONTARGET and ALTITUDE, combination therapy reduced albuminuria, and despite the favorable change in this surrogate marker of renal function, it did not result in a reduction in risk. This should be the last study undertaken with combination RAS inhibitor therapy.
Additional Considerations in Cardiorenal Disease with Combination RAS Inhibitor Therapy
There have been several areas where dual RAS inhibition has been considered as a suitable treatment option including difficult to manage hypertension, use in patients with high risk of vascular disease, post-MI, reduced EF forms of HF, and proteinuric forms of CKD. The use of dual RAS inhibitor therapy for resistant hypertension has not been studied in any sort of systematic manner and, as such, when used in this manner it has been empiric making it difficult to interpret observed responses [33]. There are currently no guidelines/treatment algorithms that support the use of dual RAS inhibitor therapy in the patient with resistant hypertension. Of note, as an example of the limited amount of information on this topic, patients with BP > 160/100 mmHg at entry were excluded from ONTARGET, thus limiting the applicability of these results to the treatment of significant hypertension [28].
Ramipril and telmisartan given together in ONTARGET did not afford a mortality or CVR benefit over and above ramipril therapy and, as such, did not provide any supporting data for the use of dual RAS inhibition in patients at high risk for vascular disease [28]. In addition, in the Valsartan in Acute Myocardial Infarction (VALIANT) trial, the combination of captopril and valsartan, together and individually given, was studied in a cohort within 10 days of acute MI. No additional survival benefits were seen with combination therapy, and the dual therapy group clearly experienced the greatest number of side effects [34].
The benefit of dual RAS inhibition, however, still remains a topic of some considerable interest in two areas, reduced EF forms of HF and proteinuric forms of CKD. In the USA, the ARBs, candesartan and valsartan, have a labeled indication for add-on use to ACE inhibitor therapy in patients with reduced EF forms of HF [35, 36]. Early HF treatment guidelines had recommended “addition of an ARB in patients with HF and a left ventricular ejection fraction ≤ 40 %, who remained symptomatic despite optimal treatment with an ACE and a beta-blocker’’ [37]. More recently, this recommendation has been revised restricting ARB add-on use to patients who are unable to tolerate an ARA [38]. A meta-analysis addressing this issue of the best next drug to add to standard HF therapy found the risk benefit ratio to favor the addition of an ARA over an ARB or a DRI, albeit with an appreciation for a greater risk of developing hyperkalemia [39].
A number of studies have found that there is an incremental benefit for reduction in proteinuria, regression to normoalbuminuria, reducing BP, and increasing the rate of reaching BP goals with combination RAS inhibition [40]. Not surprisingly, these same studies have shown more short-term declines in BP, a greater frequency of hyperkalemia, and more frequent occurrences of hypotension. The NEPHRON-D study, which evaluated combination ACE inhibitor/ARB therapy in proteinuric patients with diabetic nephropathy, was prematurely terminated based on these same specific safety concerns [30]. The results from NEPHRON-D make combination RAS inhibitor therapy an ill-advised treatment option in the patient with proteinuric diabetic nephropathy. Of note, ARAs reduce proteinuria and BP in adults who have mild-to-moderate CKD treated with an ACE inhibitor or ARB (or both), but increase the risk of developing hyperkalemia [41, 42]. Whether adding an ARA to an ACE inhibitor and/or an ARB reduces the risk of major CV events or ESRD in this population is unknown [41].
Current Status of Combination RAS Inhibitor Therapy in Stroke
Dual RAS blockade, at least on the initial cut of the data from the ALTITUDE trial, was associated with a higher rate of stroke. The rate of stroke, which was mostly ischemic stroke, was numerically higher with aliskiren, although the overall difference did not reach statistical significance (3.4 vs. 2.8 %; HR, 1.25; 95 % CI, 0.98–1.60; P = 0.07) [29]. It has been conjectured that this increase in stroke rate might be due to sensitization of the Bezold–Jarisch reflex with ensuing withdrawal of sympathetic tone, prolonged bradycardia and hypotension, and/or merely a chance finding [43]. A recent meta-analysis examining the risk of stroke with dual RAS blockade versus individual renin-angiotensin-aldosterone system (RAAS) monotherapy did not identify a signal for increased risk [44]. These findings together with the failure to prevent strokes despite lower BP with combination RAS blockade argue against any sort of routine use of these combination therapies in the primary or secondary prevention of stroke.
Side Effects with Combined RAAS Inhibitor Therapy
Hypotension is not a specific side effect with dual RAS inhibition; rather, it is a broadening of the physiologic action of these drugs that occurs most commonly when a patient becomes volume contracted. Dual RAS inhibitor therapy-related hypotension can present as a first-dose phenomenon or anytime in the course of chronic therapy, and in the latter instance being prompted by intercurrent illnesses that lead to volume contraction and/or a lessening of sodium intake [45, 46] . If dual RAS inhibition is sufficiently prolonged, a meaningful drop in the GFR will often occur, which reflects a form of functional renal insufficiency. Predisposing conditions to this process include dehydration, HF, nonsteroidal anti-inflammatory drug use, and/or either micro- or microvascular renal disease all of which would not have been thought of as being uncommon occurrences in the target populations enrolled in any of the dual RAS inhibitor trials.
Hyperkalemia is an additional dual RAS inhibitor-associated side effect that has a strong physiologic basis and like all forms of hyperkalemia is highly definitional in nature [45]. Once a specific definitional threshold value has been reached during dual RAS inhibitor therapy, a specific criterion will be satisfied and the patient then counts as an affected case. It is axiomatic in the use of dual RAS inhibitor therapy to always anticipate an increase in serum potassium values, and the frequency with which hyperkalemia is detected will in part be protocol driven according to the frequency of sampling. Study populations consisted of those with diabetes, older age, CKD, and/or HF are inherently at a greater risk for the development of hyperkalemia. As such, subjects in the NEPHRON-D population who were diabetics with nephropathy and a reduced GFR would ostensibly have a greater risk for hyperkalemia development in comparison to a less at risk population studied in ONTARGET [28, 30, 47].
A recent meta-analysis by Makani et al. found that dual RAAS inhibitor therapy compared with RAAS monotherapy was associated with a 55 % increase in the risk of hyperkalemia (P < 0.001), a 66 % increase in the risk for hypotension (P < 0.001), and a 41 % increase in the risk for renal failure (P = 0.01), as well as a 27 % increase in the risk of withdrawal due to an adverse event (P < 0.001) [48]. This constellation of findings would strongly suggest that the risk to benefit ratio for such therapy is too high for any sort of routine use of dual RAAS inhibition therapy.
Regulatory Bodies and Combined RAAS Inhibitor Therapy
The European Medicines Agency (EMA) recently warned that no two drug classes that act separately on the RAAS should be used in combination and this was viewed as particularly the case in patients with diabetic nephropathy. The EMA further advised if such combination therapy use is viewed as a critical treatment option, including the use of candesartan or valsartan, with ACE inhibitor therapy in patients with HF, then a proper specialist should supervise the use. Comments from EMA further add that “the combination of aliskiren with an ARB or ACE inhibitor is strictly contraindicated in those with kidney impairment or diabetes [49].” The 2014 Evidence-Based Guidelines for the Management of High Blood Pressure in Adults unambiguously state that ACE inhibitors and ARBs should not be used together [50]. The US Food and Drug Administration (FDA), however, has not reviewed the concerns or issued any warnings on the combined use of these drug classes beyond what was has been advised for the use of either an ACE inhibitor or an ARB with aliskiren. Of note, in that regard the fixed dose combination of valsartan/aliskiren (Valturna®) approved for use in the USA in September 2009 was voluntarily removed from marketing by Novartis in July 2012 as per safety concerns originating from the ALTITUDE trial.
Conclusions
There have been multiple commentaries on the topic of combined RAS blockade as relates to renal disease/proteinuria, HF, and use in the instance of CVD, and all have reached a similar conclusion that such a therapeutic approach is no longer advisable [51–53]. Once again, enthusiasm for an attractive pharmacologic concept, such as “blocking” the RAS as much as possible, in the hope that incremental outcome benefits would be garnered, abjectly failed. The alluring nature of a concept, such as combination RAS inhibitor therapy, is just one example of the ways in which the clinician is sidetracked from simpler and more easily accomplished ways to improve BP control and outcomes such as system-based approaches to hypertension management as are employed in the Veterans Administration system and endeavoring to ensure medication compliance.
References
Elliott WJ. Therapeutic trials comparing angiotensin converting enzyme inhibitors and angiotensin II receptor blockers. Curr Hypertens Rep. 2000;2:402–11.
Friedrich S, Schmieder RE. Review of direct renin inhibition by aliskiren. J Renin Angiotensin Aldo Syst. 2013;14:193–6.
Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9.
Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–60.
Lebovitz HE, Wiegmann TB, Cnaan A, et al. Renal protective effects of enalapril in hypertensive NIDDM: role of baseline albuminuria. Kidney Int (Suppl). 1994;45:S150–5.
Keane WF, Brenner BM, de Zeeuw D, et al. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int. 2003;63:1499–507.
Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med. 2000;342:145–53.
Fox KM, EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2003;362:782–8. (the EUROPA study).
Lindholm LH, Ibsen H, Dahlof B. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:1004–10.
Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with valsartan- or amlodipine-based regimens: VALUE, a randomised trial. Lancet. 2004;363:2021–31.
Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative group on ACE inhibitor trials. JAMA. 1995;273:1450–6.
Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-alternative trial. Lancet. 2003;362:772–6.
Sica DA. Pharmacotherapy review—part 2. Angiotensin-receptor blockers. J Clin Hypertens. 2005;7:681–4.
Sica DA, Gehr TWB, Frishman WH. The renin-angiotensin axis: angiotensin converting enzyme inhibitors and angiotensin-receptor blockers. In: Frishman W, Sonnenblick S, Sica DA, editors. Cardiovascular pharmacotherapeutics. 2nd edn. New York: McGraw-Hill; 2003. pp. 131–56.
Moniwa N, Varagic J, Ahmad S, et al. Hemodynamic and hormonal changes to dual renin-angiotensin system inhibition in experimental hypertension. Hypertension. 2013;61:417–24.
Hanon S, Vijayaraman P, Sonnenblick EH, Le Jemtel TH. Persistent formation of angiotensin II despite treatment with maximally recommended doses of angiotensin converting enzyme inhibitors in patients with chronic heart failure. J Renin Angiotensin Aldosterone Syst. 2000;1:147–50.
Balcells E, Meng QC, Johnson WH Jr, et al. Angiotensin II formation from ACE and chymase in human and animal hearts: methods and species considerations. Am J Physiol. 1997;273:H1769–74.
Chan JC, Ko GT, Leung DH, et al. Long-term effects of angiotensin-converting enzyme inhibition and metabolic control in hypertensive type 2 diabetic patients. Kidney Int. 2000;57:590–600.
Cagnoni F, Njwe CA, Zaninelli A, et al. Blocking the RAAS at different levels: an update on the use of the direct renin inhibitors alone and in combination. Vasc Health Risk Manag. 2010;6:549–59.
Bomback AS, Rekhtman Y, Klemmer PJ, Canetta PA, Radhakrishnan J, Appel GB. Aldosterone breakthrough during aliskiren, valsartan, and combination (aliskiren + valsartan) therapy. J Amer Soc Hypertens. 2012;6:338–45.
Saklayen MG, Gyebi LK, Tasosa J, Yap J. Effects of additive therapy with spironolactone on proteinuria in diabetic patients already on ACE inhibitor or ARB therapy: results of a randomized, placebo-controlled, double-blind, crossover trial. J Investig Med. 2008;56:714–9.
Werner C, Poss J, Bohm M. Optimal antagonism of the renin-angiotensin-aldosterone system: do we need dual or triple therapy. Drugs. 2010;70:1215–30.
Sica DA. Combination ACE inhibitor and angiotensin receptor blocker therapy—future considerations. J Clin Hypertens. 2007;9:78–86.
Dusing R, Brunel P, Baek I, Baschiera F. Sustained blood pressure-lowering effect of aliskiren compared with telmisartan after a single missed dose. J Clin Hypertens. 2013;15:41–7.
Doulton TW, He FJ, MacGregor GA. Systematic review of combined angiotensin-converting enzyme inhibition and angiotensin receptor blockade in hypertension. Hypertension. 2005;45:880–6.
Laverman GD, Navis G, Henning RH, de Jong PE, de Zeeuw D. Dual renin-angiotensin system blockade at optimal doses for proteinuria. Kidney Int. 2002;62:1020–5.
Menard J, Campbell DJ, Azizi M, Gonzalves MF. Synergistic effects of ACE inhibition and Ang II antagonism on blood pressure, cardiac weight, and renin in spontaneously hypertensive rats. Circulation. 1997;96:3072–8.
Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–59.
Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal endpoints in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;2204–13.
Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892–903.
Messerli FH. The sudden demise of dual renin-angiotensin system blockade or the soft science of the surrogate end point. J Am Coll Cardiol. 2009;53:468–70.
Heart and Stroke Foundation. Guideline alert for blood pressure patients as treatment combo [press release, January 16, 2009]. http://www.heartandstroke.com/site/apps/nlnet/content2.aspx?c†=ikIQLcMWJtE&b=3485819&ct=6501933. Accessed 30 June 2014.
Wong J. Is there benefit in dual renin-angiotensin-aldosterone system blockade? No, yes, and maybe: a guide for the perplexed. Diabetes Vasc Dis Res. 2013;10:193–201.
Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–906.
Cohn JN, Tognoni G, Valsartan heart failure investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–75.
McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-added trial. Lancet. 2003;362:767–71.
Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29:2388–442.
McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–847.
Bangalore S, Kumar S, Messerli FH. When conventional heart failure therapy is not enough: angiotensin receptor blocker, direct renin inhibitor, or aldosterone antagonist. Cong Heart Fail. 2013;19:107–15.
Susantitaphong P, Sewaralthahab K, Balk EM, et al. Efficacy and safety of combined versus single renin-angiotensin-aldosterone system blockade in chronic kidney disease: a meta analysis. Am J Hypertens. 2013;26:424–41.
Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014;4:CD007004. doi:10.1002/14651858.CD007004.
Van Burn PN, Adams-Huet B, Nguyen M, Molina C, Toto RD. Potassium handling with dual renin angiotensin inhibition in diabetic nephropathy. Clin J Am Soc Nephrol. 2014;9:295–301.
Sever P. Hypotension and ischaemic stroke associated with aliskiren in the ALTITUDE trial: sensitization of the Bezold-Jarisch reflex? J Renin Angiotensin Aldosterone Syst. 2013;14:1–2.
Mankani H, Bangalore S, Sever P, Messerli FH. Is dual renin-angiotensin system blockade associated with increased risk of stroke. J Am Coll Cardiol: Heart Failure. 2013;1:454–8.
Sica DA. Angiotensin-converting enzyme inhibitors side effects—physiologic and non-physiologic considerations. J Clin Hypertens. 2004;6:410–6.
Laragh JH, Sealey JE. Sodium depletion in patients in clinical trials may account for the increased cardiovascular risk of dual blockade of the renin-angiotensin system. BMJ. 2013;346:f1685.
Heerspink HJ, Gao P, Zeeuw Dd, et al. The effect of ramipril and telmisartan on serum potassium and its association with cardiovascular and renal events: results from the ONTARGET trial. Eur J Prev Cardiol. 2014;21:299–309.
Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ. 2013;346:f360.
European Medicines Agency. PRAC recommends against combined use of medicines affecting the renin-angiotensin (RAS) system [press release]. April 11, 2014.
James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–20.
de Boer RA, Azizi M, Danser J, et al. Dual RAAS inhibition: recent developments and implications in light of the ALTITUDE study. J Renin Ang Aldo Sys. 2012;13:409–12.
Messerli FH, Bangalore S. ALTITUDE trial and dual RAS blockade: the alluring but soft science of the surrogate endpoint. Am J Med. 2014;126:e1–3.
Luft F. Perspective on combination RAS blocking therapy: Off-TARGET, Dis-CORD, Map-to-Nowhere, low ALTITUDE, and Nephron-D. Am J Nephrol. 2014;39:46–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Sica, D. (2015). Dual Inhibitors: RAAS Blockers/Combination Therapies: What Do All These Trials Mean?. In: Weir, M., Lerma, E. (eds) Chronic Kidney Disease and Hypertension. Clinical Hypertension and Vascular Diseases. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-1982-6_6
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1982-6_6
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-1981-9
Online ISBN: 978-1-4939-1982-6
eBook Packages: MedicineMedicine (R0)