Abstract
Endocrine studies that investigate the interplay between hormones, behavior, and social and ecological environment are critical for our understanding of proximate, physiological mechanisms underlying the biology and sociality of a species. Nonetheless, only recently have endocrine studies been incorporated into research on howler monkeys (Alouatta spp.), and only few aspects of endocrinology in 6 out of 12 species have been addressed. These include androgen and estrogen profiles of juvenile A. palliata, and progestin and estrogen profiles of the ovarian cycle of A. arctoidea, A. caraya, and A. pigra. In addition, socioendocrine studies in A. pigra and A. palliata have investigated how male androgen levels and male and female glucocorticoid levels are influenced by intra- and extragroup male–male competition, whereas ecologically oriented endocrine studies have revealed that in A. pigra, A. palliata, A. belzebul, and A. seniculus male and female glucocorticoid levels are influenced by a scarcity of high-quality food resources, habitat fragmentation, human disturbance, and translocation. Endocrine studies have shed light on howler monkey biology and sociality that were not anticipated based on behavioral data alone. This includes a nonaggressive form of intragroup male–male competition over access to females, a more prominent reliance on high-quality food resources such as fruits for these primarily folivorous primates, and an apparent higher sensitivity to social and ecological stress in females than in males. Additional endocrine studies across howler monkey species are needed to further elucidate relationships among diet, mating competition, and social interactions.
Resumen
Estudios endocrinológicos que investigan la interacción entre las hormonas, el comportamiento, y el contexto social y ecológico son fundamentales para nuestra comprensión de los mecanismos fisiológicos subyacentes a la biología y al sistema social de una especie. Sin embargo, sólo recientemente estudios endocrinológicos han sido incorporado en la investigación de los monos aulladores (Alouatta), y sólo unos pocos aspectos de la endocrinología en seis de las 12 especies han sido abordado. Estos incluyen investigar los perfiles de andrógenos y estrógenos de juveniles de A. palliata, y los perfiles de progesterona y estrógeno de los ciclos ováricos en A. arctoidea, A. caraya, y A. pigra. Además, los estudios socio-endocrinológicos de A. pigra y A. palliata han investigado como los niveles de andrógenos en machos y los niveles de glucocorticoides en machos y hembras se ven influidos por la competencia entre machos adentro y entre grupos, mientras que los estudios endocrinológicos en A. pigra, A. palliata, A. belzebul, y A. seniculus con un enfoque en factores ecológicos han revelado que los niveles de glucocorticoides de machos y hembras son afectados por la disponibilidad de alimentos de alta calidad, la fragmentación del hábitat, perturbación humana y el estrés acumulado durante eventos de translocación. Estudios endocrinológicos en el mono aullador han revelado aspectos de su biología y su sistema social que no fueron anticipados en base a sólo datos de comportamiento. Esto incluye una forma de competición no agresivo entre machos por el acceso a hembras, una dependencia más prominente en los recursos alimenticios de alta calidad como las frutas para estos primates principalmente folívoros, y una sensibilidad superior al estrés social y ecológico en hembras en comparación con machos. Otros estudios endocrinológicos entre más especies de monos aulladores serán necesarios para elucidar aún más el vínculo entre la dieta, la competición reproductiva, y las interacciones sociales.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Endocrine studies that investigate the interplay between hormones, behavior, and social and ecological environment are critical for our understanding of proximate, physiological mechanisms underlying individual, age, and sex-based variation in behavior. Studies of primate socioendocrinology provide a framework for identifying factors that regulate differential reproductive success among individuals (Bercovitch and Ziegler 2002). Recent advances in noninvasive techniques for measuring hormonal profiles in feces and urine have provided primatologists with the opportunity to investigate a wide range of endocrine systems and their influence on behavior in both captive and wild primates (Strier and Ziegler 2005). For example, endocrine studies are central in our understanding of reproductive maturation (i.e., puberty), which is driven by an increase in gonadal activity (Plant and Witchel 2006; Saltzman et al. 2011). In addition, reproductive endocrinology is essential for understanding the basic biology of reproduction, because “the occurrence and timing of largely concealed reproductive events such as ovulation, conception, pregnancy, and natural abortions can only be accurately and reliably detected through hormone analyses” (Lasley and Savage 2007:357). Similarly, socioendocrinology examines how the social environment influences the feedback loop between hormones and behavior, and has the power to enhance our understanding of male and female reproductive strategies (Bercovitch and Ziegler 2002; Anestis 2010), whereas ecological endocrinology investigates how the physical environment modulates behavioral and hormonal responses to fluctuating ecological factors such as seasonality in food availability, photoperiod, or weather conditions (Bradshaw 2007). Furthermore, endocrine studies may contribute importantly to the conservation of endangered primate species by using hormone studies on wild populations living in undisturbed habitats as baseline information to evaluate how the interplay between behavior and hormones is affected under conditions of demographic, ecological, and behavioral disruption resulting from habitat alteration due to human activity (Cockrem 2005).
Despite the important contributions that endocrine studies have made to our understanding of primate behavior and ecology, such studies only recently have been incorporated into research on howler monkeys (Alouatta spp.). Hormonal studies have been conducted on six howler monkey species: the ursine howler monkey (A. arctoidea), the red-handed howler monkey (A. belzebul), the black-and-gold howler monkey (A. caraya), the mantled howler monkey (A. palliata), the black howler monkey (A. pigra), and the red howler monkey (A. seniculus), and these have focused principally on questions of pubertal development, female reproduction, male competition, the influence of ecological stressors, and conservation management (Table 8.1).
2 Method Validation
Of the 22 endocrine studies on howler monkeys (Table 8.1), 21 studies used noninvasively collected fecal samples, and 1 study used noninvasively collected urine samples (Herrick et al. 2000). Both fecal and urine samples contain very low or no concentrations of active hormones but are characterized by high concentrations of hormone metabolites (Bahr et al. 2000; Heistermann et al. 2006). This presents an opportunity for biased results and therefore samples need to be validated to ensure that the substance measured from urine or feces is a metabolite derived from the hormone in question, and not a substance that cross-reacts with the antibody of the respective immunoassay. This would render the hormone profile meaningless (Whitten et al. 1998; Ziegler and Wittwer 2005). The procedures required for validation include providing a stimulus to an individual that will cause an increase in circulatory levels of the hormone in question, such as injecting adrenocorticotropic hormone (ACTH) or gonadotropin-releasing hormone (GnRH) when interested in glucocorticoids or gonadal steroids, respectively, or injecting radioactive-labeled forms of the particular hormone, and subsequently assess whether concentrations of the metabolites in feces and urine vary in parallel with changes in blood hormone concentrations (Palme 2005).
When such physiological validations are impossible to perform because of a lack of captive individuals or ethical restraints, biological validations are suitable alternatives. For example, patterns in production of gonadal steroids can be biologically validated by comparing steroid concentrations from samples collected during different reproductive stages (e.g., juveniles versus adults). Similarly, Martínez-Mota et al. (2008) validated the detection and measurement of glucocorticoids in fecal samples using enzyme immunoassays (EIA) in black howler monkeys before and after the application of a stressor, i.e., anesthesia. Levels of fecal glucocorticoid metabolites measured in four adults, residing at the Chapultepec Zoo in Mexico City, reached peak concentrations 24–96 h after anesthesia, parallel to an increase in circulating glucocorticoid concentrations, suggesting that the assay correctly measured glucocorticoid metabolites. Similarly, fecal glucocorticoid metabolites increased 21 and 24 h after the anesthesia of wild mantled howler monkeys (one male and two females, Gómez-Espinosa et al. 2013) and wild red howler monkeys (one male, Rimbach et al. 2013a), respectively. Wasser et al. (2010) validated the measurement of thyroid hormones in fecal samples using radioimmunoassay methods in two adult male and three adult female mantled howler monkeys that were temporarily kept in captivity during a translocation project. Thyroid concentrations decreased significantly post-capture in females but not in males. The authors argued that endocrine differences documented in this study corresponded to the limited food intake exhibited by females compared to males, suggesting that the measured thyroid levels accurately represented to metabolic state of these individuals.
Besides biological validations, the efficiency by which hormonal metabolites are recovered from samples using different extraction methods may need to be assessed to optimize endocrine studies, as has been done for estrogen and progesterone in black howler monkeys (Torres-Pelayo et al. 2011). These authors assessed whether the type of substrate (moist versus lyophilized feces), organic solvent (80 or 100 % ethanol versus methanol), and extraction method (agitation versus ebullition) affected the extraction efficiency of estrogens and progestins in fecal samples of black howler monkeys. Their findings suggested considerable variation in percentages of recovered steroids according to the substrate, solvent, and extraction method, with an ebullition extraction method as the most efficient (Torres-Pelayo et al. 2011).
3 Endocrinology of Puberty
Puberty in primates is characterized by behavioral, morphological, neurological, and endocrine changes, along with the development of distinctive secondary sex characteristics (reviewed in Plant and Witchel 2006; Saltzman et al. 2011). The onset of puberty in both males and females is marked by the increase in the pulsative release of GnRH from the hypothalamus, due to the diminishing inhibitory effect of the neurotransmitter GABA on the GnRH neurons. This causes a dramatic elevation in circulatory levels of luteinizing hormone (LH), and to a lesser extent follicle-stimulating hormone (FSH), released from the pituitary gland. The surge in LH stimulates testicular growth, the production of gonadal androgens, and the initiation of spermatogenesis in males, and the initiation of cyclic ovarian activity and the production of ovarian steroids in females (Plant and Witchel 2006). The onset of ovarian cycles is typically associated with a period of “adolescent infertility” characterized by anovulatory or irregular cycles prior to the onset of fertile ovarian cycles. Limited information is available on the onset of sexual maturation in howler monkeys. Based on behavioral observations, without the underlying endocrine confirmation, male howler monkeys are believed to be sexually mature between 2 and 5.5 years of age, while females are believed to be sexually mature between 2.9 and 4.5 years of age and experience their first parturition at 3.4–5.0 years of age (Table 8.2).
In mantled howler monkeys that usually live in groups with a large number of resident adult males, male puberty is also characterized by the descent of the testes from the inguinal canal into the scrotum around 3 years of age (Glander 1980), while in other howler monkey species, testes descend during infancy (Crockett and Eisenberg 1987). Prior to the descent of the testes, the non-pendulous scrotum of juvenile male mantled howlers cannot be visually differentiated from female juvenile genitalia, and juveniles can only be reliably sexed when they are newborn or by external palpation of captured individuals (Glander 1980; Clarke et al. 2007). In order to determine whether developmental differences in hormone concentrations can be used to differentiate between juvenile males and females, Clarke et al. (2007) collected fecal samples from 31 mantled howler juveniles (0.8–3.5 years old) of known sex (19 males and 12 females; juveniles were captured or positively sexed as infants) from five social groups at Hacienda La Pacifica, Costa Rica, during three 1-month field studies across 3 years. Mean fecal androgen and estrogen concentrations did not differ significantly between 1-year-old, 2-year-old, and 3-year-old males and females. Male fecal androgen levels increased during puberty with 3-year-old males (N = 6) having significantly higher fecal androgen levels than younger males (N = 13). However, three 3-year-old males who were observed to be actively evicted from their natal group by other group members had lower fecal androgen levels than 3-year-old males who remained in their natal group (N = 3), and these hormonal differences could have resulted from increased aggression and harassment received and a decrease in competitive ability prior to eviction (Bernstein et al. 1979).
Clarke et al. (2007) argued that juvenile monomorphic genitalia in mantled howler monkeys might serve to prolong the period that juveniles remain in their natal group because aggressive eviction from the natal group frequently occurred immediately after the appearance of adult genitalia. Because other howler monkeys do not exhibit juvenile genitalia monomorphy, comparative data on hormonal profiles underlying juvenile development, puberty, and eviction from their natal group across other howler monkey species are needed to better understand whether hormonal profiles observed in mantled howler juveniles are unique or represent a common pattern in Alouatta. Furthermore, given that dichromatism has evolved in four howler taxa (A. caraya, A. guariba clamitans, A. seniculus puruensis, and A. ululata) and males change to their adult coat color starting at 6 months of age (Bicca-Marques and Calegaro-Marques 1998), it would be important to study the interplay between hormonal profiles and adult–juvenile social interactions during changes from natal to adult coat coloration in these sexually dichromatic howler species, to assess whether natal pelage patterns serve as sexual mimicry during puberty. This is especially true when considering that a delay in the onset of coat color change observed in two black-and-gold howler juvenile males might have been linked to the increased probability of being evicted from their natal group due to the reduced size (0.3 ha) of the forest fragment they resided in compared to other groups with lower ecological and demographic pressures (Bicca-Marques and Calegaro-Marques 1998). The authors suggested that the delay in color change might be a mechanism allowing a juvenile male to remain in their natal group for a longer period attaining larger body sizes before being forcefully evicted, similar to mantled howler juvenile genitalia mimicry.
4 Reproductive Endocrinology
Information on estrogen and progestin profiles of the howler ovarian cycle is limited to three species (A. arctoidea, A. caraya, and A. pigra, Table 8.1). Steroids were measured from urinary (A. arctoidea) or fecal samples (A. caraya and A. pigra), and the steroid profiles of the ovarian cycle followed the pattern unique to platyrrhines. In all platyrrhines examined to date, the urinary and fecal estrogen and progestin profiles do not match the profiles present in circulating steroid hormones in that the urinary and fecal estrogen profiles do not exhibit a follicular surge prior to ovulation (reviewed in Ziegler et al. 2009a). Instead, urinary and fecal estrogen levels show a sustained increase during the luteal phase of the cycle, similar to urinary and fecal progestin profiles. Several studies have indicated that the onset of the sustained increase in urinary and fecal progestin concentrations occurs shortly after the serum luteinizing hormone (LH) peak, which triggers ovulation, while the delay in the excretion of estrogens is more variable (Fig. 8.1, Ziegler et al. 2009a). Therefore, in New World monkeys urinary and fecal progestin profiles are more reliable to estimate days of ovulation than estrogen profiles, and days of ovulation are estimated as the sample preceding the first sustained rise in urinary and fecal progestin levels. The length of the ovarian cycle is calculated as the time interval between two consecutive estimated days of ovulation (Van Belle et al. 2009a). A representative hormonal profile of a black howler female is shown in Fig. 8.2.
Mean ± SE of fecal estrogen and progesterone levels across the ovarian cycle, aligned relative to estimated day of ovulation (=day 0, n = 18) in four wild female black howler monkeys (A. pigra). The average profile shows that fecal progesterone concentrations decrease to baseline levels during the follicular phase when the ovum is growing. The rise in fecal progesterone levels indicates that ovulation has occurred. Fecal estrogen levels remain relatively constant during ovulation but show an increase about 2–4 days later
The fecal hormonal profile for a female black howler monkey (A. pigra) at Palenque National Park, Mexico Van Belle et al. 2009a
Following these methods, the mean ovarian cycle length for three adult black-and-gold howler females living in captivity was 19.1 ± SD 2.1 days (range = 9–47 days, N = 18; Kugelmeier et al. 2011). For four wild multiparous black howler females, mean ovarian cycle length was 18.4 ± SE 1.4 days (range = 13–25 days, N = 12; Van Belle et al. 2009a), and for two wild adult ursine howler females, mean ovarian cycle length was 29.5 ± SE 1.5 days (range = 28–31 days, N = 2; Herrick et al. 2000). The average ovarian cycle length of 18–19 days observed in black howler and black-and-gold howler monkeys is similar to the average ovarian cycle lengths estimated from patterns of observed copulations in ursine howler monkeys (mean = 17 days, range = 11–27 days, Crockett and Sekulic 1982) and mantled howler monkeys (mean = 15.8 days, range = 10–24 days, Glander 1980; Jones 1985). The longer estimates of ovarian cycles from urinary progestin profiles in ursine howler monkeys (Herrick et al. 2000) could reflect an artifact of limited sample size.
The fecal steroid profiles of three black howler females who had lost their young infants showed a resumption in cyclicity 1–3 weeks later, indicating an almost immediate physiological switch from lactational amenorrhea to ovarian cyclicity (Van Belle et al. 2009a). Nonetheless, the first cycle these females experienced after losing their infant may have been anovulatory because they were noticeably shorter (9–11 days) and had lower fecal estrogen and progesterone levels (Van Belle et al. 2009a). This delay in returning to reproductive condition might be linked to the replenishment of maternal energetic reserves as has been noted for several other primates (reviewed in Ziegler et al. 2009a).
Mean ovarian cycle length has also been estimated from cytological profiles of vaginal swabs in captive black-and-gold howler females. Based on the recurrence of squamous epithelial cells, Colillas and Coppo (1978) estimated a mean cycle length of 19.7 ± SD 1.0 days (range = 17–24 days), with ovulation estimated to occur immediately after the surge of squamous cells. Kugelmeier et al. (2011) estimated a mean ovarian cycle length of 19.8 ± SD 0.9 days (range = 18–22 days, N = 4) based on profiles of erythrocytes in vaginal swabs with a mean bleeding period of 4.1 ± SD 1.0 days (range = 1–7 days), coinciding with basal levels of both fecal progesterone and estrogen.
A reliable estimate of the day of ovulation and peak fertility based on hormonal profiles allows for mapping copulations and male and female sexual solicitations onto profiles of the ovarian cycle to provide critical insights into male and female reproductive strategies and reproductive success. Examining the occurrence of these behaviors during the periovulatory period, when conception is most likely to occur, compared to outside the periovulatory periods, allows one to assess the degree to which dominant or central howler males successfully mate-guard fertile females and the extent to which cycling females might undermine a male’s ability to monopolize reproductive opportunities through female mate choice. This type of study has only been conducted in black howler monkeys, and revealed that female sexual solicitations, male mate-guarding efforts, male’s monitoring of a female’s reproductive state by sniffing her genitalia, and copulations were largely confined to the periovulatory period (Van Belle et al. 2009a, reviewed in Van Belle and Bicca-Marques 2014).
5 Socioendocrinology
Socioendocrine studies in howler monkeys have investigated how male androgen levels and male and female glucocorticoid levels are influenced by intra- and extragroup male–male competition. The challenge hypothesis posits that an increase in adult male androgen levels, above baseline levels required for spermatogenesis and full display of sexual behavior, are closely associated with levels of male intrasexual competition for access to fertile females (Wingfield et al. 1990). Evidence of the challenge hypothesis in primates has been found in species in which males aggressively compete for social rank resulting in dominant males having higher androgen levels than subordinate males throughout the year (e.g., chimpanzees, Pan troglodytes, Muehlenbein et al. 2004; Muller and Wrangham 2004a; mandrills, Mandrillus sphinx, Setchell et al. 2008). Because of the high costs and potentially detrimental effects (e.g., suppression of immune system, suppression of parental care, reduced survival) associated with chronically elevated androgen levels (Braude et al. 1999; Muehlenbein and Bribiescas 2005; Hau 2007), rank-related differences in androgen levels may be expected to only coincide with periods of heightened male–male aggression during social instability due to rank reversal, immigration events, or reproductive competition (e.g., Verreaux’s sifakas, Propithecus verreauxi, Brockman et al. 2001; chacma baboons, Papio ursinus, Bergman et al. 2005). Consistent with the challenge hypothesis, androgen levels were found not to differ among males in species with limited aggressive competition over access to fertile females (e.g., Northern muriquis, Brachyteles hypoxanthus, Strier et al. 1999; tufted capuchin monkeys, Sapajus nigritus, Lynch et al. 2002; moustached tamarins, Saguinus mystax, Huck et al. 2005).
The stress response is characterized by a marked increase in circulating glucocorticoids released from the adrenal gland that accelerates carbohydrate metabolism and leads to an increased availability of glucose in the bloodstream, which in turn enables individuals to deal with short- and long-term threats (Sapolsky 2002). Short-term stress responses are generally thought to be beneficial in that they mobilize energy reserves. In contrast, chronic stress can result in the suppression of reproduction, the immune system, growth, and muscle wasting (reviewed in Sapolsky 2005). Potential threats or stressors include predation, nutritional deficiencies, and conspecific agonism. The frequency and degree of aggressive interactions among conspecifics, as well as the degree of social support available to individuals, are expected to contribute to variation in glucocorticoid concentrations within and among individuals (Abbott et al. 2003; Goymann and Wingfield 2004). As such, dominant individuals are expected to have higher glucocorticoid levels than subordinates when they are frequently challenged by others and need to regularly assert their high social status (e.g., female ring-tailed lemurs, Lemur catta, Cavigelli et al. 2003; male chimpanzees, Muller and Wrangham 2004b), or subordinates might have higher glucocorticoid levels than dominant individuals when subordinates endure frequent attacks by higher ranking individuals and have limited social support such as alliances and grooming partners to cope with these social stressors (e.g., chacma baboons, Sapolsky 1993). In other species, dominant and subordinate individuals might not differ in their glucocorticoid levels because the stressors faced by dominants are similar to those faced by subordinates (e.g., long-tailed macaques, Macaca fascicularis, van Schaik 1991; mountain gorillas, Gorilla beringei, Robbins and Czekala 1997) or social status may not be directly related to frequent intragroup aggression (e.g., northern muriquis, Strier et al. 1999; tufted capuchin monkeys, Lynch et al. 2002; moustached tamarins, Huck et al. 2005).
The social system of howler monkeys is characterized by very low levels of intragroup male–male competition with low frequencies of resident male–male aggression (A. pigra: mean rate = 0.007 interactions/dyad/h, Van Belle et al. 2008; mean rate = 0.04 interactions/ind/h, Rangel-Negrín et al. 2011; A. palliata: mean rate = 0.018 interactions/ind/h, Wang and Milton 2003; reviewed in Kowalewski and Garber 2014). Overt male–male aggression is principally confined to immigration events and intergroup encounters, which may result in injuries or death (A. arctoidea: Sekulic 1983; Crockett and Pope 1988; A. palliata: Glander 1992; Cristóbal-Azkarate et al. 2004; Dias et al. 2010; A. pigra: Horwich et al. 2000; Van Belle et al. 2008; A. seniculus: Izawa and Lozano 1991; Izawa 1997). Both single males or pairs of males have been observed to successfully immigrate into established groups during which none, one, several, or all of the resident males might be evicted (A. arctoidea: Pope 1990; Crockett and Pope 1993; Agoramoorthy and Rudran 1995; Crockett and Sekulic 1984; A. palliata: Clarke 1983; Glander 1992; Dias et al. 2010; A. pigra: Horwich et al. 2000; Van Belle et al. 2008; A. seniculus: Izawa and Lozano 1991; Kimura 1992; Izawa 1997). Take-over attempts may be accompanied by infanticidal attacks (reviewed in Crockett 2003) and both take-over attempts and intergroup encounters may involve extragroup copulations (reviewed in Van Belle and Bicca-Marques 2014), indicating that encounters with extragroup males may pose substantial threats to the reproductive success of resident males and females.
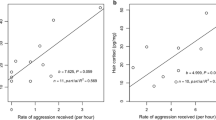
Cristóbal-Azkarate et al. (2006, 2007) investigated how male fecal androgen and male and female fecal glucocorticoid levels were influenced by the threat of solitary males in ten mantled howler monkey groups living in six forest fragments at Los Tuxtlas Biosphere Reserve, Mexico. Groups had, on average, 3.4 adult males (range = 1–6) and 5.4 adult females (range = 2–9). A total of 31 samples were collected from 17 males, with a mean of 3.1 (range = 1–7) male fecal samples per group, while a total of 35 samples were collected from 18 different adult females and 12 unidentified adult females, with a mean of 3.5 (range = 1–10) female fecal samples per group. Hormonal levels were averaged across all sampled males and all sampled females per group. No encounters between social groups and solitary males were observed during the study. Nonetheless, mean male fecal androgen levels per group were positively correlated with the number of extragroup males living in the same forest fragment, suggesting that mantled howler males on average exhibited a hormonal response proportional to the potential threat posed by solitary males (Cristóbal-Azkarate et al. 2006). Fecal glucocorticoid levels averaged across all sampled males per group were not significantly correlated, while those averaged across all sampled females per group were positively correlated with the number of solitary males living in the same forest fragment (Cristóbal-Azkarate et al. 2007). The authors suggested that differences in the ways in which males and females cope with stressful, unpredictable situations posed by the presence of extragroup males in their forest fragment may account for different mean fecal glucocorticoid levels among resident males and females. The passive response displayed by resident mantled howler females towards solitary males might be associated with an activation of the hypothalamic–pituitary–adrenal (HPA) axis resulting in chronically elevated fecal glucocorticoid levels, whereas the more active and aggressive response displayed by resident males seemed not to be associated with the activation of the HPA axis resulting in lower fecal glucocorticoid levels (Cristóbal-Azkarate et al. 2007), similar to differential glucocorticoid levels associated with passive versus active coping styles in captive rodents (Koolhaas et al. 1999; Ebner et al. 2005).
Similarly, a study of five black howler monkey groups living in five different sites in the state of Campeche, Mexico that were each observed for two sampling periods of 4 weeks separated by 3–8 months provided evidence that extragroup male–male competition also may be reflected in male fecal androgen levels in this species (Rangel-Negrín et al. 2011). In this study, mean fecal androgen levels averaged across samples per male were significantly higher for males living in one-male groups (N = 2 adult males) compared to males living in two-male or three-male groups (N = 7 adult males). Furthermore, two of the three multimale groups changed from multimale to unimale groups between the first and the second observation period, and mean fecal androgen levels of both males who remained in their respective groups increased significantly when being the sole male in a unimale group compared to when part of a multimale group. Because the probability of resident males being evicted by extragroup males during take-over attempts might be higher for unimale compared to multimale groups (A. arctoidea, Agoramoorthy and Rudran 1995; Pope 2000; A. pigra, Horwich et al. 2000; Van Belle et al. 2008), Rangel-Negrín et al. (2011) argued that unimale groups might be more attractive targets to dispersing males, and resident males of unimale groups might manifest elevated androgen levels in response to possible confrontations with extragroup males and increased risk of being ousted from their groups.
In contrast, a 14-month study of two multimale–multifemale black howler groups in Palenque National Park, Mexico, suggested that intragroup male–male competition, but not extragroup male–male competition, modulated male fecal androgen and glucocorticoid concentrations (Van Belle et al. 2009b). During this study, both focal groups underwent several changes in male group membership. These included (1) three take-over events during which three coalitions of two extragroup males each took over one of the study groups and evicted all adult resident males (N = 2 males per event) and (2) three male immigration events during which a single extragroup male joined either of the two study groups that had one, two, or three resident males at the time of immigration. This did not result in the eviction of resident males, except for one case in which one of the three adult resident males was evicted (for more details see Van Belle et al. 2008). Immigrant males (N = 9) had no significant differences in their fecal glucocorticoid and androgen levels during week 1 and week 2 following the take-over/immigration compared to week 3 and week 4. Similarly, resident males (N = 5) did not differ in their fecal glucocorticoid and androgen levels 2 weeks before and 2 weeks after a male immigration event (Van Belle et al. 2009b). Also, the five resident females in the two study groups did not have consistently higher fecal glucocorticoid levels 2 weeks after compared to 2 weeks before changes in male membership in their social group (Van Belle, unpubl. data; see Appendix 1). Furthermore, biweekly rates of encounters with either adjacent social groups or extragroup males were not correlated with changes in male hormonal levels (Van Belle et al. 2009b). These findings suggest that actual events of male–male competition over group membership, as opposed to potential threat, are not readily reflected in the fecal hormonal levels of black howler males and females in Palenque National Park. It is possible, however, that the unusually rapid turnover of male group membership in one of the study groups (7 changes in 6 months) might have affected the researchers’ ability to evaluate the influence of male immigration on male and female hormonal levels. Additional studies are needed to elucidate and compare changes in hormonal profiles during extragroup male–male competition across howler monkey species and groups living in both unimale and multimale social groups to better understand the social and demographic factors mediating hormonal responses to extragroup male–male competition.
Instead of extragroup male–male competition, Van Belle et al. (2009b) found that intragroup male–male competition is reflected in male fecal steroid levels in black howler monkeys. Although, resident black howler males seldom engaged in intrasexual agonistic (mean rate = 0.007 interactions/h/dyad) or affiliative interactions (mean rate = 0.009 interactions/h/dyad) and no agonistic dominance hierarchy could be discerned (Van Belle et al. 2008), one resident adult male per group, referred to as the “central” male, was found to monopolize almost all mating opportunities, spent significantly more time in close proximity to females, and engaged in affiliation at significantly higher rates with cycling females than did “noncentral” males (Van Belle et al. 2009b). Noncentral males had very limited mating opportunities and accounted for only 4 % of copulations (Van Belle et al. 2008). In addition, central males had significantly higher fecal androgen and glucocorticoid levels compared to noncentral males, suggesting that their efforts of fostering social relationships with females might represent a nonaggressive form of male–male competition over sexual access to females. This nonaggressive form of male–male competition might be socially challenging to central males as indicated by their higher fecal glucocorticoid levels (Van Belle et al. 2009b).
During this study, central males had significantly higher hormonal levels than noncentral males during resident female periovulatory and nonperiovulatory periods of ovarian cycles, as well as when none of the resident females were cycling (Van Belle et al. 2009b). This suggests that central males had elevated steroid levels throughout the year, even during periods when resident females were not sexually active. Furthermore, central male hormonal levels did not increase during the times when at least one resident female was cycling or during periovulatory periods of cycling females, despite higher copulation rates and heightened efforts by central males to spend time close to and groom cycling females compared to noncycling females (Van Belle et al. 2009a, b). In contrast, noncentral males had significantly lower mean fecal androgen levels during resident female periovulatory periods, which might be indicative of some suppression of testicular endocrine function at times when resident females are most likely to conceive (Van Belle et al. 2009b). Yet, it is unlikely that these lower androgen levels fully suppressed the sexual function of noncentral males because at least one noncentral male was observed copulating during the periovulatory period of a cycling female (Van Belle et al. 2009a). Furthermore, a study that investigated male reproductive physiology and sperm production in three adult male and three juvenile male black-and-gold howler monkeys in captivity revealed that even low levels of fecal androgens were sufficient for normal sperm count, quality, and motility (Moreland et al. 2001). Additionally, small testes size relative to body size characteristic for black howler monkeys suggests an overall weak level of sperm competition in this howler monkey species (Kelaita et al. 2011).
In Rangel-Negrín et al.’s (2011) study of A. pigra, mean fecal androgen levels in males of two focal groups for which copulations were observed were not significantly higher in weeks when copulations were observed compared to weeks without observed copulations. One of these two study groups contained only one adult male, while the other study group had two adult males. In the latter group, both males were observed to copulate with the resident females. In contrast to the findings of Van Belle et al. (2009b), Rangel-Negrín et al. (2011) did not observe significant differences in mean fecal androgen levels between central and noncentral males in three multimale–multifemale groups. These different results could reflect variable hormonal responses underlying individual male reproductive strategies associated with group size, group composition, number of reproductively active females, female mate choice, or demographic factors such as the number of solitary males in the local area.
Further studies are needed to elucidate hormonal profiles underlying alternative male and female reproductive strategies in this and other howler monkey species. This is especially true when considering that the genus Alouatta is characterized by a highly flexible social system including great variability in group size and composition with a mixture of unimale and multimale bisexual groups in most populations regardless of the species (Di Fiore et al. 2011). As such, the reproductive strategies and the underlying endocrine correlates of individual males and females may be influenced by the number of resident adult males and females, population density, the number of extragroup males, the intensity of intra- and intergroup male–male competition over access to the fertile females, individual social status, age, kinship patterns, the degree to which females exert mate choice, and dispersal opportunities (Fig. 8.3). It remains unclear exactly how each of these social and demographic factors differentially affect male and female reproductive strategies across howler monkeys species. What is clear is that endocrine studies will help elucidate subtle distinctions not revealed by behavioral observations alone.
Hypothetical, social, and demographic context of male hormonal profiles underlying male and female reproductive strategies in howler monkeys. Population density is considered central as it mediates both group composition and encounter rates with opponents. The gray boxes indicate social factors that have been observed to influence male hormonal profiles in howler monkeys
6 Ecological Endocrinology
The physiological stress response of increased circulating glucocorticoids and subsequent elevated glucose levels is also affected by a wide range of environmental factors. For example, elevated glucocorticoid levels is characteristic of individuals facing ecological challenges such as food scarcity (e.g., ring-tailed lemurs, Pride 2005), cold temperatures (e.g., chacma baboons, Weingrill et al. 2004), prolonged daylight duration (e.g., gray mouse lemurs, Microcebus murinus, Génin and Perret 2000), high gastrointestinal parasite load (red colobus monkeys, Piliocolobus tephrosceles, Chapman et al. 2006), increased risk of predation (e.g., gray-cheeked mangabey, Lophocebus albigena, Arlet and Isbell 2009), and habitat fragmentation and degradation (e.g., red colobus monkeys, Chapman et al. 2006; spider monkeys, Ateles geoffroyi yucatanensis, Rangel-Negrín et al. 2009). In addition, elevated glucocorticoid levels can be experienced by individuals residing in atypically large or small social groups (e.g., ring-tailed lemurs, Pride 2005) or by females during the energetically demanding period of lactation (e.g., chacma baboons, Weingrill et al. 2004).
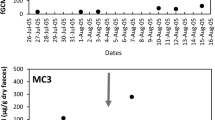
Female primates face different energetic demands across different reproductive stages, with lactation exerting the highest energetic demands (Dufour and Sauther 2002). To offset the energetic demands of lactation, black howler females are reported to increase caloric intake, rest less, and range further distances during the first 10 months of lactation compared to adult non-lactating females (Dias et al. 2011, see also Serio-Silva et al. 1999). In parallel with these behavioral changes, black howler females also may increase their metabolic rate by experiencing significantly higher fecal glucocorticoid levels, and therefore purportedly higher level of circulating glucose, when lactating compared to less energetic demanding reproductive stages (Fig. 8.4, Van Belle, unpubl. data; see Appendix 1). Elevated fecal glucocorticoid metabolites in pregnant and lactating females compared with nonreproducing females were also reported for mantled howler monkeys (Dunn et al. 2013). These elevated glucocorticoid levels in pregnant and lactating females might be partially a result of an increased stress response towards psychosocial stressors such as agonistic interactions during periods when energetic demands are high, as was observed for eight mantled howler females from two social groups (Gómez-Espinosa et al. 2013).
Using a large set of fecal samples (n = 350) collected from ten social groups during a 21-month study spread across 4 years, Behie et al. (2010) investigated variation in fecal glucocorticoid levels in the black howler population at Monkey River, Belize in relation to group size, presence of tourists, and maximum monthly temperature. Only lactating females were considered in this study to control for the effect that different reproductive stages have on glucocorticoid concentrations. These authors found that fecal glucocorticoid levels of both males and females were not influenced by group size and maximum monthly temperature, but were positively influenced by the presence of tourists. The two study groups that were frequently visited by tourists had significantly higher fecal glucocorticoid levels than other groups that experienced limited human contact. Behie et al. (2010) suggested that either the unpredictability of tourist visits or the intrusive and noisy behavior by tourists might account for the elevated fecal glucocorticoid levels in these groups. However, a group of red-handed howler monkeys exposed to loud noises from nearby mining practices did not have significantly higher fecal glucocorticoid levels compared with a nearby group living away from these mining practices (Monteiro et al. 2013). Similarly, in two mantled howler groups residing in a recreational forest reserve of which a section is a zoological park open to tourism and that had been exposed to humans throughout their lives, daily number of people visiting the park did not influence fecal glucocorticoid levels (Aguilar-Melo et al. 2013). In addition, fecal glucocorticoid levels in 31 red howler monkey groups residing in 10 different forest fragments also were not influenced by the level of human disturbance (i.e., minimal, logging, hunting, and logging and hunting), suggesting that howler monkeys might have a lower sensitivity to anthropogenic disturbance compared to other species such as spider monkeys (Rimbach et al. 2013b).
During their 21-month study, Behie and colleagues also measured fruit production of 200 trees belonging to the top 12 food species for the black howler monkeys at Monkey River and assessed parasite load in fecal samples. Infection by multiple parasite species, but not parasite prevalence, negatively influenced fecal glucocorticoid levels, suggesting greater physiological stress on the body when it is parasitized by more than one species that compete with each other for the host’s nutrients and energy (Behie and Pavelka 2013). Fruit availability also influenced male and female fecal glucocorticoid levels with elevated levels when fruits were scarce. These data suggest that black howler monkeys may be more dependent on fruit than previously believed, resulting in a subtle form of feeding competition in this primarily folivorous primate species (Behie et al. 2010; Behie and Pavelka 2013).
Similarly based on data collected during an 8-month study, limited fruit availability in particular, or low food quality in general, may have partially accounted for the higher mean fecal glucocorticoid levels found in two black howler groups inhabiting small forest fragments (<2 ha, Ejido Leona Vicario, Balancán, Mexico) compared to two black howler groups living in a more continuous forest (>1,400 ha, Calakmul Biosphere Reserve, Mexico; Martínez-Mota et al. 2007). Groups inhabiting the forest fragments rested, fed, and socially interacted with group members at similar rates as those in the continuous forest, but traveled more frequently. Members of one of the howler groups living in forest fragments spent 11 % of their traveling time walking on the ground, which may have increased their exposure to terrestrial predators including dogs or coyotes, or their contact with fecal material and increased their susceptibility to infection from anthropogenic diseases such as gastrointestinal parasites (Martínez-Mota et al. 2007). However, the precise factors that resulted in elevated fecal glucocorticoid levels in these howler groups remain unclear.
Fecal glucocorticoid levels in two groups of mantled howler monkeys residing in forest fragments of 244 ha and 7 ha, respectively, at Los Tuxtlas Biosphere Reserve were significantly and positively correlated to the percentage of time traveling, providing critical insight into the proximate mechanisms mediating elevated glucocorticoid levels in primates in fragments (Dunn et al. 2013). Although fecal glucocorticoid levels were not significantly influenced by fruit consumption per se, increased travel time was associated with reduced fruit consumption, decreased time feeding in large trees, and decreased time feeding on primary food resources resulting in increased feeding efforts in the study group living in the small and more disturbed forest fragment than the study group living in the more conserved fragment (Dunn et al. 2009, 2010). As such, in 31 groups of red howler monkeys living in 10 forest fragments ranging in size from 4 to 500 ha in Colombia, fragment size was not a significant predictor of fecal glucocorticoid levels (Rimbach et al. 2013b). The authors suggested that the apparent absence of physiological stress in the small forest fragments may be due to the fact that the study region has been undergoing fragmentation recently (<10 years), and hence that drastic changes in food availability might not have occurred yet.
A subsequent 4-month study spread evenly over the dry and wet season observing two additional mantled howler groups living in forest fragments of 230 ha and 15 ha, respectively, at the Los Tuxtlas Biosphere Reserve demonstrated that the group in the small fragment had higher fecal glucocorticoid levels during the dry season, but not the wet season, than the study group in the larger forest fragment (Gómez-Espinosa et al. 2013). However, this was not due to decreased food availability in the small forest fragment, nor were time spent traveling and ranging distance significant predictors of weekly glucocorticoid levels in these study groups, contrasting with the results by Dunn et al. (2013). Instead, Gómez-Espinosa et al. (2013) hypothesized that anthropogenic disturbance concentrated during the dry season when humans visited the river running through the small forest fragment more regularly might better explain the seasonal pattern of glucocorticoid levels in the group members residing in the small forest fragment. These inconsistent patterns of glucocorticoid profiles in howler monkeys inhabiting forest fragments reveal that multiple factors, including fragment size and history, food availability and associated feeding efforts, and the level of human disturbance, mediate the stress response in howler monkeys in fragments.
Endocrine studies also contribute directly to conservation programs, especially when researchers actively monitor glucocorticoid levels during translocation projects. One Mexican mantled howler group, composed of two adult males and two adult females, was translocated from a 4.9 ha forest fragment that was scheduled to be converted to agricultural land. The group was moved to an 80 ha protected forest 50 km away from their pre-translocation area (Aguilar-Cucurachi et al. 2010). The translocation followed a soft-release protocol in which the four adults were first held in captivity close to the post-translocation site and were provided with food for 1 month. They were then released into a 0.18 ha outdoor enclosure where the monkeys could forage on natural vegetation for 1 month, and finally released into the wild at the post-translocation site and monitored for an additional month. Each stage of the process from their pre-translocation site, to captivity, to semi-captivity, to their post-translocation site involved recapturing the four individuals, and fecal samples were collected during all four stages. Male fecal glucocorticoid levels remained stable throughout the translocation process, whereas female fecal glucocorticoid levels progressively increased from pre-translocation, to captivity, to semi-captivity, after which their glucocorticoid levels dropped below those recorded at the pre-translocation site once released at the post-translocation site and human handling was minimal. Females had significantly higher fecal glucocorticoid levels than males during all translocation stages, except during the pre-translocation stage, suggesting that female mantled howler monkeys were more sensitive to stressors. In addition, the data suggest that the increase in female howler stress response during translocation to a new habitat were lower than their response to being captured, handled, confined, and in close proximity to humans (Aguilar-Cucurachi et al. 2010). Possible ways to reduce stress during translocation include minimizing human handling and the period of confinement by curtailing the period in captivity in favor of semi-captivity.
7 Conclusion
Howler monkey studies that included the examination of hormonal profiles underlying behavioral strategies have revealed several important insights into their biology, social interactions, and mating strategies that were not anticipated based on behavioral data alone. For example, endocrine studies have suggested a nonaggressive form of intragroup male–male competition over access to fertile females in black howler monkeys based on elevated androgen and glucocorticoid levels in central compared to noncentral males (Van Belle et al. 2009b), despite otherwise neutral male–male social relationships based on tolerance and avoidance (Van Belle et al. 2008). A direct relationship between male steroid levels and male–female social and sexual interactions also has been observed in tufted capuchin monkeys (Lynch et al. 2002), Japanese macaques (Macaca fuscata, Barrett et al. 2002), and bonobos (Pan paniscus, Surbeck et al. 2012), suggesting that social relationships of males with cycling females may be a more pervasive driver of male endocrine function than generally thought. In this regard, howler monkeys are not unique but fit a broader pattern and may serve as an important instructive model for examining questions regarding male endocrinology underlying male–female relationships. In addition, the two endocrine studies investigating the influence of inter- and intragroup male–male competition on male androgen profiles in black howler monkeys (Van Belle et al. 2009b; Rangel-Negrín et al. 2011) revealed considerable differences in hormonal profiles, most likely reflecting distinct male reproductive strategies in different social and demographic settings. This suggests that howler endocrine function is highly adaptable and responds to subtle proximate changes in the social environment.
Endocrine studies also revealed that glucocorticoid levels were higher during periods of fruit scarcity compared to periods of fruit abundance in a black howler population recovering from a population collapse and habitat destruction caused by a hurricane in Belize. This suggests more prominent reliance on fruits as a staple food resource than was previously thought for howler monkeys (Behie et al. 2010; Behie and Pavelka 2013; also see Behie and Pavelka 2014 and Garber et al. 2014 on the importance of fruit in the diet of several howler species). At a proximate level, increased feeding effort by spending more time traveling between feeding sites may result in elevated glucocorticoid levels during periods of fruit scarcity or in degraded forest fragments (Dunn et al. 2013). Such findings can be incorporated into conservation programs for howler monkeys by examining fruit species diversity and seasonal availability in howler monkey habitats that have been degraded and fragmented because of human activity. For example, effective conservation policies could include the planting of particular fruiting species in fragmented habitats occupied by howler monkeys.
Finally, comparison of glucocorticoid levels between males and females has revealed that females of mantled howler monkeys appear to be more sensitive than males to either social (e.g., threat of extragroup males in habitat; Cristóbal-Azkarate et al. 2007) or ecological stressors (e.g., translocation and human handling; Aguilar-Cucurachi et al. 2010), information that could only have been identified by combining behavioral observations with endocrine studies.
I advocate for additional endocrine–behavioral–ecological studies on other howler monkey species and populations encompassing a wider range of environmental conditions, such as environments exhibiting prominent seasonality in food resources, rainfall, or temperatures, and a wider range of social/demographic conditions, including groups containing one male versus multiple males, groups with adults coresiding with kin versus nonkin, or well-established groups versus newly formed groups. Such studies are needed to assess how social, demographic, and ecological fluctuations affect male and female hormonal profiles and reproductive strategies. Although a limited number of studies of howler monkeys have focused on ovarian cycles, the effects of male–male competition, fruit availability, fragmentation, and translocation, information is extremely limited on hormonal profiles during infant development, the onset of sexual maturation, pregnancy, and patterns of hormonal fluctuation in response to seasonal changes in food availability across disturbed and undisturbed habitats. In addition, endocrine studies exploring the nature and formation of social bonding among male–male coalitions, mother–offspring bonds, or the role of paternal care during infant social development focusing on peptide hormones like oxytocin, prolactin, and vasopressin (e.g., Schradin and Anzenberger 2004; Seltzer and Ziegler 2007; Ziegler et al. 2009b; Anestis 2010; Moscovice and Ziegler 2012) are needed, especially in order to compare differences between howler groups or species in which collective action, male–male tolerance, and female–female tolerance, and female mate choice have been reported (Pope 1990, 2000; Van Belle et al. 2008, 2009a, b, 2011; Kowalewski and Garber 2010; Garber and Kowalewski 2011).
References
Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland TJ, Sapolsky RM (2003) Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav 43:67–82
Agoramoorthy G, Rudran R (1995) Infanticide by adult and subadult males in free-ranging red howler monkeys, Alouatta seniculus, in Venezuela. Ethology 99:75–88
Aguilar-Cucurachi MAS, Dias PAD, Rangel-Negrín A, Chavira R, Boeck L, Canales-Espinosa C (2010) Preliminary evidence of accumulation of stress during translocation in mantled howlers. Am J Primatol 72:805–810
Aguilar-Melo AR, Andresen E, Cristóbal-Azkarate J, Arroyo-Rodríguez V, Chavira R, Schondube J, Serio-Silva JC, Cuarón AD (2013) Behavioral and physiological responses to subgroup size and number of people in howler monkeys inhabiting a forest fragment used for nature-based tourism. Am J Primatol 75:1108–1116
Anestis SF (2010) Hormones and social behavior in primates. Evol Anthropol 19:66–78
Arlet ME, Isbell LA (2009) Variation in behavioral and hormonal responses of adult male gray-cheeked mangabeys (Lophocebus albigena) to crowned eagles (Stephanoaetus coronatus) in Kibale National Park, Uganda. Behav Ecol Sociobiol 63:491–499
Bahr NI, Palme R, Möhle U, Hodges JK, Heistermann M (2000) Comparative aspects of the metabolism and excretion of cortisol in three individual non-human primates. Gen Comp Endocrinol 117:427–438
Barrett GM, Shimizu K, Bardi M, Asaba S, Mori A (2002) Endocrine correlates of rank, reproduction, and female-directed aggression in male Japanese macaques (Macaca fuscata). Horm Behav 42:85–96
Behie AM, Pavelka MSM (2013) Interacting roles of diet, cortisol levels, and parasites in determining population density of Belizean howler monkeys in a hurricane damaged forest fragment. In: March LK, Chapman CA (eds) Primates in fragments: complexity and resilience. Springer Press, New York
Behie AM, Pavelka SM (2014) Fruit as a key factor in howler monkey population density: conservation implications. In: Kowalewski M, Garber P, Cortés-Ortiz L, Urbani B, Youlatos D (eds) Howler monkeys: behavior, ecology and conservation. Springer, New York
Behie AM, Pavelka MSM, Chapman CA (2010) Sources of variation in fecal cortisol levels in howler monkeys in Belize. Am J Primatol 71:1–7
Bercovitch FB, Ziegler TE (2002) Current topics in primate socioendocrinology. Annu Rev Anthropol 31:45–67
Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM, Whitten PL (2005) Correlates of stress in free-ranging male chacma baboons, Papio hamadryas ursinus. Anim Behav 70:703–713
Bernstein IS, Rose RM, Gordon TP, Grady CL (1979) Agonistic rank, aggression, social context, and testosterone in male pigtail monkeys. Aggress Behav 5:329–339
Bicca-Marques JC, Calegaro-Marques C (1998) Behavioral thermoregulation in a sexually and developmentally dichromatic Neotropical primate, the black-and-gold howling monkey (Alouatta caraya). Am J Phys Anthropol 106:533–646
Bradshaw D (2007) Environmental endocrinology. Gen Comp Endocrinol 152:125–141
Braude S, Tang-Martinez Z, Taylor G (1999) Stress, testosterone, and the immunoredistribution hypothesis. Behav Ecol 10:345–350
Brockman DK, Whitten PL, Richard AF, Benander B (2001) Birth season testosterone levels in male Verreaux’s sifaka, Propithecus verreauxi: insight into socio-demographic factors mediating seasonal testicular function. Behav Ecol Sociobiol 49:117–127
Cavigelli SA, Dubovick T, Levash W, Jolly A, Pitts A (2003) Female dominance status and fecal corticoids in a cooperative breeder with low reproductive skew: ring-tailed lemurs (Lemur catta). Horm Behav 43:166–179
Chapman CA, Wasserman MD, Gillespie TR, Speirs ML, Lawes MJ, Saj TJ, Ziegler TE (2006) Do food availability, parasitism, and stress have synergistic effects on red colobus populations living in forest fragments? Am J Phys Anthropol 131:525–534
Clarke MR (1983) Infant-killing and infant disappearance following male takeovers in a group of free-ranging howling monkeys (Alouatta palliata) in Costa Rica. Am J Primatol 5:241–247
Clarke MR, Zucker EL, Ford RT, Harrison RM (2007) Behavior and endocrine concentrations do no distinguish sex in monomorphic juvenile howlers (Alouatta palliata). Am J Primatol 69:477–484
Cockrem JF (2005) Conservation and behavioral neuroendocrinology. Horm Behav 48:492–501
Colillas O, Coppo J (1978) Breeding Alouatta caraya in Centro Argentino de Primates. In: Chivers DJ, Lane-Petter W (eds) Recent advances in primatology, 2. Conservation. Academic, London
Cristóbal-Azkarate J, Dias PAD, Veà JJ (2004) Causes of intraspecific aggression in Alouatta palliata mexicana: evidence from injuries, demography, and habitat. Int J Primatol 25:939–953
Cristóbal-Azkarate J, Chavira R, Boeck L, Rodríguez-Luna E, Veà JJ (2006) Testosterone levels of free-ranging resident mantled howler monkey males in relation to the number and density of solitary males: a test of the challenge hypothesis. Horm Behav 49:261–267
Cristóbal-Azkarate J, Chavira R, Boeck L, Rodríguez-Luna E, Veà JJ (2007) Glucocorticoid levels in free ranging resident mantled howlers: a study of coping strategies. Am J Primatol 69:866–876
Crockett CM (2003) Re-evaluating the sexual selection hypothesis for infanticide by Alouatta males. In: Jones CB (ed) Sexual selection and reproductive competition in primates: new perspectives and directions, vol 3, American Society of Primatology: Special Topics in Primatology. The American Society of Primatologists, Norman, Oklahoma
Crockett CM, Eisenberg JF (1987) Howlers: variations in group size and demography. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) Primate societies. The University of Chicago Press, Chicago
Crockett CM, Pope TR (1988) Inferring patterns of aggression from red howler monkey injuries. Am J Primatol 15:289–308
Crockett CM, Pope TR (1993) Consequences of sex differences in dispersal for juvenile red howler monkeys. In: Pereira MA, Fairbanks LA (eds) Juvenile primates: life history, development and behavior. Oxford University Press, New York
Crockett CM, Sekulic R (1982) Gestation length in red howler monkeys. Am J Primatol 3:291–294
Crockett CM, Sekulic R (1984) Infanticide in red howler monkeys (Alouatta seniculus). In: Hausfater G, Hrdy SB (eds) Infanticide: comparative and evolutionary perspectives. Aldine, New York
Di Fiore A, Link A, Campbell CJ (2011) The atelines: behavioral and socioecological diversity in a New World radiation. In: Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK (eds) Primates in perspective, 2nd edn. Oxford University Press, Oxford
Dias PAD, Rangel-Negrín A, Veà JJ, Canales-Espinosa D (2010) Coalitions and male-male behavior in Alouatta palliata. Primates 51:91–94
Dias PAD, Rangel-Negrín A, Canales-Espinosa D (2011) Effects of lactation in the time-budgets and foraging patterns of female black howlers (Alouatta pigra). Am J Phys Anthropol 145:137–146
Dufour DL, Sauther ML (2002) Comparative and evolutionary dimensions of human pregnancy and lactation. Am J Hum Biol 14:585–602
Dunn JC, Cristóbal-Azkarate J, Veà J (2009) Differences in diet and activity pattern between two groups of Alouatta palliata associated with the availability of big trees and fruit of top food taxa. Am J Primatol 71:654–662
Dunn JC, Cristóbal-Azkarate J, Veà J (2010) Seasonal variations in the diet and feeding effort of two groups of howler monkeys in different sized forest fragments. Int J Primatol 31: 887–903
Dunn JC, Cristóbal–Azkarate J, Schulte-Herbrüggen B, Chavira R, Veà JJ (2013) Travel time predicts fecal glucocorticoid levels in free-ranging howlers (Alouatta palliata). Int J Primatol 34:246–259
Ebner K, Wotjak CT, Landgraf R, Engelmann M (2005) Neuroendocrine and behavioral response to social confrontation: residents versus intruders, active versus passive coping styles. Horm Behav 47:14–21
Garber PA, Kowalewski MM (2011) Collective action and male affiliation in howler monkeys (Alouatta caraya). In: Sussman RW, Cloninger CR (eds) Origins of altruism and cooperation. Springer Publishers, New York
Garber P, Righini N, Kowalewski M (2014) Evidence of alternative dietary syndromes and nutritional goals in the genus Alouatta. In: Kowalewski M, Garber P, Cortés-Ortiz L, Urbani B, Youlatos D (eds) Howler monkeys: behavior, ecology and conservation. Springer, New York
Génin F, Perret M (2000) Photoperiod-induced changes in energy balance in gray mouse lemurs. Physiol Behav 71:315–321
Glander KE (1980) Reproduction and population growth in free-ranging mantled howling monkeys. Am J Phys Anthropol 53:25–36
Glander KE (1992) Dispersal patterns in Costa Rican mantled howling monkeys. Int J Primatol 13:415–436
Gómez-Espinosa E, Rangel-Negrín A, Chavira R, Canales-Espinosa D, Dias PAD (2013) The effect of energetic and psychosocial stressors in glucocorticoids in mantled howler monkeys (Alouatta palliata). Am J Primatol 76(4):362–373
Goymann W, Wingfield JC (2004) Allostatic load, social status and stress hormones: the costs of social status matter. Anim Behav 67:591–602
Hau M (2007) Regulation of male traits by testosterone: implications for the evolution of vertebrate life-histories. Bioessays 29:133–144
Heistermann M, Palme R, Ganswindt A (2006) Comparison of different enzyme immunoassays for assessment of adrenocortical activity in primates based on fecal analysis. Am J Primatol 68:257–273
Herrick JR, Agoramoorthy G, Rudran R, Harder JD (2000) Urinary progesterone in free-ranging red howler monkeys (Alouatta seniculus): preliminary observations of the estrous cycle and gestation. Am J Primatol 51:257–263
Horwich RH, Brockett RC, Jones CB (2000) Alternative male reproductive behaviors in the Belizean black howler monkey (Alouatta pigra). Neotrop Primates 8:95–98
Huck M, Löttker P, Heymann EW, Heistermann M (2005) Characterization and social correlates of fecal testosterone and cortisol excretion in wild male Saguinus mystax. Int J Primatol 26:159–179
Izawa K (1997) Social changes within a group of red howler monkeys, VI. Field Stud Fauna and Flora, La Macarena, Colombia 11:19–34
Izawa K, Lozano HM (1991) Social changes within a group of red howler monkeys (Alouatta seniculus), III. Field Stud New World Monkeys, La Macarena, Colombia 5:1–16
Jones CB (1985) Reproductive patterns in mantled howler monkeys: estrus, mate choice and copulation. Primates 26:130–142
Kelaita M, Dias PAD, Aguilar-Cucurachi MS, Canales-Espinosa D, Cortés-Ortiz L (2011) Impact of intrasexual selection on sexual dimorphism and testes size in the Mexican howler monkeys Alouatta palliata and A. pigra. Am J Phys Anthropol 146:179–187
Kimura K (1992) Demographic approach to the social group of wild red howler monkeys (Alouatta seniculus). Field Stud New World Monkeys, La Macarena, Colombia 7:29–34
Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MA, Blokhuis HJ (1999) Coping styles in animals: current status in behavior and stress physiology. Neurosci Biobehav Rev 23:925–935
Kowalewski MM, Garber PA (2010) Mating promiscuity and reproductive tactics in female black and gold howler monkeys (Alouatta caraya) inhabiting an island on the Parana River, Argentina. Am J Primatol 72:734–748
Kowalewski M, Garber P (2014) Solving the collective action problem during intergroup encounters: the case of black and gold howler monkeys. In: Kowalewski M, Garber P, Cortés-Ortiz L, Urbani B, Youlatos D (eds) Howler monkeys: behavior, ecology and conservation. Springer, New York
Kugelmeier T, del Rio Do Valle R, de Barros Vaz Guimarães MA, Carneiro Muniz JAP, Barros Monteiro FO, Alvarenga de Oliveira C (2011) Tracking the ovarian cycle in black-and-gold howlers (Alouatta caraya) by measuring fecal steroids and observing vaginal bleeding. Int J Primatol 32:605–615
Lasley BL, Savage A (2007) Advances in the understanding of primate reproductive endocrinology. In: Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK (eds) Primates in Perspective. Oxford University Press, New York
Lynch JW, Ziegler TE, Strier KB (2002) Individual and seasonal variation in fecal testosterone and cortisol levels of wild male tufted capuchin monkeys, Cebus apella nigritus. Horm Behav 41:275–287
Martínez-Mota R, Valdespino C, Sánchez-Ramos MA, Serio-Silva JC (2007) Effects of forest fragmentation on the physiological stress responses of black howler monkeys. Anim Conserv 10:374–379
Martínez-Mota R, Valdespino C, Rivera Rebolledo AR, Palme R (2008) Determination of fecal glucocorticoid metabolites to evaluate stress response in Alouatta pigra. Int J Primatol 29:1365–1373
Monteiro FOB, Kugelmeier T, Valle RR, Lima ABF, Silva FE, Martins SS, Pereira LG, Dinucci KL, Viau P (2013) Evaluation of the fecal steroid concentrations in Alouatta belzebul (Primates, Atelidae) in the national forest of Tapirape-Aquiri in Pará, Brazil. J Med Primatol 42:325–332
Moreland RB, Richardson ME, Lamberski N, Long JA (2001) Characterizing the reproductive physiology of the male Southern black howler monkey, Alouatta caraya. J Androl 22:395–403
Moscovice LR, Ziegler TE (2012) Peripheral oxytocin in female baboons relates to estrous state and maintenance of sexual consortships. Horm Behav 62(5):592–597
Muehlenbein MP, Bribiescas RG (2005) Testosterone-mediated immune functions and male life histories. Am J Hum Biol 17:527–558
Muehlenbein MP, Watts DP, Whitten PL (2004) Dominance rank and fecal testosterone levels in adult male chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda. Am J Primatol 64:71–82
Muller MN, Wrangham RW (2004a) Dominance, aggression and testosterone in wild chimpanzees: a test of the “challenge hypothesis”. Anim Behav 67:113–123
Muller MN, Wrangham RW (2004b) Cortisol, aggression, and stress in wild chimpanzees (Pan troglodytes schweinfurthii). Behav Ecol Sociobiol 55:332–340
Palme R (2005) Measuring fecal steroids: guidelines for practical application. Ann NY Acad Sci 1046:75–80
Plant TM, Witchel SF (2006) Puberty in nonhuman primates and humans. In: Neill JD (ed) Knobil and Neill’s physiology of reproduction, vol 2, 3rd edn. Elsevier, St. Louis, MO
Pope TR (1990) The reproductive consequences of male cooperation in the red howler monkey: paternity exclusion in multi-male and single-male troops using genetic markers. Behav Ecol Sociobiol 27:439–446
Pope TR (2000) The evolution of male philopatry in Neotropical monkeys. In: Kappeler PM (ed) Primate males . Cambridge University Press, Cambridge, pp 219–235
Pride ER (2005) Foraging success, agonism, and predator alarms: behavioral predictors of cortisol in Lemur catta. Int J Primatol 26:295–319
Rangel-Negrín A, Alfaro JL, Valdez RA, Romano MC, Serio-Silva JC (2009) Stress in Yucatan spider monkeys: effects of environmental conditions on fecal cortisol levels in wild and captive populations. Anim Conserv 12:496–502
Rangel-Negrín A, Dias PAD, Chavira R, Canales-Espinosa D (2011) Social modulation of testosterone levels in male black howlers (Alouatta pigra). Horm Behav 59:159–166
Rimbach R, Heymann EW, Link A, Heistermann M (2013a) Validation of an enzyme immunoassay for assessing adrenocortical activity and evaluation of factors that affect levels of fecal glucocorticoid metabolites in two New World primates. Gen Comp Endocrinol 191:13–23
Rimbach R, Link A, Heistermann M, Gomez-Posada C, Galvis N, Heymann EW (2013b) Effects of logging, hunting, forest fragment size on physiological stress levels of two sympatric ateline primates in Colombia. Conserv Physiol 1. doi:10.1093/conphys/cot031
Robbins MM, Czekala NM (1997) A preliminary investigation of urinary testosterone and cortisol levels in wild male mountain gorillas. Am J Primatol 43:51–64
Saltzman W, Tardif SD, Rutherford JN (2011) Hormones and reproductive cycles in primates. In: Norris DO, Lopez KH (eds) Hormones and reproduction of vertebrates, vol 5, Mammals. Academic, San Diego
Sapolsky RM (1993) The physiology of dominance in stable versus unstable social hierarchies. In: Mason WA, Mendoza SP (eds) Primate social conflict. State University of New York Press, Albany
Sapolsky RM (2002) Endocrinology of stress response. In: Becker JB, Breedlove SM, Crews D, McCarthy M (eds) Behavioral endocrinology, 2nd edn. MIT Press, Cambridge
Sapolsky RM (2005) The influence of social hierarchy on primate health. Science 308:648–652
Schradin C, Anzenberger G (2004) Development of prolactin levels in marmoset males: from adult son to first-time father. Horm Behav 46:670–677
Sekulic R (1983) Male relationships and infant deaths in red howler monkeys (Alouatta seniculus). Behaviour 81:38–54
Seltzer LJ, Ziegler TE (2007) Non-invasive measurement of small peptides in the common marmoset (Callithrix jacchus): a radiolabeled clearance study and endogenous excretion under varying social conditions. Horm Behav 51:436–442
Serio-Silva JC, Hernández-Salazar LT, Rico-Gray V (1999) Nutritional composition of the diet of Alouatta palliata mexicana females in different reproductive states. Zoo Biol 18:507–513
Setchell JM, Smith T, Wickings EJ, Knapp LA (2008) Social correlates of testosterone and ornamentation in male mandrills. Horm Behav 54:365–372
Shoemaker AH (1982) Fecundity in the captive howler monkey, Alouatta caraya. Zoo Biol 1:149–156
Strier KB, Ziegler TE (2005) Advances in field-based studies of primate behavioral endocrinology. Am J Primatol 67:1–4
Strier KB, Ziegler TE, Wittwer DJ (1999) Seasonal and social correlates of fecal testosterone and cortisol levels in wild male muriquis (Brachyteles arachnoides). Horm Behav 35:125–134
Surbeck M, Deschner T, Weltring A, Hohmann G (2012) Social correlates of variation in urinary cortisol in wild male bonobos (Pan paniscus). Horm Behav 62:27–35
Torres-Pelayo VR, Rovirosa-Hernández MJ, García-Orduña F, Chavira-Ramírez RD, Boeck L, Canales-Espinosa D, Rodríguez-Landa JF (2011) Variation in the extraction efficiency of estradiol and progesterone in moist and lyophilized feces of the black howler monkeys (Alouatta pigra): alternative methods. Front Physiol 2:97. doi:10.3389/fphys.2011.00097
Van Belle S, Bicca-Marques JC (2014) Insights into reproductive strategies and sexual selection in howler monkeys. In: Kowalewski MM, Garber PA, Cortés-Ortiz L, Urbani B, Youlatos D (eds) Howler monkeys: behavior, ecology and conservation. Springer, New York
Van Belle S, Estrada A, Strier KB (2008) Social relationships among male Alouatta pigra. Int J Primatol 29:1481–1498
Van Belle S, Estrada A, Ziegler TE, Strier KB (2009a) Sexual behavior across ovarian cycles in wild black howler monkeys (Alouatta pigra): male mate guarding and female mate choice. Am J Primatol 71:153–164
Van Belle S, Estrada A, Ziegler TE, Strier KB (2009b) Social and hormonal mechanisms underlying male reproductive strategies in black howler monkeys (Alouatta pigra). Horm Behav 56:355–363
Van Belle S, Estrada A, Strier KB (2011) Insights into social relationships among female black howler monkeys Alouatta pigra at Palenque National Park, Mexico. Curr Zool 57:1–7
van Schaik CP (1991) A pilot study of the social correlates of levels of urinary cortisol, prolactin, and testosterone in wild long-tailed macaques (Macaca fascicularis). Primates 32:345–356
Wang E, Milton K (2003) Intragroup social relationships of male Alouatta palliata on Barro Colorado Island, Republic of Panama. Int J Primatol 24:1227–1243
Wasser SK, Cristóbal-Azkarate J, Booth RK, Hayward L, Hunt K, Ayres K, Vynne C, Gobush K, Canales-Espinosa D, Rodríguez-Luna E (2010) Non-invasive measurement of thyroid hormone in feces of a diverse array of avian and mammalian species. Gen Comp Endocrinol 168:1–7
Weingrill T, Gray DA, Barrett L, Henzi SP (2004) Fecal cortisol levels in free-ranging female chacma baboons: relationship to dominance, reproductive state and environmental factors. Horm Behav 45:259–269
Whitten PL, Brockman DK, Stavisky RC (1998) Recent advances in noninvasive techniques to monitor hormone-behavior interactions. Am J Phys Anthropol 41:1–23
Wingfield JC, Hegner RE, Dufty AMJ, Ball GF (1990) The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat 136:829–846
Ziegler TE, Wittwer DJ (2005) Fecal steroid research in the field and laboratory: improved methods for storage, transport, processing, and analysis. Am J Primatol 67:159–174
Ziegler TE, Strier KB, Van Belle S (2009a) The reproductive ecology of South American primates: ecological adaptations in ovulation and conception. In: Garber PA, Estrada A, Bicca-Marques JC, Heymann EW, Strier KB (eds) South American primates: comparative perspectives in the study pg behavior, ecology, and conservation. Springer, New York
Ziegler TE, Prudom SL, Zahed SR, Parlow AF, Wegner F (2009b) Prolactin’s mediative role in male parenting in parentally experienced marmosets (Callithrix jacchus). Horm Behav 56:436–443
Acknowledgments
I thank Martín M. Kowalewski, Paul A. Garber, Liliana Cortés-Ortiz, Bernardo Urbani, and Dionisios Youlatos for the invitation to contribute to this volume and to Martín M. Kowalewski, Paul A. Garber, and an anonymous reviewer for constructive suggestions that improved the quality of the chapter. I was supported by a postdoctoral fellowship from Universidad Nacional Autónoma de México (UNAM) during the writing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix: The Effect of Male Migration and Reproductive Status on Black Howler Female Fecal Glucocorticoid Levels
Appendix: The Effect of Male Migration and Reproductive Status on Black Howler Female Fecal Glucocorticoid Levels
1.1 Methods
Two multimale–multifemale black howler (A. pigra) groups were studied in Palenque National Park, Mexico from June 2006 through July 2007. The Balam group had three adult females at the onset of the study, but one female (MI) disappeared on October 18, 2006. The Motiepa group had two adult females throughout the study period. Both groups underwent several changes in adult male group membership (Balam, n = 7; Motiepa, n = 2; for detailed description, see Van Belle et al. 2008). Fresh fecal samples were collected from each adult female, on average, every 4.1 ± 1.4 days, resulting in a total of 246 samples. Methods used for sample storage, hormone extraction, assay validation, and glucocorticoid EIA are described in detail in Van Belle et al. (2009b). Values of hormone concentrations were log10 transformed to normalize the distribution and equalize the variance (Kolmogorov–Smirnov tests and Levene’s tests, P > 0.05), allowing the use of parametric tests.
General linear mixed models (GLMM) were used to analyze whether glucocorticoid levels of females differed between the 2 weeks prior to and the 2 weeks after male migration events in their groups. Random factors in the GLMM included female identity to account for the repeated sampling of the same individual, which was nested within groups to account for the possibility that coresiding females had correlated hormone levels and migration events nested within groups to account for the possibility that coresiding females changed hormonal levels similarly to the same migration events. To examine whether female glucocorticoid levels changed according to their reproductive status, a GLMM was used with female identity nested within groups as random factors and reproductive status as predictor variable. Female reproductive status was classified as acyclic, cycling, pregnant, or lactating based on their fecal estrogen and progestin profiles and presence of young offspring (Van Belle et al. 2009a).
1.2 Results
Adult females did not differ in their fecal glucocorticoid levels between the 2 weeks before and after male migrations in their groups (MeanBefore = 0.99 ± SE 0.09, MeanAfter = 1.05 ± 0.07, F 2,39.6 = 0.650, P = 0.527). However, female fecal glucocorticoid levels across different reproductive states were significantly different with females having higher fecal glucocorticoid levels when lactating compared to other reproductive states (Fig. 8.3, F 3,240.7 = 10.48, P < 0.001)
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Van Belle, S. (2015). Endocrinology of Howler Monkeys: Review and Directions for Future Research. In: Kowalewski, M., Garber, P., Cortés-Ortiz, L., Urbani, B., Youlatos, D. (eds) Howler Monkeys. Developments in Primatology: Progress and Prospects. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1957-4_8
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1957-4_8
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1956-7
Online ISBN: 978-1-4939-1957-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)