Abstract
One of the most striking features of howler monkeys’ natural history is their loud call, which gives the genus Alouatta its common name in English. However, the disproportionate focus on functional aspects of those calls has driven attention away from other relevant issues related to their vocal behavior. In this chapter, we review the studies of acoustic structure conducted so far on these peculiar calls, highlighting the variation among and within the species of this genus. The variation we uncover runs against the notion of uniformity among howler monkeys, but we do find that the relationship between loud call structure and phylogeny compliments genetic work in this genus. We also show how the anatomy of howler monkey’s vocal organs can explain the unusual features of their loud calls and possibly the variation found between species, while also pointing to the various gaps that exist in our knowledge regarding the role of the several components of their highly specialized vocal apparatus. Additionally, we review some basic concepts about sound propagation and geographic variation in long-distance communication. Unlike loud calls, we know relatively little about the low-amplitude calls of howler monkeys. Such calls have received a great deal of attention in the literature, particularly in Old World monkeys, because they can offer insights into the social lives of these animals. Because few comparable studies have been conducted on howler monkeys, we propose some lines of future research that we deemed potentially interesting. We conclude with some methodological approaches to recording howler monkey calls in the field and for sharing vocalizations with other researchers.
Resumen
Una de las características más llamativas de la historia natural de los monos aulladores son sus vocalizaciones de larga distancia, las cuales son responsables del nombre popular en inglés, y algunos nombres en español, para el género Alouatta. Sin embargo, el enfoque desproporcionado que ha recibido la funcionalidad de estas vocalizaciones, ha desviado la atención de otros aspectos relevantes del comportamiento vocal de los monos aulladores. En este capítulo revisamos los estudios llevados a cabo hasta el momento, sobre la estructura acústica de estas peculiares voces, remarcando la variación de las mismas entre y dentro de las diferentes especies del género. Las variaciones que aquí dejamos al descubierto desafían la noción de uniformidad en estos primates y muestran una relación entre la estructura vocal y las relaciones filogenéticas que complementan estudios genéticos recientes realizados en este género. También mostramos cómo la anatomía de los órganos vocales de los monos aulladores puede explicar tanto las características inusuales de sus vocalizaciones de larga distancia, como posiblemente la variación entre las diferentes especies, y señalamos los vacíos existentes en el conocimiento acerca del papel que poseen diversos componentes –altamente especializados- de los aparatos vocales de estos primates. Adicionalmente, revisamos conceptos básicos sobre la propagación del sonido y la variación geográfica en la comunicación a grandes distancias. Sonidos de baja amplitud producidos en otros grupos taxonómicos, particularmente en monos del Viejo Mundo han recibido gran atención en la literatura debido a que ofrecen una mirada interna a la vida social de estos animales. Debido a que pocos estudios comparables se han llevado a cabo en monos aulladores, proponemos algunas futuras investigaciones que consideramos potencialmente interesantes. Finalmente concluimos con aproximaciones metodológicas para grabar voces de monos aulladores en el campo y para compartir las grabaciones obtenidas con otros investigadores.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Structure of loud calls
- Morphology of vocal apparatus
- Call design
- Sound propagation
- Geographic variation
- Soft calls
- Recording methods
1 Introduction
When it comes to loud calling, howler monkeys do not stand alone in the primate world (Mitani and Stuht 1998). In fact, even in the broader mammalian world, many species produce loud calls—lions (Panthera leo (McComb et al. 1994)), wolves (Canis lupus (Mech 1966)), and African elephants (Loxodonta africana (Leighty et al. 2008)), to name just a few. On the other hand, howler monkeys do stand out in this noisy crowd. They utter the most powerful primate vocalization in the Neotropics, probably rivaled only by jaguars (Panthera onca) and bellbirds (Procnias spp.). In fact, if we consider both call duration and amplitude per body size, then competitors, even worldwide, lag far behind. Such a striking feature of their natural history gives the genus Alouatta its common name in several languages.
As one would expect, a modified and specialized anatomy of the vocal apparatus, with the most noteworthy component being the greatly enlarged hyoid bone (Schön 1971; Schön Ybarra 1988), is associated with the production of these calls. It has even been suggested that this anatomical commitment might affect other aspects of the howler monkeys’ lives, such as positional behavior (Schön Ybarra 1984). Such an anatomical suite of characters, coupled with the time and presumably the energy invested in loud calling, contrasting with their otherwise phlegmatic lifestyle suggests an important role for these calls in the lives of howler monkeys. As in other species, howler monkey loud calls probably play a vital role in fitness in that they are involved in intergroup competition, mate attraction, or defense, and predator avoidance. Such functional aspects of the loud calls will be dealt with in Kitchen et al. (2014, this volume), where the issue will be analyzed at different explanatory levels.
In this current chapter, we consider loud calls from a proximate, structural perspective, including acoustic features, the specialized anatomy of the vocal apparatus, long-range propagation issues, and a consideration of geographical variation on call structure. We also give attention to the neglected female loud calls and the quieter/soft calls in the repertoire. We conclude with a brief rough guide to howler monkey vocal research, in which we address various methodological issues.
In both this chapter and in Kitchen et al. (2014), we attempt to critically review studies conducted on these peculiar vocalizations in order to highlight the variation present among the different howler monkey species. In addition to varying body mass, degree of sexual dimorphism, coat color variation, and other aspects of their behavior and ecology (see chapters throughout these volumes), we argue that the structure and putative functions of howler monkey loud calls may also vary widely across different Alouatta species.
2 Structure of Male Loud Calls
Since Carpenter’s (1934) work with A. palliata, most authors have described two main categories of howler monkey loud calls: barks and roars. The presence of both has been confirmed for every species of howler monkey studied so far. Altmann (1959), followed by Baldwin and Baldwin (1976), named the male forms of these calls the A series (roars) and C series (barks or woofs). Categorizing these calls in series stresses the high degree of variation found in each type of howler monkey loud call, reflecting high levels of gradation from “incipient” (low amplitude: Baldwin and Baldwin 1976) to very loud emissions of each form (Neville et al. 1988; Drubbel and Gautier 1993; Oliveira 2002). These low-frequency, harsh/atonal sounds also have a common structure, with marked peaks of amplitude at stable frequency bands (Schön Ybarra 1986; Drubbel and Gautier 1993; Whitehead 1995; Oliveira 2002; da Cunha 2004). Female forms of these vocalizations were labeled the B (roars or “roar accompaniments”) and D (barks) series, and they are clearly distinct from the male forms (Baldwin and Baldwin 1976). Because most studies have focused on male calling behavior, there are scarce bioacoustic analyses of female repertoires (see Sect. 13.3).
We believe it is important to stress at the onset that in addition to diversity among all species, we will reveal clear distinctions in roar types used and temporal patterns of loud calling between two groups: the two Central American species, A. pigra and A. palliata, compared to the remaining South American species. Given that Cortés-Ortiz and colleagues (2003) found closer phylogenetic relationships within than between these two clades, the acoustic and structural trends we describe below do not conflict with this taxonomic hypothesis.
2.1 Incipient Forms of Roaring and Barking
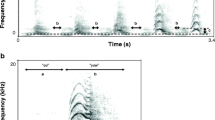
Because they can be only perceived at short range, the “incipient” forms of barks and roars cannot officially be viewed as loud calls. However, they usually have a clear structural relationship with the louder forms and are often emitted during loud calling bouts. The “incipient roar” (Figs. 13.1a, e) is made up of very short pulses (“strings of short subunits” (Drubbel and Gautier 1993); “a gruff, popping” noise (Altmann 1959)). At least for some South American species (A. caraya (da Cunha RGT unpubl. data); A. guariba Footnote 1 and A. belzebul (Oliveira 2002); A. macconnelli (formerly A. seniculus (Drubbel and Gautier 1993)), a series of incipient roars (e.g., this phase lasts 24–114 s in A. macconnelli (Drubbel and Gautier 1993)) often precede full roaring bouts, as a kind of warming-up phase where pulses gradually become louder and uttered at shorter intervals in the transition to “full roars.”
Roars. (a) A. guariba, incipient roars with two inhalatory sounds (i) between successive exhalations (e); (b) A. guariba, loud roars, with inhalatory (i) and exhalatory (e) phases and faster respiratory cycles at the climax in amplitude (i*: an apparent inhalation, but without frequency modulation); (c) A. guariba, brief roar (r), preceded and followed by single-pulsed barks (sb); (d) A. guariba, roar ending, with two normal cycles indicated by their inhalatory and exhalatory phases, followed by an oodle-like roar (olr), an oodle (od), and three coughs (c); (e) A. caraya, incipient roars, with two emissions (r) and an oodle (od) between them; (f) A. caraya, loud roars, with inhalatory (i) and exhalatory (e) phases and faster respiratory cycles at the climax in amplitude, followed by oodle-like roaring (olr) (i*, an apparent inhalation; e*, an apparent exhalation); (g) A. belzebul, roar, with exhalatory (e) and inhalatory (i) phases; (h) A. belzebul, brief roar; (i) A. pigra, a roar with exhalation (e) and inhalation (i), preceded by a single-pulsed bark (sb) and followed by a faint exhalation; (j) A. palliata, single roar, with inhalatory (i) and exhalatory (e) phases and ending in an oodle (od)
Although Baldwin and Baldwin (1976) describe incipient roars in A. palliata, these calls are apparently not as frequently produced by the Central American species (A. palliata and A. pigra) and are instead heard as a short burst of popping or an “aw” or “er” sound (Baldwin and Baldwin 1976) typically at the beginning of roars (Kitchen DM unpubl. data). Schön Ybarra (1986) also gave a similar description of this use of incipient roars at the onset of “brief roars” (defined below) in A. arctoidea (formerly A. seniculus).
A. palliata (Baldwin and Baldwin 1976) and A. guariba (Oliveira 2002) have also been described as producing “incipient barks” (Fig. 13.2a)—short-range, simple pulses usually emitted with a closed mouth. Similar muffled sounds have been observed in A. caraya, both before barking and on their own (da Cunha pers. obs.). In the Central American species, Baldwin and Baldwin (1976) describe this in A. palliata as a muffled “unf unf unf” sound, and these calls often occur before the onset of loud calling in A. pigra (also referred to as “grunting” (Kitchen pers. obs.)).
Barks. (a) A. guariba, incipient, single-pulsed barks (sb); (b) A. guariba, series of loud barks, including both single-pulsed (sb) and double-pulsed (db) calls; (c) A. guariba, five longer barks, followed by a composite roar (cr) and an oodle (od) (i*: sigh-like sound, perhaps an inhalatory sound); (d) A. caraya, multiple callers, notice more tonal voice (tb) in one caller (probably a female), while remaining barks are typically harsh calls (hb); (e) A. belzebul, single-pulsed (sb) and double-pulsed (db) barks; (f) A. pigra, five barks (1–5) or a five-pulsed single bark, interspersed with roars (r); (g) A. palliata, double-pulsed bark (db) followed by single-pulsed barks (sb)
2.2 Full Roars
Common features of howler monkey roars or “howls” are their high amplitude (up to 90 dB sound pressure level (SPL) at 5 m of distance (Whitehead 1995)), low frequency, and harshness. In the South American species analyzed so far, full roars are composed of two sections: a longer exhalatory phase and a shorter inhalatory one, with higher frequencies of the dominant band occurring in the inhaling periods (Whitehead 1995). For example, the pattern in A. guariba (Fig. 13.1b) is that short inhalatory sounds, varying in structure from tonal with low fundamental frequency (90–150 Hz) to harsh and usually with an ascendant modulation, can occur intercalated with incipient roars (Oliveira 2002). These inhalations acquire a harsh structure and merge with the exhalatory pulses (derived from the popping incipient roar but with an ascending modulation of the lower dominant band) to produce full roars (Oliveira 2002). During a roaring bout, these respiratory cycles become faster and louder until they reach a climax in amplitude (Oliveira 2002). This alternating pattern allows most South American howler monkey species to utter continuous emissions of roars lasting up to several minutes. For example, A. caraya long roars (Fig. 13.1f: da Cunha 2004) last up to 1 min 43 s (Whitehead 1995), in fact much more, da Cunha pers. obs.) and the long roars of A. macconnelli have a median duration of 3 min 28 s (range, 1–10 min (Drubbel and Gautier 1993)). The respiratory cycles of A. belzebul roars are marked by a higher degree of frequency modulation than found in other species (Fig. 13.1g) and have the longest periods of uninterrupted calling, lasting up to 12 min (Oliveira 2002).
South American species that produce these long, continuous roars also sometimes emit short-duration “brief roars” (A. caraya: A. belzebul (Oliveira 2002; da Cunha unpubl. data; Fig. 13.1h); A. guariba (Oliveira 2002, Fig. 13.1c); A. macconnelli (Drubbel and Gautier 1993)). Oliveira (2002) found a range of 2–18 s for brief roars in A. belzebul and 2–8 s in A. guariba. Some studies discuss only brief forms of roaring in A. arctoidea (up to 8 s (Schön Ybarra 1986); median value of 19 s (Sekulic and Chivers 1986)), but Drubbel and Gautier (1993) confirm the presence of both brief (“short calls,” average duration of 11 s; range 1–40 s) and continuous (“long calls” more than 60 s) forms of roaring in A. macconnelli. Bouts consisting only of brief roars can last up to 20 min (A. guariba (Oliveira D unpubl. data); A. macconnelli (Drubbel and Gautier 1993)).
The Central American species, A. palliata (Sekulic and Chivers 1986; Whitehead 1995; Fig. 13.1j) and A. pigra, are the exception in that they emit only brief roars lasting a few seconds. While Whitehead (1995) describes A. pigra as a species capable of continuous roaring, they actually produce clear pauses between consecutive “brief” roars (Kitchen unpubl. data). The false impression by Whitehead is likely because this species can emit loud calls in quick succession and their loud calling bouts overall last much longer than in the southern species.
Although superficially different, the roars of A. pigra are similar to A. palliata, except the syllables are much longer, and far fewer syllables are produced per roar in the former species (Kitchen DM, Bergman TJ, Cortés-Ortiz L unpubl. data). Individual roars by A. pigra consist of a single long exhalatory emission (lasting 2.2 s on average: Kitchen 2000), sometimes preceded by a short inhalation, followed by a shorter low-amplitude inhalatory sound (Fig. 13.1i and 13.2f). For A. palliata, Baldwin and Baldwin (1976) describe solo male roars as a series of 1–4 respiratory cycles (“exhaled separated by shorter inhaled syllables”), while roars emitted in choruses, the most frequent form, are usually longer and more variable with 2–14 cycles per emission. Whitehead (1987, 1989) describes the roars of A. palliata in a similar way, with the typical roar consisting of a legato series of cycles (“notes”), increasing in duration and intensity, followed by a single note (probably a single exhalation phase) with maximum duration and intensity, and ending usually with a diminuendo of progressively shorter notes (similar to an “oodle”; see below). Sekulic and Chivers (1986) report that the series of notes that make up a roar in A. palliata lasts an average of 3.5 s.
We want to emphasize one important message in this section so far: brief roars are rare in several South American species, whereas they are the only roars produced by the Central American species. However, despite the difference in how frequently they are produced, there may be overall similarities in the brief roars in the two clades. For example, the description of the brief roars of A. arctoidea provided by Schön Ybarra (1986) is similar to what Whitehead (1987, 1989) characterized as normal roars in A. palliata—a crescendo, followed by a climax and a short, low-intensity coda (diminuendo). Perhaps these brief roars are the ancestral form of roaring (Oliveira and Ades 2004) given that they most closely resemble the loud vocalizations found in other primate species in terms of the duration of elements (colobus monkeys (Teichroeb and Sicotte 2010); Mentawai macaques, langurs, leaf monkeys, and gibbons (Schneider et al. 2008); gibbons (Geissmann 2002); guenons (Gautier 1989)).
Besides differences in structural pattern, there is also some interspecific variation in the acoustic structure of the howls. In Table 13.1, the analysis is focused on the exhaling phase of roars, since the inhaling phase is usually shorter and modulated in variable patterns (Drubbel and Gautier 1993; Whitehead 1995; Oliveira 2002), making its description less precise. Specifically, although all species have their lowest emphasized bands in the 200–700 Hz range, A. caraya and A. palliata produce some of the lowest peak frequencies. Alouatta belzebul and A. pigra produce some of the highest peak frequencies, and they are the only two species who have a peak that is higher in frequency than their second most emphasized frequency (Table 13.1; Whitehead 1995). Whitehead (1995) also found that the peak frequencies for A. belzebul and A. pigra were higher than the second most emphasized frequency during the inhalation phase, whereas the reverse pattern was seen in the other species (see also Drubbel and Gautier 1993; Oliveira 2002)—the sole exception was A. palliata, which always emphasized their lowest frequency band in both phases. In the power spectra of roars of all species studied to date, there is a confounding factor caused by the lack of distinction between more precise amplitude peaks (dominant frequencies) and wider bands (“frequency clusters”: Drubbel and Gautier 1993). The wider bands (usually two) cover hundreds of Hz and contain one or more amplitude peaks each.
The most striking pattern was found in the roars of A. belzebul of the Atlantic rainforest of northeastern Brazil (Oliveira 2002), whose high-pitched roars presented a single dominant peak with the widest variation observed among howler monkeys: values ranging from 550 to 1,100 Hz (average values: 740 Hz exhaling phase; 920 Hz inhaling phase). However, data for the same species in Brazilian Amazon region show the typical pattern of other South American species, with two dominant peaks per phase (Table 13.1; average values inhaling phase: 732 and 823 Hz (Whitehead 1995)). The fact that both A. belzebul populations are regarded as the same subspecies (Cortés-Ortiz et al. 2003) makes this difference in pattern intriguing.
2.3 Barks
As in roars, barks or “woofs” (Baldwin and Baldwin 1976; Neville et al. 1988) have a large degree of gradation, ranging from shorter, single pulses of low amplitude (incipient forms, Fig. 13.2a) to double pulses of increasing duration, rate, and amplitude (Fig. 13.2b–g). In A. guariba (Oliveira 2002; Fig. 13.2b, c), the duration of double-pulsed barks ranges from 100 to 800 ms, and sonograms available from other species fall within this range (Baldwin and Baldwin 1976; Schön Ybarra 1986; da Cunha 2004). However, even the shortest double-pulsed barks have a longer duration (>100 ms) than the single pulses of incipient roars (usually <70 ms (Schön Ybarra 1986; Oliveira 2002)).
There are of course variations on this pattern. For example, Schön Ybarra (1986) describes the presence of triple pulses of barks for A. arctoidea. InA. pigra, barks can have multiple pulses in a sonographically continuous emission that, however, sounds like distinct emissions given that the amplitude variation is observed, with the lower-amplitude periods being quiet enough to be possibly misconstrued as silent “breaks” (Kitchen unpubl. data; Fig. 13.2f). In A. caraya, da Cunha (2004) describes male barks as having double or single pulses of a similar frequency structure to that found in roars (Fig. 13.2d) and that bouts of barking by dominant males usually include a roar climax-like vocalization, similar to the “composite roars” in A. guariba (described below). Regardless of the nature of the pulses themselves, the usual emission pattern is one made up of a long to a very long string of pulses.
Although only scarcely described acoustically, available data on barks shows that their dominant frequencies are similar to those found in the roars of the same species (Eisenberg 1976; Baldwin and Baldwin 1976; Schön Ybarra 1986; Whitehead 1995; Oliveira 2002). The barks of Central American howler monkeys, however, have even greater structural resemblance to their roars (A. pigra (Kitchen 2000); A. palliata (Baldwin and Baldwin 1976)) than those in South American species, perhaps reflecting a lower degree of functional divergence between call types (see also Sect. 13.2.5).
Since the calls are akin in their frequency spectra and high amplitude, barking is likely generated through similar processes as roaring (see Sect. 13.4). However, barks are not produced continuously as roaring can be in South American species, rendering the complex respiratory maneuvers found in sustained roaring unnecessary. Schön Ybarra (1986) noticed that most A. arctoidea barks appeared to be uttered in exhalation, but the incorporation of inhalation phases could explain the merging of longer barks, which coalesce into the loud, composite roars described below (Oliveira 2002).
2.4 Oodles and Roar Variants
There are many loud calls in howler monkey repertoires that do not seem to fall exactly, or sometimes at all, into either graded series of barks or roars. For example, Drubbel and Gautier (1993) describe “oodles” in A. macconnelli as “blowing sounds,” occurring as short-range sounds after the coda (ending phase) of a long-lasting roaring period. In A. caraya, oodle calls seem to be unvoiced (not generated by vibrating vocal folds or other anatomical structures, such as in whisper), since they have a muffled nature (da Cunha RGT unpubl. data; see also Sect. 13.4). They are heard at the end of sessions or before a brief pause that is followed by the resumption of the continuous roaring session (Fig. 13.1e). In A. guariba, oodles are found in the ending of long, continuous roars or in pauses between them (Oliveira 2002; Fig. 13.1d). Additionally, A. guariba produces a loud, roar-like call with an oodle quality at its ending, typically emerging as a fusion of very loud and long barks, usually heard in intense barking bouts (Oliveira 2002; Fig. 13.2c). We will refer to these calls as “composite roars,” as their characteristics are intermediary between regular roars, barks, and oodles. This call also resembles a brief roar but has faster cycles that sound muffled during the ending phase, just like the oodles that usually follow them.
Central American species also produce similar oodles (Kitchen unpubl. data; Fig. 13.1j) during pauses between roars. Baldwin and Baldwin (1976) discuss a “roar terminus” in A. palliata as a series of fast cycles of usually declining pitch that frequently occurs at the end of normal roars, sometimes grading into oodles. This is likely similar to the harsher and louder form of oodle that is often described as occurring at the end of a roar in A. palliata (Altmann 1959; Whitehead 1987, 1989) and A. pigra (Kitchen 2000) and is part of the complex gradation found in the loud call repertoire of these two species. Another example of this complexity is the “roar variant” in A. palliata, characterized by a start as a sudden intense note that is followed by a trailing off, without the oodle-like ending (Whitehead 1987, 1989).
2.5 Pattern of Loud Calling Bouts
Among the South American species we have been able to analyze, there seems to be a distinction between roaring and barking bouts. Pauses (defined as <1 min by Oliveira 2002) followed by either a gradual or a sudden return to full, continuous roaring can occur in roaring bouts, but otherwise (not including the “warm up phase”) males emit full roars the entire time (A. caraya (da Cunha unpubl. data); A. guariba (Oliveira 2002); A. macconnelli (Drubbel and Gautier 1993)). In contrast to these fairly ritualized roaring bouts, the barking bouts of A. guariba (one of the few South American species where this data is available) are more variable, with frequent diminuendos and crescendos in amplitude, duration, and rate of bark pulses (Oliveira 2002). However, the scarce evidence found in the literature indicates that the barking bouts of other South American species may be more stable, with uniformity in the bark pulses emitted, at least during some periods (A. belzebul (Oliveira 2002); A. arctoidea (Schön Ybarra 1986)). Barking bouts can also have a much longer duration than a roaring bout and, although a composite roar or some kind of roar-like call can sometimes constitute a climax of amplitude in these bouts, barking bouts typically do not contain full or brief roars. For example, in A. caraya, a barking bout can be sustained for around 40 min, and during some periods the calls are stable, interspersed with something like roar climaxes, and then going back through diminuendo/crescendo phases (da Cunha unpubl. data).
Although Central American species produce some bouts with only barks, roaring bouts always include at least some barks and variants of both roar and bark vocalizations (A. pigra (Kitchen 2000); A. palliata (Baldwin and Baldwin 1976)). As we said above, this mixed pattern may be occasionally observed but is apparently not typical of any South American species (Schön Ybarra 1986; Oliveira 2002). Thus, the patterns of these mixed roar/bark bouts of Central American howler monkeys are much more variable than the stereotyped roaring bouts of the South American species.
A few trends are common to both A. pigra and A. palliata—bouts are often preceded by a quieter build-up phase (e.g., incipient barks/grunts) followed by “loud calling periods” (defined by Kitchen (2000) as including any loud calls and short “breaks” of <1 s). Roars become less frequent and pauses (<1 min as defined by Kitchen (2000)) between loud calling periods get longer toward the end of a bout. Additionally, loud calling periods/roars occur at a faster rate in bouts when another group is nearby. Entire bouts (including loud calling periods and silent periods) can last over an hour in both species (Kitchen DM, Bergman TJ, Cortés-Ortiz L unpubl. data).
2.6 Male Loud Calls: Concluding Remarks
There is wide variation in acoustic properties of calls, the temporal patterning of calling bouts, and the nature and duration of such bouts in the howler monkey species studied so far. Perhaps the clearest trend is a division between Central and South American species in that features of their loud calls parallel the two identified phylogenetic clades of the genus Alouatta (Cortés-Ortiz et al. 2003; Villalobos et al. 2004). Both A. palliata and A. pigra produce only simple, short-duration roars (a few seconds each), their barks are essentially just shorter syllables of their species-typical roars, and both barks and roars usually occur in the same bout of loud calling. However, although the individual vocalizations are shorter than in South American species, they are produced during bouts that last much longer than in the southern species, with pauses between calls. On the other hand, barks and roars are much more easily distinguished in South American species and the two call types are not typically combined in the same bout. These species produce roar vocalizations in both brief and long-lasting forms, and bouts of the latter consist of continuous emissions (up to several minutes) of inhalatory and exhalatory phases. Such respiratory cycles can also be noticed on roars of Central American species, but the inhalatory phase has a much lower amplitude compared to the South American species and may not play a role in long-distance communication (Kitchen DM pers. obs.).
We found that one major difficulty in making comparisons across species is due to the fact that authors vary widely both in how they define call types and in what is considered a “bout.” Some researchers define a bout from a functional perspective; that is, sessions close in time but apparently related to the same triggering stimulus are considered part of the same bout. Others choose some arbitrary period of silence as the criteria to define a new bout. Therefore, a determinant future step in the study of howler monkey vocalizations is to unify criteria and establish a nomenclature valid for all species based on clear and objective criteria. We believe this review is a first step in that direction.
Despite decades of research on howler monkeys, their most salient vocal feature – loud calling – remains undescribed in some species and awaits more detailed acoustic data for almost all species. For example, although the calls of A. belzebul and of some species of the A. seniculus group (A. arctoidea and A. macconnelli) have been described, those taxa have wide distributions with several discrete populations (Cortés-Ortiz et al. 2003, 2014; Gregorin 2006; Rylands and Mittermeier 2009). Given some of the distinctiveness among populations (e.g., the populations of A. belzebul described above), the study of the vocal repertoire of these taxa, as well as the study of hybrid vocalizations (see Sect. 13.5), may shed light on their taxonomic relationships.
3 The Structural Features of Female Loud Calls
We have dealt so far with an issue we believe is crucial in understanding howler monkeys’ loud calls: variation. Another source of variation, a quite neglected one in fact, lies between the sexes. Howler monkeys are fairly unusual among nonmonogamous primates given that both males and females produce loud calls. Actually, it might be more accurate to say that females often utter a moan-like call, albeit a call that is clearly related to male roars in structural terms (“roar accompaniment”: Baldwin and Baldwin 1976). Females can “roar,” together with the alpha male, or they can remain silent. Furthermore, male and female calls are commonly emitted (but not always) at the same time during group sessions. This duet-like pattern is normally found in monogamous species that jointly defend a border, such as the titi monkeys (Callicebus moloch (Robinson 1979)) and the hylobatids (Geissmann 2002), but it is otherwise rare in nonhuman primates.
The paucity of studies dealing with either structural or functional aspects of female calls probably relates to the difficulty in isolating female calls—they are much lower in amplitude than male calls and are nearly always masked by the overlapping sounds of males during a chorus. In fact, because of the extent of vocal overlap in some species, many authors that have worked with howler monkeys are unable to differentiate among any of the participants in a given chorus.
Still, the structure of female “roars” has been described for A. palliata (Baldwin and Baldwin 1976), A. arctoidea (Sekulic 1982), and A. guariba (Oliveira 2002). Female roars and barks are generally higher pitched than male loud calls (Baldwin and Baldwin 1976; Eisenberg 1976; Sekulic 1982). This is not surprising, given the sexual dimorphism in body size and hyoid volume in howler monkeys (Hershkovitz 1949; Gregorin 2006). Female roars are also reported to be more intense when uttered in roar choruses as an accompaniment to male roars (Baldwin and Baldwin 1976; Sekulic 1982; Oliveira 2002). Analyzing isolated emissions of A. guariba female roars, Oliveira (2002) demonstrated variation from tonal to harsh structure, with intense and irregularly oscillating frequency modulation in the tonal sections of these vocalizations (Fig. 13.3). However, besides from the previous example, spectrograms of female calls are absent or of medium or poor quality in the literature.
Female barks are usually simple pulses of lower intensity than male barks in A. guariba (Oliveira 2002), although female A. caraya sometimes produce more intense forms with greater frequency modulation than male barks (da Cunha pers. obs.). Incipient barks (simple pulses usually emitted with closed mouth) are described for A. palliata (Baldwin and Baldwin 1976) and A. guariba (Oliveira 2002) and are frequently produced by females and juveniles.
We need better recordings and descriptions of female calls before we can further advance the study of their structure. A possible solution to circumvent the drawbacks of their softer calls that are obscured during choruses could be the use of a small microphone attached to a collar, so as to capture the sound more directly. However, despite the lack of data, one can still speculate about structural issues of female vocalizations. For example, given the existence of interspecific differences in hyoid size and shape (Gregorin 2006), one could predict there will be variation in the structure of female calls similar to that observed in males, especially in the formant frequencies (see Sect. 13.2). Of particular interest would be to investigate if differences among species in female vocalizations merely mirror interspecific male differences or if female differences follow a different pattern. In the first case, females’ hyoids may simply be species-typical but smaller versions of the male ones, and their calls might accordingly be simply softer and higher-pitched versions of the male calls, with more widely spaced formant frequencies. However, given the many socioecological and behavioral differences between males and females, we predict that female interspecific differences in vocalizations may not simply mirror those of males but may follow a distinct pattern. For example, the differences between males of two species could reflect the fact that one species has stronger intrasexual selection than another, whereas interspecies differences between females could instead reflect the fact that there is infanticide risk in one species but not in the other and females might be either quieter or more aggressive when facing such a risk. These questions remain open for further studies.
4 Morphology and Vocal Production
Although the peculiar anatomical features of the howler monkey’s vocal apparatus clearly shape their unusual sounds, the phonation mechanisms underlying these calls are complex and have been poorly studied. The hyoid bone is a large, inflated, and hollow structure (the “hyoid bulla”), accommodated within the large, expanded mandibula (Fig. 13.4a, b) and positioned below the tongue (Hershkovitz 1949; Schön 1970; Fig. 13.4c). A pair of lateral air sacs borders the bulla (Kelemen and Sade 1960; Schön 1970). The “tentorium” is a subchamber of the hyoid bulla formed by a folding at the upper border of the hyoid opening (Fig. 13.4b–d). This structure is absent in A. palliata, rudimentary in A. caraya, and variably developed and shaped in the remaining South American species (Hershkovitz 1949). The most developed tentorium is present in the A. seniculus group, in which individuals have large hyoids and inflated tentorium chambers containing bony lateral partitions or trabeculae (Hershkovitz 1949; Gregorin 2006).
Vocal anatomy of A. guariba. (a) lateral view of adult male (left) and adult female (right) skulls, both showing an enlarged mandible (ma) that houses an inflated hyoid bulla (hy) but also remarkable sexual dimorphism; (b) same structures in ventral view, notice the hyoid aperture (ha) and the upper tentorium (te) subchamber; (c) longitudinal view of adult male vocal apparatus, the inside view (left) shows the subglottal chamber (sc), a large vocal fold (vf), the lateral aperture (la) that probably leads to a lateral air sac (not confirmed for the species), the contorted supraglottal vocal tract (vc), the hyoid chamber (hy*), with the tentorium subchamber (te*) and the sectioned tongue (to*), while the outside view (right) shows the large thyroid cartilage (th), hyoid bulla, and tongue (to) after removal of layers of muscle and connective tissue; (d) the inside view of the same adult male vocal apparatus (left) compared to the same structure from an adult female (right). The ruler in the images shows scale in centimeters. All photos by Júlio César de Souza Júnior
Kelemen and Sade (1960) attributed the loudness of howler monkey calls to the presence of rigid cavities formed by the hyoid bulla (Fig. 13.4c) and nonrigid lateral air sacs (Fig. 14.3c shows lateral aperture probably leading to an air sac in A. guariba). Since then, most phonation studies address the role of the hyoid as a Helmholtz resonator, amplifying the glottal source (Schön Ybarra 1986, 1988; Riede et al. 2008; de Boer 2009).
The large glottis (Fig. 13.4c, showing an enlarged vocal fold) can produce loud, low-frequency sounds that are further amplified by the resonators (hyoid, air sacs) and the constrictions in the post-glottal structures (Fig. 13.4c depicts narrow and curved supraglottal vocal tract), features that reduce the velocity of the air flow, elevating its pressure and, consequently, raising its volume (Schön Ybarra 1988, 1995). Recent modeling studies (Riede et al. 2008; de Boer 2009) have also indicated that the hyoid is largely responsible for the low frequency of the first formant in howler monkey vocalizations and allows a greater efficiency in the generation of loud sounds.
The sound produced at the larynx encounters a contorted pathway before reaching the mouth, given the enlargement of several structures (hyoid, cartilages, vocal folds—Fig. 13.4c, enlarged subglottic chamber). Forced air passage would also result in the generation of irregular, noisy vibrations—at least partially responsible for the harshness found in roars and barks (Schön Ybarra 1986, 1995). Whitehead (1995) suggested that the acoustic features of howler monkey loud calls were derived both from hyoid involvement and sub- and supraglottal maneuvers. As an example, he mentions the generation of the broadband (noisy) bursts of loud calling by an increase in subglottal pressure and a coupling of the extra-laryngeal structures (hyoid bulla, lateral air sacs) with the supraglottal air tract, leading to wide frequency fluctuations. We have found no mention in the literature to subglottal mechanisms, but a possible way to generate a sound so high at the laryngeal source is the production of large abdominal pressures. Anecdotally, one of us (RGTC) observed the eversion of tissue in the anal region during the exhalatory phase of A. caraya roars, a likely indication of extremely high abdominal pressure.
Kelemen and Sade (1960) argued that the rigidity of the laryngeal organ, containing large ossified cartilages (see thyroid cartilage in Fig. 13.4c) restricted the modulatory capacity in howler monkeys when compared to human and ape larynges. However, Schön Ybarra (1986, 1988) argued that howler monkeys could show some vocal plasticity through changes in the width and length of the mouth chamber and that even the hyoid position could be changed by the action of some muscles (Schön 1964). As another form of modulation, Riede and colleagues (2008) suggested that the hyoid creates interactions between the vocal cords and the vocal tract that could explain the dynamic changes usually found in roar pitch.
Few studies have focused on the role of the elastic, inflatable air sacs (Kelemen and Sade 1960; Schön Ybarra 1988). Drubbel and Gautier (1993) interpreted the oodles (“blowing sounds”), usually occurring at the end or pauses of continuous roaring in South American species, as a product of the emptying of air sacs. “Coughs” are also reported at these times (Schön Ybarra 1986) and may be a kind of choking sound caused by swallowing saliva (Oliveira D pers. obs.), which often dribbles from an individual’s mouth during the bouts (Schön Ybarra 1986). Whether these phenomena occur in the Central American species is unknown.
In sum, our present knowledge of the mechanisms underlying loud call production in howler monkeys is still very limited. Although recent modeling approaches are promising (Riede et al. 2008; de Boer 2009), conceiving a way of examining phonation in living animals would be valuable as it would allow closer investigation of the dynamic processes involved in call modulation. Additionally, the few studies on morphology and phonation published to date have centered on just A. palliata (Kelemen and Sade 1960) and on the A. seniculus group (Schön 1970, 1971; Schön Ybarra 1988). The high degree of interspecific variation found in the morphology of the vocal apparatus and the structure, duration, and temporal patterning of calls highlights the need to investigate vocal production in other species.
The large variation in hyoid size and shape among different howler monkey species has implications for systematic arrangements (Hershkovitz 1949; Gregorin 2006) and since Ihering (1914) has been used as a taxonomic character (e.g., Lönnberg 1941). Hershkovitz (1949) regarded the smaller hyoid found in A. palliata as an ancestral state, from which the larger and complex hyoids found in other Alouatta species diverged; however, genetic evidence now places A. palliata as part of a clade with the other Central American species, A. pigra, being no longer considered basal for the genus (Cortés-Ortiz et al. 2003). Alouatta palliata hyoids are also less sexually dimorphic than other species (Hershkovitz 1949; Gregorin 2006; Fig. 13.4b, d), including A. pigra (Cortés-Ortiz L. pers. comm.).
Sekulic and Chivers (1986) proposed that loud calls in A. palliata are shorter in duration than in A. arctoidea because of the presence of a smaller hyoid with a smaller air reservoir. However, this explanation is unlikely given the hyoid’s rigid structure and the fact that roars, produced during the whole respiratory cycle, do not need an air reservoir. Although both Central American species, particularly A. palliata, have shorter, simpler roars than other howler monkeys, there are traits unique to only A. palliata such as the absence of significant energy above 1,000 Hz (other species have spectral energy to 2,000 Hz: Whitehead 1995). Although Thorington and colleagues (1984) suggested that large hyoid sizes meant lower frequencies, this hypothesis has not been supported (e.g., A. palliata produce low-frequency calls as compared to A. belzebul, a species with a large hyoid that produces some of the highest frequency calls in the genus (Gregorin 2006)). Thus, it remains unclear whether and how the atypical calls produced by A. palliata are linked to their distinctive hyoid morphology.
5 Sound Propagation and Geographic Variation
Howler monkeys, like many other primate species, produce loud calls to communicate over long distances. The acoustic structure of any sound can be altered and degraded as it travels, due to physical phenomena such as attenuation (intensity of acoustic signals generally decreases 6 dB each time the distance from the source is doubled, due to factors such as atmospheric absorption and sound scattering: Wiley and Richards 1978; Brenowitz 1982) and reverberation (when sound is reflected and scattered by stationary objects during propagation: Naguib and Wiley 2001). However, sounds with most of their energy concentrated at low frequencies (a common feature of primate loud calls) are less degradable by attenuation than are higher-frequency sounds (frequency-dependent attenuation (Waser and Waser 1977; Mitani and Stuht 1998; Naguib and Wiley 2001)). One exception is that the ground may cause relatively large attenuation effects, particularly in low frequencies (<1 kHz), but this effect becomes negligible above 1 m from the ground (Roberts et al. 1977; Mitani and Stuht 1998; Nelson 2003; Maciej et al. 2011).
How the different types of habitat influence the sound over distances (e.g., due to vegetation absorption and/or reverberation) is debated and the evidence is mixed (Date and Lemon 1993; Naguib 1996; Daniel and Blumstein 1998; Blumenrath and Dabelsteen 2004; Schneider et al. 2008). Contrary to intuitive expectations, sound is less scattered and travel farther distances (at almost every frequency) in closed than in open habitats (Wiley and Richards 1982; Waser and Brown 1986). In contrast, reverberation is stronger in closed habitats and constrains long-range communication (Waser and Brown 1986). However, calls of certain frequencies, given from particular heights and/or at specific times of the day, can transmit over long distances in closed habitats almost free of attenuation (Morton 1975; Marten et al. 1977; Waser and Brown 1986; Brown and Handford 2000). A sound window (frequency range that attenuates less and propagates farther in a given habitat (Morton 1975; Waser and Brown 1986)) of between 100 and 400 Hz exists in rainforests for sounds produced around 7–8 m above the ground. Howler monkey roars, with their high amplitudes, relatively low emphasized frequencies (between 300 and 1,000 Hz, well within the forest sound window), and harshness (noisy, atonal sound structure), are among the primate vocalizations capable of propagating the greatest distances (at least 1 km (Baldwin and Baldwin 1976; Schön Ybarra 1986; Whitehead 1989; Whitehead 1987; Whitehead 1995)). However, future howler monkey research might focus on how acoustic degradation in different habitats and under different conditions affects their vocalizations.
Many forest primate species seem to concentrate their long-distance calls around dawn, and howler monkeys are no exception (Sekulic 1982; Whitehead 1995; da Cunha and Byrne 2006). A commonly cited reason is that sound propagation is better during this “time window” (Gautier and Gautier 1977; Horwich and Gebhard 1983; Waser and Brown 1986; Brown and Handford 2000; Cornick and Markowitz 2002), despite increased background noise (Wiley and Richards 1982). However, there may also be other proximate explanations for such a temporal pattern; for example, research on birds found that calling at daybreak allowed animals to avoid heat stress (Ricklefs and Hainsworth 1968; see also Sekulic 1982). On the other hand, A. pigra (Horwich and Gebhard 1983; Cornick and Markowitz 2002) and perhaps other species (e.g., A. arctoidea: Braza et al. 1981) seem to have a bimodal pattern with a secondary peak at afternoon/sunset and with reductions at midday. Sekulic (1982) also reported a reduction in the midday calling activity in A. arctoidea in Venezuela, possibly the time of the day with the worst environmental conditions for sound propagation (Wiley and Richards 1982). Conversely, Drubbel and Gautier (1983) reported that A. macconnelli in Guyana frequently produce long roaring choruses at night (also heard frequently in A. pigra (Kitchen pers. obs.)), when temperature gradients are favorable and wind turbulence is scarce, helping sound propagation (Wiley and Richards 1982). A fourth pattern is a notable absence of a dawn chorus in A. guariba at several sites (Chiarello 1995; Oliveira 2002; Steinmetz 2005; da Cunha and Jalles-Filho 2007; Holzmann et al. 2012). Whether the lack of a dawn chorus in this species, or the lack of a secondary afternoon peak in species with a dawn chorus, is the result of varying environmental conditions or other factors, such as population densities, requires further investigation.
To evaluate how well howler monkey long-distance calls are adapted to local conditions, both in their structure and in their timing, it will be necessary to explore geographic variation between populations of the same species. Because different degradation processes act differently in diverse types of habitats or under different conditions, we might expect interpopulation variation due to selective pressures such as (1) vegetation structure of local environment (e.g., closed vs. open habitats (Wiley and Richards 1978)), (2) social factors such as population density (Delgado 2006), and (3) other environmental sound characteristics mostly based on local biota and local conditions (like wind and rain) that provoke sound interference (Martin 1981; Brenowitz 1982; Sorjonen 1986; Waser and Brown 1986; de la Torre and Snowdon 2002). Given that many Alouatta species concentrate calling at dawn and dusk, a noisy time in tropical forests, the frequency window is likely the most important mechanism to cope with interspecific acoustic competition, although this possibility has yet to be tested.
Rather than arising due to selection for particular call features, geographic variation in vocalizations could also arise indirectly due to differences between populations in anatomy (e.g., body size: Bowman 1979), genetics (as a result of reproductive isolation between populations of the same species: Wich et al. 2008; Thinh et al. 2011), or flexible adjustments to local conditions (e.g., increasing amplitude in a noisy habitat: Lombard 1911). A howler monkey species with a wide distribution range, present in different types of habitats (e.g., A. caraya, A. arctoidea, or A. palliata), would be an ideal model to test these different hypotheses related to geographical variation in long-distance calls.
Since howler monkey roars have been proposed to function in intergroup spacing, judging the distance from a caller can be very important (Whitehead 1987, 1989). Out of a set of sound degradation phenomena that potentially provide receivers with distance information, reverberation is the only one that might apply to howler monkeys, given the characteristics of their calls and habitats (following Wiley and Richards 1978). By manipulating this parameter in a series of playback experiments, Whitehead (1987) demonstrated that howler monkeys were able to perceive approaches and withdrawals based on barks alone (see Sect. 13.6). However, Naguib and Wiley (2001) proposed that longer barks could simulate the reverberation of shorter pulses, providing the basis for potential deceptive communication of distance in howler monkeys. To date, no one has explored a possible test between these somewhat opposing hypotheses about honesty and deception.
In summary, there are many interesting questions that remain unexplored in relation to sound propagation in howler monkeys. For example, little has been done to explore inter- and intraspecific variation in long-distance calls based on aspects things such as habitat differences. One potential confounding effect in such studies is that structural variation can also occur within a population based on individual variation. More studies should focus on uncovering the existence of individual variation between same sex individuals. For example, based on spectrographic analysis of roars, researchers found evidence for individuality in the acoustic features from two different populations of A. pigra in Belize (Bocian et al. 1999; Kitchen 2000). Additionally, we have not yet scratched the surface in understanding the ultimate and proximate factors governing the timing features of howler monkey calls. Of particular interest would be to investigate deviations from the most common timing patterns in parallel with the function of loud calls. For example, is the absence of dawn chorus in some species (such as A. guariba) related to functional, habitat, or call structure differences? Are there intraspecific differences in timing? If so, what causes them?
6 Going Soft: The Neglected Calls
Howler monkeys prodigious loud calls, as impressive and theoretically interesting as they are, have a downside. They have drawn attention away from the rich repertoire of more subtle calls. Yes, howler monkeys can and do call quietly. What is more, they have a broad repertoire of such calls, and some species are actually highly vocal in this category (A. caraya (da Cunha pers. obs., Holzmann 2012)). In this section, we will review the available work conducted on this topic and point to some lines of research we believe could be particularly fruitful. In our review, we mainly discuss studies that focused either on the entire repertoire or just soft calls. An attempt to survey all published works to uncover sources where soft calls were mentioned en passant was not feasible.
In Table 13.2, we summarize the scarce information on soft calls. The few classic published studies that have dealt with low-amplitude calls are restricted in scope, mainly descriptive, conducted only on A. palliata and A. caraya (but see A. guariba (Holzmann 2012)), with no or poor spectrograms and with functional interpretations that are not solidly grounded (Carpenter 1934; Altmann 1959; Baldwin and Baldwin 1976; Calegaro-Marques and Bicca-Marques 1997). These limitations impose serious restrictions on comparative work.
Although a number of these calls might provide interesting research projects, we chose to discuss three categories of soft calls whose study in howler monkeys we believe could be particularly fruitful. These are some of the most commonly produced call types. They have been discussed in at least some previous literature, and they pose interesting theoretical issues of potentially broader relevance: contact calls, immature calls, and alarm calls.
6.1 Contact Calls
In primates, one of the most ubiquitous categories of calls is that used to promote or retain spatial cohesion, particularly when group members become spread out or separated (see da Cunha and Byrne (2009) for a review on Neotropical primates). A variety of specific functions have been proposed for these calls, commonly labeled as contact, isolation, or “lost” calls: maintaining contact at close, visual range (Epple 1968; Pook 1977) or at longer ranges in situations likely to lead to separation, such as rapid travel or dispersed foraging, regaining contact (Daschbach et al. 1981; Byrne 1981; Palombit 1992; Harcourt et al. 1993; Halloy and Kleiman 1994), monitoring the position of others (Caine and Stevens 1990), initiating and directing or coordinating group travel (Boinski 1991, 1993), and attracting others in particular circumstances (Dittus 1988; Mitani and Nishida 1993). Before proceeding, a cautionary note: although conventional, terms like “contact call” and “alarm call” are functional labels and, as such, not adequate until appropriate studies have been conducted (Martin and Bateson 2007).
In the case of howler monkeys, contact calls have only been partially studied in a few species. Given their ubiquity, it is surprising that references to these calls are so scant in the literature (see also Kitchen et al. 2014, regarding loud contact calls), even more so if we exclude those calls performed by infants when separated from their mothers (more properly included within the subsection on immature calls below). The best examples we found included a report that A. palliata individuals emit whimpers in a variety of situations, including group progressions (Baldwin and Baldwin 1976). Also, in their brief report on A. caraya calls, Calegaro-Marques and Bicca-Marques (1997) mention a vocalization (“cry”) emitted in stressful situations including circumstances in which the caller was away from the group. Finally, based on a 19-month fieldwork study on the vocal behavior of a wild A. caraya group, da Cunha and Byrne (2013) suggested that a low-amplitude vocalization, the “moo” call, served a contact function. Based on ad libitum and anecdotal information, these authors (Byrne 2000; da Cunha and Byrne 2009) propose that “moo” calls among A. caraya individuals represent a genuine call-and-answer system, albeit one based on first-order intentionality (i.e., without comprehension of others’ mental states (Dennett 1978)). The hypothesis that “moos” are produced in antiphonal exchanges awaits rigorous testing (e.g., baboons (Cheney et al. 1996)). Besides, we call the attention that primate contact calls are ideal for studying intentionality in animal communication.
Thus, it is clear that there is a fundamental need for detailed repertoire studies, as the foundation of more advanced studies. Just with regard to contact calls, there are many interesting basic questions to focus on, for example, do other howler monkey species produce contact calls? Are contact calls structurally similar between different howler monkey species? Are there acoustic differences between contact calls produced in slightly different contexts (e.g., by isolated animals vs. those maintaining regular contact during minor spread)?
6.2 Immature Calls
Another ubiquitous kind of primate vocalization category is those calls emitted by infants and juveniles in stressful or care-related situations, usually labeled as “distress calls,” “cries,” “tantrum calls,” or just “infant calls” (see Newman (1995) for a review). Once again, information on immature howler monkey calls is scarce and concentrated mostly on A. palliata (see Table 13.2). However, in A. caraya, it was possible to identify a group of structurally related calls that perform some role related to infant distress situations (da Cunha 2004; Holzmann 2012). Similar calls were reported in A. guariba infants (Holzmann 2012; Oliveira unpubl. data). Nevertheless, such calls are so variable and graded that it is difficult to categorize them in a precise way.
6.3 Alarm Calls
Notwithstanding the undeniable importance of alarm calls in the primate bioacoustics literature, evidence for such calls in howler monkeys is even scarcer than for the two previous types of vocalizations. As seen in Table 13.2, several quiet vocalizations are produced in a variety of alert or alarm situations; however, no call types have been rigorously described, so once again more recordings in a range of contexts will be necessary in order to uncover consistent patterns. One promising example among the quiet calls is the low-amplitude “incipient barks” that Oliveira (2002) reported were emitted, usually by A. guariba females and juveniles, in mild-alarm contexts induced by the close proximity of a human observer. This author reports that these calls are also frequently emitted by females during group choruses of loud barks, possibly functioning to incite male barking, and this might be viewed as a similar context to alarm, given that they are potentially stressful situations.
In Kitchen et al. (2014), we also address the possibility that howler monkeys use loud calls as alarms. Regardless of whether, from the signaler’s perspective, quiet or loud calls produced in such contexts are affective responses to stress, referential, or both, these calls may function to alert receivers about danger (e.g., Seyfarth and Cheney 2003; but see Owren et al. 2010), and there may even be different calls for different predators/situations, as is true in other primate species (Seyfarth et al. 1980; Zuberbuhler 2000, 2001; Arnold and Zuberbuhler 2004; Casar et al. 2012). Playback experiments will ultimately be necessary to test these questions.
7 The Rough Guide to Recording and Sharing Vocalizations
Regardless of interest levels for researching the soft calls, howler monkey loud calls will certainly keep attracting abundant attention from scientists. Not only are these calls fascinating because they are peculiar in their production and stand out in the jungle soundtrack but also because their functional significance remains unsettled. Thus, we felt we could contribute to the advancement of research on this topic by briefly proposing some guidelines for their study. By doing so, we do not want to imply that this is the only or the best way to tackle the issue. We simply felt others could profit from some of our tips to avoid common mistakes.
First and foremost, authors should make clear which call type they are referring to and do so using the nomenclature already employed in the literature. One important aspect of this is that there is so much variation among species that it becomes difficult for a researcher familiar with, for example, the calls of the Central American species to understand the written description of calls from a South American species. After working together on this chapter, the four of us authors have firsthand experience with this issue. The basis for the classification should be variables extracted from good spectrograms, and, thus, more high-quality spectrograms need to be provided in the literature. Several kinds of free software are capable of producing high-quality images (e.g., Raven Lite: Bioacoustic Research Program 2011, Praat: Boersma and Weenink 2012). Another crucial point is to clearly define what authors consider a bout and, even more importantly, to show data (in the most possible raw form) on both call durations and inter-call intervals, so that others can examine these and make comparisons.
When journals offer such an option, authors should also take advantage of using online supplemental materials to upload audio examples of calls. Such examples should include both isolated individual call types and short sections of longer bouts, in order to demonstrate patterns. Although multi-animal choruses are interesting, solo calling bouts are even more useful (but rare to capture in many species). Sample recordings should also be shared with archives such as that offered by the Macaulay Library from Cornell University (see http://macaulaylibrary.org) or the sound archive of the British Library (http://sounds.bl.uk). Within the first website, it is possible to browse and use their collection for research or education, as long as proper citations are used.
The above websites also provide tips for purchasing equipment, for making proper field recordings, and for documenting information about the caller (see also Geismann and Parsons 2011). In the tropics, researchers need to consider the use of durable recorders that record in high quality without being susceptible to humidity, dust, and the occasional falls during a forest trek. Although it is common sense for most field workers to make recordings using systematic methods and professional equipment, there are common mistakes made both by people recording vocalizations for first time and by those with years of experience (including ourselves). Many important steps can be forgotten during the excitement of recording an intense calling bout. For example, not using headphones when recording causes observers to miss some of the noise that is picked up by strong directional microphones made by their own body movements, leaves under their feet when they adjust their position, and colleagues talking (even at a distance). Additionally, headphones assist the recordist in monitoring the input level (along with level meters on most recorders)—because different calls within a howler monkey bout can range so extensively in amplitude, a common mistake is to record too loudly and this causes clipping and distortion. When using headphones, we recommend in some situations the recordist keeps one ear free in order to locate individual callers—otherwise, directional microphones can be disorienting when both ears are covered. When recording, only practice helps to avoid talking over recordings while also recording information in real time about the identity of the caller, so that individuals can be compared later. This is especially important if observers want to try to capture isolated calls from individuals during group choruses.
Finally, we urge researchers who are not focusing on vocalizations in their projects to still consider carrying recording equipment with them into the field. Once familiar with recording protocols, the real-time recording ability of modern equipment can help with a variety of data collection beyond just vocalizations. And by increasing the number of recordists in the field, we may ultimately be better able to compare the repertoires of different species and to ask questions about the context of infrequently produced calls.
Notes
- 1.
All studies on A. guariba (formerly A. fusca) loud calls are restricted to the southern subspecies, A. g. clamitans. The authors found no reference to studies on the more restricted and lesser known northern subspecies, A. g. guariba.
Abbreviations
- dB:
-
Decibels
- e.g.,:
-
For example
- Hz:
-
Hertz
- i.e.,:
-
In other words
- kHz:
-
Kilohertz
- m:
-
Meters
- min:
-
Minutes
- ms:
-
Milliseconds
- pers. comm.:
-
Personal communication
- pers. obs.:
-
Personal observation
- s:
-
Seconds
- SPL:
-
Sound pressure level
- unpubl. data:
-
Unpublished data
References
Altmann SA (1959) Field observations on a howling monkey society. J Mammal 40:317–330
Baldwin JD, Baldwin JI (1976) Vocalizations of howler monkeys (Alouatta palliata) in Southwestern Panama. Folia Primatol 26:81–108
Bioacoustics Research Program (2011) Raven Pro: interactive sound analysis software, version 1.4 [Computer software]. Cornell Lab of Ornithology, Ithaca. http://www.birds.cornell.edu/raven. Accessed 11 April 2012
Blumenrath SH, Dabelsteen T (2004) Degradation of great tit (Parus major) song before and after foliation: implication for vocal communication in a deciduous Forest. Behaviour 141:935–958
Bocian D, Aday C, Gavazzi A, Markowitz H, Baptista L (1999) Can spectrographic analysis of roars be used to identify individual male black howler monkeys (Alouatta pigra)? Am J Primatol 49:36–37
Boersma P, Weenink D (2012) Praat: doing phonetics by computer, version 5.3.13 [Computer software]. University of Amsterdam. http://www.praat.org/. Accessed 11 April 2012
Boinski S (1991) The coordination of spatial position: a field study of the vocal behaviour of adult female squirrel monkeys. Anim Behav 41:89–102
Boinski S (1993) Vocal coordination of troop movement among white-faced capuchin monkeys, Cebus capucinus. Am J Primatol 30:85–100
Bowman RI (1979) Adaptive morphology of song dialects in Darwin’s finches. J Ornithol 120:353–389
Braza F, Alvarez F, Azcarate T (1981) Behaviour of the red howler monkey (Alouatta seniculus) in the llanos of Venezuela. Primates 22:459–473
Brenowitz EA (1982) The active space of red-winged blackbird song. J Comp Physiol 147:511–522
Brown TJ, Handford P (2000) Sound design for vocalizations: quality in the woods, consistency in the fields. Condor 102:81–92
Byrne RW (1981) Distance vocalisations of Guinea baboons (Papio papio) in Senegal: an analysis of function. Behaviour 78:283–312
Byrne RW (2000) How monkeys find their way: leadership, coordination, and cognitive maps of African baboons. In: Boinski S, Garber PA (eds) On the move: how and why animals travel in groups. University of Chicago Press, Chicago
Caine NG, Stevens C (1990) Evidence for a “monitoring call” in red-bellied tamarins. Am J Primatol 22:251–262
Calegaro-Marques C, Bicca-Marques JC (1997) Vocalizações de Alouatta caraya (Primates, Cebidae). In: Ferrari SF, Schneider H (eds) A primatologia no Brasil, vol 5. SBPr/UFPA, Belém
Carpenter CR (1934) A field study of the behavior and social relations of howling monkeys Alouatta palliata. Comp Psychol Monogr 10:1–168
Casar C, Byrne R, Young RJ, Zuberbuehler K (2012) The alarm call system of wild black-fronted titi monkeys, Callicebus nigrifrons. Behav Ecol Sociobiol 66:653–667
Cheney DL, Seyfarth RM, Palombit RA (1996) The function and mechanisms underlying baboon ‘contact’ barks. Anim Behav 52:507–518
Chiarello AG (1995) Role of loud calls in brown howlers, Alouatta fusca. Am J Primatol 36:213–222
Cornick LA, Markowitz H (2002) Diurnal vocal patterns of the black howler monkey (Alouatta pigra) at Lamanai, Belize. J Mammal 83:159–166
Cortés-Ortiz L, Bermingham E, Rico C, Rodrı́guez-Luna E, Sampaio I, Ruiz-Garcı́a M (2003) Molecular systematics and biogeography of the Neotropical monkey genus, Alouatta. Mol Phylogenet Evol 26:64–81
Cortés-Ortiz L, Rylands AB, Mittermeier R (2014) The taxonomy of howler monkeys: integrating old and new knowledge from morphological and genetic studies. In: Kowalewski M, Garber P, Cortés-Ortiz L, Urbani B, Youlatos D (eds) Howler monkeys: adaptive radiation, systematics, and morphology. Springer, New York
da Cunha RGT (2004) A functional analysis of vocalisations of black howler monkeys (Alouatta caraya). PhD Dissertation, University of St. Andrews, St. Andrews
da Cunha RGT, Byrne RW (2006) Roars of black howling monkeys (Alouatta caraya): evidence for a function in inter-group spacing. Behaviour 143:1169–1199
da Cunha RGT, Byrne RW (2009) The use of vocal communication in keeping the spatial cohesion of groups: intentionality and specific functions. In: Garber PA, Estrada A, Bicca-Marques JC, Heymann EW, Strier KB (eds) South American primates. Comparative perspectives in the study of behavior, ecology, and conservation. Springer, New York
da Cunha RGT, Byrne RW (2013) Age-related differences in the use of the “moo” call in black howlers (Alouatta caraya). Int J Primatol 34:1105–1121
da Cunha RGT, Jalles-Filho E (2007) The roaring of southern brown howler monkeys (Alouatta guariba clamitans) as a mechanism of active defense of borders. Folia Primatol 78:259–271
Daniel JC, Blumstein DT (1998) A test of acoustic adaptation hypothesis in four species of marmots. Anim Behav 56:1517–1528
Daschbach NJ, Schein MW, Haines DE (1981) Vocalizations of the slow loris, Nycticebus coucang (Primates, Lorisidae). Int J Primatol 2:71–80
Date EM, Lemon RE (1993) Sound transmission: a basis on dialects in birdsongs? Behaviour 124:291–312
de Boer B (2009) Acoustic analysis of primate air sacs and their effect on vocalization. J Acoust Soc Am 126:3329–3343
de la Torre S, Snowdon CT (2002) Environmental correlates of vocal communication of wild pygmy marmosets, Cebuella pygmaea. Anim Behav 63:847–856
Delgado RA (2006) Geographic variation in the long calls of male Orangutans (Pongo spp.). Ethology 113:487–498
Dennett DC (1978) Beliefs about beliefs. Behav Brain Sci 1:568–569
Dittus W (1988) An analysis of toque macaque cohesion calls from an ecological perspective. In: Todt D, Goedeking P, Symmes D (eds) Primate vocal communication. Springer, Berlin
Drubbel RV, Gautier JP (1993) On the occurrence of nocturnal and diurnal loud calls, differing in structure and duration, in red howlers (Alouatta seniculus) of French Guyana. Folia Primatol 60:195–209
Eisenberg JF (1976) Communication mechanisms and social integration in the black spider monkey, Ateles fusciceps robustus, and related species. Smithson Contrib Zool 213:1–108
Epple G (1968) Comparative studies on vocalization in marmoset monkeys (Hapalidae). Folia Primatol 8:1–40
Gautier JP (1989) A redrawn phylogeny of guenons based upon their calls: biogeographical implications. Bioacoustics 2:11–21
Gautier JP, Gautier A (1977) Communication in Old World monkeys. In: Sebeok T (ed) How animals communicate. Indiana University Press, Bloomington
Geismann T, Parsons S (2011) Recording primate vocalizations. In: Setchell JM, Curtis DJ (eds) Field and laboratory methods in primatology: a practical guide, 2nd edn. Cambridge University Press, Cambridge
Geissmann T (2002) Duet-splitting and the evolution of gibbon songs. Biol Rev 77:57–76
Gregorin R (2006) Taxonomy and geographic variation of species of the genus Alouatta Lacépède (Primates, Atelidae) in Brazil. Rev Bras Zool 23:64–144
Halloy M, Kleiman DG (1994) Acoustic structure of long calls in free-ranging groups of golden lion tamarins, Leontopithecus rosalia. Am J Primatol 32:303–310
Harcourt AH, Stewart KJ, Hauser MD (1993) Functions of wild gorilla “close” calls. I. Repertoire, context, and interspecific comparison. Behaviour 124:89–122
Hershkovitz P (1949) Mammals of northern Colombia. Preliminary report no. 4: monkeys (Primates), with taxonomic revisions of some forms. Proc U S Nat Mus 98:323–327
Holzmann I (2012) Distribución geográfica potencial y comportamiento vocal de dos especies de mono aullador (Alouatta guariba clamitans y A. caraya). PhD Dissertation, Universidad Nacional de La Plata.
Holzmann I, Agostini I, Di Bitetti M (2012) Roaring behavior of two syntopic howler species (A. caraya and A. guariba clamitans): evidence supports the mate defense hypothesis. Int J Primatol 33:338–355
Horwich RH, Gebhard K (1983) Roaring rhythms in black howler monkeys (Alouatta pigra) of Belize. Primates 24:290–296
Ihering HV (1914) Os bugios do gênero Alouatta. Rev Mus Paulista 9:231–280
Kelemen G, Sade J (1960) The vocal organ of the howling monkey, Alouatta palliata. J Morphol 107:123–140
Kitchen DM (2000) Aggression and assessment among social groups of Belizean black howler monkeys (Alouatta pigra). PhD Dissertation, University of Minnesota, Minneapolis
Kitchen DM, da Cunha RGT, Holzmann I, Oliveira DAG (2014) Function of loud calls in howler monkeys. In: Kowalewski M, Garber P, Cortés-Ortiz L, Urbani B, Youlatos D (eds) Howler monkeys: adaptive radiation, systematics, and morphology. Springer, New York
Leighty KA, Soltis J, Wesolek CM, Savage A (2008) Rumble vocalizations mediate interpartner distance in African elephants, Loxodonta africana. Anim Behav 67:125–139
Lombard E (1911) Le signe de l’élévation de la voix. Annales Des Maladies de l’Oreille et Du Larynx 37:101–119
Lönnberg E (1941) Notes on members of the genera Alouatta and Aotus. Ark Zool 33A:1–44
Maciej P, Fischer J, Hammerschmidt K (2011) Transmission characteristics of primate vocalizations: implications for acoustic analyses. PLoS One 6:e23015. doi:10.1371/journal.pone.0023015
Marten K, Quine D, Marler P (1977) Sound transmission and its significance for animal vocalization. Behav Ecol Sociobiol 2:291–302
Martin GR (1981) Avian vocalizations and the sound interference model of Robert’s et al. Anim Behav 29:632–633
Martin P, Bateson P (2007) Measuring behaviour: an introductory guide. Cambridge University Press, Cambridge
McComb K, Packer C, Pusey A (1994) Roaring and numerical assessment in contests between groups of female lions, Panthera leo. Anim Behav 47:379–387
Mech LD (1966) The wolves of Isle. Royale Fauna Series 7. U.S. Department Printing Office, Washington, DC
Mitani JC, Nishida T (1993) Contexts and social correlates of long-distance calling by male chimpanzees. Anim Behav 45:735–746
Mitani JC, Stuht J (1998) The evolution of nonhuman primate loud calls: acoustic adaptation for long-distance transmission. Primates 39:171–182
Morton ES (1975) Ecological sources of selection on avian sounds. Am Nat 109:17–34
Naguib M (1996) Ranging by song in Carolina wrens Thryothorus ludovicianus: effects of environmental acoustics and strength of song degradation. Behaviour 133:541–559
Naguib M, Wiley RH (2001) Estimating the distance to a source of sound: mechanism and adaptations for long-range communication. Anim Behav 62:825–837
Nelson BS (2003) Reliability of sound attenuation in Florida scrub habitat and behavioral habitat implications. J Acoust Soc Am 113:2901–2911
Neville MK, Glander KE, Braza F, Rylands AB (1988) The howling monkeys, genus Alouatta. In: Mittermeier RA, Rylands AB, Coimbra-Filho A, Fonseca GAB (eds) Ecology and behavior of neotropical primates, vol 2. World Wildlife Fund, Washington, DC
Newman JD (1995) Vocal ontogeny in macaques and marmosets: convergent and divergent lines of development. In: Zimmermann E, Newman JD, Jürgens U (eds) Current topics in primate vocal communication. Plenum, New York
Oliveira DAG (2002) Vocalizações de longo alcance de Alouatta fusca clamitans e Alouatta belzebul belzeul: estrutura e contexto. PhD Dissertation, Universidade de São Paulo, São Paulo
Oliveira DAG, Ades C (2004) Long-distance calls in Neotropical primates. An Acad Bras Cienc 76:393–398
Owren MJ, Rendall D, Ryan MJ (2010) Redefining animal signaling: influence versus information in communication. Biol Philos 25:755–780
Palombit RA (1992) A preliminary study of vocal communication in wild long-tailed macaques (Macaca fascicularis): II. Potential of calls to regulate intragroup spacing. Int J Primatol 13:183–207
Pook AG (1977) A comparative study of the use of contact calls in Saguinus fuscicollis and Callithrix jacchus. In: Kleiman DG (ed) The biology and conservation of the Callitrichidae. Smithsonian Institution Press, Washington, DC
Ricklefs RE, Hainsworth FR (1968) Temperature dependent behavior of the cactus wren. Ecology 49:227–233
Riede T, Tokuda IT, Munger JB, Thomson SL (2008) Mammalian laryngeal air sacs add variability to the vocal tract impedance: physical and computational modeling. J Acoust Soc Am 124:634–647
Roberts J, Kacelnik A, Hunter ML Jr (1977) A model of sound interference in relation to acoustic communication. Anim Behav 27:1271–1272
Robinson JG (1979) Vocal regulation of use of space by groups of titi monkeys Callicebus moloch. Behav Ecol Sociobiol 5:1–15
Rylands AB, Mittermeier RA (2009) The diversity of the new world primates (Platyrrhini). In: Garber PA, Estrada A, Bicca-Marques JC, Heymann EW, Strier KB (eds) South American primates: comparative perspectives in the study of behavior, ecology, and conservation. Springer, New York
Schneider C, Hodges K, Fischer J, Hammerschmidt K (2008) Acoustic niches of Siberut primates. Int J Primatol 29:601–613
Schön MA (1964) Possible function of some pharyngeal and lingual muscles of the howling monkey (Alouatta seniculus). Acta Anat 58:271–283
Schön MA (1970) On the mechanism of modulating the volume of the voice in howling monkeys. Acta Oto Laryngol 70:443–447
Schön MA (1971) The anatomy of the resonating mechanism in howling monkeys. Folia Primatol 15:117–132
Schön Ybarra MA (1984) Locomotion and postures of red howlers in a deciduous forest-savanna interface. Am J Phys Anthropol 63:65–76
Schön Ybarra MA (1986) Loud calls of adult male red howling monkeys (Alouatta seniculus). Folia Primatol 47:204–216
Schön Ybarra MA (1988) Morphological adaptations for loud phonations in the vocal organ of howling monkeys. Primate Rep 22:19–24
Schön Ybarra MA (1995) A comparative approach to the non-human primate vocal tract: implications for sound production. In: Zimmermann E, Newman JD, Jürgens U (eds) Current topics in primate vocal communication. Plenum, New York
Sekulic R (1982) Daily and seasonal patterns of roaring and spacing in four red howler Alouatta seniculus troops. Folia Primatol 39:22–48
Sekulic R, Chivers DJ (1986) The significance of call duration in howler monkeys. Int J Primatol 7:183–190
Seyfarth RM, Cheney DL (2003) Signalers and receivers in animal communication. Annu Rev Psychol 54:145–173
Seyfarth RM, Cheney DL (2011) Meaning, reference, and intentionality in the natural vocalizations of monkeys. In: Maran T, Martinelli D, Turovski A (eds) Readings in zoosemiotics. Walter de Gruyter GmbH, Berlin
Seyfarth RM, Cheney DL, Marler P (1980) Vervet monkey alarm calls: semantic communication in a free-ranging primate. Anim Behav 28:1070–1094
Sorjonen J (1986) Factors affecting the structure of song and singing behaviour of some Northern European passerine birds. Behaviour 98:286–304
Steinmetz S (2005) Vocalizações de longo alcance como comunicação intra-grupal nos bugios (Alouatta guariba). Neotrop Primates 13:11–15
Teichroeb JA, Sicotte P (2010) The function of male agonistic displays in ursine colobus monkeys (Colobus vellerosus): male competition, female mate choice or sexual coercion? Ethology 116:366–380
Thinh VN, Hallam C, Roos C, Hammerschmidt K (2011) Concordance between vocal and genetic diversity in gibbons. BMC Evol Biol 11:36
Thorington RW, Ruiz JC, Eisenberg JF (1984) A study of black howler monkeys (Alouatta caraya) populations in northern Argentina. Am J Primatol 6:357–366
Villalobos F, Valerio AA, Retana AP (2004) A phylogeny of howler monkeys (Cebidae: Alouatta) based on mitochondrial, chromosomal and morphological data. Rev Biol Trop 52:665–677
Waser PM, Brown CH (1986) Habitat acoustics and primate communication. Am J Primatol 10:135–154
Waser PM, Waser MS (1977) Experimental studies of primates vocalization: specializations for long-distance propagation. Z Tierpsychol 43:239–263
Whitehead JM (1987) Vocally mediated reciprocity between neighbouring groups of mantled howling monkeys, Alouatta palliata palliata. Anim Behav 35:1615–1627
Whitehead JM (1989) The effect of the location of a simulated intruder on responses to long-distance vocalizations of mantled howling monkeys, Alouatta palliata palliata. Behaviour 108:73–103
Whitehead JM (1995) Vox Alouattinae: a preliminary survey of the acoustic characteristics of long distance calls of howling monkeys. Int J Primatol 16:121–144
Wich SA, Schel AM, de Vries H (2008) Geographic variation in Thomas langur (Presbytis thomasi) loud calls. Am J Primatol 10:566–574
Wiley RH, Richards DG (1978) Physical constraints on acoustic communication in the atmosphere: implications for the evolution of animal vocalisations. Behav Ecol Sociobiol 3:69–94
Wiley RH, Richards DG (1982) Adaptations for acoustic communication in birds: sound transmission and signal detection. In: Kroodsma DE, Miller EH, Oullet H (eds) Acoustic communication in birds, vol 2. Academic, New York
Zuberbuhler K (2000) Referential labelling in Diana monkeys. Anim Behaviour 59:917–927
Zuberbuhler K (2001) Predator-specific alarm calls in Campbell’s guenons. Behav Ecol Sociobiol 50:414–422
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
de Cunha, R.G.T., de Oliveira, D.A.G., Holzmann, I., Kitchen, D.M. (2015). Production of Loud and Quiet Calls in Howler Monkeys. In: Kowalewski, M., Garber, P., Cortés-Ortiz, L., Urbani, B., Youlatos, D. (eds) Howler Monkeys. Developments in Primatology: Progress and Prospects. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1957-4_13
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1957-4_13
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1956-7
Online ISBN: 978-1-4939-1957-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)