Abstract
In recent years, prostate cancer-specific death rates have fallen significantly in industrialized nations, contributed in part by the widespread application of curative intent treatment and early disease detection afforded by PSA screening [1]. Unfortunately, this progress has been accompanied by the overtreatment of many patients likely to have never died of their prostate cancer. Distinguishing between clinically significant and indolent cancers is a major focus of current inquiry. Contemporary clinical screening regimens utilizing serum PSA and systematic transrectal ultrasound-guided biopsy suffer from poor sensitivity and specificity [2, 3] and ultimately lead to both overdetection of low-risk indolent disease as well as missed cancers of more clinical significance. This clinical uncertainty is reflected in the high upgrading rate of approximately 30 % between clinical diagnosis and radical prostatectomy [4]. As a result, neither clinician nor patient can rely on clinical staging information with certainty in order to predict behavior. As a result, many men turn to more invasive and radical treatments even in the setting of predicted low-risk cancer, which while effective in controlling oncologic risk are associated with significant morbidity and cost.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Radical Prostatectomy
- Magnetic Resonance Spectroscopic Image
- Endorectal Coil
- Central Gland
- Multiparametric Magnetic Resonance Image
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
In recent years, prostate cancer-specific death rates have fallen significantly in industrialized nations, contributed in part by the widespread application of curative intent treatment and early disease detection afforded by PSA screening [1]. Unfortunately, this progress has been accompanied by the overtreatment of many patients likely to have never died of their prostate cancer. Distinguishing between clinically significant and indolent cancers is a major focus of current inquiry. Contemporary clinical screening regimens utilizing serum PSA and systematic transrectal ultrasound-guided biopsy suffer from poor sensitivity and specificity [2, 3] and ultimately lead to both overdetection of low-risk indolent disease as well as missed cancers of more clinical significance. This clinical uncertainty is reflected in the high upgrading rate of approximately 30 % between clinical diagnosis and radical prostatectomy [4]. As a result, neither clinician nor patient can rely on clinical staging information with certainty in order to predict behavior. As a result, many men turn to more invasive and radical treatments even in the setting of predicted low-risk cancer, which while effective in controlling oncologic risk are associated with significant morbidity and cost.
Much investigative work has been performed in the hopes to improve and possibly remove this uncertainty. Recent advances in prostate cancer imaging, specifically those protocols which utilize 3 T Magnetic Resonance Imaging (MRI) coupled with an endorectal coil, have significantly improved the signal to noise ratio of image acquisition. As a result, a revolutionary improvement in temporal and spatial resolution has been achieved [5]. This imaging clarity has finally offered adequate insight into the three-dimensional anatomy of the gland to allow identification and characterization of individual prostate tumor lesions within the gland.
Moreover, by combining conventional anatomic MR imaging with advanced functional MR sequences (known as multiparametric imaging, including diffusion-weighted imaging, dynamic contrast-enhanced imaging, and spectroscopy), additional biophysical information can be gathered which allows true radiologic discernment between individual prostate cancer lesions and adjacent benign areas of the prostate [6, 7]. Initial reports at centers utilizing MRI in guiding diagnostic biopsy have shown it to be superior to established techniques of random sampling in the clinical diagnosis of disease [8, 9]. In this chapter, we aim to provide an overview of mpMRI of the prostate.
Multiparametric Magnetic Resonance Imaging
Multiparametric Magnetic Resonance Imaging (MP-MRI) is a noninvasive imaging technique with superior diagnostic characteristics in comparison to other imaging modalities such as ultrasound and computed tomography. Recent technologic advancements including high field strength magnets (3 T and greater) and new magnetic coil designs (including endorectal coil and multichannel surface coils) as well as advancements in software and computational algorithms have allowed the addition of more complex functional imaging to clinical imaging. Here we describe the four component parameters of a contemporary mp-MRI study.
T2-Weighted MRI (Anatomic Imaging)
T2-weighted anatomic imaging is the most commonly used and widely available imaging sequence. This modality provides excellent delineation of prostate zonal anatomy, gland borders, and visualization of surrounding tissues (Fig 3.1) [6]. Normal prostatic tissue exhibits relative high T2 signal intensity in the prostate peripheral zone (the origin of most adenocarcinoma lesions), and lower signal intensity in the central gland. Classically, prostate cancer lesions are noted to exhibit low signal intensity, a characteristic most easily visualized in the peripheral zone due to its normally higher signal intensity. Thus, rare transitional zone and central gland lesions are much more difficult to identify in this sequence. In addition, many benign conditions can mimic this appearance of low signal intensity (such as inflammation). As a result, when relying on only this parameter approximately half of lesions are missed [10]. Also, not surprisingly, lesion size has significant impact on detection, with larger tumors (1 cm) nearly always visualized and smaller tumors (<5 mm) much more likely missed [11].
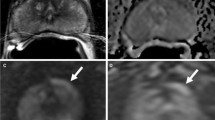
Axial MRI images of the prostate: (a) Axial T2-weighted image of the prostate shows a large decreased signal intensity lesion in the right anterior mid-transitional zone (arrow). (b) DWI ADC map of the prostate shows restricted diffusion within this lesion (arrow). (c) DCE-MRI subtracted contrast image clearly outlines the lesion (arrow). (d) DCE-MRI color map overlay indicates that the lesion is hypervascular with rapid contrast wash-in and wash-out (arrow)
Owing to the detail of T2 imaging, it is the most helpful sequence for assessing local invasion into surrounding tissues. Detection of this local invasion has clinical relevance as it decreases the likelihood of cure from local therapy. Such invasion can be seen most overtly as direct invasion into the periprostatic fat. In addition, other findings suggestive of local invasion are irregularity of the gland margin, capsular bulge, and a low signal area within the seminal vesicles (which normally exhibit very high signal intensity). The results from such local staging predictions are not perfect, however, and absence of findings may occur in the setting of true disease with reports ranging with diagnostic sensitivity of 50–60 % [12, 13].
Diffusion-Weighted MRI
Diffusion-Weighted MRI (DW-MRI) sequences can detect and quantify the Brownian motion of water within tissue in vivo [14]. As this relates to cellular density, cell permeability, and free water diffusion within the interstitial spaces, DW-MRI can assess tissue structural architecture and differentiate benign tissue from malignant tissue. Benign tissue exhibits high signal intensity as it normally allows free water to diffuse with relative ease. In the malignant setting, relative higher nuclear:cytoplasmic ratio and loss of extracellular spaces due to cellular proliferation results in decreased free water diffusion and thus relative decreased signal intensity on DW-MRI [15]. Furthermore, DW-MRI findings have been significantly correlated to underlying histopathologic grade and clinical risk scores [16], which allows some prediction of tumor histopathologic behavior based on radiologic findings. Downsides of DW-MRI include its relatively poor spatial resolution (especially in comparison to T2-weighted MRI) which limits the ability to evaluate staging using this sequence in isolation. In addition, DW-MRI is more challenging to interpret in the central gland as the presence of BPH-associated nodules in this area of the prostate can mimic the low signal intensity of malignant lesions [17]. Despite this, addition of DW-MRI to standard anatomic T2-weighted imaging has been demonstrated to improved diagnostic accuracy [10].
Dynamic Contrast-Enhanced Magnetic Resonance Imaging
Dynamic Contrast-Enhanced Magnetic Resonance Imaging (DCE-MRI) allows assessment of tissue vascular supply. This is accomplished by acquiring T1-weighted images continuously before, throughout, and continuing after the injection of an MRI detectable contrast agent (i.e., gadolinium). Signal increase during this protocol results from blood supply to the tissue of interest. Differentiation between normal and malignant tissue is possible, as cancers have a typical imaging signature owing to their disordered angiogenesis. Malignant tissue is correlated with early uptake and early washout (temporal imaging) of vascular contrast [18]. These changes are most easily seen in larger lesions and in lesions which are of higher grade. In addition, similar to T2-weighted and DWI-MRI, lesions in the central gland are more challenging to differentiate as BPH nodules themselves can show early uptake, though they do not classically have the rapid washout typical of malignant lesions. DCE-MRI has been shown to have higher diagnostic power than T2W sequences alone, especially in lesions larger than 5 mm [19]. Similar to DWI, DCE-MRI sequences have relatively poor spatial resolution (in comparison to T2-weighted MRI) which limits the ability to evaluate staging using this sequence in isolation. However, it is felt to be most useful in assessment treatment effect in settings where the prostate gland remains in situ.
Magnetic Resonance Spectroscopic Imaging
Nuclear magnetic resonance spectroscopy is possible in situ using Magnetic Resonance Spectroscopic Imaging (MRSI) which allows relative quantification of metabolites within the tissue of interest. In this technique, the tissue of interest is divided into discrete areas (volumes of interest) known as voxels. For each voxel, a spectra of EM radiation is acquired which represents a fingerprint of the composition of the volume. This data can be used to differentiate benign from malignant tissue. Benign prostate typically harbors high levels of citrate, which can be detected as a specific peak on MRSI spectra. In the setting of cancer, the increased cellular turnover results in a relatively high concentration of choline, also detectable on the MRSI spectral curve. From this data, the relative concentration of choline:citrate can be calculated, with increased ratios signifying malignant changes. The addition of MRSI has been shown to improve diagnostic accuracy over T2-weighted imaging alone, with especially high specificity [20]. Some challenges to this technique are that inflammation can mimic citrate:choline signal changes, and that spatial resolution (similar to DWI and DCE-MRI) is not as good as T2-weighted MRI in the aid of local staging. In addition, some centers report that it is technically challenging and on some platforms it increases acquisition times limiting its widespread utilization.
MP-MRI: Combining Imaging Parameters for Improved Diagnostic Power
As each individual parameter is capturing orthogonal data, the combination of them has been demonstrated to have improved diagnostic power over each individual in isolation. Using careful histopathologic correlation of radical prostatectomy specimens, it has been demonstrated that a lesion identified has a positive predictive value of 98 %, with excellent sensitivity especially in larger lesions of clinical significance (>5 mm) [20].
Harnessing the Diagnostic Power of MRI: MRI Targeted Biopsy
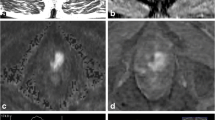
A number of strategies have been employed to take advantage of this additional diagnostic information from MRI. While in gantry biopsy has been performed to directly sample areas of suspicion, the added imaging time and need for specialized non-ferrous equipment makes it difficult to implement widely and in a cost-effective manner. Most contemporary strategies target areas of suspicion in an outpatient setting following a priori evaluation of MRI imaging by an experienced radiologist. The most popular methods employ software-based co-registration systems, known as fusion MRI-US biopsy (Fig 3.2). These systems utilize mechanically encoded biopsy arms or electromagnetic tracking to guide the needle to aforementioned areas of suspicion using software calculations which correlate MRI findings with real-time US data. Preliminary reports demonstrate excellent diagnostic power utilizing these strategies with improved sensitivity, specificity, and decreased upgrading rate [21]. In addition, manual targeting has been performed (so called “cognitive” biopsy), and in experienced hands, has been able to approximate the improved diagnostic power of computer-aided fusion-based systems [22].
While data is still preliminary, early results of the performance of MR imaging have been promising. Such strategies have proved useful in challenging situations such as persistent clinical suspicion in the setting of prior negative biopsy [9], as well as more accurately characterizing appropriate candidates considering active surveillance [23].
Conclusion
MRI of the prostate has offered additional diagnostic certainty in the setting of prostate cancer diagnosis over established standard of care methods. Contemporary experience is still very preliminary; however, it is likely that MRI will be utilized in all stages of prostate cancer diagnostics including staging, guiding of therapy, and follow-up after treatment.
References
Jemal A, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Ouzaid I, et al. A direct comparison of the diagnostic accuracy of three prostate cancer nomograms designed to predict the likelihood of a positive initial transrectal biopsy. Prostate. 2012;72:1200–6.
Haas GP, et al. Needle biopsies on autopsy prostates: sensitivity of cancer detection based on true prevalence. J Natl Cancer Inst. 2007;99(19):1484–9.
Tilki D, et al. Clinical and pathologic predictors of Gleason sum upgrading in patients after radical prostatectomy: results from a single institution series. Urol Oncol. 2011;29(5):508–14.
Lagemaat MW, Scheenen TW. Role of high‐field MR in studies of localized prostate cancer. NMR Biomed. 2014;27(1):67–79.
Turkbey B, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection-histopathologic correlation. Radiology. 2010;255(1):89–99.
Panebianco V, et al. Conventional imaging and multiparametric magnetic resonance (MRI, MRS, DWI, MRP) in the diagnosis of prostate cancer. Q J Nucl Med Mol Imaging. 2012;56(4):331–42.
Pinto PA, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186(4):1281–5.
Vourganti S, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol. 2012;188(6): 2152–7.
Haider MA, et al. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am J Roentgenol. 2007;189(2):323–8.
Nakashima J, et al. Endorectal MRI for prediction of tumor site, tumor size, and local extension of prostate cancer. Urology. 2004;64(1):101–5.
Park BK, et al. Comparison of phased-array 3.0-T and endorectal 1.5-T magnetic resonance imaging in the evaluation of local staging accuracy for prostate cancer. J Comput Assist Tomogr. 2007;31:534–8.
Futterer JJ, et al. Staging prostate cancer with dynamic contrast-enhanced endorectal MR imaging prior to radical prostatectomy: experienced versus less experienced readers. Radiology. 2005;237:541–9.
Issa B, et al. In vivo measurement of the apparent diffusion coefficient in normal and malignant prostatic tissues using echo-planar imaging. J Magn Reson Imaging. 2002;16(2):196.
Lim KS, et al. Diffusion weighted MRI of adult male pelvic cancers. Clin Radiol. 2012;67:899.
Turkbey B, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011;258(2):488–95.
Oto A, et al. Prostate cancer: differentiation of central gland cancer from benign prostatic hyperplasia by using diffusion weighted and dynamic contrast enhanced MR imaging. Radiology. 2010; 257:715.
Verma S, et al. Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR Am J Roentgenol. 2012;198(6): 1277–88.
Puech P, et al. Dynamic contrast-enhanced-magnetic resonance imaging evaluation of intraprostatic prostate cancer: correlation with radical prostatectomy specimens. Urology. 2009;74(5):1094–9.
Turkbey B, et al. Multiparametric 3 T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186(5):1818–24.
Le JD et al. MRI-ultrasound fusion biopsy for prediction of final prostate pathology. J Urol. 2014;pii: S0022-5347(14)03509-5
Wysock JS et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: The PROFUS Trial. Eur Urol. 2013;pii:S0302-2838(13)01186-X
Stamatakis L, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer. 2013;119(18):3359–66.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Vourganti, S., Wojtowycz, A.R. (2015). Magnetic Resonance Imaging in Prostate Cancer Diagnosis. In: Khanna, R., Bratslavsky, G., Stein, R. (eds) Surgical Techniques for Prostate Cancer. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1616-0_3
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1616-0_3
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1615-3
Online ISBN: 978-1-4939-1616-0
eBook Packages: MedicineMedicine (R0)