Abstract
Prostate cancer is the second leading cause of cancer death in men in the United States. With the introduction of PSA in the early 1990s, prostate cancer is detected more often at earlier stages with subsequent increase in overdiagnosis and overtreatment as many of these men may have indolent cancer. Nevertheless, there is no current clinical test that will accurately differentiate men harboring indolent cancer, affecting quality of life or shortened life expectancy due to cancer progression. Despite improvements in technology and technique with established treatment options, such as radical prostatectomy and radiation therapy, incontinence and erectile dysfunction still impacts the quality of life of some men. Focal ablation therapy may be suitable for men with low and intermediate risk prostate cancer who are not candidates for active surveillance or who wish to undergo treatment. This approach selectively treats the tumor or tumors within the prostate while sparing normal tissue. Different energy sources have been used for that purpose; Cryoablation, High-intensity focused ultrasound (HIFU), Photodynamic therapy, and Laser ablation. While cryosurgery is commercially available in the United States, the others are still being investigational, and most are still considered experimental.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Prostate cancer is the second leading cause of cancer death in men in the United States. Since the introduction of PSA, there has been a progressive downward stage migration, with more new cases presenting as clinically localized, low volume, low grade disease. Ninety percent of all prostate cancers are found when the disease is confined to the prostate [1, 2].
Radical prostatectomy (RP) and radiation therapy (RT) aim to treat the whole prostate and seminal vesicles. Major side effects include urinary incontinence and erectile dysfunction in 5–20 % and 30–70 %, respectively [3]. While these sequelae have decreased with improvements in technique and technology, these morbidities have a significant impact on quality of life. PSA has contributed to the reduced prostate cancer mortality observed in the past decade, but at the same time increased the detection of potentially clinical insignificant cancers leading to overdiagnosis and overtreatment of some men [4]. The estimated overtreatment rate for prostate cancer is at least 30 % [5]. Men, who may have been overtreated for their early, potentially clinical insignificant disease, are at risk of lifelong morbidities derived from treatment.
In recent years focal therapy (FT) has emerged as a new alternative treatment for early prostate cancer. FT consists of completely ablating clinically significant cancer foci within the prostate while preserving normal tissue, the urinary sphincter, and the neurovascular bundles with the goal of minimizing side effects [4].
The selection of appropriate candidates for FT presents a challenge for the clinician. Prostate mapping biopsies and newer imaging technologies have been utilized to help select individuals with localized disease who may benefit from focal treatment.
Cryotherapy, high-intensity focus ultrasound (HIFU), Laser ablation, and photodynamic therapy (PDT) are current ablative modalities under investigation.
Diagnosis and Patient Selection for Focal Therapy
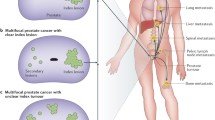
While the concept of focal therapy is simple, its application poses several challenges including optimal patient selection; localization, visualization, and characterization of significant cancer foci; accurate guidance of ablative energy in the area to be treated; follow-up and surveillance of untreated areas. A concern with FT is the multi-focality of prostate cancer as two thirds of patients with newly diagnosed prostate cancer present with more than one focus of cancer within the prostate. However, approximately 33 % will have unifocal tumor [6]. Additionally, approximately 40–80 % of multifocal tumors measure less than 0.5 mL in volume, which some investigators consider as clinically insignificant cancers [7–9]. This led to the concept of only treating the significant cancer foci (index lesion). Some reports conclude that the index lesion represents the main tumor volume, the highest Gleason score, and the potential site of extracapsular disease [2, 9].
At present, there is no agreement on which criteria should be applied for selecting optimal candidates for FT. Two multidisciplinary expert panels have reported on their selection criteria. Eggener et al. published the consensus of the International Task Force on Prostate Cancer and the Focal Lesion Paradigm (ITF-FLP) in 2007. They used clinical findings, biopsy, and imaging studies to define the criteria for patient selection. Clinical criteria included stage T1 or T2a, PSA less than 10 ng/mL, PSA density less than 0.15 ng/mL/cm3, and PSA velocity less than 2 ng/mL yearly in the years prior to diagnosis. Biopsy criteria required obtaining a minimum of 12 cores and findings of a Gleason score 3 + 3 or less, less than 20 % of cancer in each core, and less than 33 % of total cores with cancer. Imaging criteria included single lesion with a maximum of 12 mm size, <10 mm of capsular contact, and no evidence of extraprostatic extension or seminal vesicle invasion [10]. In 2010, de la Rosette et al. published the consensus from an international expert meeting, the 2nd International Workshop on Focal Therapy, and Imaging in Prostate and Kidney Cancer (IWFTI). They concluded that patients appropriate for FT should have unilateral low to intermediate risk disease, clinical stage T2a or less, PSA <20 ng/mL, Gleason score 4 + 3 or less, and life expectancy of ten or more years. They recommended evaluation with transperineal mapping biopsies and excluded patients with anterior or apical tumors [11]. Other authors have reported that FT is suitable only for patients with low-risk disease (clinical stage T1c-T2a, Gleason grade 3 + 3, and PSA < 10 ng/mL). Lindner et al. estimated that 45–85 % of patients fall into this category [12].
MRI technology is emerging as the most important imaging tool for identifying low-volume prostate cancers, assisting in risk stratification, and allowing for targeted biopsies [13, 14]. The sensitivity and specificity for identification of a significant cancer focus (>0.5 cm3) was 86 % and 94 %, respectively [15]. MRI imaging remains the most important available imaging tool for identifying early prostate cancers and enabling focused use of energy ablative modalities.
Transperineal mapping biopsy (TPMB) has been proposed as a more accurate way to determine tumor focality and is being advocated as the preferred approach to select appropriate men for FT. Onik et al. compared the traditional biopsy technique to TPMB and found a large discrepancy [6, 16]. Barqawi et al. prospectively studied 3D mapping biopsy. They reported that a significant portion of men initially diagnosed with apparently low-risk disease harbored clinically significant cancer. These results demonstrate how 3D mapping biopsy may be applied to improve patient selection for FT [17].
Follow-Up After FT
After FT, verification of complete ablation of known cancer foci and detection of any de novo cancer in the untreated prostate gland should be assessed. Defining recurrence is another challenge evaluating the efficacy of focal therapy. As FT preserves prostatic tissue, PSA is not expected to become undetectable. Accepted criteria for biochemical recurrence after radiation therapy such as the ASTRO (three consecutive PSA rises after a nadir PSA) and Phoenix (nadir PSA + 2) criteria are not applicable to FT since they were not designed for use in this setting [5]. Despite no defined PSA cut point to evaluate treatment success, it is recommended that PSA should be continuously monitored during follow-up and rising PSA should be further investigated. Some investigators suggest defining biochemical failure as a PSA nadir + 50 % rise on follow-up [18].
Most investigators include post-treatment biopsy and imaging studies as part of their follow-up of patients treated with FT.
Focal Cryotherapy
Overview
Cryotherapy was initially reported in the 1960s by Cooper and Lee. They developed the first cryotherapy probe system using liquid nitrogen. The inclusion of urethral warmers, use of transrectal ultrasound (TRUS) for real time visualization of the ice ball, replacement of liquid nitrogen by argon gas for cooling and helium for warming, as well as thinner cryoneedles, allow surgeons for more accurate targeting enhancing its effectiveness while reducing potential side effects.
Initially cryotherapy was used to destroy the whole gland. More recently it has been investigated as a tool for FT. Focal cryotherapy is a modification of the standard technique, aiming to only treat the portion of the gland which has the clinically significant disease.
Mechanism of Action
The use of freezing temperatures and thawing cycles results in cell destruction by direct injury to the cells as well as secondary injury from the inflammatory response. The current technology uses argon gas flowing through hollow needles to freeze the prostate and helium gas to actively warm after freezing via the Joule-Thompson effect. There are three treatment parameters that correlate with cancer cell destruction: cooling rate, low temperature achieved, and duration of the freeze cycle.
After reaching a tissue temperature of less than 0 °C, the extracellular fluid starts to crystallize. Formation of crystals causes hyperosmotic pressure of the unfrozen portion of the extracellular fluid compartment leading to water shifting from the intracellular space to the extracellular space. The water loss induces intracellular dehydration and pH change; this is followed by cell shrinkage and denaturing of cellular proteins. With further drops in temperature, beyond −15 °C, intracellular crystallization takes place and cell metabolism begins to fail. This leads to mechanical breaks of the cellular membrane and cell apoptosis is induced after the thermal injury. Complete cell death is likely to occur at temperatures lower than −40 °C after two cycles.
Vasodilatation around the targeted tissue occurs after thawing causing hyperpermeability of vessel walls. This leads to endothelial damage and microthrombi formation resulting in regional tissue hypoxia and secondary necrosis of the tissue [19].
Procedure
After induction of adequate general anesthesia, the patient is placed in the lithotomy position. A TRUS probe is inserted per rectum and affixed to a fixation device. A template grid is placed in front of the perineum secured to the fixation device. Two to four cryoprobes are introduced through the perineum under imaging guidance. Catheter warmer is placed per urethra and placed on continuous warmer irrigation. Double freeze-thaw cycles are delivered with the goal of bringing the temperature below −40 °C. Argon and helium gases are used for freezing and thawing, respectively. After the two cycles are completed, the needles are removed and the urethral warmer keep running for 20 additional minutes. The urethra warmer is then removed and a Foley catheter inserts and left indwelling for 5–7 days. Visualization of the ice ball in real time using ultrasonography allows treating the focal cancer zone, minimizing injury to adjacent structures (Figs. 10.1, 10.2, 10.3, and 10.4).
Current Studies: Oncologic and Functional Outcomes
Cryotherapy is the most studied ablative therapy. Onik et al. were first to report outcomes of FT in 2002 followed by an update of their experience in 2008. They reported on 48 patients with a mean follow-up of 54 months (range 2–10 years). Ninety-four percent had no evidence of cancer according to ASTRO (American Society of Therapeutic Radiology and Oncology) criteria. Potency was maintained in 36 of 40 patients (90 %) and all were continent after treatment [20].
Ellis et al. reported on 60 patients treated with focal cryotherapy. The mean follow-up was 15 months. 84 % of the patients were biochemically disease-free (ASTRO criteria) and 3.6 % reported urinary incontinence [21].
In 2007, Lambert et al. reported on 25 men treated with hemiablation of the gland. Mean follow-up was 28 months. Eighty-four percent had experienced no biochemical failure, defined as 50 % PSA increase over nadir level. Seven patients underwent a repeat biopsy. One patient had prostate cancer in the area of previous cryoablation and 2 patients in the contralateral gland [18].
Bahn et al. reported on 31 patients. Biochemical disease-free rate by ASTRO criteria was 92.8 %. Biopsy in one patient with biochemical recurrence demonstrated cancer at the apex of the untreated side. Potency preservation rate was 88.9 % (40.7 % with PDE-5 inhibitors) [22].
More recently Ward et al. published an update from the Cryo On Line Database (COLD) registry; biochemical disease-free rate was 75.7 % (ASTRO criteria) at 36 months, urinary continence was 98.4 %, and preservation of spontaneous erections 58.1 % [23].
Overall, biochemical disease-free rate is 75–94 % [18, 20–23]. Nevertheless definitions of biochemical recurrence and patient’s selection criteria were variable between studies. These studies are limited due to small number of patients and short follow up. The reported functional outcomes are encouraging, with a good potency and urinary continence rates. No other significant morbidities were reported (Table 10.1).
Future Direction
Focal Cryotherapy is a promising treatment option for selected patients with early prostate cancer. Future research should be directed towards establishing better means of characterizing clinically significant disease and developing improved image technologies to target treatment.
High-Intensity Focused Ultrasound
Overview
HIFU was first described in 1995 as a technique to treat localized prostate cancer. Most of the current reports describe whole prostate gland treatments. HIFU can also be used for focal tumor ablation with the goal of sparing normal gland and minimizing the adverse effects of whole gland treatment. There are currently two devices available for treatment: The Ablatherm HIFU device (EDAP S.A., Lyon, France) and The Sonable 500 (Focus surgery, IN, USA). Both devices are widely used in Europe, Canada, and Japan. HIFU is still considered investigational in the United States and is not currently approved by the Federal Drugs Administration (FDA). Several trials are currently in progress to establish the efficacy and safety of HIFU.
Mechanism of Action
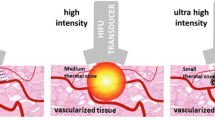
During HIFU ultrasound waves are emitted from a transducer and absorbed in the target area inducing necrosis. Two main mechanisms are involved in the HIFU ablation effect: A thermal effect is heat generation due to absorption of the acoustic energy with a rapid elevation of temperature in the targeted tissues, which denatures proteins, destroys lipid-based membranes, and finally results in instantaneous and irreversible coagulative necrosis. This is the primary mechanism for tumor cell destruction. The mechanical effect leads to cavitation causing additional damage to the prostate and periprostatic tissue. The treatment area is heated for 3 s and cooled for 6 s using real-time images. Surrounding tissue is minimally affected as the energy decreases sharply outside the target zone [24–26].
Procedure
Ablatherm ® after induction of general anesthesia, the patient lies on his left side, thighs, and legs flexed 90° on the trunk. A transrectal HIFU probe is inserted per rectum. The probe delivers a beam of high-focused convergent ultrasounds, causing heat and tissue destruction. The ultrasound beam absorption creates an immediate increase in temperature (85–100 °C). The treatment is performed using contiguous HIFU shots 1.8 mm apart with 4-s shot duration and a 12-s interval between shots. At the end of the procedure an 18 F Foley catheter is placed for 1–2 weeks.
Sonablate ® 500 after induction of general anesthesia, the patient is placed in the lithotomy position and HIFU probe is introduced per rectum. Treatment is monitored with real-time TRUS. After the procedure, an 18 F Foley catheter is placed and left for 1–2 weeks.
Current Studies, Oncologic, and Functional Outcomes
In 2008, Muto et al. reported on 29 patients who underwent transrectal HIFU (Sonablate 500). Two years biochemical disease-free rates by ASTRO criteria in patients with low and intermediate risk prostate cancer were 83.3 % and 53.6 % respectively [25].
Ahmed et al. reported a prospective study phase I/II trial in 20 men with prostate cancer who underwent a transrectal hemiablation of the prostate with the Sonablate 500 device. Patients were divided into low (n = 5) and intermediate risk (n = 15). Follow-up included MRI; TRUS-guided biopsies and PSA measurement at 1 month after the procedure and every 3 months thereafter. There was no histological evidence of cancer in 89 % of treated lobe. A trifecta status (pad-free, leak-free continence, erections sufficient for intercourse and cancer control) was achieved in 89 % at 12 months [26]. Ahmed et al. reported their results on 41 men treated between 2007 and 2010; using the Sonable 500 device and who were diagnosed by a combination of multiparametric MRI and Transperineal Template Mapping Biopsies (TTMP). Follow-up was scheduled every 3 months after treatment. Questioners were used to assess potency and incontinence. Eighty-nine percent (31 of 35 patients) described erections sufficient for penetration at 12 months. Fourteen required phosphodiestrerase-5 inhibitors. Of 38 men with no urinary leak at baseline 100 % were leak-free by 9 months. Thirty-nine of 41 patients underwent postoperative biopsy. Nine (23 %) had evidence of cancer. MRI at 6 months showed residual cancer in the treated areas in nine men; seven of whom had cancer confirmed on biopsy. Of those with positive biopsies, four patients underwent retreatment and none showed significant disease at 12 months on MRI [27]. These studies demonstrate good morbidity outcomes and promising cancer control rates. Limitations to these studies included small number of patients and short-term follow-up. Some authors considered hemiablation as a focal therapy with no consensus on definition regardless of grade, volume, or location of the tumor. Hemiablation may represent overtreatment since low-volume and low-grade lesions may be treated with more focused therapy.
Future Direction
Additional studies and more conclusive findings are needed. Trials are currently ongoing (NCT01194648, NCT00988130, NCT00987675) to establish the safety and efficacy of HIFU.
Photodynamic Therapy
Overview
The first report describing PDT for prostate cancer with light-sensitive agent using a transurethral approach was published in 1990 [28]. PDT is an experimental ablative technology which employs photosensitizing properties selectively taken up by prostate cancer cells and produces free oxygen radicals upon exposure to light of a specific wavelength which results in the destruction of the tissue.
As photosensitizers accumulate in some organs including skin and eyes, patients require light protection until the photosensitizer is no longer present. PDT is theoretically more tissue-specific and could preserve neurovascular bundle better than other FT. Recent advances in PDT have led to improvements of the synthesis of new-generation photosensitizers with better stability, shorter half-lives, and faster metabolism. The rapid clearance of these new agents from the circulation could avoid prolonged photosensitivity. Vascular photodynamic therapy (VTP) utilizes more recent photosensitizers derived from chlorophyll, such as WST09 (Tookad), to induce vascular damage leading to thrombosis and necrosis of the target tissue [29, 30].
Mechanism of Action
A Photosensitizer is injected intravenously and is distributed throughout the body; during treatment, small energy-delivering probes are placed in the prostate through optical fibers that deliver low power laser light to activate the administered drug. VTP usually uses WST09 that absorbs light near to infrared wavelength with maximum light energy absorption at 763 nm. This long light absorption wavelength allows deeper light penetration into tissues. The photosensitizer enhances sensitivity of the tumor vasculature to light energy. Damage to the vascular endothelium is followed by platelet aggregation and vascular coagulation around the tip of the fiber with subsequent localized tissue necrosis.
Procedure
The photosensitizer is given intravenously and accumulates in prostate tissue. The drug is then activated 2–5 days later by light of a specific wavelength from laser. Drug dose and light doses are variable and most are still under investigation. Manipulation of drug and light can result in varied volumes of ablation. A transperineal approach, using a brachytherapy template, guides insertion of optical fibers that deliver low power laser light.
Currents Studies and Future Direction
Few studies have been published regarding PDT. Trial NCT01310894 is currently under way to evaluate this treatment modality. PDT research focuses on determining the optimal type and dose of photosensitizing agent as well as the optimal light exposure time for treatment.
Focal Laser Ablation
Overview
A new source of energy that applied for FT is Laser Ablation (FLA). Low-power laser delivers luminous energy guided by real-time imaging; FLA produces a coagulative necrosis zone within a controlled area, reducing the risk of damaging adjacent structures.
Mechanism of Action
FLA is based on a photothermal effect which results from the absorption of radiant energy by tissue-receptive chromophores, which induces heat energy in a very short time. Increased temperature may cause irreversible damages and tissue destruction. The thermal effect depends on the amount of heat energy delivered but also on the depth of light distribution. For this reason, deep tissue damage is dependent on the wavelength of the laser used, usually a range between 590 and 1,064 nm. The extension of thermal tissue damage depends on both temperature and duration. Irreversible protein denaturation will occur around 60 °C, while over 60 °C, coagulation is quasi-instantaneous. Macroscopic appearance of coagulation areas of FLA corresponds to well-demarcated foci of necrosis surrounded by a small rim of hemorrhage with no viable glandular tissue after vital staining, based on immunoreactivity with cytokeratin [31, 32].
Procedure
The patient is placed under general anesthesia and in dorsal lithotomy position. A 2-way urethral catheter is inserted at the beginning of the procedure. A modified brachytherapy template is used for transperineal placement of the laser fibers. Depending on the size of the planned treatment volume, 1 or 2 fibers will be used. Wavelengths in the range of 590–1,064 nm are the most adequate to induce photothermal effect. An optimal fiber location is monitored with real-time ultrasound or other imaging modalities.
Currents Studies and Future Direction
There is currently very limited data available for FLA. Lindner et al. reported a pilot study in 4 patients, addressing feasibility. They correlated MRI findings with histopathology after radical prostatectomy (RP). No viable cells were found in treated regions and MRI findings correlated well with pathology reports [33]. The same group also reported their findings on image-guided focal laser ablation in 12 patients. Six patients (50 %) had negative biopsies 3–6 months following treatment and 67 % were free of tumor in targeted area. No relevant morbidities were reported [34]. Larger trials are currently in progress (NCT00805883, NCT01377753) addressing feasibility. Laser technology is improving and may lead to better focal therapy.
Conclusion
Early detection of prostate cancer has led to overdiagnosis of clinically insignificant tumors. We currently lack reliable tools to select optimal candidates for definitive treatment. With improved diagnostic modalities and optimal focal therapies the rate of complications may be markedly diminished with excellent cancer control. Men diagnosed with low-risk disease will continue to seek treatment despite excellent outcomes with active surveillance in appropriately selected patients. Researchers continue to develop new approaches to treat low-grade prostate cancer while minimizing side effects. Focal Therapy is emerging as a new treatment modality that could provide a bridge between active surveillance and more aggressive treatments for patients with low-risk tumors, achieving cancer control while minimizing morbidity. Several energy sources are being tested for this indication. The available literature is limited regarding focal therapies. Most evidence is derived from case series and small phase I trials. Ablative modalities such as VTP and FLA have only demonstrated technical feasibility to date. To make this approach valid, further research to establish patient selection criteria, new and more accurate imaging parameters, and regular follow-up protocols are needed. It is expected that new energy source will be introduced in the near future for focal therapy.
References
American Cancer Society. Cancer facts and figures 2012. Atlanta, GA: American Cancer Society; 2012.
Borofsky MS, Ito T, Rosenkrantz AB, et al. Focal therapy for prostate cancer—where are we in 2011? Ther Adv Urol. 2011;3(4):183–92.
Wilt TJ, MacDonald R, Rutks I, et al. Systematic review: comparative effectiveness and harms of treatment for clinically localized prostate cancer. Ann Inter Med. 2008;148:435–48.
Crawford ED, Barqawi A. Targeted focal therapy: a minimally invasive ablation technique for early prostate cancer. Oncology. 2007;21(1):27–32.
Scattoni V, Zlotta A, Montironi R, et al. Extended and saturation prostatic biopsy in the diagnosis and characterization of prostate cancer: a critical analysis of the literature. Eur Urol. 2007;52:1309–22.
Onik G, Miessau M, Bostwick DG. Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. J Clin Oncol. 2009;27(26):4321–6.
Djavan B, Susani M, Bursa B, et al. Predictability and significance of multifocal prostate cancer in the radical prostatectomy specimen. Tech Urol. 1999;5(3): 139–42.
Karavitakis M, Winkler M, Abel P, et al. Histological characteristics of the index lesion in whole-mount radical prostatectomy specimens: implications for focal therapy. Prostate Cancer Prostatic Dis. 2011; 14(1):46–52.
Noguchi M, Stamey TA, Mcneal JE, et al. Prognostic factors for multifocal prostate cancer in radical prostatectomy specimens: lack of significance of secondary cancers. J Urol. 2003;170:459–63.
Eggener SE, Scardino PT, Carroll PR, et al. Focal therapy for localized prostate cancer: a critical appraisal of rationale and modalities. J Urol. 2007; 178(6):2260–7.
de la Rosette J, Ahmed H, Barentsz J, et al. Focal therapy in prostate cancer-report from a consensus panel. J Endourol. 2010;24(5):775–80.
Lindner U, Trachtenberg J. Focal therapy for localized prostate cancer-choosing the middle ground. Can Urol Assoc J. 2009;3(4):333–5.
Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterization of prostate cancer: recommendations from a European consensus meeting. Eur Urol. 2011;59(4):477–94.
Ouzzane A, Puech P, Lemaitre L, et al. Combined multiparametric MRI and targeted biopsies improve anterior prostate cancer detection, staging, and grading. Urology. 2011;78(6):1356–62.
Puech P, Potiron E, Lemaitre L, et al. Dynamic contrastenhanced-magnetic resonance imaging evaluation of intraprostatic prostate cancer: correlation with radical prostatectomy specimens. Urology. 2009;74(5):1094–9.
Onik G, Barzell W. Transperineal 3D mapping biopsy of the prostate: an essential tool in selecting patients for focal prostate cancer therapy. Urol Oncol. 2008;26(5):506–10.
Barqawi AB, Rove KO, Gholizadeh S, et al. The role of 3-dimensional mapping biopsy in decision making for treatment of apparent early stage prostate cancer. J Urol. 2011;186(1):80–5.
Lambert EH, Bolte K, Masson P, et al. Focal cryosurgery: encouraging health outcomes for unifocal prostate cancer. Urology. 2007;69(6):1117–20.
Babaian RJ, Donnelly B, Bahn D, et al. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol. 2008;180:1993–2004.
Onik G, Vaughan D, Lotenfoe R, et al. The “male lumpectomy”: focal therapy for prostate cancer using cryoablation results in 48 patients with at least 2-year follow-up. Urol Oncol. 2008;26(5):500–5.
Ellis DS, Manny Jr TB, Rewcastle JC. Focal cryosurgery followed by penile rehabilitation as primary treatment for localized prostate cancer: initial results. Urology. 2007;70:9–15.
Bahn DK, Silverman P, Lee F, et al. Focal prostate cryoablation: initial results show cancer control and potency preservation. J Endourol. 2006;20(9): 688–92.
Ward JF, Jones JS. Focal cryotherapy for localized prostate cancer: a report from the national Cryo On-Line Database (COLD) Registry. BJU Int. 2012;109(11):1648–54.
Maestroni U, Ziveri M, Azzolini N, et al. High intensity focused ultrasound (HIFU): a useful alternative choice in prostate cancer treatment. Preliminary results. Acta Biomed. 2008;79(3):211–6.
Muto S, Yoshii T, Saito K, et al. Focal therapy with high-intensity-focused ultrasound in the treatment of localized prostate cancer. Japan J Clin Oncol. 2008;38(3):192–9.
Ahmed HU, Freeman A, Kirkham A, et al. Focal therapy for localized prostate cancer: a phase I/II trial. J Urol. 2011;185(4):1246–54.
Ahmed HU, Hindley RG, Dickinson L, et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol. 2012;13(6):622–32.
Windahl T, Andersson SO, Lofgren L. Photodynamic therapy of localized prostatic cancer. Lancet. 1990;336:1139.
Arumainayagam N, Moore CM, Ahmed HU, et al. Photodynamic therapy for focal ablation of the prostate. World J Urol. 2010;28(5):571–6.
Moore C, Pendse D, Emberton M. Photodynamic therapy for prostate cancer a review of current status and future promise. Nat Clin Pract Urol. 2009;6(1): 18–30.
Lindner U, Lawrentschuk N, Trachtenberg J. Focal laser ablation for localized prostate cancer. J Endourol. 2010;24(5):791–7.
Colin P, Mordon S, Nevoux P, et al. Focal laser ablation of prostate cancer: definition, needs, and future. Adv Urol. 2012;2012:589160.
Lindner U, Lawrentschuk N, Weersink R, et al. Focal laser ablation for prostate cancer followed by radical prostatectomy: validation of focal therapy and imaging accuracy. Eur Urol. 2010;57(6):1111–4.
Lindner U, Weersink RA, Haider MA, et al. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. J Urol. 2009;182(4): 1371–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Pow-Sang, J.M., Sverrisson, E.F., Valderrama, O.M. (2015). Focal Ablation for Prostate Cancer. In: Khanna, R., Bratslavsky, G., Stein, R. (eds) Surgical Techniques for Prostate Cancer. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1616-0_10
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1616-0_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1615-3
Online ISBN: 978-1-4939-1616-0
eBook Packages: MedicineMedicine (R0)