Abstract
Microglia are the first responders to central nervous system (CNS) trauma. They are thought to be a central player in initiating and orchestrating a cascade of changes that lead to an elaborate inflammatory response at the site of injury. This inflammatory response also includes the influx of peripheral immune cells into the injured CNS. In this chapter we will focus our attention on the microglial response to spinal cord injury, but where possible we will include discussion of microglial responses after traumatic brain injury. We will discuss the differences in the early and late responses of microglia to CNS injury; the signaling molecules, cytokines and other factors that modulate their responses, the evidence for their beneficial and detrimental effects, and the effects of their activation at the epicenter of the injury and in sites distal to the injury. Attention will also be focused on the evidence of microglial changes in chronic spinal cord and brain trauma. We will highlight the preclinical evidence for targeting some aspects of the microglial response to treat spinal cord and brain trauma and take a look at some future directions to pursue.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Microglia
- Spinal cord injury
- Traumatic brain injury
- Purinergic signaling
- Damage-associated molecular patterns
- Toll-like receptors
- Cytokines
- Free radicals
- Wallerian degeneration
- Regenerative sprouting
-
Microglia respond within minutes by sending processes towards the injury.
-
The early microglial response to micro-lesions is protective and mediated by ATP-purinergic receptor, damage-associated molecular patterns (DAMPs)-Toll-like receptors (TLRs) signaling, and other factors.
-
DAMP-TLR signaling in microglia leads to pro-inflammatory cytokine expression.
-
Microglia, like peripheral macrophages entering the injured spinal cord, become polarized into cytotoxic M1 and non-cytotoxic M2 states.
-
M1 polarization is predominant in the injured spinal cord.
-
Microglia respond very slowly in areas of CNS white matter undergoing Wallerian degeneration the reasons for which are not fully understood.
-
Microglia in grey matter regions far removed from the lesion site also respond.
-
Microglia play a role in synaptic pruning and reorganization of circuits after regenerative sprouting.
-
Chronic activation of microglia can contribute to pain and functional disability.
-
Chronic microglial activation generates free radicals and other toxic mediators that can impair neurological function even years after injury.
1 Introduction
Microglia, which are the resident tissue macrophages of the central nervous system (CNS), are derived from the yolk sac and populate the CNS during early embryonic development (Ginhoux et al. 2010). They are widely distributed throughout the CNS (Lawson et al. 1990; Vela et al. 1995). Their number and distribution pattern varies between different regions of the CNS and also within the same CNS region, e.g., in the cerebellum they are more numerous in the deep nuclei as compared to the molecular layer or granule cell layer (Lawson et al. 1990; Vela et al. 1995). In general they appear to be more abundant in the grey than the white matter (Lawson et al. 1990). Microglia, which are located in the CNS parenchyma, have a ramified morphology in the normal, uninjured CNS. In contrast, macrophages in the leptomeninges and choroid plexus, which share many antigenic markers with microglia, are rounded or ameboid in shape (David and Kroner 2011; Perry et al. 1985). The shape and extent of microglial ramification is also region-specific, with those in the cerebral cortex and hippocampus having extensively branched morphologies, while those in the white matter have long processes and slender-shaped cell bodies (Lawson et al. 1990). The overall shape of these cells appears to reflect the cytoarchitecture of the region in which they are found. Microglia in lower vertebrates such as zebrafish also have a similar branching shape (Sieger et al. 2012). In some grey matter regions of the mammalian CNS, microglia are so abundant that their processes almost touch each other to give the appearance of “tiling”. This relatively close distribution means that microglia in these regions would not need to move far in response to perturbations, unlike those in the developing zebrafish that migrate to regions of damage (Sieger et al. 2012).
The characteristic branching morphology of microglia gives the appearance that these cells are surveying and monitoring the CNS tissue. Two-photon imaging of microglia in real-time in the normal mouse cerebral cortex and spinal cord white matter revealed rapid extension and retraction of microglial processes from 2 to 8 μm within seconds to minutes (Davalos et al. 2005; Dibaj et al. 2010) without movement of the cell soma (Dibaj et al. 2010). These data indicate that the cytoplasmic processes of these cells are dynamic and play an active role in constantly monitoring their microenvironment. Microglia are quick to respond to any type of perturbation of the CNS, whether it be injury or disease (discussed below). Although they often respond to CNS alterations by retracting their processes, such morphological changes are not an essential feature of all microglial responses as phenotype switching in terms of cytokine expression can be induced without morphological changes as seen in brains of mice with prion disease after peripheral lipopolysaccharide challenge (Perry et al. 2007). Microglia in the normal uninjured brain can also be identified by the expression of various cell surface and intracellular antigens including CD11b, CD45low, CX3CR1high, Ly6Clow, Gr-1low (David and Kroner 2011). Against this backdrop, damage or injury to the CNS causes rapid changes in microglial morphology, and expression of cell surface or intracellular antigens and synthesis/release of cytokines. Like inflammation elsewhere, the inflammatory response initiated and mediated by microglia after CNS injury is largely meant to control infection and initiate wound healing. This response, which is inherently protective as it ensures survival against sepsis, can also exacerbate tissue damage and worsen recovery. In this chapter we will discuss some of the salient features of the microglial response to CNS injury and its implications for CNS repair, with greater focus on spinal cord injury (SCI) and the relationship of microglia to macrophages derived from blood monocytes during the onset of intraspinal inflammation.
2 Early Responses: Minutes to Hours After Injury
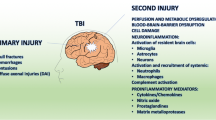
Morphological and phenotypic changes at the lesion epicenter: Extreme physical forces including blunt trauma, penetrating bone or projectiles (e.g., knife, bullet), or rapid acceleration/deceleration (e.g., blast, whiplash) causes shearing of axons, dendrites, and blood vessels. At the primary site of injury, bleeding and necrotic and apoptotic cell death occur creating a “hot zone” that is often referred to as the injury epicenter. Micro-lesions in the brain and spinal cord generated by either high-powered laser pulses or with a glass micropipette and then imaged in real-time by two-photon live imaging showed striking and unexpectedly rapid changes in motility of microglial processes. The first paper using this approach showed that instead of process retraction, microglia respond within 10–15 min by extending their cytoplasmic processes towards the lesion (Davalos et al. 2005). By 20–60 min these microglial processes form a dense network tightly around the lesion (Davalos et al. 2005). Other groups have reported similar responses in the cerebral cortex in slice cultures (Hines et al. 2009) and in vivo in spinal cord white matter microglia (Dibaj et al. 2010). The studies on the spinal cord laser lesions showed that microglia located 50–100 μm from the lesion respond by extending their processes towards the lesion, while at the same time process retraction occurred from regions of the cell facing away from the lesion (Dibaj et al. 2010; Hines et al. 2009). The processes that were retracted were restored after ~40 min (Hines et al. 2009). In experiments done in the spinal cord, some microglia soma in white matter were found to migrate towards the lesion within 2 h, sometimes along degenerating axons (Dibaj et al. 2010). A consistent finding is that these microglial responses, regardless of the CNS region, create within minutes a dense meshwork of microglial processes that wall-off the lesion border. Blocking or preventing this response in slice cultures by selective laser ablation of surrounding microglia, preventing actin polymerization, or chloride channel blocking causes the lesion to expand three to fourfold (Hines et al. 2009), indicating that this early and rapid microglial response within the first hour after injury is beneficial and protective. It makes sense that this first and earliest response of microglia would be protective, perhaps by participating in lesion containment or by rapidly surveying regions where the likelihood of pathogen entry is enhanced (e.g., regions of bleeding or tissue barrier compromise). In contrast to these small, well-defined laser or micropipette lesions, contusion injuries to spinal cord or brain cause more extensive damage and it is not known whether microglia respond similarly to small or large lesions, mostly because comparative real-time studies of this type have not been completed.
Molecular control of the rapid microglial response to injury: Glia-derived ATP, and damage-associated molecular patterns (DAMPs) from dying and necrotic cells and plasma proteins, most notably fibrinogen, are among the most prominent early microglia activating factors (Davalos et al. 2012; Kigerl and Popovich 2009; Popovich and Longbrake 2008). The rapid microglial process extension towards cortical laser or traumatic micro-lesions was shown to be mediated by ATP, as it could be inhibited by an ATP-degrading enzyme, or be induced by injection of ATP into the cortex (Davalos et al. 2005). The injury-induced response but not normal resting state motility of microglial processes is mediated by P2Y12 G-protein-coupled purinergic receptors (Haynes et al. 2006). In addition, the injury response but not the resting state motility of microglial processes also requires functional volume-sensitive chloride channels (Hines et al. 2009). However, both types of process extension require actin polymerization (Hines et al. 2009). Interestingly, microglial process motility in the normal brain and injury-induced response is mediated by ATP released from astrocytes and can be prevented by blocking connexin hemichannels expressed in astrocytes (Davalos et al. 2005). These findings indicate functional communication between astrocytes and microglia that mediate microglial behavior in the normal and injured CNS. Nitric oxide gradients at the lesion also contribute to microglial process extension and soma migration, which can be augmented by tissue ATP (Dibaj et al. 2010). In the developing zebrafish brain, where microglia are more sparsely distributed, laser lesions induce microglia located at a distance to sense signals originating from the lesion and migrate towards the site of damage (Sieger et al. 2012). These authors have shown that glutamate released by damaged cells induce influx of intracellular calcium (Ca2+) and the spread of Ca2+ waves in astrocytes that leads to release of ATP that is sensed by microglia located at a distance via the P2Y12 receptors (Sieger et al. 2012). Such mechanisms may also operate in regions of the mammalian CNS in which microglia are sparsely distributed such as the white matter in the brain (Lawson et al. 1990) and spinal cord (Carlson et al. 1998).
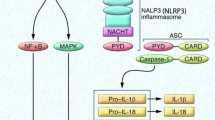
DAMPs-TLR-mediated responses: As microglia extend processes towards the site of injury, they are likely to interact with intracellular molecules and fragments of extracellular molecules (ECM) generated from damaged cells and tissue. These DAMPs can signal microglia via binding and activation of various pattern recognition receptors (PRRs). In response to DAMPs that are liberated by sterile injury, such as in non-penetrating, contusion or compression type CNS injury, or pathogens that enter the CNS, microglial PRRs respond in a similar manner with the ultimate goal of preventing infection and promoting tissue repair. Toll-like receptors (TLRs) are one of the major classes of PRRs through which DAMPs signal microglial responses. Some of the known DAMPs include intracellular molecules released after injury such as heat-shock proteins (Hsp 22, 60, 70 and 72), some members of the S100 family of calcium-binding proteins, mRNA and single-stranded RNA (ssRNA); fragments of ECM molecules fibronectin, tenascin, versican, heparan sulfate, and hyaluronic acid; and serum amyloid (Piccinini and Midwood 2010). Except for mRNA and ssRNA, the other DAMPs listed signal via TLR2 and TLR4. Activation of TLR2 and 4 via the MyD88-dependent pathway leads to NFκB activation and expression of pro-inflammatory cytokines such as interleukin 1 β (IL-1β), TNFα, as well as expression of cyclooxygenase (COX), inducible nitric oxide synthase, and matrix metalloproteinases, all of which are increased after CNS injury. In a model of autoimmune demyelination, DAMP-mediated activation of microglia via TLR4 and integrin receptors (CD11b/CD18) results in the production of inflammatory chemokines and destructive oxidative bursts (Davalos et al. 2012; Smiley et al. 2001). In vivo two-photon imaging of the spinal cord in experimental autoimmune encephalomyelitis (EAE) showed microglia clusters co-localized to zones of fibrinogen leakage in the perivascular space (Davalos et al. 2012). These changes preceded axonal injury and demyelination, which could be inhibited by blocking reactive oxygen species (ROS) or deleting the fibrinogen binding site in microglia CD11b receptors (Davalos et al. 2012). A similar response is likely to occur after SCI or traumatic brain injury (TBI) since fibrinogen, albumin, and other as yet to be defined factors in blood persist at and near the epicenter for extended periods of time (days to weeks post-injury).
The binding of DAMPs to TLRs induces inflammatory signaling. When such signals persist or when active resolution of inflammation is impaired, chronic inflammation and pathology ensue resulting in conditions such as rheumatoid arthritis, and inflammatory lung or bowel disease (Piccinini and Midwood 2010). Spinal cord injuries result in an immediate and abundant availability of DAMPs and robust induction of TLRs (Kigerl et al. 2007). DAMP-TLR signaling likely continues chronically after SCI as the period of secondary tissue damage can extend for weeks or months, and as components of extracellular matrix molecules involved in tissue repair can also act as DAMPs. In addition, resolution of inflammation is impaired in injured spinal cord (Pruss et al. 2011). After spinal cord contusion injury in mice, there is increased expression of TLR1, 2, 5, and 7 mRNA immediately after spinal cord contusion injury (1–14 days after SCI), in addition to increased MyD88 and NFκB (Kigerl et al. 2007). Interestingly, mice lacking TLR2 and TLR4 showed a subtle but noticeable worsening of locomotor control, unusual patterns of myelin and axon pathology, and a poorly formed glial limitans after SCI (Kigerl et al. 2007). In wild-type mice after spinal cord contusion injury, macrophages that appear “foamy” due to the breakdown of phagocytosed material (Fig. 19.1) often remain clustered in the central core of the lesion within a well-defined glial boundary. In contrast, TLR-deficient SCI mice had smaller clusters of phagocytic macrophages scattered in the ventral white matter beyond the normal extent of the glial limitans (Kigerl et al. 2007). This distribution of macrophages in TLR null mice may be due to failure of normal wound healing in the CNS that includes the formation of a glia limitans. The reformation of the glia limitans prevents immune cells from entering damaged CNS tissue (Bush et al. 1999). These findings suggest that DAMP-TLR signaling immediately after SCI prior to the formation of the glia limitans may be protective. Whether this is due to reparative programs that are activated in microglia or macrophages by TLR signaling or to the effects of TLR signaling in astrocytes which also express TLR2 and 4 (Kigerl et al. 2007) is not clear at present. Furthermore, the effects of prolonged DAMP-TLR signaling beyond the acute period after SCI cannot be known from these studies because signaling mutants or conventional knockout mice were used. Studies using conditional deletion TLR2 and 4 in microglia/macrophages after the first week after injury may help to better define the role of TLR signaling in these cell types after SCI.
Upper: Morphology of microglia prior to injury (resting state) and changes in their morphology over time after SCI. Lower: The bottom part of the figure is a representation of the spatial distribution of these cells in the contused spinal cord about 2 weeks after contusion injury. “Resting” microglia: CD11b, CD45low, CX3CR1high, Ly6Clow, Gr-1low. “Activated” microglia (and newly infiltrating monocytes) at and near lesion: CD11b, CD45high, CX3CR1low, CD68, MHC class II, Ly6Chigh, Gr-1low. Microglia located at a distance from lesion: CD11b, CD45, CXCR3, CCL21, IL-6, MHC class II
Expression of pro-inflammatory cytokines: DAMP-TLR signaling can be expected to lead to the early expression of IL-1β and TNFα in microglia after injury. In fact, increased expression of these cytokines is seen soon after spinal cord contusion injury (Pineau and Lacroix 2007; Rice et al. 2007). The IL-1β mRNA expressing cells increase in number 6 h after contusion injury, peak at 12 h then return to normal levels by 48 h (Pineau and Lacroix 2007). At the peak of expression this cytokine is expressed mainly by microglia and astrocytes (Pineau and Lacroix 2007). IL-1 receptor antagonist (IL-1ra) is also up-regulated after SCI but at much lower levels than IL-1β (Pan et al. 2002). Intrathecal treatment with IL-1ra for the first 3 days after SCI in rats prevented apoptosis and caspase-3 expression (Nesic et al. 2001) and improved locomotor recovery (Zong et al. 2012). After TBI, activated microglia also increased expression of IL-1β (Bye et al. 2007). Following brain ischemic injury, treatment with IL-1ra reduces infarct volume (Loddick and Rothwell 1996; Relton et al. 1996), while treatment with exogenous IL-1β worsens brain injury (Lawrence et al. 1998; Loddick and Rothwell 1996). Furthermore, neutralizing IL-1β with function-blocking antibodies reduces tissue damage and improves cognition after brain injury (Clausen et al. 2011).
TNFα mRNA expressing cells detected by in-situ hybridization are seen within 15 min after spinal cord contusion injury, reach peak expression at 1 h, and return to baseline levels after 2 days. In addition, there is a second period of TNFα expression between 14 and 28 days (the maximum time examined) (Pineau and Lacroix 2007). During the period immediately after injury, TNFα is expressed by microglia rather than invading monocytes because of the rapid expression within 1 h, which precedes entry of monocytes (Pineau and Lacroix 2007). In addition to microglia, astrocytes, oligodendrocytes, and neurons also expressed TNFα at this early period after injury (Pineau and Lacroix 2007). During the second peak of expression at 2–4 weeks after injury, microglia and macrophages appear to be the main cells expressing TNFα (Pineau and Lacroix 2007). In the injured human spinal cord, TNFα protein is also detected early at 1–3 h after injury in microglia and neurons (Yang et al. 2005). There is also evidence that IL-1β and TNFα produced by microglia are associated with white matter damage around the ventricles in brain hypoxia injury (Deng et al. 2011). Blocking TNFα with a monoclonal antibody (Infliximab) or a TNF receptor–Fc fusion protein (Etanercept) improves histological and locomotor outcomes after SCI (Genovese et al. 2006, 2008).
Is the early microglial response beneficial or detrimental: The two-photon imaging studies showing that microglial ablation or prevention of microglial process extension towards the site of lesion enlarged lesion volume (Hines et al. 2009) indicate that this early response is beneficial. The latter work was done with micro-lesions. Whether microglial process extension occurs after large lesions such as spinal cord contusion injury is not known. Furthermore, if microglial process extension was to occur to any extent after contusion injuries, it would be important to assess if it is beneficial in limiting the size of the lesion. The early DAMP-TLR signaling response, which serves to alert the body of tissue damage, is thought to be involved in restoring tissue homeostasis and effect repair. The evidence obtained from conventional TLR knockout mice indicates that lack of TLR2 and 4 results in noticeable worsening of locomotor control, and a poorly formed glial limitans at the injury site with spreading of clusters of phagocytic macrophages beyond the normal extent of the glial limitans that surround the lesion core (Kigerl et al. 2007). The expression of the pro-inflammatory cytokines IL-1β and TNFα by injured CNS tissue has been shown in a variety of experiments to be detrimental and contributing to secondary damage.
3 Late Responses that Occur Days and Weeks After Injury
Phenotypic and functional polarization of microglia/macrophages: Within 2–3 days after SCI, circulating monocytes infiltrate the injury epicenter where they differentiate into tissue macrophages, i.e., monocyte-derived macrophages (MDMs). Within a few days after SCI microglia begin to retract their processes and become phagocytic at the site of lesion (Fig. 19.1). Within 1 week post-injury, routine phenotypic or morphological criteria cannot be used to distinguish between macrophages derived from activated microglia or MDMs. In general, a large proportion of macrophage/microglia that are phagocytic (foamy-looking cells) migrate into a cluster at the very center of the lesion, while activated microglia with short processes are seen in the surrounding areas (Fig. 19.1). As these macrophage/microglia accumulate, factors in the extracellular milieu will modulate their phenotype with discrete effects on neuron survival and axon growth (Kigerl et al. 2009; Stout et al. 2005). For example, cytokines and TLR agonists (e.g., DAMPs) can promote the differentiation of classically activated “M1” or alternatively activated “M2” macrophages. The canonical in vitro model for stimulating M1 differentiation is exposure of naive myeloid cells to LPS and IFNγ or, to promote M2 differentiation, cells are stimulated with IL-4 (Gordon and Taylor 2005). M1 macrophages were originally described as potent microbicidal effector cells; a function associated with release of inflammatory cytokines and ROS. M2 macrophages were found to have enhanced phagocytic capacity and release cytokines and growth factors that promote revascularization and tissue formation (Gordon and Taylor 2005). Both types of macrophages can phagocytose dying cells and tissue debris and their phagocytic activity is likely influenced by the tissue environment. SCI and TBI induce a heterogeneous macrophage response that can be defined by the increased expression of M1 and M2 phenotypic markers (Hsieh et al. 2013; Kigerl et al. 2009). After SCI, M1 macrophages dominate at the injury epicenter and nearby penumbra, overwhelming a comparatively smaller number and transient M2 macrophage response (Kigerl et al. 2009). M1 macrophages likely contribute to neurotoxicity and axonal “die-back”. Depletion or inhibition of the acute macrophage response, which are likely to be M1 macrophages, is neuroprotective and reduces axonal retraction at the site of injury (Blight 1994; Gris et al. 2004; Horn et al. 2008; Popovich et al. 1999). In vitro, M2 macrophages can promote long-distance axon growth without causing neurotoxicity (Kigerl et al. 2009). Therefore, in theory, any manipulation that biases the endogenous response toward an M2 phenotype could limit tissue damage and/or enhance tissue repair and perhaps axonal growth/regeneration. In support of this hypothesis, recent data show that post-injury administration of substance P, neural stem cells, granulocyte-colony-stimulating factor (G-CSF), mesenchymal stem cells or acidic-fibroblast growth factor laced peripheral nerve grafts improve recovery and/or promote repair in the injured spinal cord, and in each case, these interventions enhanced intraspinal M2 macrophage reactions (Guo et al. 2013; Jiang et al. 2012; Nakajima et al. 2012). A direct effect of M2 macrophages on mediating tissue repair or improving functional recovery has not been proved unequivocally in SCI or TBI models.
Phenotypic changes: Morphological transformation of microglia is accompanied by de novo expression of new membrane and cytoplasmic proteins. Since microglial phenotype varies throughout the CNS (de Haas et al. 2008), the phenotypic changes induced by injury, whether the injury is to brain or spinal cord, will vary as a function of injury location. For example, in brain and spinal cord, microglia express CD11b, CD40, CD45, CD80, CD86, F4/80, TREM-2b, CXCR3, and CCR9, albeit at relatively different levels indicating increased migration, phagocytic activity, antigen presentation, T and B cell interactions, inflammatory responses, and others. CD11b and CD45 are expressed by both microglia and MDMs, but overall levels of CD45 are reduced in microglia. Differential expression of CD45 can be detected using flow cytometry and is often used to distinguish between microglia and MDMs, but such differences cannot be distinguished by immunofluorescence in tissue sections. Microglia constitutively express various surface antigens common to other cells of the myeloid cell lineage including CX3CR1 and complement receptors. Activated microglia increase expression of these proteins and begin to express other antigens including major histocompatibility complex class II antigens (MHC class II). After SCI and TBI in rats, mice, non-human primates and humans, MHC class II expression is increased primarily on microglia found within white matter undergoing degeneration distal to the site of injury called, Wallerian degeneration (Kigerl et al. 2006; Popovich et al. 1993; Schmitt et al. 2000; Watanabe et al. 1999). There is no obvious relationship between MHC class II expression and demyelination in these regions, signifying diverse roles for MHC class II in microglia.
Mechanisms and functional implications of microglial activation distal to the injury epicenter: Axonal degeneration and plasticity also occur at sites distal to the epicenter, either as a result of physical severing of axons or diffuse axonal injury. Severed axonal segments located distal to the epicenter, whether they are located in spinal cord, brain, or peripheral nerve, undergo Wallerian degeneration. Wallerian degeneration is an active process that invariably transforms the phenotype of microglia. Several factors produced by Wallerian degenerating axons or their surrounding glia activate microglia. Complement proteins, members of the phospholipase A2 superfamily of enzymes, galectins, and PRRs (e.g., TLRs), among others, all have been implicated in microglial activation and/or recruitment of MDMs (Boivin et al. 2007; Gaudet et al. 2009; Lopez-Vales et al. 2008; Mietto et al. 2013). Although microglia and MDMs phagocytose degenerating axons and myelin debris, whether these cells also cause collateral damage to intact axons or directly promote regeneration of injured axons remains controversial (Gaudet et al. 2011). Clearly, effective regeneration of injured peripheral nerve requires inflammatory signaling in macrophages (Boivin et al. 2007) that promote rapid clearance of myelin and axonal debris, and the secretion of growth promoting factors. In the CNS, the macrophage/microglial response that underlie Wallerian degeneration is significantly delayed (George and Griffin 1994; Vargas and Barres 2007). This delay in Wallerian degeneration is thought to contribute to the failure of axon regeneration in the CNS.
Microglial activation also occurs in grey matter regions remote from the site of injury and can have pathological consequences. For example, after a mid-thoracic SCI (e.g., T9), remote activation of microglia occurs over a period of several weeks post-injury in lumbar spinal cord (~10 segments below the level of injury), sensory relay nuclei in the brainstem (e.g., cuneate nucleus), and the ventral posterolateral (VPL) nucleus of the thalamus (Detloff et al. 2008; Hains and Waxman 2006; Koshinaga and Whittemore 1995). The microglia in these regions remain process-bearing (Fig. 19.1) but display other immunohistochemical evidence of activation. These responses occur within regions that process and relay sensory information, including pain. After SCI, activated microglia are implicated in the onset and progression of neuropathic pain (Hulsebosch et al. 2009; Tsuda et al. 2005) (see Chap. 11). A novel mechanism for transynaptic microglial activation has been described in which injured neurons increase synthesis of CCL21, a chemokine that is packaged into vesicles and is transported to presynaptic terminals and activates microglia via interactions with CXCR3 (de Jong et al. 2005). Transynaptic increases in CCL21 after SCI may also regulate microglial contributions to neuropathic pain. In a model of mid-thoracic SCI, CCL21 levels increase in neurons and microglia below the level of injury and in the VPL of the thalamus (Zhao et al. 2007b). Blocking axonal transmission rostral to the injury site or locally applying anti-CCL21 antibodies reduces CCL21 in the VPL, impairs microglial activation, and attenuates neuropathic pain (Zhao et al. 2007b). Cortical contusion injuries also cause remote microglia activation in cerebellum and spinal cord (Czeiter et al. 2008; Fukuda et al. 1996). These findings suggest that delay in microglial activation in white matter tracts undergoing Wallerian degeneration distal to the site of injury may contribute to the lack of regeneration in the CNS, while rapid and prolonged activation of microglia in grey matter regions distal to the injury site contribute to the generation of neuropathic pain.
Implications of chronic microglia and macrophage activation: Microglia and macrophages within the epicenter and in regions distal to the injury site remain activated for months or even years post-injury. Immunohistochemical analysis of postmortem human SCI specimens reveal activated phagocytic macrophage/microglia in degenerating white matter distal to the injury epicenter where they cluster or assume rounded phagocytic morphologies. These cells increase de novo expression of MHC class II glycoproteins or the lysosomal protein CD68, which may be functionally related to phagocytosis (Fleming et al. 2006; Schmitt et al. 2000). Identical responses are evident in white matter of SCI rats and mice several weeks after injury (Kigerl et al. 2006; Popovich et al. 1997). SCI also causes chronic microglial activation in remote regions of spinal cord gray matter and in the thalamus (Detloff et al. 2008; Zhao et al. 2007a, b). Expression of inflammatory signaling proteins including p38 mitogen-activated protein kinase (MAPK) and phosphorylated extracellular signal regulated kinase 1/2 (pERK1/2) increases in microglia in lumbar spinal cord gray matter, several segments below the level of a thoracic SCI (Detloff et al. 2008; Zhao et al. 2007a). pERK1/2 is an upstream regulator of prostaglandin E2 (PGE2), a lipid inflammatory mediator that is produced downstream of pERK1/2 and COX. Pharmacological blocking of PGE2 release in chronically injured spinal cord lowers the firing threshold for dorsal horn neurons resulting in aberrant sensory function including the onset of neuropathic pain (Zhao et al. 2007a). The neuronal chemokine CCL21 which activates microglia is increased in the dorsal horn of the spinal cord and VPL of the thalamus after SCI and was shown to induce pain-related behaviors (Zhao et al. 2007b). IL-6 levels are increased 3 weeks and later in regions caudal to the site of SCI and may be involved in the maintenance of pain (Detloff et al. 2008). These findings suggest that microglial activation in regions far removed from the site of SCI can contribute to pain and functional disability.
Interestingly, blockade of the C5a fragment of complement, a potent microglia activator and leukocyte chemoattractant, impairs recovery of locomotor function and exacerbates pathology at the site of injury when given beginning at 2 weeks post-injury (Beck et al. 2010). These data may indicate a role for complement in regulating inflammatory-mediated repair processes or, alternatively, the importance of complement in signaling microglia to participate in the surveillance and maintenance of spinal circuitry. Microglial phagocytosis of synapses is triggered by synaptic complement proteins expressed by neurons during postnatal development (Schafer et al. 2012) (see Chap. 9). If microglia function is impaired, the formation and refinement of new circuits resulting from regenerative sprouting or therapeutic intervention could be adversely affected (Paolicelli et al. 2011; Schafer et al. 2012; Stephan et al. 2012). In the adult CNS, microglia strip and phagocytose axon-somatic synapses on facial motor neurons after nerve injury (Kreutzberg 1993).
Chronic activation of microglia or macrophages may predispose the injured brain and spinal cord to excess oxidative stress and inflammatory signaling. Gene expression profiling studies show that a subset of inflammatory genes expressed early after SCI or TBI persist for up to 6 months post-injury and are translated into proteins that may have pathogenic significance (Byrnes et al. 2006). For example, p22Phox and gp91Phox are components of NADPH oxidase and are increased early after injury but expression is maintained for up to 6 weeks (latest time examined). NADPH oxidase produces ROS which in turn activates NFκB and MAPK-dependent inflammatory signaling. Chronic activation of microglial NADPH oxidase can cause neurotoxicity in vivo (Lull and Block 2010). Inhibition of NADPH oxidase during the first week post-contusive SCI with diphenylene iodonium reduces the normally high levels of NADPH oxidase, as well as other inflammatory genes and proteins, and is associated with a decrease in lesion volume (Byrnes et al. 2011).
Chronic oxidative stress also produces a pool of lipid inflammatory mediators that persist in chronically injured spinal cord. Oxidative metabolism of arachidonic acid (AA) yields leukotrienes (LTs) and prostaglandins (PGs). LTs and PGs are elevated in injured rat spinal cord for up to 9 months post-injury (Dulin et al. 2013). These inflammatory mediators are potent activators of microglia and macrophages and can elicit inflammatory signaling that lowers the threshold for neuronal depolarization and can result in neuropathic pain. Licofelone, a novel inhibitor of COX and 5-lipoxygenase (5-LOX), i.e., the enzymes responsible for LT and PG synthesis, normalized the oxidative and inflammatory microenvironment associated with chronic SCI and reduced post-injury allodynia. Licofelone did not improve motor recovery (Dulin et al. 2013).
Despite evidence that acute neuroinflammatory cascades are distinct in injured brain and spinal cord (Batchelor et al. 2008; Schnell et al. 1999), microglia and macrophage reactions in the chronic lesion environment of traumatically injured brain are remarkably similar to those described above for injured spinal cord. In non-human primates, lesions in the primary motor cortex elicit microglial activation for up to 1 year post-injury; microglia in cortex exhibit an activated phenotype (CD68+) with prolonged expression of brain-derived neurotrophic factors (BDNF) and tyrosine receptor kinase B (TrkB) receptors evident in cortical and spinal cord microglia (Nagamoto-Combs et al. 2007). Activated microglia were detected in subcortical grey and white matter in humans 11 months or longer after TBI using positron emission tomography (PET) for the translocator protein (TSPO), which is found in high levels in the mitochondria of activated microglia (Ramlackhansingh et al. 2011). They found that the magnitude of microglial activation in thalamus correlated with cognitive impairment but not structural brain damage (Ramlackhansingh et al. 2011). In addition, in a post-mortem study of humans with single TBI, activated microglia detected by immunohistochemistry for CR3-43 and CD68 were seen in 28 % of cases in which there was also evidence of white matter damage (Johnson et al. 2013). Although correlative, these data indicate that chronically activated microglia could contribute to damage and long-term neurologic impairment. If true, microglia-specific therapeutic intervention may still be effective for several years post-injury.
4 Future Directions
Microglia and MDMs are derived from distinct myeloid precursors with discrete transcriptional requirements (Ginhoux et al. 2010; Kierdorf et al. 2013); monocytes develop from hematopoietic stem cells in bone marrow, a process that requires the transcription factor Myb (Schulz et al. 2012). Conversely, microglial development from yolk sac myeloid progenitors is Myb-independent (Schulz et al. 2012). Additional cell-specific differences are likely to be discovered and will yield new genetic tools that will allow scientists to independently manipulate these distinct myeloid cell lineages, further refining our knowledge about how these myeloid cell subsets contribute to CNS injury and repair.
How aging affects microglia and CNS repair is also an important experimental and clinical variable for future consideration, especially since aging affects microglial function (Streit 2006) (see Chap. 13). Aging can affect hematopoiesis, and activation of these and other innate immune cells may affect macrophage and microglial function (Kovacs et al. 2009; Shaw et al. 2010). Aging has become an area of intense preclinical study and a significant clinical concern after SCI and TBI, especially since the effects of injury may accelerate aging of some organ systems such as the cardiovascular, endocrine, and musculoskeletal (Hitzig et al. 2011). There is little known about how aging affects microglia and MDMs after SCI, although this is likely to be an area of research that will receive increased attention (Kumamaru et al. 2012).
Why microglia and macrophages remain activated in chronically injured brain and spinal cord is also unclear. It is probable that specific “on” signals including ATP, DAMPs, degenerating axons, myelin debris, and cytokines persist indefinitely or reappear slowly over time creating a pool of ligands that can stimulate microglia and macrophages (Biber et al. 2007). Chronic activation may also be explained by the loss of “off” signals including CD200L or CX3CL1. These proteins are normally found on or are released by healthy neurons and effectively inhibit or silence microglia effector functions (Biber et al. 2007). The presence of “off” signals indicates that the intact nervous system actively regulates microglial function. However, after SCI or TBI, inflammation does not appear to be self-limiting (Pruss et al. 2011).
It seems intuitive that limiting chronic activation of microglia and MDMs would yield better outcomes after SCI or TBI. However, a more in-depth understanding about the functional heterogeneity of these cell types in both the acute and chronic lesion microenvironment is needed. As treatment and care for SCI and TBI individuals improve, lifespan is increasing. Thus, the consequences of an aging microglia repertoire also need to be considered in order to optimize CNS repair.
References
Batchelor PE, Tan S, Wills TE, Porritt MJ, Howells DW (2008) Comparison of inflammation in the brain and spinal cord following mechanical injury. J Neurotrauma 25:1217–1225
Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ (2010) Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 133:433–447
Biber K, Neumann H, Inoue K, Boddeke HW (2007) Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci 30:596–602
Blight AR (1994) Effects of silica on the outcome from experimental spinal cord injury: implication of macrophages in secondary tissue damage. Neuroscience 60:263–273
Boivin A, Pineau I, Barrette B, Filali M, Vallieres N, Rivest S, Lacroix S (2007) Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J Neurosci 27:12565–12576
Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV (1999) Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23:297–308
Bye N, Habgood MD, Callaway JK, Malakooti N, Potter A, Kossmann T, Morganti-Kossmann MC (2007) Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp Neurol 204:220–233
Byrnes KR, Garay J, Di Giovanni S, De Biase A, Knoblach SM, Hoffman EP, Movsesyan V, Faden AI (2006) Expression of two temporally distinct microglia-related gene clusters after spinal cord injury. Glia 53:420–433
Byrnes KR, Washington PM, Knoblach SM, Hoffman E, Faden AI (2011) Delayed inflammatory mRNA and protein expression after spinal cord injury. J Neuroinflammation 8:130
Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L (1998) Acute inflammatory response in spinal cord following impact injury. Exp Neurol 151:77–88
Clausen F, Hanell A, Israelsson C, Hedin J, Ebendal T, Mir AK, Gram H, Marklund N (2011) Neutralization of interleukin-1beta reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur J Neurosci 34:110–123
Czeiter E, Pal J, Kovesdi E, Bukovics P, Luckl J, Doczi T, Buki A (2008) Traumatic axonal injury in the spinal cord evoked by traumatic brain injury. J Neurotrauma 25:205–213
Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8:752–758
Davalos D, Ryu JK, Merlini M, Baeten KM, Le Moan N, Petersen MA, Deerinck TJ, Smirnoff DS, Bedard C, Hakozaki H, Gonias Murray S, Ling JB, Lassmann H, Degen JL, Ellisman MH, Akassoglou K (2012) Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun 3:1227
David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12:388–399
de Haas AH, Boddeke HW, Biber K (2008) Region-specific expression of immunoregulatory proteins on microglia in the healthy CNS. Glia 56:888–894
de Jong EK, Dijkstra IM, Hensens M, Brouwer N, van Amerongen M, Liem RS, Boddeke HW, Biber K (2005) Vesicle-mediated transport and release of CCL21 in endangered neurons: a possible explanation for microglia activation remote from a primary lesion. J Neurosci 25:7548–7557
Deng YY, Lu J, Ling EA, Kaur C (2011) Role of microglia in the process of inflammation in the hypoxic developing brain. Front Biosci (Schol Ed) 3:884–900
Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM (2008) Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol 212:337–347
Dibaj P, Nadrigny F, Steffens H, Scheller A, Hirrlinger J, Schomburg ED, Neusch C, Kirchhoff F (2010) NO mediates microglial response to acute spinal cord injury under ATP control in vivo. Glia 58:1133–1144
Dulin JN, Karoly ED, Wang Y, Strobel HW, Grill RJ (2013) Licofelone modulates neuroinflammation and attenuates mechanical hypersensitivity in the chronic phase of spinal cord injury. J Neurosci 33:652–664
Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC (2006) The cellular inflammatory response in human spinal cords after injury. Brain 129:3249–3269
Fukuda K, Aihara N, Sagar SM, Sharp FR, Pitts LH, Honkaniemi J, Noble LJ (1996) Purkinje cell vulnerability to mild traumatic brain injury. J Neurotrauma 13:255–266
Gaudet AD, Leung M, Poirier F, Kadoya T, Horie H, Ramer MS (2009) A role for galectin-1 in the immune response to peripheral nerve injury. Exp Neurol 220:320–327
Gaudet AD, Popovich PG, Ramer MS (2011) Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation 8:110
Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Bramanti P, Cuzzocrea S (2006) Immunomodulatory effects of etanercept in an experimental model of spinal cord injury. J Pharmacol Exp Ther 316:1006–1016
Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Esposito E, Bramanti P, Cuzzocrea S (2008) TNF-alpha blockage in a mouse model of SCI: evidence for improved outcome. Shock 29:32–41
George R, Griffin JW (1994) Delayed macrophage responses and myelin clearance during Wallerian degeneration in the central nervous system: the dorsal radiculotomy model. Exp Neurol 129:225–236
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330:841–845
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5:953–964
Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC (2004) Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci 24:4043–4051
Guo Y, Zhang H, Yang J, Liu S, Bing L, Gao J, Hao A (2013) Granulocyte colony-stimulating factor improves alternative activation of microglia under microenvironment of spinal cord injury. Neuroscience 238:1–10
Hains BC, Waxman SG (2006) Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci 26:4308–4317
Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9:1512–1519
Hines DJ, Hines RM, Mulligan SJ, Macvicar BA (2009) Microglia processes block the spread of damage in the brain and require functional chloride channels. Glia 57:1610–1618
Hitzig SL, Eng JJ, Miller WC, Sakakibara BM (2011) An evidence-based review of aging of the body systems following spinal cord injury. Spinal Cord 49:684–701
Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J (2008) Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci 28:9330–9341
Hsieh CL, Kim CC, Ryba BE, Niemi EC, Bando JK, Locksley RM, Liu J, Nakamura MC, Seaman WE (2013) Traumatic brain injury induces macrophage subsets in the brain. Eur J Immunol 43:2010–2022
Hulsebosch CE, Hains BC, Crown ED, Carlton SM (2009) Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev 60:202–213
Jiang MH, Chung E, Chi GF, Ahn W, Lim JE, Hong HS, Kim DW, Choi H, Kim J, Son Y (2012) Substance P induces M2-type macrophages after spinal cord injury. Neuroreport 23:786–792
Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W (2013) Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136:28–42
Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Holscher C, Muller DN, Luckow B, Brocker T, Debowski K, Fritz G, Opdenakker G, Diefenbach A, Biber K, Heikenwalder M, Geissmann F, Rosenbauer F, Prinz M (2013) Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci 16:273–280
Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG (2009) Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29:13435–13444
Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PG (2007) Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J Neurochem 102:37–50
Kigerl KA, McGaughy VM, Popovich PG (2006) Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J Comp Neurol 494:578–594
Kigerl KA, Popovich PG (2009) Toll-like receptors in spinal cord injury. Curr Top Microbiol Immunol 336:121–136
Koshinaga M, Whittemore SR (1995) The temporal and spatial activation of microglia in fiber tracts undergoing anterograde and retrograde degeneration following spinal cord lesion. J Neurotrauma 12:209–222
Kovacs EJ, Palmer JL, Fortin CF, Fulop T Jr, Goldstein DR, Linton PJ (2009) Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends Immunol 30:319–324
Kreutzberg GW (1993) Dynamic changes in motoneurons during regeneration. Restor Neurol Neurosci 5:59–60
Kumamaru H, Saiwai H, Ohkawa Y, Yamada H, Iwamoto Y, Okada S (2012) Age-related differences in cellular and molecular profiles of inflammatory responses after spinal cord injury. J Cell Physiol 227:1335–1346
Lawrence CB, Allan SM, Rothwell NJ (1998) Interleukin-1beta and the interleukin-1 receptor antagonist act in the striatum to modify excitotoxic brain damage in the rat. Eur J Neurosci 10:1188–1195
Lawson LJ, Perry VH, Dri P, Gordon S (1990) Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39:151–170
Loddick SA, Rothwell NJ (1996) Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab 16:932–940
Lopez-Vales R, Navarro X, Shimizu T, Baskakis C, Kokotos G, Constantinou-Kokotou V, Stephens D, Dennis EA, David S (2008) Intracellular phospholipase A(2) group IVA and group VIA play important roles in Wallerian degeneration and axon regeneration after peripheral nerve injury. Brain 131:2620–2631
Lull ME, Block ML (2010) Microglial activation and chronic neurodegeneration. Neurotherapeutics 7:354–365
Mietto BS, Jurgensen S, Alves L, Pecli C, Narciso MS, Assuncao-Miranda I, Villa-Verde DM, de Souza Lima FR, de Menezes JR, Benjamim CF, Bozza MT, Martinez AM (2013) Lack of galectin-3 speeds Wallerian degeneration by altering TLR and pro-inflammatory cytokine expressions in injured sciatic nerve. Eur J Neurosci 37:1682–1690
Nagamoto-Combs K, McNeal DW, Morecraft RJ, Combs CK (2007) Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury. J Neurotrauma 24:1719–1742
Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT, Johnson WE, Baba H (2012) Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma 29:1614–1625
Nesic O, Xu GY, McAdoo D, High KW, Hulsebosch C, Perez-Pol R (2001) IL-1 receptor antagonist prevents apoptosis and caspase-3 activation after spinal cord injury. J Neurotrauma 18:947–956
Pan JZ, Ni L, Sodhi A, Aguanno A, Young W, Hart RP (2002) Cytokine activity contributes to induction of inflammatory cytokine mRNAs in spinal cord following contusion. J Neurosci Res 68:315–322
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333:1456–1458
Perry VH, Cunningham C, Holmes C (2007) Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 7:161–167
Perry VH, Hume DA, Gordon S (1985) Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience 15:313–326
Piccinini AM, Midwood KS (2010) DAMPening inflammation by modulating TLR signalling. Mediators Inflamm 2010. DOI: 10.1155/2010/672395
Pineau I, Lacroix S (2007) Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol 500:267–285
Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT (1999) Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol 158:351–365
Popovich PG, Longbrake EE (2008) Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci 9:481–493
Popovich PG, Streit WJ, Stokes BT (1993) Differential expression of MHC class II antigen in the contused rat spinal cord. J Neurotrauma 10:37–46
Popovich PG, Wei P, Stokes BT (1997) Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol 377:443–464
Pruss H, Kopp MA, Brommer B, Gatzemeier N, Laginha I, Dirnagl U, Schwab JM (2011) Non-resolving aspects of acute inflammation after spinal cord injury (SCI): indices and resolution plateau. Brain Pathol 21:652–660
Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ (2011) Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol 70:374–383
Relton JK, Martin D, Thompson RC, Russell DA (1996) Peripheral administration of interleukin-1 receptor antagonist inhibits brain damage after focal cerebral ischemia in the rat. Exp Neurol 138:206–213
Rice T, Larsen J, Rivest S, Yong VW (2007) Characterization of the early neuroinflammation after spinal cord injury in mice. J Neuropathol Exp Neurol 66:184–195
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74:691–705
Schmitt AB, Buss A, Breuer S, Brook GA, Pech K, Martin D, Schoenen J, Noth J, Love S, Schroder JM, Kreutzberg GW, Nacimiento W (2000) Major histocompatibility complex class II expression by activated microglia caudal to lesions of descending tracts in the human spinal cord is not associated with a T cell response. Acta Neuropathol 100:528–536
Schnell L, Fearn S, Klassen H, Schwab ME, Perry VH (1999) Acute inflammatory responses to mechanical lesions in the CNS: differences between brain and spinal cord. Eur J Neurosci 11:3648–3658
Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336:86–90
Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM (2010) Aging of the innate immune system. Curr Opin Immunol 22:507–513
Sieger D, Moritz C, Ziegenhals T, Prykhozhij S, Peri F (2012) Long-range Ca2+ waves transmit brain-damage signals to microglia. Dev Cell 22:1138–1148
Smiley ST, King JA, Hancock WW (2001) Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol 167:2887–2894
Stephan AH, Barres BA, Stevens B (2012) The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 35:369–389
Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J (2005) Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol 175:342–349
Streit WJ (2006) Microglial senescence: does the brain’s immune system have an expiration date? Trends Neurosci 29:506–510
Tsuda M, Inoue K, Salter MW (2005) Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci 28:101–107
Vargas ME, Barres BA (2007) Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci 30:153–179
Vela JM, Dalmau I, Gonzalez B, Castellano B (1995) Morphology and distribution of microglial cells in the young and adult mouse cerebellum. J Comp Neurol 361:602–616
Watanabe T, Yamamoto T, Abe Y, Saito N, Kumagai T, Kayama H (1999) Differential activation of microglia after experimental spinal cord injury. J Neurotrauma 16:255–265
Yang L, Jones NR, Blumbergs PC, Van Den Heuvel C, Moore EJ, Manavis J, Sarvestani GT, Ghabriel MN (2005) Severity-dependent expression of pro-inflammatory cytokines in traumatic spinal cord injury in the rat. J Clin Neurosci 12:276–284
Zhao P, Waxman SG, Hains BC (2007a) Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci 27:2357–2368
Zhao P, Waxman SG, Hains BC (2007b) Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J Neurosci 27:8893–8902
Zong S, Zeng G, Wei B, Xiong C, Zhao Y (2012) Beneficial effect of interleukin-1 receptor antagonist protein on spinal cord injury recovery in the rat. Inflammation 35:520–526
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
David, S., Popovich, P.G. (2014). Spinal Cord and Brain Trauma. In: Tremblay, MÈ., Sierra, A. (eds) Microglia in Health and Disease. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1429-6_19
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1429-6_19
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1428-9
Online ISBN: 978-1-4939-1429-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)