Abstract
Multiple sclerosis (MS) is an inflammatory disease characterized by demyelination and axonal degeneration in the central nervous system (CNS). Although MS is considered an autoimmune disease against myelin antigens, its pathogenesis still remains unclear. Microglia are macrophage-like cells in the CNS which play a critical role in innate immunity, in addition to activating pathways associated with adaptive immunity. Microglia produce pro-inflammatory and anti-inflammatory mediators, including cytokines and chemokines, and phagocytose various types of cellular debris. In MS, microglia critically contribute to the inflammatory milieu, but also participate in disrupting the blood–brain barrier integrity, thus inducing the migration of various types of immune cells such as T and B lymphocytes, macrophages, and neutrophils into the CNS. In this disease, microglia may additionally behave as antigen-presenting cells and function as effector cells causing demyelination and axonal degeneration. However, recent evidence also indicates that microglia could play a beneficial role in remyelination and neuroprotection in MS. In this chapter, we will discuss about microglial involvement in MS, with an emphasis on the experimental autoimmune encephalomyelitis (EAE) animal model and describe the cellular and molecular mechanisms which could be specifically implicated in the pathogenesis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Microglia
- Inflammation
- Cytokine
- Chemokine
- Blood–brain barrier
- Antigen presentation
- Demyelination
- Neurodegeneration
- Multiple sclerosis
- Experimental autoimmune encephalomyelitis
-
Microglia, macrophage-like cells in the central nervous system (CNS), play a substantial role in the pathogenesis of multiple sclerosis (MS).
-
In MS, microglia contribute to the development of neuroinflammation by producing both pro-inflammatory and anti-inflammatory mediators.
-
Microglia also promote the migration of peripheral immune cells into the CNS and might additionally behave as antigen-presenting cells in MS.
-
Microglia could lastly influence by their effector functions the demyelination and axonal degeneration observed in MS.
1 Introduction
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system (CNS) resulting from an autoimmune response against myelin antigens. It affects approximately 2.5 million people worldwide, with a predominance in women (ratio of females to males, 2:1). The disease is characterized by a progressive loss of neurological functions caused by the destruction of axonal myelin sheaths throughout the brain and spinal cord white matter. The loss of myelin translates into clinical symptoms ranging from paralysis, muscle spasms, and optic neuritis, to neuropathic pain. Pathological features of MS lesions include increased blood–brain barrier (BBB) permeability, axonal degeneration, glial scar formation, and the prevalence of peripheral immune cells such as T and B lymphocytes, macrophages, and neutrophils within the CNS (Williams et al. 2007; Jadidi-Niaragh and Mirshafiey 2011). The etiology of MS is still unclear. Genetic factors like variations in the HLA-DRB1 gene coding for the major histocompatibility complex (MHC) class II complex (DRB1-9 beta chain), and the IL-7R gene coding for the interleukin (IL)-7 receptor, as well as environmental factors such as exposure to the Epstein-Barr virus, low levels of vitamin D, and smoking, have been associated with an increased risk of developing MS (Haines et al. 1996; Sawcer et al. 1996; Teutsch et al. 2003; Oreja-Guevara et al. 2014). Approximately 85 % of MS patients repeatedly undergo relapse followed by partial or complete recovery periods (or remissions), a form of the disease which is termed relapsing-remitting MS. In more than 50 % of the relapse-remitting MS patients, the disease progressively worsens with minor remissions, reaching a stage of secondary progressive MS. In the remaining 10–15 % of MS patients, however, the disease only advances without remission, a form of the disease which is termed primary progressive MS (Thompson et al. 1997; Haines et al. 2011).
1.1 Inflammatory Mechanisms in MS/EAE
Experimental autoimmune encephalomyelitis (EAE) is commonly used as an animal model of MS, being similarly associated with axonal degeneration and chronic demyelination, primarily in the spinal cord, resulting in tail and hindlimb paralysis. However, similar to MS, the disease symptoms reflect the anatomical location of the inflammatory lesions and may also include emotional instability, sensory loss, ataxia, muscle weakness, and spasms. EAE is generally induced in rodents by immunization with myelin peptides, such as myelin basic protein (MBP), myelin proteolipid protein (PLP), and myelin oligodendrocyte glycoprotein (MOG), emulsified in an adjuvant (typically complete Freund’s adjuvant) to enhance the immune response. EAE is also produced by adoptive transfer of myelin-reactive T lymphocytes expressing CD4 glycoproteins on their surface (CD4+ T cells), isolated from mice immunized with myelin peptides, and further stimulated in vitro with myelin peptides. Depending on the nature of the antigens, and on the background of the animals, an acute stretch of EAE, a relapsing-remitting form, or chronic EAE can be induced (Rangachari and Kuchroo 2013).
In both EAE and MS, the infiltration of T and B lymphocytes, macrophages, and neutrophils is pronounced around the demyelinating lesions in situ, within the perivascular space and/or parenchyma. In addition, oligoclonal IgGs are commonly detected in the cerebrospinal fluid of EAE mice and MS patients (Mehta et al. 1985; Tomioka and Matsui 2014), thus suggesting the presence of an immune response in the CNS. Although the initiation mechanisms of EAE still remain unclear, they were shown to be mediated by Th effector T cells, a phenotype of CD4+ cells resulting from their activation by antigen-presenting cells (APCs) (Montero et al. 2004; reviewed in Kawakami et al. 2012) (see Chap. 5 for further reading on antigen presentation).
1.2 Microglia in MS/EAE
It is usually difficult to discriminate microglia from infiltrated macrophages in the postmortem brains of MS patients. However, several lines of evidence have suggested that microglia could play a pivotal role in mediating neuroinflammation in MS. Microglia are macrophage-like cells that reside in the CNS and contribute in various manners to maintaining CNS integrity. In the inflamed CNS, microglia can also function as immunocompetent cells, particularly involved with the production of inflammatory mediators and/or the presentation of antigens, depending on the context (Ransohoff et al. 2003; Tran and Miller 2003; Raivich and Banati 2004; Chastain et al. 2011).
During the development of EAE and MS, microglia display several signs of ‘activation’ at the morphological and gene expression levels. For instance, microglia have larger cell bodies, accumulate around lesions sites, and show immunoreactivity for MHC class II and CD68, a lysosomal marker also named ‘macrosialin’ in mouse that is upregulated during inflammation (Minagar et al. 2002; Jack et al. 2005; Marik et al. 2007; also see Chap. 10 for additional reading about CD68). In the postmortem brains of progressive MS patients, demyelination and neuronal damage reportedly correlate with an increased density and clustering of CD68 or MHC class II positive cells, a pathological feature commonly referred to as ‘microglial nodules’ (Prineas et al. 2001; Singh et al. 2013). In addition, microglia have been proposed to behave as APCs in MS. They were shown to express MHC class II, display antigens on their cellular surface, and colocalize with CD4+ cells before the onset of EAE, and the infiltration of myeloid cells in bone marrow chimera in vivo (Ponomarev et al. 2005). These observations suggest a possible role for microglia in the activation of T cells in MS, or in their reactivation following antigen presentation in the periphery (see Chap. 5 for more information on both processes), although direct evidence remains to be shown. In addition, microglia and macrophages contained phagocytosed myelin debris around the white matter demyelinating lesions in MS postmortem samples, highlighting their possible involvement, whether detrimental or beneficial, to the demyelination and axonal degeneration (Tanaka et al. 1975; Bauer et al. 1994; also see Napoli and Neumann 2010).
Moreover, it has been reported that preventing microglial activation could repress the development of EAE in vivo (Heppner et al. 2005). In particular, Heppner and colleagues have generated a mouse model expressing the herpes simplex virus thymidine kinase (HSVTK) specifically in microglia/macrophages, under the CD11b promoter, thus rendering these cells susceptible to ganciclovir cytotoxicity. Following transplantation of wild-type bone-marrow cells, to spare the peripheral myeloid cell population from ganciclovir treatment, and the subsequent peripheral injection of ganciclovir, microglial transformation to amoeboid morphologies was found to be arrested, a phenomenon referred to as “microglial paralysis”, which resulted in delayed EAE onset and reduced clinical score (Heppner et al. 2005). These findings strongly suggested that microglia could play a significant role in the pathogenesis of EAE and MS. The focus of this chapter is on microglial implication in multiple immunological aspects of the disease pathogenesis, including antigen presentation, inflammation, demyelination, and neurotoxicity.
2 Microglia as Antigen-Presenting Cells
Dendritic cells (DCs), which are monocyte-derived cells considered as ‘professional’ APCs, are often encountered in the leptomeninges and white matter lesions of MS patients (Ganguly et al. 2013; Nuyts et al. 2013). However, microglial cells could also behave as APCs in MS as will be discussed below (Smith et al. 1998) (see Chap. 5 for further reading). After the phagocytosis of antigens, such as myelin peptides, APCs become engaged in antigen presentation through MHC class II signaling to CD4+ cells, which express the cognate T cell receptor, leading to their activation (or reactivation) and differentiation into various Th effector T cell subsets, such as the pro-inflammatory, encephalitogenic Th1 and Th17 cells. Costimulatory molecules such as CD80 and CD86 on APCs, or the CD40 ligand (CD40L) expressed on T cells further contribute to activating Th cells via CD28 (member of the B7 family), or to activating APCs via CD40, to promote cellular expansion and survival. Conversely, the costimulatory molecules-programmed cell death-ligand 1 (PD-L1) and PD-L2 suppress Th cell activation by acting on their programmed cell death 1 (PD-1) receptor (Keir et al. 2008; Elgueta et al. 2009).
During normal physiological conditions, microglia express undetectable to low levels of MHC class II molecules (Wong et al. 1984; Suzumura et al. 1987) and constitutively express low levels of CD80 and high levels of CD86 (Satoh et al. 1995; Dangond et al. 1997). Over the course of EAE, however, the expression of MHC class II, CD80 and CD86, was found to be upregulated in CD11b+CD45low microglial cells, isolated from the brains of EAE mice by flow cytometry (Ponomarev et al. 2005; Murphy et al. 2010). Expression of MHC class II is also induced in cultured microglia/macrophages upon stimulation with interferon (IFN)-γ (Suzumura et al. 1987). This pro-inflammatory cytokine produced by Th1 cells, T cells expressing the CD8 glycoprotein (CD8+ T cells), macrophages, DCs, and microglial cells in culture, is well known for promoting immune responses against viral and bacterial infection, as well as the development of tumors (Munder et al. 1998; Kawanokuchi et al. 2006; Vremec et al. 2007). Additionally, treatment of cultured microglial cells with the supernatant from IFNγ-producing Th1 cell lines, specific for MBP, particularly induced microglial expression of MHC class II, CD80, CD86, and CD40 in vitro (Seguin et al. 2003). The binding of CD40 to CD40L induces APCs to produce pro-inflammatory mediators such as TNF-α, IL-6, and IL-12 in vitro (Aloisi et al. 1999; Rezai-Zadeh et al. 2008). When cultured in the presence of IFNγ-stimulated microglial cells, the proliferative capacity of Th cells is additionally increased and accompanied by an enhanced production of IL-2 and IFNγ in vitro (Aloisi et al. 1998). On the other hand, IFNγ also induces the expression of PD-L1 on microglia, while suppressing Th cell activation and the production of IFNγ in vitro (Magnus et al. 2005). Together, these findings suggest that IFNγ-stimulated microglial cells may not only activate Th cells via the induction of CD80 and CD86, or CD40, but also suppress T cell activation via the induction of PD-L1 expression, at least in vitro.
The cytokine granulocyte macrophage colony-stimulating factor (GM-CSF), which is secreted by T cells, as well as astrocytes and macrophages in vitro (Ohno et al. 1990; Shi et al. 2006), may also play a critical role in the induction of APCs functions in microglia (Matyszak et al. 1999). During EAE, MHC class II expression is considerably reduced ex vivo in microglial cells derived from GM-CSF-deficient mice, compared to wild-type mice (Ponomarev et al. 2007; Codarri et al. 2011), suggesting that GM-CSF regulates microglial expression of MHC class II. Interestingly, GM-CSF also promotes DCs-like properties in microglia ex vivo and in vivo, enhancing their expression of the DCs marker CD11c, as well as MHC class II, CD80, and CD86 (Schermer and Humpel 2002; Li et al. 2011). GM-CSF-stimulated microglia also have the ability to induce the proliferation and reactivation of CD4+ T cells in vitro (Fischer et al. 1993; Aloisi et al. 2000). In GM-CSF-stimulated microglia, however, the levels of MHC class II are lower than in DCs and associated with reduced Th cells proliferation (Lambert et al. 2008), in agreement with the view that microglia have limited antigen-presenting capacity (Ransohoff and Engelhardt 2012). Therefore, GM-CSF-stimulated microglia could be used for stimulating adaptive immune responses, such as antigen presentation, in EAE and MS, albeit with a limited capacity as compared to monocyte-derived DCs.
3 Microglia as Inflammatory Cells
Activated microglial cells produce a variety of cytokines and monokines (i.e., cytokines mainly produced by monocytes/macrophages) involved in mediating neuroinflammation. In particular, the secretion of IL-1β, IL-6, and tumour necrosis factor α (TNFα) (Fig. 16.1) is upregulated in cultured microglia upon direct contact with MBP-primed T cells (Dasgupta et al. 2005), while their production of TNFα, IL-6, and IL-12 is enhanced by antigen presentation to CD4+ T cells via the CD40-CD40L signaling pathway in vitro (Rezai-Zadeh et al. 2008; Aloisi et al. 1998). These findings suggest that microglial interactions with T cells could influence their contribution to the neuroinflammatory milieu in EAE and MS, specifically by modulating their release of TNFα, IL-1β, IL-6, and IL-12 as discussed below.
The role of microglia in neuroinflammation. Microglia produce monokines including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 when stimulated with CD40 ligand (CD40L) expressed on activated T cells and/or T cell-derived cytokines. In addition, activated microglia produce a variety of chemokines and induce inflammatory cell infiltration. Monokines activate microglia and astrocytes to induce the production of chemokines, leading to the infiltration of inflammatory cells including T cells, monocytes/macrophages, and neutrophils. IL-1β further disrupts blood–brain barrier (BBB) and induces the production of the chemokine monocyte chemoattractant protein (MCP)-1, which contributes to the infiltration of inflammatory cells. However, TNF-α also induces microglial production of IL-10 via the TNF receptor (TNFR)2, thus exerting anti-inflammatory responses as well
-
1.
TNFα is produced in the MS/EAE brain by microglia as well as macrophages (Renno et al. 1995) and functions as a pro-inflammatory mediator in the CNS, by inducing the production of chemokines (or chemotactic cytokines; mediating the attraction of their responsive cells) such as IL-8, macrophage inflammatory protein (MIP)-1α and MIP-1β in cultured human microglia (Ehrlich et al. 1998; McManus et al. 1998), and the production of monocyte chemoattractant protein (MCP)-1 and regulated on activation, normal T cell expressed and secreted (RANTES) in cultured rat astrocytes (Guo et al. 1998). MIP-1α, MIP-1β, MCP-1, and RANTES are chemotactic for T cells, macrophages, and microglia. Interestingly, in mice devoid of CNS expression of TNF receptor 1 (TNFR1), the recruitment of macrophages and granulocytes is reduced over the course of EAE, induced using MOG-reactive T cells. The levels of MCP-1 and MIP-2, i.e., a chemotactic factor for neutrophils produced by macrophages and microglia, were also found to be reduced in the CNS of these mice (Gimenez et al. 2006). These findings suggest that TNFα could enhance neuroinflammation in EAE and MS by acting on the infiltration of inflammatory cells from the periphery, and the induction of additional chemokines in microglia or astrocytes. Consistently, transgenic mice over-expressing TNFα were shown to spontaneously develop an inflammatory demyelinating disease characterized by the activation of astrocytes and microglial cells, together with the infiltration of CD4+ and CD8+ T cells into the meninges and CNS parenchyma (Probert et al. 1995). TNFα-deficient mice also displayed a delayed EAE onset, but similar to higher levels of EAE severity were observed in later phases of the disease (Kassiotis et al. 1999), thus suggesting that TNFα could exert distinct roles, either detrimental or beneficial, depending on the stage of disease progression.
The functions of TNFα are exerted via either TNFR1 or TNFR2 expressed on various types of cells (Dopp et al. 1997; Baker and Reddy 1998; Tracey et al. 2008; Martin et al. 2014). In the healthy CNS, these receptors are found on neurons, astrocytes, and oligodendrocytes (Yang et al. 2002; Kuno et al. 2006; Faustman and Davis 2013). In EAE/MS, the infiltrated lymphocytes, neutrophils, macrophages, and MHC class II positive cells additionally express TNFR1 and TNFR2 around EAE lesions (Kahn et al. 1999), while oligodendrocytes express TNFR1 around MS lesions (Probert et al. 2000). TNFR1, but not TNFR2, contains a death domain. The affinity of TNFα for TNFR1 is significantly greater than for TNFR2 (Grell et al. 1998). In previous reports, TNFR1-deficient mice were found to be resistant to MOG-induced EAE, whereas TNFR2-deficient mice displayed enhanced CD4+ and F4/80+ cells infiltration in vivo, together with an exacerbated EAE outcome (Eugster et al. 1999; Suvannavejh et al. 2000). In addition, TNFR2 stimulation promotes microglial expression of the anti-inflammatory cytokine IL-10 in vitro (Veroni et al. 2010). The findings suggest that TNFα could mediate neuroinflammation in MS and EAE, through the activation of TNFR1, and anti-inflammatory responses via TNFR2.
-
2.
IL-1β is also detected in microglial cells and macrophages during EAE (Bauer et al. 1993; Cash et al. 1994). MBP-primed T cells induce the production of IL-1β from murine microglial cells and macrophages in vitro (Dasgupta et al. 2005). Microinjection of IL-1β into the CNS reportedly increases BBB permeability, accompanied by a pronounced recruitment of neutrophils (Ferrari et al. 2004). In addition, IL-1β induces the expression of genes favoring blood vessel plasticity, such as the hypoxia-inducible factor 1α (HIF-1α) and its target, vascular endothelial growth factor (VEGF)-A, in cultured human astrocytes. This results in increased BBB permeability through downregulation of the tight junction proteins claudin 5 and occludin in endothelial cells (Argaw et al. 2012). In a mouse model of traumatic brain injury, IL-1β similarly induces the invasion of neutrophils and T cells into the CNS (Clausen et al. 2009). In vitro studies also demonstrate that IL-1β induces MCP-1, IL-8, MIP-1α, and MIP-1β expression in microglial cells (Calvo et al. 1996; Ehrlich et al. 1998; McManus et al. 1998), and that of MCP-1 and RANTES in astrocytes (Hayashi et al. 1995; Barnes et al. 1996). Thus, microglia-derived IL-1β could induce disruption of the BBB, release of chemoattractant mediators from microglia and astrocytes, and infiltration of peripheral inflammatory cells into the CNS. However, the particular involvement of microglia-derived IL-1β as a neuroinflammatory mediator in EAE and MS remains to be tested experimentally.
-
3.
IL-6 is also a potent inducer of microglia-mediated neuroinflammation. In EAE, IL-6 is produced by microglial cells as well as T cells and macrophages (Diab et al. 1997; Wlodarczyk et al. 2014). Many reports have suggested that IL-6 plays an inflammatory role in the pathogenesis of EAE (Erta et al. 2012). Neuron-targeted expression of IL-6 has been shown to induce reactive astrogliosis and microglial activation in transgenic mice in vivo (Fattori et al. 1995). Accordingly, IL-6 also induces MCP-1 mRNA expression by rat microglia in vitro (Calvo et al. 1996). In transgenic mice where the production of IL-6 is restricted to the cerebellum, MOG-induced EAE additionally resulted in the activation of infiltrated macrophages and microglia, accompanied by severe ataxia, enhanced cerebellum infiltration of neutrophils and B cells, and expression of RANTES (or CCL-5), MCP-5 (or CCL-12), and TNFα in situ (Quintana et al. 2009), suggesting its involvement in EAE and MS.
-
4.
IL-12: IFNγ induces the expression of CD40 on microglia (Aloisi et al. 1998), while binding of CD40 to its ligand CD40L induces microglial production of IL-12p70 in vitro (Aloisi et al. 1999). This heterodimeric cytokine, which is composed of the IL-12p35 and IL-12p40 subunits, is a crucial differentiation factor for pro-inflammatory Th1 cells, which can trigger inflammatory responses and activate APCs and cytotoxic T cells to attack their target cells (Knutson and Disis 2005). In addition, microglial cells stimulated with IFNγ in conjunction with the Toll-like receptor (TLR) 4 ligand, bacterial lipopolysaccharide (LPS), produced IL-12p70 and IL-23 in vitro (Suzumura et al. 1998; Sonobe et al. 2005). IL-23 is a heterodimer consisting of p19 and the IL-12p40 subunit. It induces the development of IL-17-producing Th17 cells, another type of pro-inflammatory CD4+ cells, which play a crucial role in the pathogenesis of EAE (Langrish et al. 2005). However, more recent studies have suggested that mice deficient in the IL-12p35 subunit are fully susceptible to EAE (Becher et al. 2002), indicating that factors other than IL-12p70 might be crucial for the induction of EAE. In contrast, p19-deficient mice are reportedly resistant to EAE (Cua et al. 2003), suggesting that IL-23 production is more critical than IL-12p70 production in the CNS. It is important to note that mice in which the IL-23 subunit IL-23p40 is devoid of CNS expression have decreased EAE severity, indicating that IL-23 produced by CNS-resident cells is important for the development of EAE (Becher et al. 2003). Moreover, expression of the IL-23p19 subunit was observed in APCs including microglia and macrophages localized around the lesion sites in MS postmortem brains (Li et al. 2007). Because IFNγ is also involved with the production of IL-23, IFNγ could regulate the differentiation of both Th1 and Th17 cells.
Taken together, these findings suggest that three main monokines secreted by microglia, namely TNF-α, IL-1β, and IL-6, could synergistically ‘activate’ glial cells and promote infiltration of peripheral immune cells in the CNS, as observed in MS and EAE, while IL-12 and IL-23 could mediate differentiation of the encephalitogenic Th cells. Nonetheless, the specific contribution of microglial cells to the release of these monokines, at different stages of MS and EAE, and their ultimate impact on the disease pathogenesis remain unknown.
4 Microglia in Demyelination
Microglial cells are recruited to areas of demyelination in MS/EAE, where they transform their morphology and actively proliferate. Activated phenotypes are also observed in rodents upon feeding with dietary cuprizone, a copper chelator which causes demyelination of the corpus callosum (Remington et al. 2007; Groebe et al. 2009). Recent findings suggest that microglia could be directly involved in the mechanisms of demyelination, by their release of excitotoxic glutamate, reactive oxygen species, pro-inflammatory cytokines, nitric oxide, and mediators of apoptosis as described below (see Fig. 16.2).
The role of microglia in demyelination. Activated microglia produce soluble factors including TNF-α, IL-1β, TNF-related apoptosis-inducing ligand (TRAIL), glutamate, nitric oxide (NO), and reactive oxygen species (ROS), which damage oligodendrocytes via the induction of apoptosis and/or oxidative stress. Alternatively, Fas ligand (FasL) expressed on activated microglia interacts with Fas on oligodendrocytes and induces apoptosis of oligodendrocytes. Abbreviations as in Fig. 16.1
The expression levels of glutaminase, an enzyme that converts glutamine into glutamate, were shown to be increased in microglial cells localized within the active MS lesions in situ (Werner et al. 2001), suggesting a possible role of excitotoxic glutamate released from microglia in the demyelination process. LPS-activated microglia also increase the extracellular glutamate levels and induce the death of oligodendrocytes in vitro (Domercq et al. 2007). IL-1β reportedly induces apoptosis in cultures of oligodendrocytes, astrocytes and microglia, and this effect is blocked pharmacologically by applying antagonists of the AMPA/kainate glutamate receptors (Takahashi et al. 2003). The combined findings suggest that glutamate released from microglia could be directly involved in demyelination during EAE and MS, by inducing toxicity against oligodendrocytes, although this hypothesis remains to be tested.
In the brains of MS patients, DNA oxidation and lipid peroxidation are mainly observed in the nucleus and cytoplasm of oligodendrocytes, respectively, thus suggesting an ongoing state of oxidative stress (Haider et al. 2011). Accordingly, the expression levels of several enzymes controlling the respiratory burst, including the nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) catalytic subunits p91phox, p22phox, and p47phox, were found to be upregulated in MS lesions (Fischer et al. 2012). IFNγ- or GM-CSF-stimulated microglial cells reportedly produce reactive oxygen species (ROS) in vitro (Hu et al. 1995; Smith et al. 1998), showing cytotoxicity against cultured oligodendrocytes (Schreibelt et al. 2007), thus suggesting a possible role for microglial production of ROS in demyelination.
Microglial release of TNFα and IL-1β could be specifically involved in this process. It has been reported that microglia-secreted TNFα induces the death of oligodendrocytes and their progenitor cells in vitro (Zajicek et al. 1992; Pang et al. 2010). Oligodendrocyte-specific ablation of TNFR1 also attenuates the clinical signs of EAE, suggesting that oligodendrocytic TNFR1 could be involved in demyelination (Hovelmeyer et al. 2005). On the other hand, using the cuprizone-induced demyelinating model, Arnett and colleagues revealed that TNFR2, but not TNFR1, is critical for the regeneration of oligodendrocytes in vivo (Arnett et al. 2001). These observations suggest that microglia-derived TNFα could mediate the death of oligodendrocytes via TNFR1, and their regeneration through TNFR2.
In previous reports, IL-1β was also reported to cause demyelination in vivo and in vitro, while IL-1β-stimulated microglia exerted an increased oxidative activity in vitro (Smith et al. 1998), suggesting that IL-1β could damage oligodendrocytes through the release of ROS. In addition, IL-1β degrades intracellular sphingomyelin to ceramide and induces the apoptosis of oligodendrocytes in vitro (Brogi et al. 1997). Sphingomyelin, which mainly consists of ceramide and phosphocholine, is a component of the myelin sheath. Accordingly, administration of the IL-1 receptor antagonist suppressed EAE in rats, while mice deficient in the IL-1 receptor 1 (IL-1R1) showed ameliorated symptoms of EAE (Martin and Near 1995; Sutton et al. 2009). These findings suggest that IL-1β could contribute to demyelination in MS and EAE via the induction of oligodendrocyte apoptosis. On the other hand, IL-1β-deficient mice undergoing the cuprizone-induced demyelination failed to remyelinate, following withdrawal of the dietary cuprizone, suggesting that IL-1β additionally contributes to remyelination (Mason et al. 2001). This failure of remyelination also appears to correlate with a lack of insulin-like growth factor-1 (IGF-1) production by microglia and astrocytes, as their mRNA levels were shown to be reduced in brain extracts from IL-1β-deficient mice (Mason et al. 2001). Since IGF-1 is also required for the differentiation of precursor cells into mature oligodendrocytes, it has been speculated that IL-1β could play a crucial role in remyelination through the induction of microglial and/or astrocytic IGF-1 (Mason et al. 2001). Thus, it is possible that microglial IL-1β is similarly required in MS for remyelination.
Furthermore, in vitro studies have shown that microglia stimulated with IFNγ and/or LPS produce nitric oxide (NO) and the TNF-related apoptosis-inducing ligand (TRAIL) (Chao et al. 1992; Zielasek et al. 1992; Genc et al. 2003). NO reportedly causes single-stranded DNA breaks and mitochondrial damage in oligodendrocytes in vitro (Mitrovic et al. 1994), suggesting another mechanism by which microglia could contribute to demyelination in MS and EAE. In addition, TRAIL, which is a member of the death-signaling molecule family, reportedly induces human oligodendrocyte apoptosis via TRAILR1 in vitro (Matysiak et al. 2002). Thus, IFNγ might also promote demyelination via the production of NO and TRAIL by microglia.
In MS lesions, microglia, infiltrated macrophages, and T lymphocytes were lastly found to express the Fas ligand (FasL), which belongs to the TNF family and induces apoptosis upon binding to its receptor FasR (Dowling et al. 1996; D’Souza et al. 1996). Fas ligation with an anti-Fas antibody or the Fas ligand induced oligodendrocyte cell membrane lysis and subsequent cellular death in vitro (D’Souza et al. 1996). In addition, mice lacking Fas in oligodendrocytes exclusively were reported to be partially resistant to EAE (Hovelmeyer et al. 2005). Further research will test the direct contribution of microglia-derived glutamate, ROS, TNFα, IL-1β, IGF-1, NO, TRAIL, and FasL in the processes of oligodendrocyte apoptosis and regeneration, as well as concomitant demyelination and remyelination in MS.
5 Microglia in Neurodegeneration
MS has long been considered as a chronic inflammatory demyelinating disease of the CNS. However, several lines of evidence also suggest that axonal degeneration in MS and EAE could occur independently from demyelination. Even though axonal degeneration is widespread in the corpus callosum in EAE (Mangiardi et al. 2011), the underlying molecular mechanisms still remain unclear. Howell and colleagues proposed that microglial cells could be involved, since neurodegeneration is accompanied by the presence of activated microglia in MS postmortem brains, showing thicker and shorter processes than observed in control microglia (Howell et al. 2010). The evidence from in vivo and in vitro studies that activated microglia could cause ‘inflammation-induced neurodegeneration’ in MS and EAE, via the release of NO, ROS, glutamate, and various pro-inflammatory cytokines, will be reviewed in the following section.
Neuronal degeneration is induced in cultures of microglia and neurons upon stimulation with LPS and IFNγ and reduced by the addition of NOS inhibitors (Chao et al. 1992). In addition, heat shock protein (HSP)60 induces microglial production of NO via TLR4, in addition to causing extensive axonal loss and neuronal death in vitro (Lehnardt et al. 2008). Lipoteichoic acid (LPA), an agonist of TLR2 derived from staphylococcus aureus, which is involved in pathogen recognition and has been reported to exacerbate both EAE and MS (Gambuzza et al., 2011), also promotes the production of NO and superoxide by microglial cells. LPA additionally induces neuronal death, and this process is blocked by an iNOS inhibitor and a peroxynitrite scavenger in vitro, suggesting a direct detrimental contribution of microglia-derived NO to neuronal death (Kinsner et al., 2005).
In the EAE model, Nikić and colleagues revealed that ROS and reactive nitrogen species (RNS) released from activated macrophages/microglia induce focal axonal degeneration, using two-photon in vivo imaging (Nikić et al. 2011). Microglial expression of p47 phox, a cytosolic subunit of NADPH oxidase, was further shown to be upregulated in the active lesions of MS postmortem brains (Fischer et al. 2012). Microglia stimulated with the nucleotide ATP also released superoxides via P2X7 receptor, a purinergic receptor for ATP, and elicited toxicity against cultured neurons (Mead et al. 2012). It has been shown that blockade of the P2X7 receptor ameliorates EAE (Matute et al. 2007). Interestingly, microglia activated with thrombin, which induces blood coagulation via converting fibrinogen to fibrin, induce oxidative stress resulting in hippocampal neuronal cell death (Choi et al. 2005). Prothrombin kringle-2, a domain of prothrombin distinct from thrombin, also induces the loss of cortical neurons and this effect is partially inhibited by a NADPH oxidase inhibitor (Won et al. 2009). In addition, the leakage of fibrinogen from blood vessels has been shown to activate microglia and further induce axonal damage in EAE, using two-photon in vivo imaging (Davalos et al. 2012) (see Chap. 4 for additional information on these observations). It is possible that these blood-derived proteins enter the CNS through damaged BBB, thus activating microglia and inducing neurodegeneration through the release of ROS. Furthermore, high mobility group box chromosomal protein 1 (HMGB1), a chromatin-associated nuclear protein, has been detected in active lesions of MS and EAE (Andersson et al. 2008). It reportedly induces p47 phox membrane translocation and microglial production of ROS via MAC1, which consists of integrin alpha M and beta 2 (Gao et al. 2011). These findings strongly suggest that ROS produced by microglia, possibly activated by ATP or thrombin, contribute to axonal and neurodegeneration in MS and EAE.
Moreover, microglial stimulation with Chromogranin A, a marker of neurodegeneration that is released from damaged neurons and elevated in the CSF of MS patients (Stoop et al. 2008), induced their production of NO and glutamate in vitro. The conditioned medium from Chromogranin A-stimulated microglia also killed rat cerebellar granule cells via caspase-3-dependent apoptosis, blocked with an ionotropic glutamate receptor antagonist, thus suggesting that microglia induce neuronal death via glutamate release (Kingham et al. 1999). In addition, in vitro studies have suggested that LPS and TNFα could induce neurotoxicity through the release of glutamate from activated microglia in vitro (Takeuchi et al. 2006; Yawata et al. 2008). Neuroinflammation, including microglial TNFα production, is associated with neurodegeneration in EAE (Centonze et al. 2009). Thus, it is possible that microglia-derived glutamate exerts toxicity against neurons in EAE and MS.
6 Conclusion
In EAE and MS, microglial cells function as APCs, albeit possibly at lower levels than professional APCs such as DCs (Fig. 16.3). IFNγ and GM-CSF induce expression of MHC class II and costimulatory molecules of the B7 family in microglia, activate (or reactivate) myelin-specific T cells, and enhance neuroinflammation. In addition, microglia are activated by monokines such as TNFα, IL-1β, and IL-6 early in disease. Activated microglia also produce monokines, which in turn act on microglia and astrocytes to further enhance neuroinflammation via the production of cytokines and chemokines. Microglia-derived inflammatory monokines such as, IL-1β and TNFα, and degenerative factors such as ROS, NO, and glutamate could additionally induce demyelination. Furthermore, microglia-derived degenerative factors, such as ROS, NO, and glutamate, could cause inflammation-induced neurodegeneration. Thus, microglial cells could be considered as conductors that orchestrate a plethora of neuroinflammatory phenomena involved in the pathogenesis of EAE and MS. However, many of the discussed studies were performed in vitro. Further investigation is needed to clarify the direct contribution of microglia versus the other inflammatory cells in this exciting field.
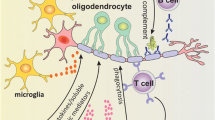
The role of microglia in neuroinflammatory diseases. Microglia are activated by CD4+ T cells via CD40L–CD40 interactions and/or soluble factors. In some cases, activated microglia express major histocompatibility complex (MHC) class II and costimulatory molecules including CD80 and CD86, and possibly behave as antigen presenting cells (APCs) that restimulate infiltrating CD4+ cells. In another cases, activated microglia produce monokines such as TNF-α, IL-1β, and IL-6, which contribute to the activation of astrocytes and bystander microglial cells. Astrocytes and microglia activated by the monokines produce chemokines including monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, MIP-1β, regulated on activation, normal T cell expressed and secreted (RANTES), and IL-8 which induce chemotaxis of various immune cells including monocytes/macrophages, neutrophils, CD4+, and CD8+ T cells. Activated microglial cells could also induce demyelination via FasL-Fas interactions, and the production of TNF-α, IL-1β, NO, glutamate, and ROS. Moreover, activated microglia could further induce neurodegeneration via the production of NO, glutamate, and ROS. Abbreviations as in Figs. 16.1 and 16.2
References
Aloisi F, Ria F, Penna G et al (1998) Microglia are more efficient than astrocytes in antigen processing and in Th1 but not Th2 cell activation. J Immunol 160(10):4671–4680
Aloisi F, Penna G, Polazzi E et al (1999) CD40–CD154 interaction and IFNgamma are required for IL-12 but not prostaglandin E2 secretion by microglia during antigen presentation to Th1 cells. J Immunol 162(3):1384–1391
Aloisi F, De Simone R, Columba-Cabezas S et al (2000) Functional maturation of adult mouse resting microglia into an APC is promoted by granulocyte-macrophage colony-stimulating factor and interaction with Th1 cells. J Immunol 164(4):1705–1712
Andersson A, Covacu R, Sunnemark D et al (2008) Pivotal advance: HMGB1 expression in active lesions of human and experimental multiple sclerosis. J Leukoc Biol 84(5):1248–1255
Argaw AT, Asp L, Zhang J et al (2012) Astrocyte-derived VEGF-A drives blood–brain barrier disruption in CNS inflammatory disease. J Clin Invest 122(7):2454–2468
Arnett HA, Mason J, Marino M et al (2001) TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 4(11):1116–1122
Baker SJ, Reddy EP (1998) Modulation of life and death by the TNF receptor superfamily. Oncogene 17(25):3261–3270
Barnes DA, Huston M, Holmes R et al (1996) Induction of RANTES expression by astrocytes and astrocytoma cell lines. J Neuroimmunol 71(1–2):207–214
Bauer J, Berkenbosch F, Van Dam AM et al (1993) Demonstration of interleukin-1 beta in Lewis rat brain during experimental allergic encephalomyelitis by immunocytochemistry at the light and ultrastructural level. J Neuroimmunol 48(1):13–21
Bauer J, Sminia T, Wouterlood FG et al (1994) Phagocytic activity of macrophages and microglial cells during the course of acute and chronic relapsing experimental autoimmune encephalomyelitis. J Neurosci Res 38(4):365–375
Becher B, Durell BG, Noelle RJ (2002) Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest 110(4):493–497
Becher B, Durell BG, Noelle RJ (2003) IL-23 produced by CNS-resident cells controls T cell encephalitogenicity during the effector phase of experimental autoimmune encephalomyelitis. J Clin Invest 112(8):1186–1191
Brogi A, Strazza M, Melli M et al (1997) Induction of intracellular ceramide by interleukin-1 beta in oligodendrocytes. J Cell Biochem 66(4):532–541
Calvo CF, Yoshimura T, Gelman M et al (1996) Production of monocyte chemotactic protein-1 by rat brain macrophages. Eur J Neurosci 8(8):1725–1734
Cash E1, Minty A, Ferrara P, Caput D, Fradelizi D, Rott O (1994) Macrophage-inactivating IL-13 suppresses experimental autoimmune encephalomyelitis in rats. J Immunol 153(9):4258–4267
Centonze D, Muzio L, Rossi S et al (2009) Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci 29(11):3442–3452
Chao CC, Hu S, Molitor TW et al (1992) Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol 149(8):2736–2741
Chastain EM, Duncan DS, Rodgers JM et al (2011) The role of antigen presenting cells in multiple sclerosis. Biochim Biophys Acta 1812(2):265–274
Choi SH, Lee DY, Kim SU et al (2005) Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: role of microglial NADPH oxidase. J Neurosci 25(16):4082–4090
Clausen F, Hanell A, Bjork M et al (2009) Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur J Neurosci 30(3):385–396
Codarri L, Gyulveszi G, Tosevski V et al (2011) RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol 12(6):560–567
Cua DJ, Sherlock J, Chen Y et al (2003) Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421(6924):744–748
D’Souza SD, Bonetti B, Balasingam V et al (1996) Multiple sclerosis: Fas signaling in oligodendrocyte cell death. J Exp Med 184(6):2361–7230
Dangond F, Windhagen A, Groves CJ et al (1997) Constitutive expression of costimulatory molecules by human microglia and its relevance to CNS autoimmunity. J Neuroimmunol 76(1–2):132–138
Dasgupta S, Jana M, Liu X et al (2005) Myelin basic protein-primed T cells of female but not male mice induce nitric-oxide synthase and proinflammatory cytokines in microglia: implications for gender bias in multiple sclerosis. J Biol Chem 280(38):32609–32617
Davalos D, Ryu JK, Merlini M et al (2012) Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun 3:1227
Diab A, Zhu J, Xiao BG et al (1997) High IL-6 and low IL-10 in the central nervous system are associated with protracted relapsing EAE in DA rats. J Neuropathol Exp Neurol 56(6):641–650
Domercq M, Sanchez-Gomez MV, Sherwin C et al (2007) System xc- and glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. J Immunol 178(10):6549–6556
Dopp JM, Mackenzie-Graham A, Otero GC et al (1997) Differential expression, cytokine modulation, and specific functions of type-1 and type-2 tumor necrosis factor receptors in rat glia. J Neuroimmunol 75(1–2):104–112
Dowling P, Shang G, Raval S et al (1996) Involvement of the CD95 (APO-1/Fas) receptor/ligand system in multiple sclerosis brain. J Exp Med 184(4):1513–1518
Ehrlich LC, Hu S, Sheng WS et al (1998) Cytokine regulation of human microglial cell IL-8 production. J Immunol 160(4):1944–1948
Elgueta R, Benson MJ, de Vries VC et al (2009) Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 229(1):152–172
Erta M, Quintana A, Hidalgo J et al (2012) Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci 8(9):1254–1266
Eugster HP, Frei K, Bachmann R et al (1999) Severity of symptoms and demyelination in MOG-induced EAE depends on TNFR1. Eur J Immunol 29(2):626–632
Fattori E, Lazzaro D, Musiani P et al (1995) IL-6 expression in neurons of transgenic mice causes reactive astrocytosis and increase in ramified microglial cells but no neuronal damage. Eur J Neurosci 7(12):2441–2449
Faustman DL, Davis M (2013) TNF receptor 2 and disease: autoimmunity and regenerative medicine. Front Immunol 4:478
Ferrari CC, Depino AM, Prada F et al (2004) Reversible demyelination, blood–brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain. Am J Pathol 165(5):1827–1837
Fischer HG, Nitzgen B, Germann T et al (1993) Differentiation driven by granulocyte-macrophage colony-stimulating factor endows microglia with interferon-gamma-independent antigen presentation function. J Neuroimmunol 42(1):87–95
Fischer MT, Sharma R, Lim JL et al (2012) NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 135(Pt 3): 886–899
Gambuzza M, Licata N, Palella E et al (2011) Targeting Toll-like receptors: emerging therapeutics for multiple sclerosis management. J Neuroimmunol 239(1–2):1–12
Ganguly D, Haak S, Sisirak V et al (2013) The role of dendritic cells in autoimmunity. Nat Rev Immunol 13(8):566–577
Gao HM, Zhou H, Zhang F et al (2011) HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci 31(3):1081–1092
Genc S, Kizildag S, Genc K et al (2003) Interferon gamma and lipopolysaccharide upregulate TNF-related apoptosis-inducing ligand expression in murine microglia. Immunol Lett 85(3):271–274
Gimenez MA, Sim J, Archambault AS et al (2006) A tumor necrosis factor receptor 1-dependent conversation between central nervous system-specific T cells and the central nervous system is required for inflammatory infiltration of the spinal cord. Am J Pathol 168(4):1200–1209
Grell M, Wajant H, Zimmermann G et al (1998) The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A 95(2):570–575
Groebe A, Clarner T, Baumgartner W et al (2009) Cuprizone treatment induces distinct demyelination, astrocytosis, and microglia cell invasion or proliferation in the mouse cerebellum. Cerebellum 8(3):163–174
Guo H, Jin YX, Ishikawa M et al (1998) Regulation of beta-chemokine mRNA expression in adult rat astrocytes by lipopolysaccharide, proinflammatory and immunoregulatory cytokines. Scand J Immunol 48(5):502–508
Haider L, Fischer MT, Frischer JM et al (2011) Oxidative damage in multiple sclerosis lesions. Brain 134(Pt 7):1914–1924
Haines JL, Ter-Minassian M, Bazyk A et al (1996) A complete genomic screen for multiple sclerosis underscores a role for the major histocompatability complex. The Multiple Sclerosis Genetics Group. Nat Genet 13(4):469–471
Haines JD, Inglese M, Casaccia P (2011) Axonal damage in multiple sclerosis. Mt Sinai J Med 78(2):231–243
Hayashi M, Luo Y, Laning J et al (1995) Production and function of monocyte chemoattractant protein-1 and other beta-chemokines in murine glial cells. J Neuroimmunol 60(1–2):143–150
Heppner FL, Greter M, Marino D et al (2005) Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med 11(2):146–152
Hovelmeyer N, Hao Z, Kranidioti K et al (2005) Apoptosis of oligodendrocytes via Fas and TNF-R1 is a key event in the induction of experimental autoimmune encephalomyelitis. J Immunol 175(9):5875–5884
Howell OW, Rundle JL, Garg A et al (2010) Activated microglia mediate axoglial disruption that contributes to axonal injury in multiple sclerosis. J Neuropathol Exp Neurol 69(10):1017–1033
Hu S, Sheng WS, Peterson PK et al (1995) Cytokine modulation of murine microglial cell superoxide production. Glia 13(1):45–50
Jack C, Ruffini F, Bar-Or A et al (2005) Microglia and multiple sclerosis. J Neurosci Res 81(3):363–373
Jadidi-Niaragh F, Mirshafiey A (2011) Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand J Immunol 74(1):1–13
Kahn MA, Dopp JM, Liva S et al (1999) Temporal kinetics and cellular phenotype of TNF p55/p75 receptors in experimental allergic encephalomyelitis. J Neuroimmunol 95(1–2):19–34
Kassiotis G, Pasparakis M, Kollias G et al (1999) TNF accelerates the onset but does not alter the incidence and severity of myelin basic protein-induced experimental autoimmune encephalomyelitis. Eur J Immunol 29(3):774–780
Kawakami N, Bartholomäus I, Pesic M et al (2012) An autoimmunity odyssey: how autoreactive T cells infiltrate into the CNS. Immunol Rev 248(1):140–55
Kawanokuchi J, Mizuno T, Takeuchi H et al (2006) Production of interferon-gamma by microglia. Mult Scler 12(5):558–564
Keir ME, Butte MJ, Freeman GJ et al (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704
Kingham PJ, Cuzner ML, Pocock JM (1999) Apoptotic pathways mobilized in microglia and neurones as a consequence of chromogranin A-induced microglial activation. J Neurochem 73(2):538–547
Kinsner A, Pilotto V, Deininger S et al (2005) Inflammatory neurodegeneration induced by lipoteichoic acid from Staphylococcus aureus is mediated by glia activation, nitrosative and oxidative stress, and caspase activation. J Neurochem 95(4):1132–1143
Knutson KL, Disis ML (2005) Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother 54(8):721–728
Kuno R, Yoshida Y, Nitta A (2006) The role of TNF-alpha and its receptors in the production of NGF and GDNF by astrocytes. Brain Res 1116(1):12–18
Lambert C, Desbarats J, Arbour N et al (2008) Dendritic cell differentiation signals induce anti-inflammatory properties in human adult microglia. J Immunol 181(12):8288–8297
Langrish CL, Chen Y, Blumenschein WM et al (2005) IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201(2):233–240
Lassmann H (2010) Axonal and neuronal pathology in multiple sclerosis: what have we learnt from animal models. Exp Neurol 225(1):2–8
Lassmann H, van Horssen J (2011) The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett 585(23):3715–3723
Lehnardt S, Schott E, Trimbuch T et al (2008) A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci 28(10):2320–2331
Li Y, Chu N, Hu A et al (2007) Increased IL-23p19 expression in multiple sclerosis lesions and its induction in microglia. Brain 130(Pt 2):490–501
Li H, Sonobe Y, Tabata H et al (2011) Tumor necrosis factor-α promotes granulocyte-macrophage colony-stimulating factor-stimulated microglia to differentiate into competent dendritic cell-like antigen-presenting cells. Clin Exp Neuroimmunol 2(1):1–11
Magnus T, Schreiner B, Korn T et al (2005) Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS. J Neurosci 25(10):2537–2546
Mangiardi M, Crawford DK, Xia X et al (2011) An animal model of cortical and callosal pathology in multiple sclerosis. Brain Pathol 21(3):263–278
Marik C, Felts PA, Bauer J et al (2007) Lesion genesis in a subset of patients with multiple sclerosis: a role for innate immunity? Brain 130(Pt 11):2800–2815
Martin D, Near SL (1995) Protective effect of the interleukin-1 receptor antagonist (IL-1ra) on experimental allergic encephalomyelitis in rats. J Neuroimmunol 61(2):241–245
Martin EM, Remke A, Pfeifer E et al (2014) TNFR2 maintains adequate IL-12 production by dendritic cells in inflammatory responses by regulating endogenous TNF levels. Innate Immun. doi:10.1177/1753425913506949
Mason JL, Suzuki K, Chaplin DD et al (2001) Interleukin-1beta promotes repair of the CNS. J Neurosci 21(18):7046–7052
Matute C, Torre I, Perez-Cerda F et al (2007) P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J Neurosci 27(35):9525–9233
Matysiak M, Jurewicz A, Jaskolski D et al (2002) TRAIL induces death of human oligodendrocytes isolated from adult brain. Brain 125(Pt 11):2469–2480
Matyszak MK, Denis-Donini S, Citterio S et al (1999) Microglia induce myelin basic protein-specific T cell anergy or T cell activation, according to their state of activation. Eur J Immunol 29(10):3063–3076
McManus CM, Brosnan CF, Berman JW (1998) Cytokine induction of MIP-1 alpha and MIP-1 beta in human fetal microglia. J Immunol 160(3):1449–1455
Mead EL, Mosley A, Eaton S et al (2012) Microglial neurotransmitter receptors trigger superoxide production in microglia; consequences for microglial–neuronal interactions. J Neurochem 121(2):287–301
Mehta PD, Patrick BA, Wisniewski HM (1985) Specificity of oligoclonal IgG bands in sera from chronic relapsing experimental allergic encephalomyelitis guinea pigs. J Immunol 134(4):2338–2342
Minagar A, Shapshak P, Fujimura R, Eisdorfer C et al (2002) The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci 202(1–2):13–23
Mitrovic B, Ignarro LJ, Montestruque S et al (1994) Nitric oxide as a potential pathological mechanism in demyelination: its differential effects on primary glial cells in vitro. Neuroscience 61(3):575–585
Montero E, Nussbaum G, Kaye JF et al (2004) Regulation of experimental autoimmune encephalomyelitis by CD4+, CD25+ and CD8+ T cells: analysis using depleting antibodies. J Autoimmun 23(1):1–7
Munder M, Mallo M, Eichmann K et al (1998) Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med 187(12):2103–2108
Murphy AC, Lalor SJ, Lynch MA et al (2010) Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun 24(4):641–651
Napoli I, Neumann H (2010) Protective effects of microglia in multiple sclerosis. Exp Neurol 225(1):24–28
Nikić I, Merkler D, Sorbara C et al (2011) A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med 17(4):495–499
Nuyts AH, Lee WP, Bashir-Dar R et al (2013) Dendritic cells in multiple sclerosis: key players in the immunopathogenesis, key players for new cellular immunotherapies? Mult Scler 19(8):995–1002
Ohno K, Suzumura A, Sawada M et al (1990) Production of granulocyte/macrophage colony-stimulating factor by cultured astrocytes. Biochem Biophys Res Commun 169(2):719–724
Oreja-Guevara C, Wiendl H, Kieseier BC et al (2014) Specific aspects of modern life for people with multiple sclerosis: considerations for the practitioner. Ther Adv Neurol Disord 7(2):137–149
Pang Y, Campbell L, Zheng B et al (2010) Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience 166(2):464–475
Ponomarev ED, Shriver LP, Maresz K et al (2005) Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res 81(3):374–389
Ponomarev ED, Shriver LP, Maresz K et al (2007) GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol 178(1):39–48
Prineas JW, Kwon EE, Cho ES et al (2001) Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol 50(5):646–657
Probert L, Akassoglou K, Pasparakis M et al (1995) Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system-specific expression of tumor necrosis factor alpha. Proc Natl Acad Sci U S A 92(24):11294–11298
Probert L, Eugster HP, Akassoglou K et al (2000) TNFR1 signalling is critical for the development of demyelination and the limitation of T-cell responses during immune-mediated CNS disease. Brain 123(Pt 10):2005–2019
Quintana A, Muller M, Frausto RF et al (2009) Site-specific production of IL-6 in the central nervous system retargets and enhances the inflammatory response in experimental autoimmune encephalomyelitis. J Immunol 183(3):2079–2088
Raivich G, Banati R (2004) Brain microglia and blood-derived macrophages: molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Res Brain Res Rev 46(3):261–281
Rangachari M, Kuchroo K (2013) Using EAE to better understand principles of immune function and autoimmune pathology. J Autoimmun 45:31–39
Ransohoff RM, Engelhardt B (2012) The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 12(9):623–635
Ransohoff RM, Kivisakk P, Kidd G (2003) Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 3(7):569–581
Remington LT, Babcock AA, Zehntner SP et al (2007) Microglial recruitment, activation, and proliferation in response to primary demyelination. Am J Pathol 170(5):1713–1724
Renno T, Krakowski M, Piccirillo C et al (1995) TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J Immunol 154(2):944–953
Rezai-Zadeh K, Ehrhart J, Bai Y et al (2008) Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J Neuroinflammation 5:41
Satoh J, Lee YB, Kim SU (1995) T-cell costimulatory molecules B7-1 (CD80) and B7-2 (CD86) are expressed in human microglia but not in astrocytes in culture. Brain Res 704(1):92–96
Sawcer S, Jones HB, Feakes R et al (1996) A genome screen in multiple sclerosis reveals susceptibility loci on chromosome 6p21 and 17q22. Nat Genet 13(4):464–468
Schermer C, Humpel C (2002) Granulocyte macrophage-colony stimulating factor activates microglia in rat cortex organotypic brain slices. Neurosci Lett 328(2):180–184
Schreibelt G, van Horssen J, van Rossum S et al (2007) Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. Brain Res Rev 56(2):322–330
Seguin R, Biernacki K, Prat A et al (2003) Differential effects of Th1 and Th2 lymphocyte supernatants on human microglia. Glia 42(1):36–45
Shi Y, Liu CH, Roberts AI et al (2006) Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don't know. Cell Res 16(2):126–133
Singh S, Metz I, Armor S et al (2013) Microglial nodules in early multiple sclerosis white matter are associated with degenerating axons. Acta Neuropathol 125(4):595–608
Smith ME, van der Maesen K, Somera FP (1998) Macrophage and microglial responses to cytokines in vitro: phagocytic activity, proteolytic enzyme release, and free radical production. J Neurosci Res 54(1):68–78
Sonobe Y, Yawata I, Kawanokuchi J et al (2005) Production of IL-27 and other IL-12 family cytokines by microglia and their subpopulations. Brain Res 1040(1–2):202–207
Stoop MP, Dekker LJ, Titulaer MK et al (2008) Multiple sclerosis-related proteins identified in cerebrospinal fluid by advanced mass spectrometry. Proteomics 8(8):1576–1585
Sutton CE, Lalor SJ, Sweeney CM et al (2009) Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31(2):331–341
Suvannavejh GC, Lee HO, Padilla J et al (2000) Divergent roles for p55 and p75 tumor necrosis factor receptors in the pathogenesis of MOG(35-55)-induced experimental autoimmune encephalomyelitis. Cell Immunol 205(1):24–33
Suzumura A, Mezitis SG, Gonatas NK et al (1987) MHC antigen expression on bulk isolated macrophage-microglia from newborn mouse brain: induction of Ia antigen expression by gamma-interferon. J Neuroimmunol 15(3):263–278
Suzumura A, Sawada M, Takayanagi T (1998) Production of interleukin-12 and expression of its receptors by murine microglia. Brain Res 787(1):139–142
Takahashi JL, Giuliani F, Power C et al (2003) Interleukin-1beta promotes oligodendrocyte death through glutamate excitotoxicity. Ann Neurol 53(5):588–595
Takeuchi H, Jin S, Wang J et al (2006) Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem 281(30):21362–21368
Tanaka R, Iwasaki Y, Koprowski H (1975) Ultrastructural studies of perivascular cuffing cells in multiple sclerosis brain. Am J Pathol 81(3):467–478
Teutsch SM, Booth DR, Bennetts BH et al (2003) Identification of 11 novel and common single nucleotide polymorphisms in the interleukin-7 receptor-alpha gene and their associations with multiple sclerosis. Eur J Hum Genet 11(7):509–515
Thompson AJ, Polman CH, Miller DH et al (1997) Primary progressive multiple sclerosis. Brain 120(Pt6):1085–1096
Tomioka R, Matsui M (2014) Biomarkers for multiple sclerosis. Intern Med 53(5):361–365
Tracey D, Klareskog L, Sasso EH et al (2008) Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 117(2):244–279
Tran PB, Miller RJ (2003) Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci 4(6):444–455
Veroni C, Gabriele L, Canini I et al (2010) Activation of TNF receptor 2 in microglia promotes induction of anti-inflammatory pathways. Mol Cell Neurosci 45(3):234–244
Vremec D, O’Keeffe M, Hochrein H et al (2007) Production of interferons by dendritic cells, plasmacytoid cells, natural killer cells, and interferon-producing killer dendritic cells. Blood 109(3):1165–1173
Werner P, Pitt D, Raine CS (2001) Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann Neurol 50(2):169–180
Williams A, Piaton G, Lubetzki C (2007) Astrocytes—friends or foes in multiple sclerosis? Glia 55(13):1300–1312
Wlodarczyk A, Løbner M, Cédile O et al (2014) Comparison of microglia and infiltrating CD11c+ cells as antigen presenting cells for T cell proliferation and cytokine response. J Neuroinflammation 11:57
Won SY, Choi SH, Jin BK (2009) Prothrombin kringle-2-induced oxidative stress contributes to the death of cortical neurons in vivo and in vitro: role of microglial NADPH oxidase. J Neuroimmunol 214(1–2):83–92
Wong GH, Bartlett PF, Clark-Lewis I et al (1984) Inducible expression of H-2 and Ia antigens on brain cells. Nature 310(5979):688–691
Yang L, Lindholm K, Konishi Y et al (2002) Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J Neurosci 22(8):3025–3032
Yawata I, Takeuchi H, Doi Y et al (2008) Macrophage-induced neurotoxicity is mediated by glutamate and attenuated by glutaminase inhibitors and gap junction inhibitors. Life Sci 82(21–22):1111–1116
Zajicek JP, Wing M, Scolding NJ et al (1992) Interactions between oligodendrocytes and microglia. A major role for complement and tumour necrosis factor in oligodendrocyte adherence and killing. Brain 115(Pt 6):1611–1631
Zielasek J, Tausch M, Toyka KV et al (1992) Production of nitrite by neonatal rat microglial cells/brain macrophages. Cell Immunol 141(1):111–120
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Sonobe, Y., Suzumura, A. (2014). Multiple Sclerosis. In: Tremblay, MÈ., Sierra, A. (eds) Microglia in Health and Disease. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1429-6_16
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1429-6_16
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1428-9
Online ISBN: 978-1-4939-1429-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)