Abstract
Surfactants are the active ingredients in personal hygiene products, and detergents for industrial and household cleaning. There are four classes (cationic, anionic, amphoteric and nonionic) based on the ionic charge (if present) of the hydrophilic portion of the surfactant in an aqueous solution. The annual global production of surfactants was 13 million metric tons in 2008; about 70 % represent the anionic ones. Surfactants are among the most important components in the group of highly toxic substances that affect environmental conditions in marine ecosystems.
Analysis of surfactants in the environment is important not only because they are toxic, but also for their biodegradation products and metabolites that are more persistent. The routine procedure for surfactant analysis is based on two-phase titration methods. While this method was sensitive, it had many disadvantages such as limitation of application to strongly colored and turbid samples, time consumption, toxicity of organic chlorinated solvent used and formation of emulsion during titration which could disturb visual end-point detection. In view of its disadvantages, other alternative analytical techniques have been developed such as spectrophotometry, thin-layer chromatography and capillary electrophoresis.
However, increasing environmental concerns have fostered the development of automated analytical systems for environmental monitoring with added features for in situ, real-time and remote operation. The use of electrochemical sensors as detectors integrated in automated flow systems has proved to achieve simple, robust and automatic analyzers for environmental monitoring.
This chapter presents an overview of electrochemical techniques applied for the determination of surfactants. Special focusing on both potentiometric and amperometric sensors and biosensors is considered.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Surfactants are the active ingredients in personal hygiene products and detergents for industrial and household cleaning. There are four classes (cationic, anionic, amphoteric, and nonionic) based on the ionic charge (if present) of the hydrophilic portion of the surfactant in an aqueous solution. The annual global production of surfactants was 13 million metric tons in 2008, about 70 % represent anionic ones. Surfactants are among the most important components in the group of highly toxic substances that affect environmental conditions in marine ecosystems.
The determination of surfactants in the environment is important not only because they are toxic, but also for their biodegradation metabolites that are more persistent. The routine procedure for surfactants analysis is based on a two-phase titration method. While this method is sensitive, it has many disadvantages such as limitation of application to strongly colored and turbid samples, time-consuming, toxicity of organic chlorinated solvents used, and formation of emulsions during titration which could disturb the visual end-point detection. In view of its disadvantages, other alternative analytical techniques have been developed such as spectrophotometry, thin layer chromatography, and capillary electrophoresis.
However, increasing environmental concerns have fostered the development of automated analytical systems for environmental monitoring with added features for in situ, real time and remote operation. The use of electrochemical sensors as detectors integrated in automated flow systems has proved to achieve simple, robust, and automatic analyzers for environmental monitoring.
This chapter presents an overview of electrochemical techniques applied for the determination of surfactants. Special focus will be put on both potentiometric and amperometric sensors and biosensors.
1 Introduction
Surfactants form a unique class of organic compounds. A surfactant molecule contains a hydrophilic head group and a hydrophobic chain (or tail). The polar or ionic head group usually interacts strongly with an aqueous environment, in which case it is solvated via dipole–dipole or ion–dipole interactions. Surfactants have a remarkable ability to influence the properties of surfaces and interfaces, and thereby have an impact on industrial processes and products. Applications of surfactants in industry area are quite diverse and have a great practical importance. Surfactants may be used in the production and processing of foods, agrochemicals, pharmaceuticals, personal care and laundry products, petroleum, mineral ores, fuel additives and lubricants, paints, coatings and adhesives, and in photographic films.1–6 They can also be found throughout a wide spectrum of biological systems and medical applications, soil remediation techniques, and other environmental, health, and safety applications.6
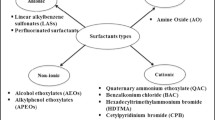
The nature of the polar head group is used to divide surfactants into different categories.6 Anionic surfactants are dissociated in water in an amphiphilic anion, and a cation (metal or quaternary ammonium ions). They are the most commonly used surfactants as they account for about 50 % of the world production. Examples for anionic surfactants include alkylbenzene sulfonates (detergents), soaps (fatty acids), lauryl sulfate (foaming agent), di-alkyl sulfosuccinate (wetting agent), lignosulfonates (dispersants), etc. On second position behind anionic surfactants, nonionic surfactants represent about 45 % of the overall industrial production. They do not ionize in aqueous solution, because their hydrophilic group is of a non-dissociable type, such as alcohol, phenol, ether, ester, or amide. A large proportion of these nonionic surfactants are made hydrophilic by the presence of a polyethylene glycol chain, obtained by polycondensation of ethylene oxide. They are called polyethoxylated nonionics. Cationic surfactants dissociate in water into an amphiphilic cation and an anion, most often of the halogen type. A very large proportion of this class corresponds to nitrogen compounds such as fatty amine salts and quaternary ammoniums, with one or several long chain of the alkyl type, often coming from natural fatty acids.
Surfactants are widely used in everyday personal care and household products as well as in a variety of industrial applications. As a result, large amounts of surfactants are commonly discharged in large quantities to sewage treatment plants or directly to the aquatic environment in areas where there is no sewage treatment. The toxicity and persistence of surfactants is now fairly predictable for a variety of environmental situations and several reviews are available.7–11 Under aerobic conditions many surfactants are readily biodegraded, while anaerobic biodegradation generally proceeds more slowly. The toxicity of surfactants naturally depends greatly upon their structure. Increasing the alkyl chain length in the hydrophobic group will generally increase the toxicity, whereas increasing the ethylene oxide (EO) numbers with the same hydrophobic group will generally decrease the toxicity.6 Because many surfactants are ubiquitous,12 the potential toxic effects of these chemicals have attracted much research attention in the past several decades.13–15
2 Determination of Surfactants
The widespread importance of surfactants in industrial applications, and scientific interest in their nature and properties have been reflected on a wealth of published literature on surfactant analysis.16–31 Probably the most common analytical method for ionic surfactants is Epton’s two-phase titration.32–34 Here, the surfactant is extracted into an organic hydrophobic solvent (CHCl3) when a lipophilic ion-pair formed with the titrant. The latter is generally a surfactant of opposite charge. The titration is carried out in the presence of an ionic dye (or a mixture of ionic dyes) which colors the organic layer differently in the presence of an excess of anionic or cationic surfactants. Nonionic surfactants can be titrated after the addition of an activator to form a charged complex.35 Although this procedure was currently used as a standard method; Gerlache et al.30 reported several drawbacks such as formation of an emulsion during titration (risks of errors in visual end-point detection), lack of efficiency for a surfactant having a short carbon chain length, turbidity of the solution when analyzing a complex sample, toxicity of the chlorinated organic solvent (CHCl3), time-consuming, and difficulty of automation. So it seems necessary to search for alternatives of the aforementioned method in order to increase the laboratory productivity and operator safety and comfort and to reduce drastically the reagents consumption and waste production.
This chapter was intended to provide an overview of different electroanalytical methods for surfactant analysis. Surfactant sensors are usually divided into potentiometric, voltammetric, and amperometric sensors and biosensors. While amperometric sensors and biosensors are very few for the determination of surfactants, potentiometric sensors are the most common due to their simplicity and versatility.
2.1 Potentiometric Surfactant Sensors
Potentiometric surfactant sensors are usually used as titration end-point indicator electrodes, although some direct potentiometric surfactant electrodes have also been reported.20–23,29–31 All surfactant titrations are based on the so-called antagonist reaction, where an ionic surfactant reacts with an oppositely charged ion (mainly surfactant, too) forming a water insoluble salt (ion-pair).21,29 Before the equivalent point, the analyte is in excess and, afterwards, the titrant is in excess. The electrode may be sensitive to the analyte or to the titrant. The electroactive material is usually an ion associate A+B− in the sensing element in which B− is an anionic surfactant and A+ is usually a cationic surfactant.21,29 Simply, the ion associate can be prepared by mixing adequate volumes of an aqueous solution containing the anionic surfactant with a solution containing an equimolar amount of the cationic one. The precipitate is filtered, washed thoroughly with distilled water, and dried under vacuum at 25 °C for at least 24 h; afterwards it is ground to a fine powder. Potentiometric sensors are suitable for the determination of ionic (cationic and anionic) as well as nonionic surfactants after activation of the latter with barium chloride.36
Investigations of surfactant-sensitive potentiometric electrodes began in the 1970s.37 Since surfactant ion-selective electrodes have been developed by Gavach and Seta,38 the development of potentiometric surfactant sensors is an area of interest. Several excellent articles20,21,29,30,39,40 review the use of different types of electrodes for surfactant analysis. When compared with other analytical methods, ion-selective electrodes (ISEs) are simple, relatively inexpensive, robust, durable, and ideal for their use in field environments. Some other advantages involve that they can be used very rapidly, that they allow continuous monitoring, and that they are not affected by turbidity or color of a sample.
In this part, different potentiometric ion-selective electrodes for the determination of surfactants will be discussed including liquid membrane electrodes, conventional polyvinyl chloride membrane electrodes (PVC), solid contact electrodes, carbon paste electrodes (CPEs), and more recently disposable screen-printed electrodes (SPEs).
2.1.1 Liquid Membrane Electrodes
The customary type of a liquid membrane electrode is a design in which the sensitive membrane is composed of a water-immiscible organic solvent containing the ion of interest in the form of ion associate. The membrane is interposed between a standard (internal) and a test (external) ion solution.41–43 In early types of liquid membrane electrodes, an organic phase as a membrane is placed between two aqueous phases in bulk or with the support of a thin, porous cellulose sheet, sintered glass, or similar lamellas. Nitrobenzene is the popular membrane solvent; other organic solvents are also applied such as o-nitrotoluene, 4-ethylnitrobenzene, 4-nitro-m-xylene, and p-nitrocoumarin.
Gavach et al.44 were probably the first to apply liquid membrane-based ion-selective electrodes (ISEs) for the titration of long chain alkyl methyl ammonium salts with sodium tetraphenyl borate. Birch et al.45,46 were also among the first researchers to use liquid ion-exchange electrodes responsive to ionic surfactants in 1972.
A solution of a surfactant ion associate dissolved in a hydrophobic organic solvent (nitroaromatic derivatives) was used as a sensitive liquid membrane electrode for the determination of surfactants.25,26 The inner body of the electrode was filled with a chloride solution of a diluted anionic surfactant and a Ag/AgCl reference electrode was dipped into this internal solution.
Ishibashi et al.47 used a Crystal Violet salt in nitrobenzene or 1,2-dichloromethane as the active sensing part for aromatic sulfonates. As reported, the presence of hydrophobic groups in the Crystal Violet molecule limited the dissolution of the sensing solution in aqueous samples.
A comparison of the results obtained with surfactant selective electrodes48 and the two-phase titration procedure reveals great accuracy for both techniques. However, the analysis of commercial cosmetic products and washing powders gave better results with the potentiometric titration method.
Sap et al.49 used an Fe (I1) bis [2,4,6-tri(2-pyridyl)]-1,3,5-triazine complex embedded in the sensor for the determination of dodecyl sulfate (DS). Linear response was in the concentration range between 7 × 10−6 and 1.5 × 10−6 mol L−1. No interference from inorganic ions was noticed while some other organic ions did. The potential jump magnitude increases with the alkyl chain length of the surfactant (formation of a more hydrophobic ion-pair).
A supported liquid and a PVC-based membrane selective for dodecyl sulfate (DS−) ion was described by Arvand-Barmchia et al.50 The electroactive element was a membrane containing a dissolved ion association complex of DS− with cetylpyridinium (CP+) cation dissolved in acetophenone. Nernstian response towards the DS− anion was achieved over the concentration range from 8.3 × 10−3 to 1.0 × 10−6 M at 25 °C. The proposed electrode also showed good selectivity and precision (RSD about 2.0 %), and was usable within the pH range of 4.0–6.8. The liquid membrane electrode could find application in the direct determination of DS by the standard addition method at pH 5.0, and exhibited useful analytical characteristics for the determination of sodium dodecyl sulfate (SDS) in detergents and real samples.
Two major problems encountered with liquid membrane electrodes are the drift of the potentiometric signal with time and a risk of membrane dissolution in the aqueous phase when the concentration of the surfactant approaches the critical micelle concentration (CMC).
2.1.2 Conventional Polymeric Membrane ISEs (PVC)
Moody et al.51 introduced a more convenient approach for liquid membrane electrodes, where the membrane components (electroactive material, supporting polymer and plasticizer) were dissolved in tetrahydrofuran or cyclohexanone at room temperature. The cocktail was poured in a Petri dish, where the slow evaporation of the solvent left a flexible master sheet of 10–100 μm thickness. The membrane was cut with a cork borer and mounted at the end of a plastic tube. The electrode body was filled with a standard internal solution of the target ion and saturated KCl as desired for establishing the potential of the internal reference electrode. Since these membranes contained ~70 % (w/w) plasticizer and because the plasticized polymer behaved like a viscous liquid, the properties of the electrode were very similar to the original liquid membrane electrode. However, the polymeric matrix could be considered as a microporous net or a close netting, respectively, the vacancies of which were occupied by the plasticizer in which the electroactive material was dissolved.41 Many polymers were used as a polymeric support such as polyvinyl chloride (PVC), polyurethane, silicone rubber, polystyrene, and polymethyl methacrylate.52 PVC was the most commonly used polymer matrix as it gave homogeneous, solid and flexible membranes with electrochemical compatibility with a host of sensor material cocktails based on liquid ion exchanger and their appropriate plasticizing solvent mediators.53 The role of plasticizers in plastic membrane electrode might be considered analogous to that of organic solvent in liquid membrane electrodes, as it would determine the electrode selectivity towards the ion of interest, the slope of the calibration graph, as well as the membrane resistance.
Moody and coworkers54,55 and Buschmann and coworkers36,39 were leaders in constructing efficient surfactant sensors. ISEs containing PVC membranes with ortho-nitrophenyloctyl ether (o-NPOE) as a plasticizer were suitable for the determination of amphoteric (ZS) and cationic (CS) surfactants. At pH < 1.5, the ZS reacts as a CS and can be titrated by a bulky counter ion such as tetraphenylborate.56 Complexation of the ethoxylated part of nonionic surfactants with a bivalent cation such as Ba2+ allows potentiometric titration with tetraphenyl borate.36 Jones et al.54 report a detection limit of 1 × 10−7 mol L−1 for Antarox 880.
A potentiometric flow injection determination method for DS− ion was proposed by utilizing a flow-through type ion-selective electrode detector. The sensing membrane of the electrode was a PVC membrane plasticized with o-NPOE without added ion exchanger.57 A linear relationship was found between peak height and logarithmic concentration of the DS− ion with a Nernstian slope of 52 mV decade−1 in a concentration range from 1.0 × 10−5 to 1.0 × 10−3 mol L−1; the detection limit was 5 × 10−7 mol L−1 and the relative standard deviation 1.3 %. The method was free from the interference of nonionic surfactants and inorganic electrolytes for the determination of the DS− ion. The same author applied a plasticized PVC membrane electrode sensitive to dodecylbenzene sulfonate (DBS) ion for the determination of anionic polyelectrolytes by potentiometric titration, using a solution of (Cat-floc) as a titrant. A linear relationship between the concentration of anionic polyelectrolytes and the end-point volume of the titrant existed in the concentration range from 2 × 10−5 to 4 × 10−4 mol L−1 for potassium polyvinyl sulfate (PVSK), alginate, and carrageenan.58 In a more recent publication, a PVC membrane electrode sensitive to octadecylammonium (stearyltrimethylammonium) ion for the determination of cationic polyelectrolytes or Cat-floc by potentiometric titration was published.51 A linear relationship between the concentration of cationic polyelectrolyte and the end-point volume of the titrant existed in the concentration range from 2 × 10−5 to 4 × 10−4 mol L−1 for Cat-floc, glycol chitosan, and methylglycol chitosan.
A sensor for anionic surfactants with a membrane consisting of 33 % (m:m) PVC, 66 % dioctylphthalate (DOP) plasticizer, and 1 % tridodecylmethylammonium chloride (TDMAC) was developed and used for flow injection analysis.59 The sensor displayed a working response range from 5 × 10−7 to 5 × 10−3 mol L−1 DBS with a Nernstian slope of 58.5 ± 0.2 mV decade−1, a response time of 30 s and a detection limit of 1.5 × 10−7 mol L−1. The sensor was used to measure anionic surfactants (DBS) in different wastewater samples, commercial detergent products, and for monitoring the rate of surfactant biodegradation in sewage treatment plants. The results obtained agreed fairly well with the data obtained by the standard spectrophotometric method.
Three other kinds of ion-selective polymeric membranes (ISEs) sensitive to cationic surfactants were fabricated and characterized by Campanella et al.60 They use benzyldimethylhexadecylammonium-reineckate, dodecyltrimethylammonium-reineckate, or hexadecyl-pyridinium-phosphotungstate as exchanger. ISEs displayed a linearity range for common cationic surfactants between about 10−6 and 10−4 mol L−1, satisfactory fast response (60 s) and moderately sub-Nernstian slope values. An electrode based on PVC membrane containing trioctylhydroxybenzene sulfonic acid as an ion exchanger and dibutylphthalate (DBP) as a plasticizer was used for the determination of low concentrations of cationic surfactants in antiseptic formulations, by potentiometric titration with sodium tetraphenyl borate.61
A cetylpyridinium chloride (CPC)-selective membrane sensor consisting of CPC-ferric thiocyanate ion pairs in a PVC matrix plasticized with DOP was described by Mostafa.62 The electrode showed a stable, near-Nernstian response for 10−3 to 10−6 mol L−1 CPC at 25 °C over the pH range of 1–6 with a slope of 57.5 ± 0.4 mV decade−1. There is negligible interference from many cations, anions, and pharmaceutical excipients; however, cetyltrimethylammonium bromide (CTAB) interfered significantly. The direct determination of CPC in Ezafluor mouthwash gave results that compare favorably with those obtained by the British Pharmacopoeia method.
Badawy et al.63 prepared a cetyltrimethylammonium (CTA) cation sensitive polymeric membrane electrode. The electroactive material was the ion-association complex of the cation (CTA)+ with phosphotungstic acid (PTA). The electrode was applied for the determination of CTAB in aqueous solutions by standard additions and by potentiometric titration with PTA. The response was nearly Nernstian between 3.2 and 830 μM and was unaffected by pH changes. Recoveries of CTAB from a disinfectant solution were 102.8 and 97 % by the standard addition and the potentiometric method, respectively. The same group fabricated a PVC membrane electrode selective for the CTA+ ion using the CTA-trinitrobenzene sulfonate (TNBS) ion pair as electroactive material. The electrode showed a near-Nernstian response within the CTA+ concentration range from 1.08 × 10−6 to 8 × 10−4 M at 25 ± 1 °C, good selectivity and precision (RSD = 1.0 %); it was usable within the pH range 2.5–10.2. The electrode was used for the direct determination of CTAB either by the standard addition method or by potentiometric titration with TNBS at pH 7.64 In another publication, the same author65 prepared a PVC membrane electrode selective for the cetyldimethylethylammonium ion (CDEA). The active element was a plasticized PVC membrane containing the ion associate complex of CDEA with phosphotungstic acid. Near-Nernstian response within the concentration range of l0−6 to 10−4 mol L−1 CDEA was achieved.
A polyvinyl chloride (PVC) membrane66 electrode based on hexadecylpyridinium-phosphotungstate (HDP-PTA) ion associate was constructed for the determination of HDP ion in some antiseptic and disinfecting preparations by standard addition or potentiometric titration methods. The electrode showed a Nernstian response over the concentration range of 6.3 × 10−6 and 3.1 × 10−3 mol L−1 of HDP at 25 °C over a wide pH range.
Patil et al.67 fabricated PVC electrodes sensitive to dodecyltri-methylammonium ions (DTA+) and tetradecyltrimethylammonium ions (TTA+). The electrode was used for determination of critical micelle concentration of TTAB in water. Moreover the electrode was tested in the presence of nonaqueous polar solvents, i.e., dimethylformamide (DMF) and dimethylsulphoxide (DMSO).
A sensitive potentiometric surfactant sensor was prepared based on the highly lipophilic 1,3-didecyl-2-methyl-imidazolium cation in the form of its tetraphenylborate associate.68 The sensor responded fast and showed a Nernstian response for the following surfactants under investigation: CPC, CTAB, and hyamine with slope values of 59.8, 58.6, and 56.8 mV decade−1, respectively. The sensor served as an end-point detector in ion-pair surfactant potentiometric titrations using sodium tetraphenylborate as titrant. Several technical grade cationic surfactants and a few commercial disinfectant products were also titrated, and the results were compared with those obtained from a two-phase standard titration method. The results, compared to those obtained using a commercial surfactant electrode with the standard two-phase titration method, exhibited satisfactory mutual agreement.
A membrane with the hexadecyltrioctadecylammonium-tetraphenylborate (HDTA-TPB) ion pair was used for the preparation of a potentiometric sensor for anionic surfactants, such as DS−.69 The sensor exhibited a Nernstian response of 58.1 mV decade−1 between 3 × 10−7 and 3 × 10−3 mol L−1. The interfering effect of several inorganic and organic anions, most frequently used in formulated products, was investigated. The homologous series of C7–C12 alkane sulfonates and some commercial detergent products were successfully titrated.
More recently,70 a PVC membrane electrode selective to HDTA+ and DS− was constructed using modified single-walled carbon nanotubes (SWCNTs). The membrane electrodes exhibited a Nernstian response (59.5 mV/decade) for DS− and a near-Nernstian response (57.2 mV decade−1) for CTAB over a wide concentration range below their critical micelle concentrations (CMC). The electrodes showed a fast response time (t90 % = 30 s) and could be used for 3 months without any divergence in potentials. The ion-selective electrode could determine SD− down to concentrations as low as 1.9 × 10−6 mol L−1 and CTA+ to 5.2 × 10−6 mol L−1. This method for determining anionic surfactants was found to be quite accurate when compared with classical methods.
An installation for the rapid determination of surfactants in seawater using ion-selective electrodes was developed by Stepanets et al.71 The installation was tested under on-site conditions on shipboard in the course of integrated marine studies of environmental conditions in the Baltic Sea. Maximum marine pollution levels were detected in the near-shore zone; this fact can be explained by waste discharges from sites where surfactants are in wide residential and industrial use.
Generally, potentiometric sensors incorporated with ion-pair associates are plagued by limited selectivity and their applications are restricted to more challenging matrices; therefore more selective molecular recognition components are clearly required. Chemically modified electrodes (CMEs) were suggested for improving the electroanalytical performance through application of molecular recognition species selective to the target analyte.72,73 Different types of molecular recognition elements have been proposed including crown ethers, calixarenes, cyclodextrins (CDs), or porphyrins.74,75 Cyclodextrins are naturally occurring macrocyclic oligosaccharides formed of 1,4-glucosidic bond-linked d(+)glucopyranose oligomers of 6, 7, and 8 glucose units yielding α-, β-, and γ-CDs, respectively. CDs can form inclusion complexes with different types of guest molecules without the formation of chemical bonds or changing their structure, where the binding forces are attributed to a number of weak interactive forces, such as hydrophobic forces, hydrogen bonding, and other factors such as size of the cavity and shape of the guest molecule.76
Functionalized, lipophilic α-, β-, and γ-cyclodextrins were synthesized and their suitability as onium ion-selective potentiometric sensors was investigated. The proposed electrodes showed Nernstian responses for acetylcholine chloride, dopamine hydrochloride, and the surfactant myristyltrimethylammonium bromide (MTMAB). In each instance, the electrode response was substantially enhanced and stabilized by the presence of the lipophilic cyclodextrin.77
A PVC membrane ion-selective electrode suitable for the potentiometric end-point detection in the titration of cationic surfactants was constructed by Khalil et al.78 The active substance of the electrode was the neutral carrier dibenzo-18-crown-6 using diisooctyl phthalate as plasticizer. The electrode had a pH working range from 2.0 to 12.0 with a Nernstian behavior between 6.0 × 10−6 and 1.6 × 10−3 mol L−1 of hyamine 1622. Katsu and Nishimura79 evaluated eight dioxadicarboxylic diamides as ionophores in PVC membrane electrodes for the detection of hexylammonium ions. As reported, near-Nernstian response was achieved and the selectivities were much better than those of the dibenzo-18-crown-6-electrode.
A cyclic aza-oxa-cycloalkane, 7,13-bis(n-octyl)-1,4,10-trioxa-7,13-diazacyclopentadecane (L1), was characterized and its interaction with anionic surfactants studied.80 Different PVC membrane anionic surfactant-selective electrodes were prepared using L1 as ionophore and o-NPOE, bis(2-ethylhexyl) sebacate (BEHS) or DBP as plasticizers. The PVC-based membrane electrode containing o-NPOE as plasticizer showed a Nernstian response with a slope of 57.7 ± 0.2 mV decade−1 for lauryl sulfate (LS) in a concentration range from 3.3 × 10−6 to 6.7 × 10−3 mol L−1 with a detection limit of 2.2 × 10−6 mol L−1. The fabricated electrode was used for the determination of anionic surfactants in several mixtures, and the results obtained were compared to those found using a commercially available electrode. A similar ligand (7-methyl-7,13-di-octyl-1,4,10-trioxa-13-aza-7-azonia-cyclopentadecane) was used as ionophore in the development of a LS ion-selective electrodes.81 The characteristic performances of the sensor were as follows: the potentiometric response was Nernstian with 59.5 mV decade−1 in a range of concentrations from 1.3 × 10−6 to 6.8 × 10−3 mol L−1 with a detection limit of 6.0 × 10−7 mol L−1. Moreover, the cyclam derivative 1,4,8,11-tetra(n-octyl)-1,4,8,11-tetraazacyclotetradecane (L) was used as a carrier for the preparation of PVC-based membrane ion-selective electrodes for anionic surfactants in the presence of tetra-n-octylammonium bromide (TOAB) as cationic additive.82 The electrode composition was as follows: 56 % DBP, 3.4 % ionophore, 3.8 % TOAB, and 36.8 % PVC. This electrode displays a Nernstian slope of 60.0 ± 0.9 mV decade−1 in the concentration range from 2.0 × 10−3 to 7.9 × 10−6 mol L−1 DS− and a poor response to common inorganic cations and anions. The selective sequence found was DS− > ClO4 − > HCO3 − > SCN− > NO3 − ≈ CH3COO− ≈ I− > Cl− > Br− > IO3 − ≈ NO2 − ≈ SO3 2− > HPO4 2− > C2O4 2− > SO4 2−, i.e., basically following the Hoffmeister series except for the hydrophilic anion bicarbonate. The electrode could be used for 144 days without showing significant changes in the value of the slope or the working range. The electrode showed a selective response to DS and a poor response to common inorganic cations and anions.
Although symmetric ISEs have found a wide range of applications,54–83 they still have certain inherent limitations; they are mechanically complex, and thus difficult to be miniaturized. The internal reference solution increases the system’s impedance and the electrode response time, in addition to a shorter lifetime due to leaching of the electroactive material throughout both solutions in contact with the membrane, and finally due to the internal compartment, which cannot withstand high pressure.84
2.1.3 Solid Contact Electrodes
The increased interest in using ISEs has led to the development of new sensor materials that show high selectivity for a variety of anions and cations and new approaches for electrode construction. Several attempts have been made to eliminate the internal reference electrode resulting in a solid-state sensor design. Examples of these types of sensors include coated wire electrodes, graphite rods, graphite-based electrodes, and ion-selective field-effect transistors (ISFETs).
A new kind of all solid-state sensors was first reported by Cattrall and Freiser in 1971 in which the internal reference element was in direct contact with the sensing membrane and thus contained no aqueous solution. The first group of such simplified sensors was those of the so-called coated wire construction type (CWE). In this approach, a metal wire was dipped with a solution of PVC in THF containing also a suitable electroactive material. During evaporation of the solvent, a PVC film on the metal wire surface was formed.85 Although different materials such as platinum, silver or sliver chloride, and aluminum could serve as central conductors, the nature of the wire support had no substantial influence on the electrode performance if it did not react with the membrane components.86
Cottrell was the first who used a PVC-coated wire electrode for the determination of low concentrations of surfactants.87 Extensive articles and excellent overviews on CWEs for the determination of surfactants were published by Vytras’s group, who were very active in developing simple coated wire electrodes for cationic and ampholytic surfactants especially during the 1980s.21,29,43,88–95
A method was described for the determination of nonionic surfactants containing poly(oxyethylene) chains with sodium tetraphenylborate, based on the precipitation of ternary compounds in the presence of bivalent metal ions (barium salts). Titrations were monitored potentiometrically with a simple PVC membrane-coated aluminum wire electrode plasticized with 2,4-dinitrophenyloctyl ether.94
Cations of the homologous series of N-alkyl-N-ethylpyrrolidinium salts were determined with a good precision by a titration using sodium tetraphenylborate using a simple CWE.95 The magnitude of both, the potential break and sharpness at the inflexion point of the titration curve, was predetermined by the solubility of the corresponding ion-pair in the membrane solvent and was affected by the nature of the membrane plasticizer. The results showed that o-NPOE gave the highest ΔE, which might be due to its high polarity. The author reported that the value of the potentiometric titration break depended on the number of carbon atoms in the alkyl chain.
More recently, a new, simple, sensitive, low cost, and rapid potentiometric method for direct determination of ultra-trace amounts of SDS with a new DS− selective electrode was reported.96 The electrode was prepared by electropolymerization of aniline on the surface of a Pt electrode in acidified solutions containing DS− ions. The sensor showed a Nernstian behavior response of 59.0 ± 2.3 mV decade−1 over a very wide linear range from 1.0 × 10−9 to 3.0 × 10−6 mol L−1. The electrode exhibited high selectivity to DS− over other ions and could be used for 4 weeks without any major deviations in the pH range of 3.5–9.8. The proposed electrode was applied to the determination of DS− in mouth washing solution and tap water samples. The potentiometric behavior of coated wire electrodes based on DBS-doped polypyrrole (PPy-DBS) and hyamine as ion exchanger was investigated by Shafiee-Dastjerdi and Alizadeh.97 Two types of coated wire electrodes made of PVC-PPy-DBS and PVC-hyamine-DBS, plasticized with o-NPOE, showed Nernstian behavior (with respective calibration slopes of about 58 and 60 mV per decade) within a concentration range of 3.0 × 10−6 to 1.1 × 10−3 and 5.0 × 10−6 to 1.3 × 10−3 mol L−1 DBS−, respectively. The potentiometric response was independent from the pH of the test solution in the range of 3–10. The response time of electrodes was fast (10 s for both types of electrode), and the electrodes could be used for at least 3 months without any significant changes in the potential. DBS− was determined in some commercial detergents with results in satisfactory agreement with those obtained by a standard method (two-phase titration).
Data for cationic surfactant ions in common cleaners as determined with CWEs were presented by Plesha et al.98 Dinonylnaphthalene sulfonic acid, tetraphenylborate, and tetrakis (4-chlorophenyl)borate were examined as ion exchangers, from which dinonylnaphthalene sulfonic acid showed to be the favorable agent. The ISEs exhibited approximately Nernstian behavior down to the 10−6 mol L−1 with lifetimes over 50 days when used continuously, and a shelf life of over 100 days.
Selig was the first who used a spectroscopic graphite rod coated with a solution of PVC and DOP in tetrahydrofuran for the potentiometric titration process.99 An interesting membrane was constructed by Dowle and coworkers,100,101 where a graphite rod was coated with PVC containing tritolyl phosphate (TPP) and an appropriate ionophore (tetradecylammonium dodecyl sulfate) which is sensitive to anionic surfactants. The fabricated electrode showed a linear measuring range from 10−2 to 10−6 mol L−1 SDS with fast response time of 30 s. The sensor operated well in ethanolic solutions (20 % v/v) and could be used under flow conditions such as in HPLC. The disadvantages were a relatively poor lifetime and the need to calibrate the electrode several times per day.
Nineteen quaternary ammonium salts including CTAB and 25 basic dyes were potentiometrically titrated against Orange IV.102 The indicator electrode was a carbon rod coated with a PVC membrane containing triheptyldodecylammonium iodide (THDAI). The ammonium compounds containing alkyl groups of 14–18 carbon atoms showed well-defined titration curves with sensitivity in the concentration range from 10−5 to 10−4 mol L−1.
An alternative approach for construction of solid contact electrodes can be also applied by casting the sensitive membrane directly on the surface of a conductor such as silver or a conductive carbon resin. Thus, two surfactant sensors were prepared using hyamine 1622 or tetradodecylammonium (TDA) as cationic and DBS as anionic surfactant.103 The sensing materials were incorporated in a PVC matrix containing o-NPOE as a solvent mediator and applied on a support of a conductive resin without inner reference solution. The responses of these electrodes to DS and DBS as well as the interferences of several common inorganic anions and anionic surfactants were examined. The membranes showed good performance for use as a general potentiometric sensor responsive to anionic surfactants.
An automated FIA system with a throughput of 85 samples/h for the determination of low concentration levels of anionic surfactants in river water and wastewater was developed by Saad et al.104 The system used specially constructed tubular flow-through all solid-state ion-selective electrodes as potentiometric sensors and on-line pre-concentration techniques. They showed a general response to anionic surfactants with a lower detection limit of approximately 10−5 mol L−1. Potentially interfering substances such as chloride, nitrate, and nonionic surfactants were proved not to interfere. Matesic-Puac et al.105 prepared an all solid-state surfactant-sensitive electrode based on a teflonized graphite conducting substrate coated with a plasticized PVC membrane containing tetrahexyldecylammonium dodecylsulfate (THDADS) as an anionic surfactant-sensing material. The electrode was used as end-point indicator for potentiometric titrations.
An automated electronic tongue consisting of an array of potentiometric sensors and an artificial neural network (ANN) was developed to resolve mixtures of anionic surfactants. The sensor array was formed by five different flow-through sensors for anionic surfactants, based on polyvinyl chloride membranes having cross-sensitivity features.106
ISFETs work as varieties of CWEs, incorporating the ion sensing membrane directly on the gate of a FET. The construction is based on the technology to prepare small multisensor systems with multiple gates, for sensing several ions simultaneously, while their small size permits the in vivo determination of analytes. An ISFET device selective to anionic detergents, based on a PVC-sebacate membrane, containing benzyldimethylcetylammonium cholate (BSCAC) as the ion exchanger was characterized and applied for the determination of some anionic surfactants by Campanella et al.107 The linearity range extended over 3–4 concentration decades depending on the examined surfactant, within the range between about 10−6 and 10−3 mol L−1 in all cases. The proposed electrode could determine anionic surfactants in standard aqueous solutions and in authentic matrices (lake and sea water). The same author108 described three new ISFET devices based on polymeric selective membranes sensitive to cationic surfactants. These sensors use a PVC-sebacate matrix incorporating as exchanger benzyldimethylhexadecylammonium-reineckate (BDHC-RN), dodecyltrimethylammonium-reineckate (DTA-RN), and hexadecylpyridinium-phosphotungstate (HDP-PT), respectively. A characterization of the ISFETs was performed and the analytical results were compared with those obtained using other sensors such as ISEs equipped with the same exchanger or using a biosensor responsive to cationic and anionic surfactants. Determinations of cationic surfactants in aqueous matrices of environmental interest were carried out using the sensor with the best performance. Furthermore, the critical micellar concentration (CMC) of some cationic surfactants was also evaluated.
Sanchez and coworkers109 reported a new ISFET sensor for the determination of anionic surfactants by titrations. The developed devices showed a lifetime longer than 4 months, improving the reported values of PVC membrane-ISFETs. Other characteristics were Nernstian slopes from 59 to 62 mV decade−1, with detection limits of about 10−6 mol L−1. In a comparative study, there were no significant differences between the results produced with the standard two-phase titration method and the proposed potentiometric titration method. In a following publication, ISFETs based on a photocurable membrane sensitive to anionic surfactants SDBS and DS were reported.110 The determination of the concentrations of the surfactants was performed following a standard addition methodology using ISFETs as sensors without any previous separation stages.
Mainly due to the sacrifice of the internal reference solution, solid contact electrodes exhibit interesting properties: they can be machined in any shape, exhibit faster responses, and facilitate the possibility to be operated in higher pressure environments where symmetric ISEs might be damaged. Drawbacks of the above construction are the poor reproducibility of the electrode potential and a drift that can be related to the asymmetric membrane with no internal reference electrode and filling solution. There is an ill-defined thermodynamic charge transfer at the blocked interface (phase boundary) between the ion sensitive membrane with ionic conductivity and the conducting substrate with electronic conductivity.112 For this reason, CWEs are better applied under hydrodynamic conditions, e.g., in flow injection analysis, where the contact of the sample plug with the electrode surface is brief.
2.1.4 Carbon Paste Electrodes (CPEs)
Carbon paste electrodes (CPEs) were firstly reported by Adams in 1958 as a new sensor material to overcome limitations of the dropping mercury electrode (DME) caused by anodic dissolution of Hg at more positive potentials.112 Carbon pastes are usually prepared by mixing of graphite powder and a pasting liquid (binder), using an agate mortar and a pestle. The resulting paste with fine consistency is packed into a piston driven electrode holder offering easy and quick surface regeneration which is the most frequently emphasized advantage of CPEs. In addition, carbon paste has advantages of ease of bulk modification compared with surface modification which is usually more complex and time-consuming.42,113–115 The binder, or pasting liquid, behaves similar to the plasticizer of the membrane electrodes. From among the substances currently used, tricresyl phosphate (TCP) was recommended in case of ISE potentiometry.116 Earlier, CPEs were classified as a special type of solid and/or carbon electrodes. From the potentiometric point of view, the electrodes are now classified as ion-selective electrodes with a liquid membrane as the pasting liquid is present as a very thin film surrounding the carbon particles and exhibits usually good extraction ability against ion associates composed of lipophilic species.117,118
Concerning the determination of surfactants, in 1997 Vytras’s group demonstrated that carbon paste-based electrodes could also be used as potentiometric sensors to monitor titrations of surfactants.117,119 When compared to PVC and CW electrodes, CPEs had the advantages of very low Ohmic resistance, very short response time in addition to the ease of fabrication and regeneration as well as long functional lifetime. Jezkova et al.116 used perchlorate and fluoroborate ion-selective carbon paste electrodes for both direct potentiometric measurements and potentiometric titration of perchlorate or fluoroborate with 0.1 M CPC. The electrodes had a rapid response, low Ohmic resistance, and limits of detections and selectivity similar to the limits of commercial membrane electrodes. A carbon paste electrode incorporated with the ion association of CPC–thallium halo complexes was applied as indicator electrode with the potentiometric titration of surfactants.120
2.1.5 Screen-Printed Electrodes
While CPEs continued to play a major role in the development of analytical procedures applicable in laboratory or to test new analytical methodologies,113–115 their relatively large size diminishes commercialization. Over the past few years, interest has been growing in the application of simple, rapid, inexpensive disposable sensors for clinical, environmental, and industrial analysis. Screen printing seems to be one of the most promising technologies allowing sensors to be produced on a large scale with the advantages of optimized manufacturing repeatability. Mass production at low cost makes them ideal for industrial commercialization with long shelf-life time and applications in the field with portable small instruments.113,121–125 Historically, the first reports on single-use disposable strip electrodes were dealing with amperometric biosensors by Wring and coworkers.126
Screen-printed electrodes (SPEs) are devices that are produced by printing different inks on a suitable substrate. Through the application of this technique, it is possible to fabricate a chip containing working, reference, and auxiliary electrode or even multisensory array. Electrode fabrication requires an ink as a precursor, which is typically a mixture containing at least three components113 (see also Chap. 16 of Volume I):
-
1.
Conductive particles having electroactive sites.
-
2.
A polymer that can bind together these particles.
-
3.
A solvent in which the ink components are dissolved or dispersed.
The mixture is liquid at room temperature, while after printing and curing at suitable temperatures, a solid conductive film with surface electroactive sites is produced. Commercially available inks are already tested and optimized under various conditions.
In a comprehensive study, Khaled et al.127 fabricated a simple disposable potentiometric sensor for the determination of surfactants. The proposed disposable sensor, containing both working and reference electrodes, was fabricated by screen printing technology using a homemade printing ink composed of carbon particle and a plasticizer dissolved in a PVC/THF solution. It was expected that, when such sensors were immersed in a stirred aqueous suspension of the ion-pair formed during the potentiometric titration, their organic solvents (plasticizers) became gradually saturated with the ion pair and there was no need for the addition of the electroactive material to the electrode matrix.
Analytical performances of the printed electrodes were compared with those of carbon paste, coated wire, coated graphite, and PVC polymeric membrane electrodes. The printed electrodes showed very short response time reaching 3 s with adequate shelf-life (6 months). The disposable sensor was successfully applied for the potentiometric titration of five different cationic surfactants, namely CPC, hexadecyltrimethylammonium bromide (HDTAB), DTAB, didodecyldimethylammonium bromide (DDMAB), and septonex with NaTPB. Concerning the titration process, the total potential changes and potential jump at the end-point were large (between 528 and 234 mV with potential jump ranging between 2,035 and 650 mV mL−1 titrant) which allowed the determination of 90 μg surfactant. The total potential change decreased proportionally with decreasing hydrophobicity of the molecule yielding the following series: CPC > HDTAB > Septonex > DDMAB > DTAB (Fig. 10.1).
Potentiometric titration of different cationic surfactants with 10−2 mol L−1 NaTPB using SPCPEs as potentiometric sensor. (Reprint with permission from reference (127). Copyright (2008) Elsevier
The proposed disposable strips were successfully used for the potentiometric titration of cationic and anionic surfactants, pharmaceutical preparations, detergents, and water samples with sensitivities comparable with the official method; they also have the ability of field measurements using a portable titration system (Fig. 10.2).
Schematic diagram of a portable system manifold used for the potentiometric titration of surfactants in the field. (Reprint with permission from reference (127). Copyright (2008) Elsevier
Similar sensors were fabricated using screen-printed electrodes for the determination of CPC,128 CTAB,129 and Septonex130 using different thick films modified by the corresponding ion associates of the surfactant with tetraphenyl borate. The fabricated electrodes showed a stable, near-Nernstian response for 1 × 10−2 to 1 × 10−6 mol L−1 CPC at 25 °C over the pH range 2–8 with a slope of 60.66 ± 1.10 mV decade−1.
2.1.6 Commercial Surfactants Potentiometric Electrodes
In parallel to research considering surfactants sensors, some commercially surfactant selective electrodes have been launched into the market. Orion model 93-42,131 ASTEC model TSE 01:91,132 and Metrohm “High Sense Tenside” are examples with considerable long operational life time38,55 and is the most promising specimen on the market.39,56 It is stable over a wide pH range and its use permits differentiation between some detergents in mixtures simply by changing the pH of the analyzed sample.
The polyoxyethylene portion of nonionic surfactants forms pseudo-crown compounds in the presence of barium ions which can be titrated with sodium tetraphenylborate using the Orion surfactant electrode as an end-point indicator electrode. An average recovery of 96.4 % can obtained for the determination of three standard detergents.131 The described method has been used successfully in the routine determination of nonionic surfactants.
A commercially available fluoroborate ion-selective indicator electrode was used for the potentiometric titration of surfactants and soaps.132 The titrants were HDTAC, hexadecylpyridinium chloride (HDPC), and diisobutylphenoxyethoxyethyl-(dimethyl)benzylammonium chloride (DIPEBC).133 Similarly, Benoit et al.134 titrated CTAB with sodium tetraphenylborate potentiometrically using a commercial ClO4 − selective electrode. The method proved to be precise exhibiting a relative standard deviation of 1.1 %.
2.2 Voltammetric and Amperometric Sensors
A surfactant is usually not electroactive from voltammetric and amperometric view; therefore, it is necessary to apply an indirect technique using a solution with a certain amount of an electroactive species (a marker) which is oxidized or reduced at the working electrode. The current of the marker decreases in the presence of a surfactant, which inhibits the electronic transfer at the electrode surface, and the concentration of the analyte can be related to the magnitude of the current decrease. For instance, Skoog et al.135 studied different surfactants by their influence on the amperometric and cyclic voltammetric responses of hexacyanoferrate. In a flow system, they noted that the presence of nonionic surfactants had an irreversible blocking effect on the electrode response. The suppression effect by a surfactant on the adsorptive voltammetric response of the nickel dimethylglyoxime complex was also reported by Adeloju and Shaw136,137 using different types of working electrodes, such as an HMDE, a mercury-film electrode, or a dropping mercury electrode. CPC and CTAB could be determined at ultra-trace levels by indirect stripping voltammetry on a hanging Hg drop electrode. The calibration graphs were linear up to 65, 5, and 0.5–3 mg L−1 for CPC, CTAB, and DBS, respectively. The method was applied to the determination of the aforementioned surfactants in disinfectants, lozenges, and mouth wash. In some cases the sample solution was subjected to cleanup through a chromatographic column before analysis to eliminate matrix interference.
The study of the inhibition of parahydroquinone oxidation at a graphite electrode in the presence of a surfactant (phospholipid) was reported by Schmidt and Emons.138 The decrease of the parahydroquinone oxidation peak was related to the surfactant concentration. In a similar approach, using ferrocene in a CPE as a redox tracer, Kim et al.139 determined different surfactants via modification of the ferrocene redox behavior. In the presence of DS, the oxidation peak current of ferrocene was enhanced by a factor of 3.5. A limitation of the technique was the analysis time because the electrode-solution contact period was 1 h. Similarly, anionic polyelectrolytes were determined by adsorptive stripping voltammetry (ADSV) at a carbon paste electrode in the presence of 11-ferrocenyltrimethylundecyl ammonium ions.140 The ion-association complex between an anionic polyelectrolyte and the ferrocenyl cationic surfactant was adsorptively accumulated at the electrode in the absence of an applied potential. The concentration of the anionic surfactant was indirectly evaluated from the oxidation wave of the ferrocenyl cationic surfactant. By means of AdSV, levels of anionic surfactants of 10−7 mol L−1 could be measured with good selectivity.
The combination of ion-pair extraction and differential pulse polarography was shown to be a method suitable for the determination of 10−7 mol L−1 of organic quaternary ammonium bases using Orange II as an appropriate polarographically active counter ion.141 The proposed method was used for the determination of tetrapentylammonium bromide (TPAB), Septonex, and codeine. An indirect voltammetric method for determination of CPC was proposed by Simunkova via the extraction of the electroactive CP-picrate as a 1:1 ion associate into chloroform. After drying the equivalent amount of picric acid in the extracted ion associate was determined by differential pulse voltammetry (DVP) on a hanging mercury drop electrode.142 The limit of determination was 0.136 mg L−1 with a relative standard deviation less than 6 %. The CPC content in pastilles of an antiseptic medicament (“Halset”) was determined with good accuracy. Moreover, other simple extractive procedures were suggested for the determination of anionic surfactants.143,144 The methods involved the formation of an extractable complex between the synthetic surfactant anion and the bis-(ethylenediamine)-diaqua-copper(II) cation. It was extracted into chloroform and then back-extracted into dilute acid. The resulting Cu(II) ions were determined by AAS and ASV with good correlation. A limit of detection of 5.0 μg L−1 anionic surfactant was observed with a linear calibration from 5.0 to 500 μg L−1 in the sample.
In addition, few direct voltammetric procedures were suggested for the quantification of surfactants. Zhao and Zeng145 noticed that, in presence of l-cysteine, CPC exhibited a sensitive cathodic stripping peak at about ~1.2 V. (vs. SCE). The square root of the peak height was linear to the CPC concentration in the range of 2–50 μM. A flow injection voltammetric method was developed for the determination of surfactants and ethanol.146 Surfactants in waters and ethanol in alcoholic drinks were determined.
2.3 Surfactant Biosensors
During the last decades, many biosensors have been developed for monitoring environmental parameters.145–148 With respect to surfactants, several biosensors have been proposed that employ microorganisms, cells, or enzymes. In this way, a bioreactor-type electrochemical sensor for the determination of linear alkylbenzene sulfonates (LASs) was described.149 The detection principle is based on the fact that, when LASs are biodegraded by certain bacteria, their respiratory activity in solution increases with a concomitant decrease in dissolved oxygen. The latter can be followed by an oxygen electrode positioned on-line in an appropriate flow setup. A linear response up to 6 mg L−1 of LAS could be achieved in less than 15 min. Applications to the determination of surfactants in river pollution were suggested.
Bacterial degraders as the base of an amperometric biosensor for the detection of anionic surfactants were investigated by Taranova et al.150 Several strains belonging to genera Pseudomonas and Achromobacter were characterized by their ability to degrade anionic surfactants; they were tested as potential bases of microbial biosensors. For each strain the author studied the substrate specificity and stability of the sensor signals. Maximal signals were observed with anionic surfactants. The lower limit of detection for DS used as a model surfactant in the field was 1 μM for all strains. The microbial biosensor can extend the practical possibilities for rapid evaluation of surfactants in water media.
A Pseudomonas rathonis T-based amperometric biosensor was constructed for the detection of anionic surfactants.151 Microorganisms contained the plasmid for the degradation of surfactant. The sensor had high sensitivity to DS (the lower limit of DS detection was within the range from 0.25 to 0.75 mg L−1); the responses to other detergents—volgonat, decylbenzene sulfonate, metaupon, toluene sulfonate, and alkylbenzene sulfonate—were 82, 36, 20, 10, and 10 % of response to SDS, respectively. As reported, the measurement time did not exceed 5 min.
The effect of nonionic surfactants in textile and tannery wastewater on the bacterium Escherichia coli, immobilized in an Anopore membrane on the surface of a screen printed carbon electrode, was studied.152 The amperometric response of the sensor was monitored at +550 mV versus a chloridized silver wire electrode in a vial with the neutralized sample and ferricyanide as a redox mediator. Toxicity was measured by determining the degree of inhibition of the biosensor signal after an exposure of 35 min. The observed toxicity of wastewater samples was attributed to nonionic surfactants.
Some studies have recently indicated that some surfactants may inhibit cholinesterase (ChE) activity in aquatic animals.153,154 Indeed, the chemical properties of surfactants can alter enzyme activities by binding or disrupting the enzyme structure.155 Evtugyn et al.156 investigated the influence of nonionic surfactants on the response of cholinesterase-based potentiometric biosensors. The effect of surface-active compounds depended on the hydrophilic properties and porosity of the enzyme support material and the inhibition mechanism. In the range of mass per volume (m/V) ratio 0.002–0.3 % the surfactants showed a reversible inhibiting effect on the biosensor response. At lower concentrations (down to mass per volume ratio 10−4) the surfactants alter the analytical characteristics of reversible and irreversible inhibitor determination.
References
(1966–2002) Surfactant science series, vol 1–109. Dekker, New York
Wasan DT, Ginn ME, Shah DO (eds) (1988) Surfactants in chemical/process engineering. Dekker, New York
Myers D (1988) Surfactant science and technology. VCH, New York
Porter MR (1991) Handbook of surfactants. Blackie, Glasgow
Karsa DR (ed) (1999) Industrial applications of surfactants IV. Royal Society of Chemistry, Cambridge
Schramm LL, Stasiuk EN, Marangoni DG (2003) Surfactants and their applications. Annu Rep Prog Chem Sect C 99:3–34
Maddin CM (1991) Proceedings of the international conference on health safety and environment. Society of Petroleum Engineers, Richardson, TX, SPE paper 23354
Arthur D. Little, Inc (1991) Environmental and human safety of major surfactants. Vol. I. Anionic surfactants. A final report to The Soap and Detergent Assoc. Arthur D. Little, Inc, Cambridge, MA
Hutzinger O (ed) (1992) Handbook of environmental chemistry. Springer, Berlin
Swisher D (1987) Surfactant biodegradation. Dekker, New York
Karsa DR, Porter MR (eds) (1995) Biodegradability of surfactants. Blackie Academic, London
Ying GG, Williams B, Kookana R (2002) Environmental fate of alkylphenols and alkylphenol ethoxylates—a review. Environ Int 28:215–226
Abel PD (1974) Toxicity of synthetic detergents to fish and aquatic invertebrates. J Fish Biol 6:279–298
Lewis MA (1991) Chronic and sublethal toxicities of surfactants to aquatic animals: a review and risk assessment. Water Res 25:101–113
Lewis MA, Suprenant D (1983) Comparative acute toxicities of surfactants to aquatic invertebrates. Ecotoxicol Environ Saf 7:313–322
Rosen MJ, Goldsmith HA (1972) Systematic analysis of surface-active agents. Wiley, New York
Cross J (ed) (1977) Anionic surfactants—chemical analysis. Dekker, New York
Cross J (ed) (1986) Non-ionic surfactants—chemical analysis. Dekker, New York
Porter MR (ed) (1991) Recent developments in the analysis of surfactants. Elsevier, Essex
Birch BJ, Cockcroft RN (1981) Analysis of ionic surfactants in the detergent industry using ion-selective electrodes. Ion Sel Electrode Rev 4:1–41
Vytras K (1985) Potentiometry. In: Encyclopedia of pharmaceutical technology. Swarbic J, Boylan JC. Marcel Dekker (eds), New York 12:347–388
Toei K (1987) Ion association reagents. Anal Sci 3:479–488
Cullum DC, Platt P (1991) Recent developments in the analysis of surfactants, vol 32. Elsevier, New York
Aboul‐Kassim TA, Simonei BRT (1993) Detergents: a review of the nature, chemistry, and behavior in the aquatic environment. Part I. Chemical composition and analytical techniques. Crit Rev Environ Sci Technol 23:325–376
Schmitt TM (1994) Analysis of surfactants, 2nd edn. Dekker, New York
Cullum DC (ed) (2001) Introduction to surfactant analysis. Blackie, London
Schmitt TM (2001) Analysis of surfactants, vol 96, Surfactant science series. Marcel Dekker, New York
Hummel DO (2000) Handbook of surfactant analysis: chemical physico-chemical and physical methods. Wiley, New York
Vytras K (1991) Coated wire electrodes in the analysis of surfactants of various types: an overview. Electroanalysis 3:343–347
Gerlache M, Kauffmann JM, Quarin G, Vire JE, Bryant GA, Talbot JM (1996) Electrochemical analysis of surfactants: an overview. Talanta 43:507–519
Sak-Bosnar M, Grabaric Z, Grabari BS (2004) Surfactant sensors in biotechnology part 1—electrochemical sensors. Food Technol Biotechnol 42:197–206
Epton SR (1947) A rapid method of analysis for certain surface-active agents. Nature 160:795–796
Glazer J, Smith TD (1952) Determination of sodium oleate in dilute aqueous solution. Nature 169:497–498
Greenberg AF, Clesceri LS, Eaton AD (eds) (1992) Standard methods for the examination of water and wastewater, 18th edn. American Public Health Association, Washington, DC, p 5, Section 5540 C
De Caro CA (1998) Automated surfactant titration: a review of various techniques. Riv Ital Sostanze Grasse LXXV:197–205
Buschmann N, Gors U, Schulz R (1993) Comparison of different cationic titrants for the potentiometric determination of anionic surfactants. Comun Jorn Com Esp Deterg 24:469–476
International Organization for Standardization (1989) Surface active agents, detergents, determination of anionic-active matter by manual or mechanical direct two-phase titration procedure, ISO 2271. International Organization for Standardization, Geneva
Gavach C, Seta P (1970) Dosage potentiometrique des ions alkyl-trimethylammonium a longue chaine et tetrabutyl-ammonium. Anal Chim Acta 50:407–412
Buschmann N, Schultz R (1993) Comparison of different ion-sensitive electrodes for the titrimetric determination of ionic surfactants. Tenside Surf Det 30:18–23
Buschmann N, Schultz R (1992) Development of an ion-sensitive electrode for the titration of surfactants. Comun Jorn Com Esp Deterg 23:323–328
Ma TS, Hassan SSM (1982) Organic analysis using ion-selective electrode, vol 1 and 2. Academic, London
Wang J (2000) Analytical electrochemistry, 2nd edn. Wiley VCH, New York
Vytras K (1995) Potentiometry. In: Swarbic J, Boylan JC (eds) Encyclopedia of pharmaceutical technology. Marcel Dekker, New York, pp 347–388
Gavach C, Bertrand C (1971) Electrodes specifiques d’anions a longue chaine hydro-carbonee: Application au dosage potentiométriqué de détergents anioniques. Anal Chim Acta 55:385–393
Birch BJ, Clarke DE (1972) An electrode selective to the dodecyl sulphate anion: comments on the application of direct potentiometry to c.m.c. measurements. Anal Chim Acta 61:159–162
Birch BJ, Clarke DE, Lee RS, Oakes J (1974) Surfactant-selective electrodes: Part III. Evaluation of a dodecyl sulphate electrode in surfactant solutions containing polymers and a protein. Anal Chim Acta 70:417–423
Ishibashi N, Kohara H, Horinouchi K (1973) Aromatic sulphonate ion-selective electrode membrane with crystal violet as ion-exchange site. Talanta 20:867–874
Anghel DF, Popescu G, Niculescu F (1980) Analytical applications of surfactant selective electrodes-1. Cosmetic products. Tenside Surf Det 17:171–173
Sap P, Anghel DF, Luca C (1983) Electrochemical sensors for anionic surfactants. I. The electrode based on bis[2,4,6-tris(2-pyridyl)-1,3,5-triazine]iron(II) dodecyl sulfate. Rev Roum Chim 28:883–890
Arvand-Barmchia M, Mousavi ME, Zanjanchi MA, Shamsipur M (2003) A new dodecylsulfate-selective supported liquid membrane electrode based on its N-cetylpyridinium ion-pair. Microchem J 74:149–156
Moody GJ, Oke RP, Thomas JDR (1970) A calcium-sensitive electrode based on a liquid ion exchanger in a poly(vinyl chloride) matrix. Analyst 95:910–918
Fiedler U, Ruzicka J (1973) Selectrode—the universal ion-selective electrode: Part VII. A valinomycin-based potassium electrode with nonporous polymer membrane and solid-state inner reference system. Anal Chim Acta 67:179–193
Davies G, Moody GJ, Thomas JDR (1988) Optimization of poly(vinyl chloride) matrix membrane ion-selective electrodes for ammonium ions. Analyst 113:497–500
Jones DL, Moody GJ, Moody GJ, Birch BJ (1981) Barium-polyethoxylate complexes as potentiometric sensors and their application to the determination of non-ionic surfactants. Analyst 106:974–984
Alexander PHV, Moody GJ, Thomas JDR (1987) Electrode membrane and solvent extraction parameters relating to the potentiometry of polyalkoxylates. Analyst 112:113–120
Buschmann N, Schulz R (1992) Determination of cationic and zwitterionic surfactants using ion selective electrodes. Tenside Surf Det 29:128–130
Masadome T, Imato T, Hachiya H, Asano Y (1998) Flow injection determination of dodecylsulfate-selective plasticized poly (vinyl chloride) membrane electrode detector. J Flow Injection Anal 15:242–247
Masadome T, Imato T, Asano Y (1999) End point detection of potentiometric titration for anionic polyelectrolytes using an anionic surfactant-selective plasticized poly (vinyl chloride) membrane electrode and ancenioric surfactant as a marker. Fres J Anal Chem 363:241–245
Hassan SSM, Badr IHA, Abd-Rabboh HSM (2004) Potentiometric flow injection analysis of anionic surfactants in industrial products and wastes. Microchim Acta 144:263–269
Campanella L, Aiello L, Colapicchioni C, Tomassetti M (1996) New ion selective polymeric membrane for cationic surfactant analysis. Analusis 24:387–391
Egorov VV, Repin VA, Kaputskii VA (1996) Determination of cationic surface-active antiseptics by ion-selective electrodes. J Anal Chem 51:986–988
Mostafa GAE (2001) PVC matrix membrane sensor for potentiometric determination of cetylpyridinium chloride. Anal Sci 17:1043–1048
Badawy SS, Shoukry AF, Farghaly RA (1989) A cetyltrimethyl-ammonium cation-sensitive polymeric membrane electrode based on the cetyltrimethylammonium-phosphotungstate ion association. Microchem J 40:181–186
Issa YM, Badawy SS, El-Hawary WF, Ashour MS (1999) Cetyltrimethylammonium-selective PVC electrode based on its trinitrobenzene sulfonate ion-pair. Electroanalysis 11:1063–1067
Badawy SS, Issa YM, El-Hawary WF, Ashour MS (2001) Cetyldimethylethylammonium-selective PVC electrode based on its ion-associate with phosphotungstic acid. Mikrochim Acta 136:1–17
Shoukry AF, Badawy SS, Farghali RA (1988) Hexadecyl-pyridinium-phosphotungstate ion association in construction of a hexadecylpyridinium cation selective electrode. Anal Chem 60:2399–2402
Patil SR, Rokshit AK (2004) Membrane electrode sensitive to a cationic surfactant in aquo-organic media. Anal Chim Acta 518:87–91
Madunic-Cacic D, Sak-Bosnar M, Galovic O, Sakac N, Matesic-Puac R (2008) Determination of cationic surfactants in pharmaceutical disinfectants using a new sensitive potentiometric sensor. Talanta 76:259–264
Madunic-Cacic D, Sak-Bosnar M, Matešic-Puac R (2011) A new anionic surfactant-sensitive potentiometric sensor with a highly lipophilic electroactive material. Int J Electrochem Sci 6:240–253
Najafi M, Maleki L, Rafati AA (2011) Novel surfactant selective electrochemical sensors based on single walled carbon nanotubes. J Mol Liq 159:226–229
Stepanets OV, Soloveva G, Mikhailova AM, Kulapin AI (2001) Rapid determination of anionic surfactants in seawater under shipboard conditions. J Anal Chem 56:290–293
Cox JA, Tess ME, Cummings TE (1996) Electroanalytical methods based on modified electrodes: a review of recent advances. Rev Anal Chem 15:173–223
Zen J, Kumar AS, Tasi D (2003) Recent updates of chemically modified electrodes in analytical chemistry. Electroanalysis 15:1073–1086
Shahgaldian P, Pieles U (2006) Cyclodextrin derivatives as chiral supramolecular receptors for enantioselective sensing. Sensors 6:593–615
Ogoshi T, Harada A (2008) Chemical sensors based on cyclodextrin derivatives. Sensors 8:4961–4982
Astray G, Gonzalez-Barreiro C, Mejuto JC, Rial-Otero R, Simal-Gandara (2009) A review on the use of cyclodextrins in foods. Food Hydrocolloids 23:1631–1640
Bates PS, Kataky R, Parker D (1994) Functionalized cyclodextrins as potentiometric sensors for onium ions. Analyst 119:181–186
Khalil MM, Anghel DF, Luca CC (1986) Poly (vinyl chloride) containing dibenzo-18-crown-6 as an ion-selective membrane for hyamine 1622. Anal Lett 19:807–824
Katsu TK, Nishimura N (2000) Organic ammonium ion-selective electrodes using acyclic neutral carriers developed for inorganic-selective electrodes. Anal Sci 16:523–526
Segui MJ, Lizondo-Sabater J, Martınez-Manez R, Pardo T, Sancenon F, Soto J (2004) Ion-selective electrodes for anionic surfactants using a new aza-oxa-cycloalkane as active ionophore. Anal Chim Acta 525:83–90
Segui MJ, Lizondo-Sabater J, Benito A, Martınez-Manez R, Pardo T, Sancenon F, Soto J (2007) A new ion-selective electrode for anionic surfactants. Talanta 71:333–338
Lizondo-Sabater J, Martınez-Manez R, Sancenon F, Segui MJ, Soto J (2008) Ion-selective electrodes for anionic surfactants using a cyclam derivative as ionophore. Talanta 75:317–325
Masadome T, Imato T (2003) Use of marker ion and cationic surfactant plastic membrane electrode for potentiometric titration of cationic polyelectrolyte. Talanta 60:663–668
Muller B, Hauser PC (1996) Effect of pressure on the potentiometric response of ion-selective electrodes and reference electrodes. Anal Chim Acta 320:69–75
Cattrall RW, Freiser H (1971) Coated wire ion selective electrodes. Anal Chem 43:1905–1906
Vytras K, Kalous J, Simickova B, Cerna J, Silena I (1988) Potentiometric titration of palladium (II) halide complex anions based on ion-pair formation. Anal Chim Acta 209:357–361
Cotrell R (1973) IUPAC, book of papers, international symposium on ion-selective electrodes, Cardiff
Vytras K (1977) Titration of organic cations with sodium tetraphenylborate indicated by K+ ion selective electrode Crytur. Collect Czech Chem C 42:3168–3174
Vytras K, Remeš M, Kubešová-Svobodová H (1981) Coated-wire organic ion-selective electrodes in titrations based on ion-pair formation: determination of arenediazonium salts with sodium tetraphenylborate. Anal Chim Acta 124:91–98
Vytras K, Djakova M, Mach V (1981) Coated-wire organic ion-selective electrodes in titrations based on ion-pair formation: Part 2. Determination of ionic surfactants. Anal Chim Acta 127:165–172
Vytras K, Kalous J, Vosmanská M (1985) Ion-selective electrodes in titrations involving azo-coupling reactions: Part 5. Determination of arenediazonium salts of ampholytic character. Anal Chim Acta 175:313–317
Vytras K (1984) Determination of some pharmaceuticals using simple potentiometric sensors of coated-wire type. Mikrochim Acta 3:139–148
Vytras K, Kalous J, Symersky J (1985) Determination of some ampholytic and cationic surfactants by potentiometric titrations based on ion-pair formation. Anal Chim Acta 177:219–223
Vytřas K, Dvořáková V, Zeman I (1989) Titrations of non-ionic surfactants with sodium tetraphenylborate using simple potentiometric sensors. Analyst 114:1435–1441
Vytřas K, Tomáš Čapoun T, Halámek E, Souček J, Štajerová B (1990) Potentiometric ion-pair formation titrations of N-alkyl-N-ethylpyrrolidinium cations using plastic membrane electrodes. Collect Czech Chem C 55:941–950
Mousavi MF, Shamsipur M, Riahi S, Rahmanifar MS (2002) Design of a new dodecylsulfate-selective electrode based on conductive polyaniline. Anal Sci 18:137–140
Shafiee-Dastjerdi L, Alizadeh N (2004) Coated wire linear alkylbenzenesulfonate sensor based on polypyrrole and improvement of the selectivity behavior. Anal Chim Acta 505:195–200
Plesha MA, Van Wie BJ, Mullin JM, Kidwell DA (2006) Measuring quaternary ammonium cleaning agents with ion selective electrodes. Anal Chim Acta 570:186–194
Selig W (1982) A simple sensor for potentiometric titrations. Anal Lett 15:309–329
Dowle CJ, Cooksey BG, Ottaway JM, Campbell WC (1987) Development of ion-selective electrodes for use in the titration of ionic surfactants in mixed solvent systems. Analyst 112:1299–1302
Dowle CJ, Cooksey BG, Ottaway JM, Campbell WC (1988) Determination of ionic surfactants by flow injection pseudotitration. Analyst 113:117–119
Pan J, Cao L, Li B, Fan H (1986) Potentiometric titration curves of the Orange IV-basic dye or quaternary ammonium salt systems. Yingyong Huaxue 3:39–43
Baro-Roma J, Sanchez J, del Valle M, Alonso J, Bartroli J (1993) Construction and development of ion-selective electrodes responsive to anionic surfactants. Sens Actuators B Chem 15:179–183
Saad B, Ariffin MM, Saleh MI (1997) Paraquat sensors containing membrane components of high lipophilicities. Anal Chim Acta 338:89–96
Matesic-Puac R, Sak-Bosnar M, Bilic M, Grabaric S (2005) Potentiometric determination of anionic surfactants using a new ion-pair-based all-solid-state surfactant sensitive electrode. Sens Actuators B Chem 106:221–228
Ecker C, Calvo D, del Valle M (2008) Potentiometric determination of anionic surfactants using a new ion-pair-based all-solid-state surfactant sensitive electrode. J Pharm Biomed Anal 46:213–218
Campanella L, Battilotti M, Borraccino A, Colapicchioni C, Tomassetti M, Visco G (1994) A new ISFET device responsive to anionic detergents. Sens Actuators B Chem 19:321–328
Campanella L, Aiello L, Colapicchioni C, Tomassetti M (1997) Analysis of cationic surfactants in environmental aqueous matrices by new ISFET devices. Anal Lett 30:1611–1629
Sanchez J, Beltran A, Alonso J, Jimenez C, del Valle M (2000) Development of a new ion-selective field-effect transistor sensor for anionic surfactants: application to potentiometric titrations. Anal Chim Acta 382:157–164
Sanchez J, del Valle M (2001) Photocurable ISFET for anionic surfactants. Monitoring of photodegradation processes. Talanta 54:893–902
Fibbioli M, Morf WE, Badertscher M, de Rooij NF, Pretsc E (2000) Potential drifts of solid-contacted ion-selective electrodes due to zero-current ion fluxes through the sensor membrane. Electroanalysis 12:1286–1292.
Adams RN (1958) Carbon paste electrode. Anal Chem 30:1576
Kalcher K, Švancara I, Metelka R, Vytras K, Walcarius A (2006) In: Grimes CA, Dickey EC, Pishko MV (eds) Encyclopedia of sensors, vol 4. American Scientific Publishers, Stevenson Ranch, pp 283–430. ISBN 1-58883-060-8
Svancara I, Vytras K, Kalcher K, Walcarius A, Wang J (2009) Carbon paste electrodes in facts, numbers, and notes: a review on the occasion of the 50-years jubilee of carbon paste in electrochemistry and electroanalysis. Electroanalysis 21:7–28
Svancara I, Kalcher K, Walcarius A, Vytras K (2012) Electroanalysis with carbon paste electrodes. CRC Press, Boca Raton, FL
Jezkova J, Musilova J, Vytras K (1997) Potentiometry with perchlorate and fluoroborate ion-selective carbon paste electrodes. Electroanalysis 9:1433–1436
Vytras K, Kalous J, Jezkova J (1997) Automated potentiometry as an ecologic alterative to two-phase titration of surfactants. Egypt J Anal Chem 6:107–128
Svancara I, Hvizdolova M, Vytras K, Kalcher K, Novotny R (1996) A microscopic study on carbon paste electrodes. Electroanalysis 8:61–65
Vytras K, Jezkova J, Dlabka V, Kalous J (1997) Studies on potentiometric titrations using liquid membrane-based electrodes: coated wires vs. carbon paste. Sci Pap Univ Pardubice Ser A 3:307–323
Vytras K, Khaled E, Jezkova J, Hassan HNA, Barsoum BN (2000) Studies on potentiometric thallium (III)-selective carbon paste electrodes and its possible applications. Fres J Anal Chem 367:203–207
Hart JP, Wring SA (1997) Recent developments in the design and application of screen-printed electrochemical sensors for biomedical, environmental and industrial analyses. Trends Anal Chem 16:89–103
Honeychurch KC, Hart JP (2003) Screen-printed electrochemical sensors for monitoring metal pollutants. Trends Anal Chem 22:456–469
Hart JP, Crew A, Crouch E, Honeychurch KC, Pemberton RM (2004) Some recent designs and developments of screen-printed carbon electrochemical sensors/biosensors for biomedical, environmental and industrial analyses (review). Anal Lett 37:789–830
Renedo OD, Alonso-Lomillo MA, Martinez MJ (2007) Recent developments in the field of screen-printed electrodes and their related applications. Talanta 73:202–219
Li M, Li Y, Li D, Long Y (2012) Recent developments and applications of screen-printed electrodes in environmental assays—a review. Anal Chim Acta 743:31–44
Wring SA, Hart JP, Bracey L, Birch BJ (1990) Development of screen-printed carbon electrodes, chemically modified with cobalt phthalocyanine, for electrochemical sensor applications. Anal Chim Acta 231:203–212
Khaled E, Mohamed GG, Ali TA (2008) Disposal screen-printed carbon paste electrodes for the potentiometric titration of surfactants. Sens Actuators B Chem 135:74–80
Mohamed GG, Ali TA, El-Shahat MF, Al-Sabagh AM, Migahed MA, Khaled E (2010) Potentiometric determination of cetylpyridinium chloride using a new type of screen-printed ion selective electrodes. Anal Chim Acta 673:79–87
Mohamed GG, Ali TA, El-Shahat MF, Al-Sabagh AM, Migahed MA (2010) New screen-printed ion-selective electrodes for potentiometric titration of cetyltrimethylammonium bromide in different civilic media. Electroanalysis 22:2587–2599
Mohamed GG, El-Shahat MF, Al-Sabagh AM, Migahed MA, Ali TA (2011) Septonex–tetraphenylborate screen-printed ion selective electrode for the potentiometric determination of Septonex in pharmaceutical preparations. Analyst 136:1488–1495
Selig S (1980) The potentiometric titration of surfactants and soaps using ion-selective electrodes. Fres J Anal Chem 300:183–188.
Gallegos RD (1993) Titrations of non-ionic surfactants with sodium tetraphenylborate using the Orion surfactant electrode. Analyst 118:1137–1141
Szczepaniak W (1990) Mercurated polystyrene as a sensor for anionic surfactants in ion-selective polymeric membrane electrodes. Analyst 115:1451–1455
Benoit E, Leroy P, Nicolas A (1985) Use of an electrode selective for perchlorate ions in the potentiometric analysis of quaternary ammonium by tetraphenylborate ions. Ann Pharm Fr 43:177–182
Skoog M, Kronkvist K, Johansson G (1992) Blocking of chemically modified graphite electrodes by surfactants. Anal Chim Acta 269:59–64
Adeloju SB, Shaw SJ (1994) Indirect determination of surfactants by adsorptive voltammetry: Part 1: determination of trace and ultratrace concentrations of sodium dodecylbenzene sulfonate. Electroanalysis 6:639–644
Adeloju SB, Shaw SJ (1994) Indirect determination of surfactants by adsorptive voltammetry: Part 2: determination of hexadecyltrimethyl ammonium bromide and cetylpyridinium chloride in industrial and consumer products. Electroanalysis 6:645–649
Schmidt T, Emons H (1994) Tensammetric and indirect amperometric detection of phospholipids. Electroanalysis 3:543–551
Kim OS, Shiragami S, Kusuda K (1994) Increase of the redox current in cyclic voltammetry of a compound dissolved in Nujol by contacting with a surface active compound in an aqueous solution. J Electroanal Chem 367:271–273
Toshiaki H, Kenji O (2002) Determination of anionic polyelectrolytes by adsorptive voltammetry using 11-ferrocenyltrimethylundecyl ammonium ion at a carbon past. Bunseki Kagaku 51:1171–1174
Koula V, Kucova D, Gasparic J (1992) Determination of organic bases by ion-pair extraction polarography using Orange II as counter ion. Collect Czech Chem C 57:2272–2278
Simunkova-Kulova P, Kotoucek M (2003) Indirect voltammetric determination of cetylpyridinium chloride using ion pair extraction. Acta Univ Palacki Olomuc 42:51–60
Spencer MJ, Wallace GG, Crisp PT, Lewis TW (1991) Determination of anionic surfactants by bis(ethylenediamine) copper(II) extraction and anodic stripping voltammetry. Anal Chim Acta 244:197–200
John R, Lord D (1999) Determination of anionic surfactants using atomic absorption spectrometry and anodic stripping voltammetry. J Chem Edu 76:1256–1258
Zhao FQ, Zeng BZ (2002) Voltammetric determination of cetylpyridinium chloride with a silver electrode. Anal Lett 35:1603–1615
Sawamoto H, Gamoh K (1991) Determination of the compounds which are neither oxidized or reduced at electrodes by a flow injection voltammetric methods. Anal Sci 7:1707–1709
Andreescu S, Marty J (2006) Twenty years research in cholinesterase biosensors: from basic research to practical applications. Biomol Eng 23:1–15
Amine A, Mohammadi H, Bourais I, Palleschi G (2006) Enzyme inhibition-based biosensors for food safety and environmental monitoring. Biosens Bioelectron 21:1405–1423
Rogers KR (2006) Recent advances in biosensor techniques for environmental monitoring. Anal Chim Acta 568:222–231
Rodriguez-Mozaz S, de Alda MJL, Marco M, Barceló D (2005) Biosensors for environmental monitoring: a global perspective. Talanta 65:291–297
Nomura Y, Ikebukuro K, Yokoyama K, Takeuchi T, Arikawa Y, Ohno S, Karube L (1994) A novel microbial sensor for anionic surfactant determination. Anal Lett 27:3095–3108
Taranova L, Semenchuk I, Manolov T, Iliasov P, Reshetilov A (2002) Bacteria-degraders as the base of an amperometric biosensor for detection of anionic surfactants. Biosens Bioelectron 17:635–640
Reshetilov AN, Semenchuk IN, Iliasov PV, Taranova LA (1997) The amperometric biosensor for detection of sodium dodecyl sulfate. Anal Chim Acta 347:19–26
Farre M, Pasini O, Alonso MC, Castillo M, Barcelo D (2001) Toxicity assessment of organic pollution in wastewaters using a bacterial biosensor. Anal Chim Acta 426:155–165
Guilhermino L, Lacerda MN, Nogueira AJ, Soares AM (2000) In vitro and in vivo inhibition of Daphnia magna acetylcholinesterase by surfactant agents: possible implications for contamination biomonitoring. Sci Total Environ 247:137–141
Garcia LM, Castro B, Ribeiro R, Guilhermino L (2000) Characterization of cholinesterase from guppy (Poecilia reticulata) muscle and its in vitro inhibition by environmental contaminants. Biomarkers 5:274–284
Cserhati T, Forgacs E, Oros G (2002) Biological activity and environmental impact of anionic surfactants. Environ Int 28:337–348
Evtugyn GA, Budnikov HC, Nikolskaya EB (1996) Influence of surface-active compounds on the response and sensitivity of cholinesterase biosensors for inhibitor determination. Analyst 121:1911–1915
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Khaled, E., Aboul-Enein, H.Y. (2015). Surfactants. In: Moretto, L., Kalcher, K. (eds) Environmental Analysis by Electrochemical Sensors and Biosensors. Nanostructure Science and Technology. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1301-5_10
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1301-5_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1300-8
Online ISBN: 978-1-4939-1301-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)