Abstract
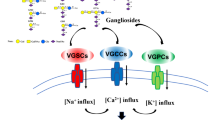
The nervous system is richly endowed with large transmembrane proteins that mediate ion transport, including gated ion channels as well as energy-consuming pumps and transporters. Transport proteins undergo N-linked glycosylation which can affect expression, location, stability, and function. The N-linked glycans of ion channels are large, contributing between 5 and 50 % of their molecular weight. Many contain a high density of negatively charged sialic acid residues which modulate voltage-dependent gating of ion channels. Changes in the size and chemical composition of glycans are responsible for developmental and cell-specific variability in the biophysical and functional properties of many ion channels. Glycolipids, principally gangliosides, exert considerable influence on some forms of ion transport, either through direct association with ion transport proteins or indirectly through association with proteins that activate transport through appropriate signaling. Examples of both pumps and ion channels have been revealed which depend on ganglioside regulation. While some of these processes are localized in the plasma membrane, ganglioside-regulated ion transport can also occur at various loci within the cell including the nucleus. This chapter will describe ion channel and ion pump structures with a focus on the functional effects of glycosylation on ion channel availability and function, and effects of alterations in glycosylation on nervous system function. It will also summarize highlights of the research on glycolipid/ganglioside-mediated regulation of ion transport.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ion channels
- Voltage-gated sodium channel
- Transporters
- GM1 and Ca2+ modulation
- Sodium-calcium exchanger
- TRP channels
15.1 Introduction
The transport of charged ions across impermeant lipid membranes is a critical physiological process in all cells. Pumps, transporters, and channels are found in the plasma membrane, as well as the membranes of organelles such as mitochondria, the endoplasmic reticulum (ER), and nucleus. Excitable cells, particularly neurons of the central and peripheral nervous system, are endowed with an unusually rich variety of voltage-gated and ligand-gated ion channels that are used for rapid electrical signaling and signal processing.
All transport proteins, and many of their auxiliary subunits, are glycosylated. As with most transmembrane proteins, changes in glycosylation affect both the expression and stability of proteins at the plasma membrane thereby changing the electrical properties of cells. In addition, the negative charges contributed by sialic acid residues in the glycan structures can affect the electrical potential near the pore of ion channels thereby influencing channel characteristics and behavior. In this chapter voltage-gated sodium channels will be used as exemplars to describe the functional effects of glycosylation on ion channel activity. We will focus on N-glycosylation of ion channels which is common to all transmembrane proteins. O-linked glycosylation has been described for a few channels (see e.g. Schwetz et al. 2011), however, it is much less well studied.
Glycolipids also contribute importantly to ion transport, gangliosides with their variety of oligosaccharide chains being the major species so engaged. As with glycoproteins, negatively charged sialic acid is crucial to such functional roles. These behave in many cases as modulatory agents through direct association with proteins that function as ion channels or pumps, while indirect modulation can also occur through association with proteins that influence ion transport downstream through appropriate signaling. Such processes are abundant in the plasma membrane but a growing number have been identified at various intracellular loci as well. The monosialo-ganglioside, GM1, has been the major focus of such activities, while the disialoganglioside, GD1a, has subsumed a supporting role as metabolic precursor to GM1. Our survey of such modulatory activities will include the especially prominent role that appears to have been assigned the GM1/GD1a duo in relation to Ca2+ transport.
15.2 Glycosylation of Ion Transport Proteins
15.2.1 Glycosylation of Voltage-Gated Na Channel
Voltage-gated Na channels are responsible for generating action potentials in neurons and other excitable cells. Structurally, they are representative of an ancient protein superfamily that includes voltage-gated channels selective for Na+, Ca2+, and K+. The sodium channel was the first member of the family of voltage-gated ion channels to be sequenced and cloned [for historical review see Catterall 2012]. Early structural studies identified a large glycoprotein containing a major α subunit as well as a noncovalently associated β1 subunit and a disulfide-linked β2 subunit (Hartshorne and Catterall 1981, 1984; Barchi 1983). Currently, there are ten known isoforms of alpha subunits (proteins Nav1.1 through Nav1.9, genes SCN1A–SCN11A), and four isoforms of beta subunits (proteins β1–β4 and genes SCNB1–SCNB4). The β1 and β3 subunits are noncovalently associated with the main subunit, while the β2 and β4subunit are disulfide-linked (Isom et al. 1992).
Sodium channels, similar to essentially all membrane proteins, undergo “N-linked” glycosylation of an asparagine residue. The target sequence is Asn-X-Ser/Thr, where X is any amino acid other than proline. Both the main α subunit and auxiliary β subunits are heavily glycosylated. Glycosylation accounts for 15–40 % of the MW of the main pore-forming α subunit, depending on factors such as the channel isoform, cell type, and developmental or pathological state (see below). For example, the mature α subunit protein isolated from rat brain has a MW of 260 kDa (Schmidt and Catterall 1987). Inhibition of glycosylation with tunicamycin lowered the apparent MW to 203 kDa, a value close to that predicted for the ~2,000 amino acid sequence. The β subunits are also heavily glycosylated. Mutation of the putative glycosylation sites of the β1 subunit reduced the MW from 38 to 22 kDa, a value predicted for the amino acid sequence (Johnson et al. 2004).
The structure of the α subunit of voltage-gated Na channels is typical of the voltage-gated ion channel family: the subunit consists of 4 domains (I–IV) each with six alpha helical transmembrane segments (S1–S6) arranged to form a cylindrical structure in the membrane (Fig. 15.1). The four sets of S5–S6 segments form the inner wall of the pore, while the corresponding S1–S4 segments are arranged around them. The long extracellular loop between S5 and S6 is called the P loop (Pore) and is reentrant: it projects extracellularly, then dips half-way into the membrane before again emerging extracellularly. The S4 segment is the main voltage-sensor for activation or opening of voltage-gated channels, while the amino acids along the inward dip of the P loop are primary determinants of ion selectivity and conductance.
Model of the primary structure of a voltage-gated sodium channel. The structure of the channel is illustrated with transmembrane α-helical regions represented as cylinders. S4 segments of domains I–IV are the primary voltage-sensing regions. S5–S6 with the re-entrant loop line the pore. Glycosylation sites on the α subunit are limited to the extracellular loop between S5 and S6 in domain I and indicated with Ψ. The lengths of lines are approximately proportional to the lengths of each extra- or intracellular poplypeptide segment. The extracellular domains of the β1 and β2 subunits are shown as immunoglobulin-like folds. Reprinted from Catterall (2012) with permission from John Wiley and Sons
Despite the modular structure of the channel, all the putative glycosylation sites of the α subunit are restricted to the large extracellular S5–S6 loop of Domain I (Bennett 2002). Deleting putative sites in the Domain I S5–S6 loop eliminated glycosylation (Bennett et al. 1997). When chimeric channels were formed by switching the Domain I S5–S6 loop of human isoform Nav1.4 (hSkM1), a heavily glycosylated isoform, with the loop from human Nav1.5 (hH1), an isoform with little glycosylation, both chimeric channels exhibited the glycosylation properties appropriate to the channel from which the loops were derived (Bennett 2002).
β subunits are single-pass transmembrane proteins of the Ig superfamily, with a short cytoplasmic C-terminus and large extracellular portion that has a V-type Ig-like fold (Fig. 15.1). The β1 subunit has 3–4 N-linked carbohydrate chains as determined by sequential treatment with neuraminidase and endoglycosidase (Messner and Catterall 1985). This corresponds well with the four putative extracellular glycosylation sites predicted by “sequence gazing” (Isom et al. 1992).
An unusual characteristic of ion channel glycosylation is the high sialic acid residue content of the glycan structures. In an analysis of carbohydrate content of the main subunit of the eel electroplax voltage-gated sodium channels, Miller et al. (1983) estimated that sialic acid residues made up 11.8 % of the alpha subunit MW and 39.7 % of total carbohydrate. Roberts and Barchi (1987) estimated that the main subunit molecule of the skeletal muscle sodium channel contained over 100 sialic acid residues. James and Agnew (1989) estimated a very high degree of polysialic acid for the same channel—~113 negative charges to the protein surface. The ratio of sialic acid residues to consensus glycosylation sites suggests that the terminal chains are well over ten sialosyl residues in length, potentially extending 10–30 nm into the extracellular environment (James and Agnew 1989). Polysialic acid in glycan structures of vertebrate proteins is rare and is most often associated with neural cell adhesion molecules (James and Agnew 1989; Rutishauser and Landmesser 1996). In electroplax membranes from Torpedo, the sodium channel is the only protein that is immunoreactive for sialic acid (James and Agnew 1989).
15.2.2 Functional Consequences of Ion Channel Glycosylation
The high degree of sialic acid content found in ion channels has consequences for their biophysical properties and function. Each sialic acid residue usually carries a negative charge at physiological pH. The cloud of negative charges created by a high density of sialic acid residues, particularly when localized just above the channel pore as it is for voltage-gated sodium channels, can have functional effects on voltage-dependent properties. Complete enzymatic removal of sialic acid from rat skeletal muscle sodium channels modified several parameters of voltage-dependent gating (Bennett et al. 1997). The voltage dependence of the time constants of channel activation and inactivation and the voltage for steady-state half-activation were ~10 mV more depolarized for neuraminidase-treated channels compared to controls. A similar shift was observed when channels were expressed in a sialylation-deficient cell line (lec2) or following deletion of likely glycosylation sites. Desialylated channels were also less sensitive to the charge screening effects of external calcium. The authors concluded that sialic acid most likely contributed to the negative surface potential and altered the electric field sensed by channel gating elements (Bennett et al. 1997).
In a later study, the same group compared two human isoforms of sodium channels: Nav1.4 from skeletal muscle (hSkM1) and Nav1.5 (hH1) from heart (Bennett 2002). Native hSkM1 has numerous sialic acid residues while hH1 does not. Consistent with the predicted role of sialic acid, neuraminidase caused a depolarizing shift in channel gating of hSkM1 but not hH1. When the S5–S6 loop of domain I was exchanged between the two isoforms, the loop determined the degree of sialylation of the chimeric channels, indicating that the primary sequence of the S5–S6 linker of domain I to some extent regulates the composition of the glycan structure attached to the channel (Bennett 2002).
In addition to the effects of sialic acid residues attached to the main pore-forming subunit, Johnson et al. (2004) reported that glycosylation of the auxiliary β subunits also modifies the activation and inactivation range of voltage-gated Na channels. The fully sialylated β1 subunit caused a hyperpolarizing shift in the voltage range of gating for the cardiac and two neuronal α subunit isoforms. Mutation of the N-glycosylation sites abolished the effects of the β1 subunit. Interestingly, the β1 subunit had no effect on the gating properties of the heavily glycosylated skeletal muscle α subunit. The authors proposed a saturating electrostatic mechanism in which a spectrum of differentially sialylated α and β subunits could modulate channel gating and influence the excitability properties of cells (Johnson et al. 2004).
Variability in glycosylation of ion channels can have profound effects on the excitability characteristics of a cell. Stocker and Bennett (2006) proposed mechanisms by which cardiac voltage-gated sodium channel gating and subsequently cardiac rhythms are modulated by changes in channel-associated sialic acids. Changes in neuronal excitability and function have been reported for various regions of the nervous system. Isaev et al. (2007) reported that the activation and inactivation properties of voltage-gated Na channels from CA3 pyramidal cells were shifted towards depolarizing potentials by treatment of hippocampal slices by neuraminidase. This led to an increase in the action potential threshold due to enhanced steady-state inactivation. Neuraminidase treatment had powerful anticonvulsive action both in vitro and in acute and chronic in vivo models of epilepsy (Isaev et al. 2007; Isaeva et al. 2011). Large diameter dorsal root ganglion (DRG) cells (Aα and β) become hyperexcitable following chronic constriction injury. The hyperexcitability of injured DRG neurons was reduced substantially by neuraminidase treatment (Peng et al. 2004). Desialylation had no effect on normal intact neurons. Such effects may indicate plasticity in the glycosylation of ion channels.
Sialic acid residues modify the function of other voltage-gated channels in addition to Na channels. The channel gating of voltage-gated Kv1.1 (potassium) channels expressed in CHO cells was shifted in a depolarizing direction by tunicamycin and neuraminidase treatment (Thornhill et al. 1996). Kv3.1, a voltage-gated potassium channel expressed throughout the nervous system, was studied in its glycosylated (wild type), partially glycosylated (N220Q or N229Q) and unglycosylated (N220Q/N229Q) states (Hall et al. 2011). Wild-type Kv3.1 channel currents had faster activation and deactivation rates than the mutated forms. When channels were expressed in the B35 neuroblastoma cell line, cells with wild-type Kv3.1 channels migrated more rapidly in a wound-healing assay (Hall et al. 2011). Potassium channel activity can also be modulated by glycosylation of auxiliary subunits. Tunicamycin blocked the glycosylation of DPP10, an auxiliary subunit of the Kv4 channel and a key determinant of cardiac and neuronal excitability (Cotella et al. 2010). This abolished the effects of DPP10 on Kv4.3 inactivation and recovery from inactivation.
Glycosylation can also affect ion channels gated by ligands or other activators. Glycosylation affects the stability, assembly, and open times of the GABAAionotropic channel (Lo et al. 2010) Although more work needs to be done, the authors postulate that the N-glycans of at least one of the channel’s glycosylation sites (N173) make stabilizing contacts with adjacent subunits or perhaps with chaperone proteins during the assembly process. A particularly intriguing observation is the role of glycosylation in regulation of TRPC6 and TRPC3, two closely related channels of the TRP family. TRPC6 has 2 glycosylation sites and is tightly regulated while TRPC3 has a single glycosylation site and is constitutively active. Mutation of the unique NX(S/T) motif in TRPC6 converted it to a constitutively active channel, while addition of the second glycosylation site to TRPC3 reduced TRPC3 basal activity (Dietrich et al. 2003). Glycosylation also regulates the ligand binding and gating properties of the TRP vanilloid 1 receptor (TRPV1; Wirkner et al. 2005). A mutant channel lacking the potential N-glycosylation site at position N604 had a depressed maximum of the dose–response curve for capsaicin, altered dependence of the capsaicin effect on extracellular pH, and decreased sensitivity to the antagonist capsazepine. Thus glycosylation affects both the gating, ligand binding, and pharmacology of TRP channels.
15.2.3 Plasma Membrane Expression
N-linked glycosylation plays an important role in the expression, insertion, and stability of many plasma membrane proteins. Expression and localization of ion channels is tightly regulated by multiple factors such as targeting to lipid rafts, anchoring to scaffolding proteins, and coassembly with essential or auxiliary subunits. Glycosylation, while important, is only one factor influencing expression. Consequently, the role of glycosylation varies for different ion channels, between cell types, and under specific physiological conditions.
As with most proteins expressed on the surface membrane, the number and density of voltage-gated Nachannels is decreased when glycosylation is inhibited by tunicamycin. In neuroblastoma cells, tunicamycin reduced the number of voltage-gated Na channels as measured by high-affnity saxitoxin binding to 20–28 % of control values over a 60 h period or by batrachotoxin-activated 22Na+ influx (Waechter et al. 1983). In embryonic rat neocortical neurons, tunicamycin application decreased the voltage-clamped Na current to ~40 % of control values (Zona et al. 1990). The effect was much more rapid for neurons that were actively growing in culture (days 5–14 after dissociation) compared to more established neurons (days 20–40 after dissociation). Currents were reduced to 40 % within 24 h for more recently plated neurons compared with 68 h for neurons that were fully grown (Zona et al. 1990).
In squid giant fiber lobe neurons of the stellate ganglion, voltage-gated Na channels are present at high density in axons but are absent from its somata in vivo. This distribution is maintained in culture except for the appearance of low-level expression in cell bodies (Gilly et al. 1990). Tunicamycin disrupted the expression of Na channels in axonal membranes in vitro with no effect on low levels in the soma. Disruption of glycosylation did not affect voltage-gated Na channel turnover, axon viability, or K channel distribution, indicating that glycosylation had a specific effect on voltage-gated Na channel localization in this system (Gilly et al. 1990).
The effect of glycosylation on other channels is highly variable. Glycosylation has profound effects on the stability of the Shaker K channel, although it is not needed for expression (Khanna et al. 2001). In pulse chase experiments, the wild-type protein was stable with little degradation after 48 h, however, a mutant form with glycosylation sites removed (N259Q, N263Q) was rapidly degraded (t1/2 ~ 18 h). Glycosylation regulates efficient multimerization and transport of the TRPM8 channel (Erler et al. 2006). Similarly, mutation of two putative glycosylation sites within α2δ, a subunit that regulates trafficking and function of voltage-gated calcium channels, decreased the number of functional charges in the plasma membrane (Sandoval et al. 2004). The pentameric nicotinic acetylcholine receptor (AChR) assembles when glycosylation is blocked, however, it is not inserted into the plasma membrane, remaining stuck in internal compartments (Sumikawa and Miledi 1989).
15.2.4 Variability of Glycosylation
The extent of glycosylation is highly variable not only for ion channel isoforms, but also for any given isoform expressed in different cells or at various developmental stages. Differences in glycosylation affect both channel expression levels and may manifest as profound alterations of biophysical properties. These, in turn, can affect neuronal excitability and function.
The degree of glycosylation can be due to differences in the primary sequence of an isoform. An example was provided earlier in the studies of the heavily glycosylated Nav1.4 channel compared to the lightly glycosylated Nav1.5 (Bennett 2002). Exchange of the domain I S5–S6 linker regions demonstrated that the extent of glycosylation was determined by the amino acids of the loop structure. Alternatively, the primary amino acid sequence distant from the glycosylation site may have an effect. Kv1.4 and Kv1.1 are two isoforms of a mammalian Shaker family channel involved in action potential repolarization. Block of N-glycosylation affected the protein trafficking, stability, and cell surface expression of Kv1.4, while Kv1.1 was unaffected. However, exchanging a trafficking pore region—a site distant from the glycosylation loop—from Kv1.4 into Kv1.1, caused changes in Kv1.1 comparable to those of Kv1.4 (Watanabe et al. 2004). The authors conclude that multiple regions of the protein must participate in proper folding, trafficking, and glycosylation of the channel.
Much of the variability in glycosylation is due to the expression and activity of the glycosylation machinery. N-linked glycosylation is a complex process that takes place within the ER and Golgi and involves numerous enzymes (see Chap. 3 for details). Briefly, the glycan structure is preformed in the endoplasmic reticulum attached to a lipid anchor. It is transferred to a target asparagine cotranslationally, while the peptide is synthesized. The N-linked glycan is further modified by removal of sugars (trimming) and re-addition of sugar residues (processing). Dozens of genes participate in this process. The enzymes are controlled through gene expression or through regulation or targeting of the proteins (Ohtsubo and Marth 2006).
Variability in the glycosylation of a single ion channel isoform can arise because of differences in expression of specific glycosylation enzymes between cells, at various stages of the developmental process, or in pathological conditions. A particularly striking example of the cell-type variability that can be observed is seen for cells derived from the various chambers of the heart. Montpetit et al. (2009) compared the expression of 239 genes coding for glycosyltransferases, glycosidases, and sugar nucleotide synthesis/transporter genes in four myocyte types: neonatal and adult atrium, and neonatal and adult ventricle. Of these, 110 glycogenes tested in mice were significantly differentially expressed among the four myocyte types (see Montpetit et al. 2009, their Fig. 15.1).
Stocker and Bennett (2006) found that the channels from neonatal atria, compared to adult atria or neonatal or adult ventricles, are much more heavily sialylated with approximately 15 more sialic acid residues attached to each alpha subunit. They showed that the difference is due to the expression of ST8sia2, a polysialyltransferase, that is expressed only in the neonatal atrium but not in adult atrium or in ventricles at any age (Montpetit et al. 2009). Comparison of action potential waveforms in neonatal atrial cells to those from ST8sia2 −/− mice showed distinct differences in time-to-peak and AP duration. These were consistent with the negative shift of gating of voltage-gated Na channels observed after ST8sia2 expression, while there was no effect on the level of expression of voltage-gated Na channels. The authors conclude that the expression of a single glycogene is sufficient to modulate cardiomyocyte excitability.
Similar changes in glycosylation and functional consequences also occur in neurons. The degree of sialylation can be developmentally regulated and accounts for variability in the biophysical properties of channels expressed at different stages of an animal’s life. The tetrodotoxin-resistant voltage-gated Na channel isoform Nav1.9 exists in two glycosylated states in neonatal rat DRG neurons (but only in a less glycosylated isoform in adult DRG (Tyrrell et al. 2001)). Deglycosylation of Nav1.9 caused an 8 mV depolarizing shift in steady-state inactivation in the neonatal but not adult DRG (Tyrrell et al. 2001). Castillo et al. (1997) demonstrated a progressive shift in the gating characteristics of forebrain Na channels from P0, P15, and adult P30/P180 rats. The shifts in the midpoint potential of activation paralleled an increase in apparent size. Both were reversed by neuraminidase treatment, indicating the increased amount of sialylation during development. In addition to variability due to glycosylation of the main pore-forming subunit of voltage-gated Na channels, glycosylation of auxiliary subunits also contributes to the complexity of effects. For example, the β4 subunit is expressed in a 35 kDa form between P0 and P6, but shifts to a heavily glycosylated 38 kDa form on P7 (Zhou et al. 2012).
15.3 Glycolipids and Ion Transport
Glycolipids influence ion transport primarily through association with and modulation of transport-associated proteins, which are often glycoconjugates themselves. Two general approaches have been employed in such investigations: (a) study of endogenous glycolipids through manipulations such as genetic alteration or structurally specific perturbing agents, and (b) application of exogenous glycolipids to isolated transport systems or cultured cells containing the transport system. The implied assumption with the exogenous approach is that the applied glycolipid inserts into the membrane or associates with the isolated transporter in a manner corresponding to its natural topography. This is often the reality, in which case the observed glycolipid-induced effects are viewed as true manifestations of their physiological function. However, exogenous glycolipids are known to associate with cellular membranes in three distinct modes: (a) a loosely attached pool removable with serum, (b) a somewhat more tightly associated pool released by trypsin, and (c) a serum- and trypsin-stable component consisting of the membrane-inserted pool (Wu and Ledeen 1994). The latter fraction, normally a small portion of associated glycolipid, is the one most likely to mimic endogenous glycolipid in relation to natural function, although the other two pools can conceivably give rise to pharmacological effects of potential therapeutic interest. With those caveats in mind, we will recount examples where the exogenous approach has been employed, while emphasizing studies based on endogenous glycolipid function for which powerful research tools have become available.
In most cases the glycolipid under study has been one or another ganglioside, the negatively charged sialic acid being crucial to their role in mediating cation movement. GM1 ganglioside, the prototypic member of the ganglio-series (Fig. 15.2) is often considered in conjunction with GD1a, the other prominent member of the a-series (Ando and Yu 1979). A primary function of this disialoganglioside is that of metabolic precursor to GM1 by virtue of neuraminidase (N’ase, also called sialidase), most forms of which remove only the terminal sialic acid. These two glycolipids have received prominent attention in regard to Ca2+ transport mechanisms. GM1 is one of the few sialoglycoconjugates in nature resistant to most types of N’ase, a property that facilitates elevation of its concentration on membrane surfaces while retaining its negative charge. The fact that it binds with high affinity and relative selectivity to the B subunit of cholera toxin (CtxB) (Schengrund and Ringler 1989) has provided a useful tool for probing its functional roles as well as its location in and within specific cells.
A rare example of a neutral glycolipid influencing ion transport is that of glucosylceramide, which was shown to increase Ca2+ mobilization from intracellular stores in the ER, via activation of the ryanodine receptor (Lloyd-Evans et al. 2003; Korkotian et al. 1999). This property, proposed as an explanation for the pathophysiology of neuronopathic forms of Gaucher disease, was not shared with galactosylceramide and several other sphingolipids. Whether this is a normal function of glucosylceramide in nonpathological cells remains to be determined.
15.3.1 Ganglioside Modulation of Na+ Transport
A role for GM1 in retaining neuronal conduction and excitability has been suggested in relation to its effect on Na+ channels. In some neurological or neuroimmunological patients, clusters of voltage-gated Na+ channels in nodes of Ranvier were shown to suffer damage by complement-mediated disruption through anti-GM1 antibodies, thought to represent disruption of axon-Schwann cell interactions at GM1 foci (Suzuki et al. 2007a). Mutant mice lacking the GM1 (ganglio) family of gangliosides due to disruption of the B4galnt1 gene (GM2/GD2 synthase) were initially shown to have a slight reduction in the neural conduction velocity of the tibial nerve (Takamiya et al. 1996), whereas subsequent studies indicated altered paranodal junctions, broadened Nav channel clusters, and aberrant Kv channel localization at the paranodes of peripheral motor nerves (Suzuki et al. 2007b). The fact that GD3 synthase gene knockout mice showed no loss of peripheral nerve conduction velocity (Handa et al. 2005) pointed to a-series gangliosides (GM1, GD1a) as the causative agents. These features further suggested that the GM1 contribution to Nav channel function resided in maintenance of microdomain (raft) integrity. However, more intimate association of GM1 with Navchannels was suggested by the observation that current densities of both tetrodotoxin-sensitive and insentitive Na+ channels were significantly decreased by CtxB (Qiao et al. 2008). That study provided evidence that endogenous GM1 plays a crucial role via modulation of Nav channels in retaining the afferent conduction velocity of not only myelinated fibers of motor nerves but also of myelinated and unmyelinated fibers of visceral afferents. Sodium transport mediated by the antiporter, Na+/K+-ATPase was shown to be activated by nmolar concentrations of GM1, an effect that was diminished at higher concentrations (Leon et al. 1981). The fact that this regulatory property was shared with other ganglio-series gangliosides suggested the possible presence of N’ase in the employed crude membrane fraction that could have produced GM1.
15.3.2 Ganglioside Modulation of Ca2+ Transport at the Plasma Membrane
Efficient regulation of free intracellular Ca2+ is essential for maintaining viability and excitability of neurons, and GM1 ganglioside has been widely implicated in regulatory roles for this ion (Ledeen and Wu 2002). One approach to elucidating the relevant mechanisms has been to elevate its endogenous level on the cell surface with applied N’ase,which triggered Ca2+ influx in Neuro2a, B104, and B50 neuroblastoma cells but not N1A-103 or N18 cells (Wu and Ledeen 1991; Fang et al. 2000). Those cells experiencing an elevation of intracellular Ca2+ in this manner extended neurites which were subsequently shown to have axonal character (Wu et al. 1998a). Both N’ase-induced Ca2+ influx and neuritogenesis were blocked by CtxB, indicating that GM1 elevation, as opposed to other effects of N’ase, was key to the changes. Activation of a specific channel type by elevated GM1 was suggested based on blockade by low concentrations of amiloride, described as a specific inhibitor of low threshold voltage dependent T type channels (Tang et al. 1988). The physiological significance of these phenomena was suggested by the involvement of endogenous plasma membrane-localized ganglioside-reactive N’ase (Miyagi et al. 1999; Monti et al. 2000) that regulates axonal growth in Neuro2a cells (Hasegawa et al. 2000) and primary hippocampal neurons (Rodriguez et al. 2001).
As mentioned, some cell lines did not respond to N’ase with Ca2+ influx and axon outgrowth, but instead showed a different regulatory mechanism mediated by GM1. Contrary to the inhibitory effect of CtxB on Neuro2a cells, N18 cells responded to CtxB with Ca2+ influx (Masco et al. 1991; Carlson et al. 1994), the response being more robust if preceded by N’ase treatment (Fang et al. 2002). Thus, N’ase-mediated elevation of surface GM1 facilitated Ca2+ entry in both mechanisms but through activation of different channel types. Similar CtxB effects analogous to those in N18 cells were observed in primary neurons of both the PNS (Milani et al. 1992) and CNS (Wu et al. 1996), but not with microglia or oligodendrocytes (Nedelkoska and Benjamins 1998) or in Schwann cells (Skoff and Benjamins 1998). In a study with cerebellar granule neurons, CtxB-induce Ca2+ influx occurred during the first 7 days in culture after which CtxB inhibited Ca2+ influx. A somewhat similar developmental sequence was observed in NG108-15 cells, these responding to CtxB with Ca2+ influx only during the initial phase of axon outgrowth (Fang et al. 2002). In addition to this CtxB-mediated effect, NG108-15 cells also showed the above N’ase-induced Ca2+ influx, indicating coexistence of both GM1-regulated Ca2+ channels (Fang et al. 2002). Both mechanisms of Ca2+ influx resulted in axon-like neurite outgrowth, in contrast to dendrite-like processes that resulted from agents (e.g., retinoic acid, dibutyrylcAMP) that did not stimulate Ca2+ influx (Wu et al. 1998a). Paradoxically, elevation of cellular ganglioside through application of exogenous gangliosides resulted in Ca2+ influx that gave rise to dendrite-like processes (Wu et al. 1990; 1998a). Exogenous gangliosides were also shown to reduce intracellular Ca2+ elevated in Neuro2a cells by ionomycin (Wu and Ledeen 1994), suggesting ganglioside promotion of Ca2+ homeostasis as part of its neuroprotective mechanism (Nakamura et al. 1992).
Whereas Ca2+ influx induced by N’ase elevation of surface GM1 appeared to involve T type channels, the GM1-regulated mechanism activated by CtxB was eventually shown to involve the TRPC5 channel (Wu et al. 2007). TRPC5 is an isoform of the canonical subgroup of mammalian genes homologous to the transient receptor potential (TRP) family in Drosophila (Montell 2004). GM1 does not associate directly with this channel but rather with α5β1 integrin heterodimers, these becoming cross-linked concurrently upon binding of CtxB to the ganglioside (Wu et al. 2007). Integrin cross-linking in this manner was shown to induce autophosphorylation of associated focal adhesion kinase, which in turn activated phospholipase Cγ and phosphoinositide-3 kinase. These effects were first revealed in NG108-15 cells following N’ase-induced elevation of cell surface GM1, which greatly enhanced the level of Ca2+ influx and neurite outgrowth induced by CtxB. This also promoted neurite outgrowth in murine cerebellar granule neurons without the need for N’ase pretreatment, this apparently being accomplished by upregulation of endogenous N’ase during neuronal differentiation. TRPC5 is prominently expressed in the soma of primary neurons and neuroblastoma cells only at an early stage of differentiation, consonant with selective activity of CtxB at that stage. The natural, endogenous cross-linking agent remained in doubt until studies with T cells in the immune system revealed that homodimeric galectin-1 exerts similar GM1 cross-linking as CtxB with similar TRPC5 Ca2+ channel activation (Wang et al. 2009a; Wu et al. 2011). Comparison of galectin-1 binding to GM1-deficient T cells vs wild type T cells suggested primary binding to GM1 (rather than glycoproteins) in that system (Wang et al. 2009a), consistent with the interaction of these two molecules in neuroblastoma cells and primary neurons (Kopitz et al. 1998; Gabius 2009). A schematic illustration has been presented (Ledeen et al. 2012) of homodimeric galectin-1 binding to the oligosaccharide structure of GM1 according to the Coulomb/van der Waals energy term obtained by computational interaction analysis (Siebert et al. 2003). Analogous to CtxB, anti-GM1 antibodies of the cross-linking IgM type were shown to induce similar Ca2+ changes (Quattrini et al. 2001) and neurite outgrowth (O’Hanlon et al. 2003) in neuroblastoma cells.
An additional example of GM1 modulation of Ca2+ influx, albeit indirectly, was seen in opioid activity of a certain type. Opioids are known to be capable of dual modulatory activities, as shown with action potential duration of sensory neurons (Shen and Crain 1989), neurotransmitter release in SK-N-SH cells (Keren et al. 1994), and calcium influx in NG108-15 cells (Jin et al. 1992). The excitatory mode, which promotes Ca2+ influx, was blocked by CtxB, implicating GM1 as facilitator of the excitatory response. This was verified in experiments showing conversion from inhibitory to excitatory mode by bath application of GM1 to CHO cells expressing the δ-opioid receptor (Wu et al. 1997a). The importance of the negatively charged carboxyl group of sialic acid was illustrated in loss of excitatory promotion by this modified GM1 (Wu et al. 1998b). Site-directed mutagenesis of the δ-opioid receptor involving replacement of the positively charged arginine residue at 192 with alanine also abolished the GM1-modulated excitatory response, suggesting this as the likely locus for charge-charge interaction of GM1 with this receptor (Wu et al. 1998b). This conformational interaction of GM1 with the δ-opioid receptor was seen as uncoupling of the receptor from Gi and facilitated coupling to Gs (Wu et al. 1997b). Neuronal Ca2+ influx stimulated by GM1 in this manner was postulated to occur through modulation of N-type Ca2+ channels (Keren et al. 1997).
Plasma membrane gangliosides have also been shown to influence Ca2+ efflux mechanisms, these often accounting for the neuroprotective activities observed with exogenously applied gangliosides (see above). Plasma membrane Ca2+-ATPase (PMCA), the high affinity mechanism for extrusion of cytosolic Ca2+, was studied in porcine brain synaptosomes and found to vary in response to different ganglioside structures in a manner reflecting the number of sialic acids: GD1b (two sialic acids) stimulated activity in contrast to GM1 (one sialic acid) which slightly reduced activity while asialo GM1 (no sialic acids) was strongly inhibitory (Zhao et al. 2004). Chain length was also considered important since GM2 and GM3 were both more inhibitory than GM1. The experimental procedure consisted of adding ganglioside to either synaptosomes or reconstituted proteo-liposomes containing purified synaptosomal PMCA followed by measurement of Ca2+ uptake, both procedures showing the same ganglioside modulatory effects. Purified PMCA was inactive due to delipidation during isolation, but was restored to full activity by reconstituting into liposomes containing phosphatidylcholine. Interestingly, a similar study with PMCA from pig erythrocytes gave very different results, all gangliosides being stimulatory up to sevenfold in the sequence: GD1b > GM1 > GM2 > GM3 = asialo-GM1 (Zhang et al. 2005). This difference was attributed to PMCA isoforms, PMCA1 and PMCA4 predominating in erythrocytes in contrast to PMCA2 and PMCA3 which are restricted to nerve cells. As these studies were carried out with applied gangliosides, it would be of interest to know whether the modulatory effect applied as well through in situ association with PMCA.
15.3.3 Ganglioside Modulation of Ca2+ Transport at Intracellular Loci
A number of studies on lysosomal storage disorders suggested a mechanistic link between ganglioside accumulation and disrupted Ca2+ homeostasis based on modulation of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) (Ginzburg et al. 2004). Thus a reduction in Ca2+-uptake via the SERCA pump was observed in neurons and brain microsomes of the Hexb−/− mouse, a model of Sandhoff disease (Pelled et al. 2003). The latter study also demonstrated reduction in the rate of Ca2+ uptake in normal brain microsomes by exogenous GM2, to a lesser extent by GM1 and least by GM3. A more detailed follow up study with brain microsomes revealed the necessity of N-acetylneuraminic acid with a free carboxyl group for inhibitory activity (Ginzburg et al. 2008). That study further proposed that the GalNAc residue of GM2 and GM1 may be an additional structural requirement for SERCA inhibition. The oligosaccharides alone had no activity. Similar studies on skeletal muscle sarcoplasmic reticulum also reported an inhibitory effect of GM1 on SERCA activity but in contrast a positive modulatory effect by GM3 (Wang et al. 1999a). Using intrinsic and time-resolved fluorescence in addition to fluorescence quenching, the conformational changes observed indicated that GM1 could render the SERCA molecules less compact in the hydrophilic domain but more compact in the hydrophobic domain; GM3 on the other hand made the enzyme more compact in both the hydrophilic and hydrophobic domains. Further study of this system employing circular dichroism showed that both GM1 and GM3 reduced the α-helical content of the protein, with GM1 causing the stronger decrease; study of the proteo-liposomes containing this Ca2+-ATPase using DPH as the probe showed that GM1 decreased membrane lipid fluidity while GM3 tended to increase it (Wang et al. 1999b). All the above SERCA studies were carried out with applied (exogenous) gangliosides, underscoring the desirability of ascertaining whether endogenous gangliosides have similar modulatory effects.
Nuclear GM1 was shown to have a prominent role in regulation of nuclear Ca2+ homeostasis through association with a sodium-calcium exchanger (NCX) in the inner nuclear membrane (Xie et al. 2002). This NCX, which mediates transfer of Ca2+ between the nucleoplasm and the luminal space of the nuclear envelope, is potently and specifically activated by GM1. Immunoblot analysis revealed an unusually tight association of GM1 with the nuclear NCX, so strong that it survived SDS-PAGE; this differed from the NCX of the plasma membrane for which a looser association with GM1 was suggested. A key feature proposed for the nuclear topology based on colocalization of GM1 and NCX in the inner nuclear membrane is interaction of the negative charge of N-acetylneuraminic acid of GM1 with the alternative splice region of the NCX loop containing positively charged amino acid(s) (Xie et al. 2004). For the plasma membrane, this NCX loop is seen as residing on the opposite side of the bilayer as GM1, thus accounting for the lower affinity association. Nuclear NCX is activated in developing neurons upon upregulation of GM1 synthesis, a process that also occurs in some but not all extraneural cell types (Xie et al. 2004). Use of a variety of cell types with and without nuclear NCX together with specific Ca2+ fluorescent indicators revealed transport of nucleoplasmic Ca2+ into the nuclear envelope followed by transfer to the ER lumen; in keeping with cytosolic Ca2+ flux through nuclear pores, the nuclear NCX/GM1 complex was shown to gate Ca2+ transfer from cytosol to ER, thus constituting an alternative mechanism to the SERCA pump for such transfer (Wu et al. 2009). As with plasma membrane NCX, the driving force for such transfer is the Na+ gradient created by Na+/K+-ATPase, and the latter transporter was shown to occur in the nuclear membrane suggesting concerted physiological coupling between these transporters (Galva et al. 2012). The relatively simple ganglioside pattern of the nuclear envelope includes GD1a which serves as metabolic precursor to GM1 owing to the presence of N’ase at the same locus (Wang et al. 2009b).
The nuclear NCX/GM1 complex was shown to serve a neuroprotective role in shielding the nucleus against prolonged elevation of nucleoplasmic Ca2+, as seen in studies with mice lacking GM1 due to deletion of GM2/GD2 synthase [B4galnt1(-/-)]. Cultured cerebellar granule neurons from such mice were shown to have lost the ability possessed by wild-type cells to regulate Ca2+ homeostasis, resulting in apoptotic death when the cells were exposed to high K+ (Wu et al. 2001). This neuroprotective role was demonstrated in vivo with the above mice which showed enhanced susceptibility to kainate-induced seizures and neuronal apoptosis (Wu et al. 2005). Seizure activity and deterioration of pyramidal neurons in the CA3 of the hippocampus were significantly alleviated by intraperitoneal administration of LIGA20, a membrane permeable analog of GM1 (Fig. 15.2). This was coincident with LIGA20 entering the brain and brain cells, including neuronal nuclear membrane, with restoration of attenuated NCX activity. GM1 itself, with limited membrane permeability, showed little benefit when administered intraperitoneally.
The above reports on SERCA and nuclear NCX, examples of intracellular glycolipid regulation of ion transport, indicate the importance of considering the whole cell, not the plasma membrane alone. Additional examples of intracellular regulation are being revealed, such as the role of GM1 in modulating Ca2+ levels in the ER, with potential for initiating a UPR-mediated apoptotic cascade (Tessitore et al. 2004). Further work in this area employing the GM1 gangliosidosis mouse model revealed GM1 accumulation in the raft fraction of mitochondria-associated ER membranes that influence Ca2+ flux between these organelles (Sano et al. 2009). The accumulated GM1 was shown to interact with the phosphorylated form of IP3 receptor-1, influencing the activity of that channel. It will be of interest to have more detail on the nature of this interaction and to know whether it occurs as well in nonpathological neurons. The numerous ways in which GM1 regulates neuronal Ca2+ homeostasis, as revealed to date, are summarized in Fig. 15.3.
Summary of GM1 modulatory roles for neuronal Ca2+. For the plasma membrane, Ca2+ influx is promoted by GM1 via T type channels and by GM1 association with α5β1 integrin, cross-linking of which leads to TRPC5 channel activation. Calcium efflux is influenced positively in the plasma membrane by GM1 association with Na+/Ca2+ exchanger (NCX) and negatively by GM1 association with plasma membrane Ca2+-ATPase (PMCA). Intracellular mechanisms include GM1 association with NCX in the inner nuclear membrane that mediates transfer of Ca2+ from nucleoplasm to the nuclear envelope (and hence the ER); also inhibition of the SERCA pump. GM1 in the ER influences Ca2+ flux from that organelle to mitochondria
15.4 Ion Transport and the Sugar Code
Considering the crucial role of ion transport in numerous aspects of nervous system functioning, it is not unexpected to find glycosylation in its multiple forms fulfilling a wide variety of essential modulatory roles. The relatively simple glycosylation patterns that characterize invertebrate nervous systems contrasts with the myriad arrays of protein and lipid glycoconjugates that occur and appear essential to the nervous systems of higher forms. The complexity of glycan configurations that mediate neural phenomena increases in tandem with complexity of evolutionary life forms. Carbohydrates, it has been pointed out, “…are second to no other class of biomolecules in the capacity of information coding using oligomers” (Gabius 2009). A more complete understanding of such coding as applied to ion transport awaits elucidation of the detailed mechanisms by which glycosylation influences channel/pump transport, conformational folding, and the stereochemistry of molecular interactions.
Abbreviations
- N’ase (also called sialidase):
-
Neuraminidase
- TRP (TRPC3, TRPC5, TRPC6, TRPV1, TRPM8):
-
Transient receptor potential channel
- AChR:
-
Nicotinic acetylcholine receptor
- CtxB:
-
Cholera toxin B subunit
- DRG:
-
Dorsal root ganglion
- ER:
-
Endoplasmic reticulum
- NCX:
-
Sodium-calcium exchanger
- PMCA:
-
Plasma membrane Ca2+-ATPase
- SERCA:
-
Sarco/endoplasmic reticulum Ca2+-ATPase
References
Ando S, Yu RK. Isolation and characterization of two isomers of brain tetrasialogangliosides. J Biol Chem. 1979;254:12224–9.
Barchi RL. Protein components of the purified sodium channel from rat skeletal muscle sarcolemma. J Neurochem. 1983;40:1377–85.
Bennett ES. Isoform-specific effects of sialic acid on voltage-dependent Na+ channel gating: functional sialic acids are localized to the S5-S6 loop of domain I. J Physiol. 2002;538:675–90.
Bennett E, Urcan MS, Tinkle SS, Koszowski AG, Levinson SR. Contribution of sialic acid to the voltage dependence of sodium channel gating. A possible electrostatic mechanism. J Gen Physiol. 1997;109:327–43.
Carlson RO, Masco D, Brooker G, Spiegel S. Endogenous ganglioside GM1 modulates l-type calcium channel activity in N18 neuroblastoma cells. J Neurosci. 1994;14:2272–81.
Castillo C, Diaz ME, Balbi D, Thornhill WB, Recio-Pinto E. Changes in sodium channel function during postnatal brain development reflect increases in the level of channel sialidation. Brain Res Dev Brain Res. 1997;104:119–30.
Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol. 2012;590:2577–89.
Cotella D, Radicke S, Bortoluzzi A, Ravens U, Wettwer E, Santoro C, et al. Impaired glycosylation blocks DPP10 cell surface expression and alters the electrophysiology of Ito channel complex. Pflugers Arch. 2010;460:87–97.
Dietrich A, Mederos y Schnitzler M, Emmel J, Kalwa H, Hofmann T, Gudermann T. N-linked protein glycosylation is a major determinant for basal TRPC3 and TRPC6 channel activity. J Biol Chem. 2003;278:47842–52.
Erler I, Al-Ansary DM, Wissenbach U, Wagner TF, Flockerzi V, Niemeyer BA. Trafficking and assembly of the cold-sensitive TRPM8 channel. J Biol Chem. 2006;281(50):38396–404.
Fang Y, Wu G, Xie X, Lu Z-H, Ledeen RW. Endogenous GM1 ganglioside of the plasma membrane promotes neuritogenesis by two mechanisms. Neurochem Res. 2000;25:931–40.
Fang Y, Xie X, Ledeen RW, Wu G. Characterization of cholera toxin B subunit-induced Ca2+ influx in neuroblastoma cells: evidence for a voltage independent GM1-associated Ca2+ channel. J Neurosci Res. 2002;57:1–10.
Gabius H-J, editor. The sugar code. Fundamentals of glycosciences. Weinheim, Germany: Wiley-VHC; 2009.
Galva C, Artigas P, Gatto C. Nuclear Na+K+-ATPase plays an active role in nucleoplasmic Ca2+ homeostasis. J Cell Sci. 2012;125:6137–47.
Gilly WF, Lucero MT, Horrigan FT. Control of the spatial distribution of sodium channels in giant fiber lobe neurons of the squid. Neuron. 1990;5:663–74.
Ginzburg L, Kacher Y, Futerman AH. The pathogenesis of glycosphingolipid storage disorders. Semin Cell Dev Biol. 2004;15:417–31.
Ginzburg L, Li S-C, Li Y-T, Futerman AH. An exposed carboxyl group on sialic acid is essential for gangliosides to inhibit calcium uptake via the sarco/endoplasmic reticulum Ca2+-ATPase: relevance to gangliosidoses. J Neurochem. 2008;104:140–6.
Hall MK, Cartwright TA, Fleming CM, Schwalbe RA. Importance of glycosylation on function of a potassium channel in neuroblastoma cells. PLoS One. 2011;6:e19317.
Handa Y, Ozaki N, Honda T, Furukawa K, Tomita Y, Inoue M, et al. GD3 synthase gene knockout mice exhibit thermal hyperalgesia and mechanical allodynia but decreased response to formalin-induced prolonged noxious stimulation. Pain. 2005;117:271–9.
Hartshorne RP, Catterall WA. Purification of the saxitoxin receptor of the sodium channel from rat brain. Proc Natl Acad Sci U S A. 1981;78:4620–4.
Hartshorne RP, Catterall WA. The sodium channel from rat brain. Purification and subunit composition. J Biol Chem. 1984;259:1667–75.
Hasegawa T, Yamaguchi K, Wada T, Takeda A, Itoyama Y, Miyagi T. Molecular cloning of mouse ganglioside sialidase and its increased expression in Neuro2a differentiation. J Biol Chem. 2000;275:8007–15.
Isaev D, Isaeva E, Shatskih T, Zhao Q, Smits NC, Shworak NW, et al. Role of extracellular sialic acid in regulation of neuronal and network excitability in the rat hippocampus. J Neurosci. 2007;27:11587–94.
Isaeva E, Lushnikova I, Savrasova A, Skibo G, Holmes GL, Isaev D. Effect of neuraminidase treatment on persistent epileptiform activity in the rat hippocampus. Pharmacol Rep. 2011;63:840–4.
Isom LL, De Jongh KS, Patton DE, Reber BF, Offord J, Charbonneau H, et al. Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science. 1992;256:839–42.
James WM, Agnew WS. Alpha-(2–8)-polysialic acid immunoreactivity in voltage-sensitive sodium channel of eel electric organ. Proc R Soc Lond B Biol Sci. 1989;237:233–45.
Jin W, Lee NM, Loh HH, Thayer SA. Dual excitatory and inhibitory effects of opioids on intracellular calcium in neuroblastoma X glioma NG108-15 cells. Mol Pharmacol. 1992;42:1083–9.
Johnson D, Montpetit ML, Stocker PJ, Bennett ES. The sialic acid component of the beta1 subunit modulates voltage-gated sodium channel function. J Biol Chem. 2004;279:44303–10.
Keren O, Garty M, Sarne Y. Dual regulation by opioids of [3H]norepinephrine release in the human neuroblastoma cell line SK-N-SH. Brain Res. 1994;646:319–23.
Keren O, Gafni M, Sarne Y. Opioids potentiate transmitter release from SK-N-SH human neuroblastoma cells by modulating N-type calcium channels. Brain Res. 1997;764:277–82.
Khanna R, Myers MP, Laine M, Papazian DM. Glycosylation increases potassium channel stability and surface expression in mammalian cells. J Biol Chem. 2001;276:34028–34.
Kopitz J, Von Reitzenstein C, Burchert M, Cantz M, Gabius H-J. Galectin-1 is a major receptor for ganglioside GM1, a product of the growth controlling activity of a cell surface ganglioside sialidase, on human neuroblastoma cells in culture. J Biol Chem. 1998;273:11205–11.
Korkotian E, Schwarz A, Pelled D, Schwarzmann G, Segal M, Futerman AH. Elevation of intracellular glucosylceramide levels results in an increase in endoplasmic reticulum density and in functional calcium stores in cultured neurons. J Biol Chem. 1999;274:21673–8.
Ledeen RW, Wu G. Ganglioside function in calcium homeostasis and signaling. Neurochem Res. 2002;27:637–47.
Ledeen RW, Wu G, André S, Bleich D, Huet G, Kaltner H, et al. Beyond glycoproteins as galectincounterreceptors: tumor-effector T cell growth control via ganglioside GM1. Ann N Y Acad Sci. 2012;1253:206–21.
Leon A, Facci L, Toffano G, Sonnino S, Tettamanti G. Activation of (Na+, K+)-ATPase by nanomolar concentrations of GM1 ganglioside. J Neurochem. 1981;37:350–7.
Lloyd-Evans E, Pelled D, Riebeling C, Bodennec J, de-Morgan A, Waller H, Schiffmann R, Futerman AH. Glucosylceramide and glucosylsphingosine modulate calcium mobilization from brain microsomes via different mechanisms. J Biol Chem. 2003;278:23594–9.
Lo WY, Lagrange AH, Hernandez CC, Harrison R, Dell A, Haslam SM, et al. Glycosylation of β2 subunits regulates GABAA receptor biogenesis and channel gating. J Biol Chem. 2010;285:31348–61.
Masco D, Van de Walle M, Spiegel S. Interaction of ganglioside GM1 with the B subunit of cholera toxin modulates growth and differentiation of neuroblastoma N18 cells. J Neurosci. 1991;11:2443–52.
Messner DJ, Catterall WA. The sodium channel from rat brain. Separation and characterization of subunits. J Biol Chem. 1985;260:10597–604.
Milani D, Minozzi MC, Petrelli L, Guidolin D, Skaper SD, Spoerri PE. Interaction of ganglioside GM1 with the B subunit of cholera toxin modulates intracellular free calcium in sensory neurons. J Neurosci Res. 1992;33:466–75.
Miller JA, Agnew WS, Levinson SR. Principal glycopeptide of the tetrodotoxin/saxitoxin binding protein from electrophorus electricus: isolation and partial chemical and physical characterization. Biochemistry. 1983;22:462–70.
Miyagi T, Wada T, Iwamatsu A, Hata K, Yoshikawa Y, Tokuyama S, et al. Molecular cloning and characterization of plasma membrane-associated sialidase specific for gangliosides. J Biol Chem. 1999;274:5004–11.
Montell C. Exciting trips for TRPs. Nat Cell Biol. 2004;6:690–2.
Monti E, Bassi MT, Papini N, Riboni M, Manzoni M, Venerando B, et al. Identification and expression of NEU3, a novel human sialidase associated to the plasma membrane. Biochem J. 2000;81:284–96.
Montpetit ML, Stocker PJ, Schwetz TA, Harper JM, Norring SA, Schaffer L, et al. Regulated and aberrant glycosylation modulate cardiac electrical signaling. Proc Natl Acad Sci U S A. 2009;106:16517–22.
Nakamura K, Wu G, Ledeen RW. Protection of Neuro-2a cells against calcium ionophore cytotoxicity by gangliosides. J Neurosci Res. 1992;31:245–53.
Nedelkoska L, Benjamins JA. Binding of cholera toxin B subunit: a surface marker for murine microglia but not oligodendrocytes or astrocytes. J Neurosci Res. 1998;53:605–12.
O’Hanlon GM, Hirst TR, Willison HJ. Ganglioside GM1 binding toxins and human neuropathy-associated IgM antibodies differentially promote neuritogenesis in a PC12 assay. Neurosci Res. 2003;47:383–90.
Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–67.
Pelled D, Lloyd-Evans E, Riebeling C, Jeyakumar M, Platt FM, Futerman AH. Inhibition of calcium uptake via the sarco/endoplasmic reticulum Ca2+-ATPase in a mouse model of Sandhoff disease and prevention by treatment with N-butyldeoxynojirimycin. J Biol Chem. 2003;278:29496–501.
Peng XQ, Zhang XL, Fang Y, Xie WR, Xie YK. Sialic acid contributes to hyperexcitability of dorsal root ganglion neurons in rats with peripheral nerve injury. Brain Res. 2004;1026(2):185–93.
Qiao GF, Cheng ZF, Huo R, Sui XH, Lu YJ, Li BY. GM1 ganglioside contributes to retain the neuronal conduction and neuronal excitability in visceral and baroreceptor afferents. J Neurochem. 2008;106:1637–45.
Quattrini A, Lorenzetti I, Sciorati C, Corbo M, Previtali SC, Feltri ML, et al. Human IgM anti-GM1 autoantibodies modulate intracellular calcium homeostasis in neuroblastoma cells. J Neuroimmunol. 2001;114:213–9.
Roberts RH, Barchi RL. The voltage-sensitive sodium channel from rabbit skeletal muscle. Chemical characterization of subunits. J Biol Chem. 1987;262:2298–303.
Rodriguez JA, Piddini E, Hasegawa T, Miyagi T, Dotti CG. Plasma membrane ganglioside sialidase regulates axonal growth and regeneration in hippocampal neurons in culture. J Neurosci. 2001;21:8387–95.
Rutishauser U, Landmesser L. Polysialic acid in the vertebrate nervous system: a promoter of plasticity in cell-cell interactions. Trends Neurosci. 1996;19:422–7.
Sandoval A, Oviedo N, Andrade A, Felix R. Glycosylation of asparagines 136 and 184 is necessary for the alpha2delta subunit-mediated regulation of voltage-gated Ca2+ channels. FEBS Lett. 2004;576:21–6.
Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, Opferman J, et al. GM1 ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca2+-dependent mitochondrial apoptosis. Mol Cell. 2009;36:500–11.
Schengrund C-L, Ringler NJ. Binding of Vibrio cholera toxin and the heat labile enterotoxin of Escherichia coli to GM1 and derivatives of GM1, and nonlipid, oligosaccharide polyvalent ligands. J Biol Chem. 1989;264:13233–7.
Schmidt JW, Catterall WA. Palmitylation, sulfation, and glycosylation of the alpha subunit of the sodium channel. Role of post-translational modifications in channel assembly. J Biol Chem. 1987;262:13713–23.
Schwetz TA, Norring SA, Ednie AR, Bennett ES. Sialic acids attached to O-glycans modulate voltage-gated potassium channel gating. J Biol Chem. 2011;286:4123–32.
Shen KF, Crain SM. Dual modulation of the action potential duration of mouse dorsal root ganglion neurons in culture. Brain Res. 1989;491:227–42.
Siebert HC, André S, Lu SY, Frank M, Kaltner H, van Kuik JA, et al. Unique conformer selection of human growth-regulatory lectin galectin-1 for ganglioside GM1 versus bacterial toxins. Biochemistry. 2003;42:14762–73.
Skoff AM, Benjamins JA. Antibodies to glycolipids and cholera toxin B subunit do not initiate Ca2+ signaling in rat Schwann cells. J Peripher Nerv Syst. 1998;3:19–27.
Stocker PJ, Bennett ES. Differential sialylation modulates voltage-gated Na+ channel gating throughout the developing myocardium. J Gen Physiol. 2006;127:253–65.
Sumikawa K, Miledi R. Assembly and N-glycosylation of all ACh receptor subunits are required for their efficient insertion into plasma membranes. Brain Res Mol Brain Res. 1989;5:183–92.
Susuki K, Rasband MN, Tohyama K, Koibuchi K, Okamoto S, Funakoshi K, et al. Anti-GM1 antibodies cause complement-mediated disruption of sodium channel clusters in peripheral motor nerve fibers. J Neurosci. 2007a;27:3956–67.
Susuki K, Baba H, Tohyama K, Kanai K, Kuwabara S, Hirata K, et al. Gangliosides contribute to stability of paranodal junctions and ion channel clusters in myelinated nerve fibers. Glia. 2007b;55:746–57.
Takamiya K, Yamamoto A, Furukawa K, Yamashiro S, Shin M, Okada M, et al. Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc Natl Acad Sci U S A. 1996;93:10662–7.
Tang C-M, Presser F, Morad M. Amiloride selectively blocks the low threshold (T) calcium channel. Science. 1988;240:213–5.
Tessitore A, del P Martin M, Sano R, Ma Y, Mann L, Ingrassia A, et al. GM1-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis. Mol Cell. 2004;15:753–66.
Thornhill WB, Wu MB, Jiang X, Wu X, Morgan PT, Margiotta JF. Expression of Kv1.1 delayed rectifier potassium channels in Lec mutant Chinese hamster ovary cell lines reveals a role for sialidation in channel function. J Biol Chem. 1996;271:19093–8.
Tyrrell L, Renganathan M, Dib-Hajj SD, Waxman SG. Glycosylation alters steady-state inactivation of sodium channel Nav 1.9/NaN in dorsal root ganglion neurons and is developmentally regulated. J Neurosci. 2001;21(24):9629–37.
Waechter CJ, Schmidt JW, Catterall WA. Glycosylation is required for maintenance of functional sodium channels in neuroblastoma cells. J Biol Chem. 1983;258:5117–23.
Wang Y, Tsui Z, Yang F. Antagonistic effect of ganglioside GM1 and GM3 on the activity and conformation of sarcoplasmic reticulum Ca2+-ATPase. FEBS Lett. 1999a;457:144–8.
Wang Y, Tsui Z, Yang F. Mechanistic study of modulation of SR Ca2+-ATPase activity by gangliosides GM1 and GM3 through some biophysical measurements. Glycoconj J. 1999b;16:781–6.
Wang J, Lu Z-H, Gabius H-J, Rolhowsky-Kochan C, Ledeen RW, Wu G. Cross-linking of GM1 ganglioside by galectin-1 mediates regulatory T cell activity involving TRPC5 channel activation: possible role in suppressing experimental autoimmune encephalomyelitis. J Immunol. 2009a;182:4036–45.
Wang J, Wu G, Miyagi T, Lu Z-H, Ledeen RW. Sialidase occurs in both membranes of the nuclear envelope and hydrolyzes endogenous GD1a. J Neurochem. 2009b;111:547–54.
Watanabe I, Zhu J, Recio-Pinto E, Thornhill WB. Glycosylation affects the protein stability and cell surface expression of Kv1.4 but not Kv1.1 potassium channels. A pore region determinant dictates the effect of glycosylation on trafficking. J Biol Chem. 2004;279:8879–85.
Wirkner K, Hognestad H, Jahnel R, Hucho F, Illes P. Characterization of rat transient receptor potential vanilloid 1 receptors lacking the N-glycosylation site N604. Neuroreport. 2005;16:997–1001.
Wu G, Ledeen RW. Stimulation of neurite outgrowth in neuroblastoma cells by neuraminidase: putative role of GM1 ganglioside in differentiation. J Neurochem. 1991;56:95–104.
Wu G, Ledeen RW. Gangliosides as modulators of neuronal calcium. Prog Brain Res. 1994;101:101–12.
Wu G, Vaswani KK, Lu Z-H, Ledeen RW. Gangliosides stimulate calcium flux in Neuro-2A cells and require exogenous calcium for neuritogenesis. J Neurochem. 1990;55:484–91.
Wu G, Lu Z-H, Nakamura K, Spray DC, Ledeen RW. Trophic effect of cholera toxin B subunit in cultured cerebellar granule neurons: modulation of intracellular calcium by GM1 ganglioside. J Neurosci Res. 1996;44:243–54.
Wu G, Lu Z-H, Ledeen RW. Interaction of the δ-opioid receptor with GM1 ganglioside: conversion from inhibitory to excitatory mode. Brain Res Mol Brain Res. 1997a;44:341–6.
Wu G, Lu Z-H, Alfinito P, Ledeen RW. Opioid receptor and calcium channel regulation of adenylyl cyclase, modulated by GM1, in NG108-15 cells: competitive interactions. Neurochem Res. 1997b;22:1281–9.
Wu G, Fang Y, Lu Z-H, Ledeen RW. Induction of axon-like and dendrite-like processes in neuroblastoma cells. J Neurocytol. 1998a;27:1–14.
Wu G, Lu ZH, Wei TJ, Howells RD, Christoffers K, Ledeen RW. The role of GM1 ganglioside in regulating excitatory opioid effects. Ann N Y Acad Sci. 1998b;845:126–38.
Wu G, Xie X, Lu Z-H, Ledeen RW. Cerebellar neurons lacking complex gangliosides degenerate in the presence of depolarizing levels of potassium. Proc Natl Acad Sci U S A. 2001;98:307–12.
Wu G, Lu ZH, Wang J, Wang Y, Xie X, Meyenhofer MF, et al. Enhanced susceptibility to kainate-induced seizures, neuronal apoptosis, and death in mice lacking gangliotetraosegangliosides: protection with LIGA20, a membrane-permeant analog of GM1. J Neurosci. 2005;25:11014–22.
Wu G, Lu Z-H, Obukhov ASG, Nowycky MC, Ledeen RW. Induction of calcium influx through TRPC5 channels by cross-linking of GM1 ganglioside associated with α5β1 integrin initiates neurite outgrowth. J Neurosci. 2007;27:7447–58.
Wu G, Xie X, Lu Z-H, Ledeen RW. Sodium-calcium exchanger complexed with GM1 ganglioside in nuclear membrane transfers calcium from nucleoplasm to endoplasmic reticulum. Proc Natl Acad Sci U S A. 2009;106:10829–34.
Wu G, Lu Z-H, Gabius H-J, Ledeen RW, Bleich D. Ganglioside GM1 deficiency in effector T cells from NOD mice induces resistance to regulatory T-cell suppression. Diabetes. 2011;60:2341–9.
Xie X, Wu G, Lu Z-H, Ledeen RW. Potentiation of a sodium-calcium exchanger in the nuclear envelope by nuclear GM1 ganglioside. J Neurochem. 2002;81:1185–95.
Xie X, Wu G, Lu Z-H, Rohowsky-Kochan C, Ledeen RW. Presence of sodium-calcium exchanger/GM1 complex in the nuclear envelope of non-neural cells: nature of the exchanger-GM1 interaction. Neurochem Res. 2004;29:2135–46.
Zhang J, Zhao Y, Duan J, Yang F, Zhang X. Gangliosides activate the phosphatase activity of the erythrocyte plasma membrane Ca2+-ATPase. Arch Biochem Biophys. 2005;444:1–6.
Zhao Y, Fan X, Yang F, Zhang Z. Gangliosides modulate the activity of the plasma membrane Ca2+-ATPase from porcine brain synaptosomes. Arch Biochem Biophys. 2004;427:204–12.
Zhou TT, Zhang ZW, Liu J, Zhang JP, Jiao BH. Glycosylation of the sodium channel β4 subunit is developmentally regulated and involves in neuritic degeneration. Int J Biol Sci. 2012;8:630–9.
Zona C, Eusebi F, Miledi R. Glycosylation is required for maintenance of functional voltage-activated channels in growing neocortical neurons of the rat. Proc R Soc Lond B Biol Sci. 1990;239:119–27.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Nowycky, M.C., Wu, G., Ledeen, R.W. (2014). Glycobiology of Ion Transport in the Nervous System. In: Yu, R., Schengrund, CL. (eds) Glycobiology of the Nervous System. Advances in Neurobiology, vol 9. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1154-7_15
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1154-7_15
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1153-0
Online ISBN: 978-1-4939-1154-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)