Abstract
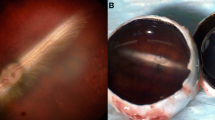
Aging of vitreous involves a degenerative process where glycosaminoglycans (GAGs) dissociate from collagen and the gel disintegrates into fluid adjacent to which collagen fibrils adhere to each other and cross-link [1–4]. In most eyes this process is accompanied by dehiscence at the vitreoretinal interface progressing to posterior vitreous detachment and nothing more happens [see chapter II.C. Vitreous aging and posterior vitreous detachment]. In several pathologic conditions, vitreous is hazy due to hemorrhage and/or cell proliferation forming membranes that contract, leading to traction retinal detachment [see chapters III.J. Cell proliferation at the vitreo-retinal interface in proliferative vitreo-retinopathy and related disorders; III.L. Proliferative diabetic vitreo-retinopathy]. Vitreoretinal surgery for these conditions includes complete removal of the vitreous gel (Figure I.F-1), removal of pre- and subretinal membranes, and injection of a tamponade such as silicone oil or expanding gases. All the presently used intravitreal tamponade substances carry different side effects making them unsuitable for permanent or long-standing use. Many authors [5–9] have suggested an ideal single artificial vitreous gel covering all needs, but no gel has yet been found to fit these demands [10].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vitreous
- Biochemistry
- Collagen
- Hyaluronan
- Artificial vitreous
- Hydrogels
- Polyalkylimide
- Polyethylene glycol
- Cross-linked hyaluronic acid hydrogels
-
1.
Vitreous is not an inert substance but has a complex biochemistry and plays an important role in ocular physiology.
-
2.
To date, vitreous substitutes have only been developed for short-term use during or, typically, after vitreoretinal surgery, without consideration for the optical and physiologic properties necessary for long-term homeostasis. Cross-linked hyaluronic acid hydrogels possess many of the needed physiologic properties without the untoward effects of polyalkylimide and polyethylene glycol.
-
3.
Future development of artificial vitreous gels must confirm biocompatibility as tested with electrophysiology, morphology, and histology both in vitro and in vivo. Future objectives will be to determine whether a synthetic posterior vitreous cortex is needed and whether “smart hydrogels” and hydrogels containing hyaluronic acid and cross-linked, enzyme-stable gels will be the best future options for an artificial vitreous.
I. Introduction
A. Rationale: Why an Artificial Vitreous?
Aging of vitreous involves a degenerative process where glycosaminoglycans (GAGs) dissociate from collagen and the gel disintegrates into fluid adjacent to which collagen fibrils adhere to each other and cross-link [1–4]. In most eyes this process is accompanied by dehiscence at the vitreoretinal interface progressing to posterior vitreous detachment and nothing more happens [see chapter II.C. Vitreous aging and posterior vitreous detachment]. In several pathologic conditions, vitreous is hazy due to hemorrhage and/or cell proliferation forming membranes that contract, leading to traction retinal detachment [see chapters III.J. Cell proliferation at the vitreo-retinal interface in proliferative vitreo-retinopathy and related disorders; III.L. Proliferative diabetic vitreo-retinopathy]. Vitreoretinal surgery for these conditions includes complete removal of the vitreous gel (Figure I.F-1), removal of pre- and subretinal membranes, and injection of a tamponade such as silicone oil or expanding gases. All the presently used intravitreal tamponade substances carry different side effects making them unsuitable for permanent or long-standing use. Many authors [5–9] have suggested an ideal single artificial vitreous gel covering all needs, but no gel has yet been found to fit these demands [10].
This chapter reviews the biochemical composition and organization of vitreous, the currently used vitreous substitutes, and the rationale as well as approaches to developing an artificial vitreous.
B. Vitreous Biochemistry
More than 95 % of the vitreous gel weight is water. About 15–20 % of this water is bound to glycosaminoglycans (GAGS) and proteins. The vitreous body of all species is composed of essentially the same extracellular matrix elements organized to fill the center of the eye with a clear viscous substance surrounded by a dense cortex that is attached to the basal laminae of surrounding cells. There are, however, species variations in the relative concentrations of the major structural components [2, 11] of vitreous, i.e., hyaluronan (HA) and collagen. These differences account for variations in the rheologic (gel-liquid) state of the vitreous body in different species. It should be emphasized that in more advanced species there are also age-related differences. Consequently, the selection of an appropriate animal with which to model human diseases for scientific investigation must take into consideration these species variations and age-related differences. HA is present in the vitreous body of all species studied except for fishes.
1. Glycosaminoglycans (GAGs)
GAGs are polysaccharides composed of repeating disaccharide units, each consisting of hexosamine (usually N-acetyl glucosamine or N-acetyl galactosamine) glycosidically linked to either uronic (glucuronic or iduronic) acid or galactose. The nature of the predominant repeating unit is characteristic for each GAG and the relative amount, molecular size, and type of GAG are said to be tissue specific [12]. A sulfated group is attached to oxygen or nitrogen in all GAGs except HA. GAGs do not normally occur in vivo as free polymers but are covalently linked to a protein core, the ensemble called a proteoglycan. There are three types of GAGs present in the vitreous body: hyaluronan (HA), chondroitin sulfate (CS) and heparan sulfate (HS).
a. Hyaluronan
Hyaluronan (HA; previously called hyaluronic acid) is the major GAG present in the human vitreous. Meyer and Palmer 1934 [13] first isolated this macromolecule from bovine vitreous. HA’s name is derived from the fact that it was first discovered in the clear, colorless vitreous (“hyalos,” means glass in Greek) and that it contains uronic acid. Balazs et al. [14, 15] documented the presence of sulfated galactosamine-containing GAGS in bovine vitreous (less than 5 % heparin sulfate). Meyer [16] and Balazs [17] first identified HA as a long, unbranched polymer of repeating disaccharide (glucuronic acid beta 1–3 N-acetylglucosamine) linked by beta l-4 bonds. HA first appears after birth and is believed to be synthesized primarily by hyalocytes. Although the synthesis of HA plateaus in the adult, there does not appear to be any extracellular degradation [18–21], so HA levels remain constant because there is escape of HA molecules from vitreous via the anterior segment of the eye [22] and there is reuptake by hyalocytes. Laurent and Fraser [23] showed that the escape of HA from the vitreous to the anterior segment is strongly molecular weight dependent, indicating a diffusion-controlled process. In contrast, disappearance of HA from the anterior chamber is independent of molecular weight, suggesting that this is controlled by bulk flow. Later studies noted a marked linear decrease of HA levels with age in human vitreous [24]. Laurent and Granath [25] used gel chromatography and found the average molecular weight of rabbit vitreous to be 2–3 × l06 and of bovine vitreous to be 0.5–0.8 × l06. These studies found age-related differences in the bovine vitreous since the HA molecular weight dropped from 3 × l06 in the newborn calf to 0.5 × l06 in old cattle.
The volume of the unhydrated HA molecule is about 0.66 cm3/g, whereas the hydrated specific volume is 2–3,000 cm3/g [15]. Thus, the degree of hydration (and any pathologic condition that alters hydration) can have a significant influence on the size and configuration of the molecular network in vitreous. Because the solution domains are so large, the long unbranched HA chains form widely open coils, which at concentrations greater than 1 mg/cm3, become highly entangled [26]. Due to its entanglement and immobilization in tissue, HA acts much like an ion-exchange resin in that an electrostatic interaction can occur between the small charges of mobile ions in the tissue and the electrostatic envelope of the stationary polyelectrolyte. This electrostatic interaction forms the basis for various properties of HA including its influence upon osmotic pressure, ion transport and distribution, and electric potentials within vitreous [27]. A compressed HA chain has extensive “interdigitations” since it interacts with nearest antiparallel as well as parallel neighbors (totalling 8 molecules), whereas extended forms only interact with 3 antiparallel neighbors [28].
b. Chondroitin Sulfate (CS)
CS is a major component in the extracellular matrix throughout the body, likewise in vitreous. In Wagner’s vitreoretinal degeneration a mutation in the gene for versican (one of the CS) was found to be the cause [29, 30]. This and other hereditary vitreo-retinopathies are discussed extensively in chapter I.C. Hereditary vitreo-retinopathies.
c. Heparan Sulfate (HS)
HS is a renewable proteoglycan present in vitreous in small amounts and presumed to maintain adequate distance between the collagen fibrils [31].
2. Proteins
The total protein content in vitreous is rather small in percentage, variable, and complex in nature. Among the soluble proteins, albumin and iron-binding proteins play the major role. Transferrin and other iron-binding proteins may have a protective capability by reducing iron toxicity in vitreous hemorrhage [32]. Collagens are the most frequent insoluble proteins and it has been shown that the Muller cells can synthesize vitreous collagens in vitro [33]. Vitreous proteins are extensively discussed in chapter I.A. Vitreous proteins. An important consideration is the interaction of collagen and HA.
3. HA-Collagen Interaction
Vitreous is composed of interpenetrating networks of HA molecules and collagen fibrils. The collagen fibrils provide a solid structure to the vitreous that is “inflated” by the hydrophilic HA. Comper and Laurent [27] found that if collagen is removed, the remaining HA forms a viscous solution; if HA is removed, the gel shrinks. There exists interaction between HA and collagen, and the structure and function of these macromolecules is influenced by this interaction [34]. In all extracellular matrices collagen–proteoglycan interaction determines the morphology of the matrix. The nature of this interaction is a function of collagen type and proteoglycan concentration. Type I collagen is associated with small amounts of proteoglycan and has a weak interaction with this proteoglycan, thus appearing as compact fibrils. The appearance is different in extracellular matrices with predominantly type II collagen, which is rich in proteoglycans and has strong collagen–proteoglycan interaction. Thus, in such extracellular matrices, the type II collagen fibrils are widely separated and the spaces between are filled with proteoglycan.
Physiologic observations also suggest the existence of an important interaction between HA and collagen. Jackson [35] was the first to propose that proteoglycans had a “stabilizing effect” upon collagen. Gelman and Blackwell and Gelman et al. [36, 37] have shown that several glycosaminoglycans, including HA, stabilized the helical structure of collagen so that the melting temperature of collagen was increased from 38 to 46 °C. Measurements of the dynamic viscoelasticity of bovine vitreous showed that the shapes of the master relaxation curves of vitreous are quite similar to those of lightly cross-linked polymer systems [38]. Notably, the behavior of these relaxation curves is different from that of solutions of HA and collagen. This suggests that the physicochemical properties of vitreous in vivo are not simply the result of a combination of these two molecular elements but that HA and collagen form a “lightly” cross-linked polymer system. Furthermore, these investigations suggest that the molecular weight at the cross-linking points is about 106. This corresponds closely to the molecular weight of HA and according to these investigators suggests that this molecule might serve as the cross-linking element in this polymer system.
HA-collagen interaction in the vitreous may be mediated by a third molecule. Swann et al. [39] have demonstrated large amount of noncollagenous protein associated with collagen in the insoluble residue fraction of vitreous. There are filaments connecting the collagen fibrils and these amorphous masses. These filaments may represent “link” structures of either a glycoprotein or proteoglycans nature. HA is known to interact with link proteins [40] as well as an HA-binding glycoprotein, hyaluronectin [41]. Thus, HA could bind to vitreous collagen fibrils via such linkage molecules, most probably in a repeating, ordered manner. This type of arrangement would not be consistent with the concept of random distribution of HA molecules spreading apart the collagen fibrils. Rather, binding to collagen fibrils of the protein core of a proteoglycan such as chondroitin sulfate would “organize” the network in a manner to keep the vitreous collagen fibrils at a critical distance apart [41] to minimize light scattering. Many investigators believe that HA-collagen interaction occurs on a physicochemical rather than chemical level. It may well be that there are several types of collagen-HA interactions that are at play in different circumstances. Further investigation must be undertaken to identify the nature of HA-collagen interaction(s) in vitreous. This question is important not only with respect to the normal physiology of vitreous and its structure but also to understand the aging phenomena of vitreous liquefaction and posterior vitreous detachment, as well as to aid in the development of an artificial vitreous.
4. Miscellaneous Components
a. Metabolites and Enzymes
Glucose and lactic acid are found in vitreous mostly due to cellular metabolism in adjacent tissues. Glucose is supposed to support the enzymatic activity in vitreous in connection with HA turnover [42]. In diabetes, there are elevated levels of glucose in vitreous [43] leading to the formation of advanced glycation at end products in the gel [44] and presumably at the vitreoretinal interface as well.
Renin–angiotensin-converting enzyme has been isolated from the vitreous [45]. Vitreous lactate seems to be a major metabolite in the human vitreous [46].
b. Ascorbic Acid
Ascorbic acid is found in higher levels in the vitreous body compared to plasma.
It is postulated that ascorbic acid is associated with liquefaction during aging and after cataract extraction [47], but it could also be an inhibitor for neovascularization [48] and increase proliferation of hyalocytes [49]. Recent studies also implicate that ascorbic acid may play an important role as antioxidant and thereby decreasing the amount of free oxygen near the lens and thus preventing cataract formation [50] [see chapter IV.B. Oxygen in vitreo-retinal physiology and pathology.]
c. Amino and Fatty Acids
Some amino acids have been detected in vitreous at the same concentration as in plasma. Unsaturated fatty acids (50 % of all fatty acids in vitreous) have been found to have a constant level throughout age [51].
d. Prostaglandins
Prostaglandins have been measured in concentration of about 100 pg/ml in the human vitreous [52].
II. Roles of an Artificial Vitreous
With an ever-increasing appreciation of the role of vitreous in ocular health [see chapters IV.A. Vitreous in ocular physiology; IV.B. Oxygen in vitreo-retinal physiology and pathology; IV.D. Physiology of accommodation and role of vitreous], there is growing interest in the development of an artificial vitreous. The diversity of demands on artificial vitreous candidates is notable (Figure I.F-2):
-
1.
Volume filler: In hypotonous and phthisical eyes where aqueous production is diminished or absent, there is a need for a volume filler to hold the form of the eye globe.
-
2.
Tamponade: In severe forms of retinal detachment, it is mandatory to seal the breaks by gel adhesion or “glueing” with a tamponade.
-
3.
Drug vehicle: In diseases such as age-related macular degeneration (AMD), a suitable vehicle for drugs such as synthetic gels capable of slow release of pharmacologic substances is desired [53–55].
-
4.
Free radical scavenger: After vitrectomy, the oxygen balance is changed and the PO2 near the lens increases causing cataract progression. With a suitable gel, cataract formation might be prevented [56–58].
-
5.
Refract incident light: Refractive error such as high myopia can be difficult to adjust with optical aid. A vitreous gel with refractive properties may be useful (compare to silicone oil).
-
6.
Hemostasis: In diabetic and other vascular retinal diseases, patients may suffer recurrent intravitreal hemorrhage. A suitable synthetic vitreous tamponade (similar to silicone oil or a gel containing anti-VEGF) might stop further bleeding.
-
7.
Maintain optical clarity: Cell migration and proliferation must be inhibited and protein exudation must be mitigated to maintain a clear medium through which photons can pass unhindered to undergo photoreception and begin vision.
III. Properties of an Artificial Vitreous
An artificial vitreous gel should possess certain physical properties such as being injectable through a small cannula without disintegration (shearing). To avoid thermal damage, the gelation process should not produce high temperature once inside the eye. The gel must be inert, have the ability to allow transfer of metabolites, be nonabsorbable or slow disintegrating, and carry high clarity and transparency. It seems that for different indications and purposes we might not be able to identify one single gel. Instead a variety of different gels with various properties (such as long or short lasting, drug delivery, glue, volume, or tamponade) for specified indications may be interesting.
A. Anatomic Considerations: Do We Need a Posterior Vitreous Cortex?
During removal of the natural vitreous in patients, we consider it necessary to induce a posterior vitreous detachment sometimes in combination with peeling of the inner limiting membrane (ILM). This is to ensure that all vitreous remnants are removed together with fibrous membranes and other traction components. We leave the retina without cover and the protection of ILM or the posterior vitreous cortex. If we inject an artificial gel into the vitreous cavity in such an eye, it will come in close contact with the neural retina and have a higher potential of toxic influences on the retinal cells. Histological findings [59] suggested a direct toxic effect at sites where a polyacrylamide hydrogel came in direct contact with the neural retina. In sections where a PVD was not complete and the natural posterior vitreous cortex persisted, the neural retina appeared normal on histology. Could it be that constructing a thin, semipermeable membrane around the artificial vitreous gel is a solution to prevent destruction of the neural retina seen in experimental studies? Gao et al. [60] tested a capsular silicone elastomer 0.01 mm thick which inside the eye on rabbits was filled with physiological balanced salt solution and included a tube–valve system for pressure regulation. During the 8 weeks the experiment lasted, there were no negative effects or malfunction detected by ERG or histology.
IV. Vitreous Substitutes in Vitreoretinal Surgery
Besides the currently used clinical vitreous substitutes (silicone oil, expanding gases, perfluorocarbon liquids, semi-fluorinated alkanes, and mixtures such as heavy silicone oil; see chapter IV.G. Physiology of vitreous substitutes), many different gels with various chemical and physical properties have been tested in vitro and in vivo in animal and humans experiments.
A. Natural and Semisynthetic Polymers and Hyaluronic Hydrogels
Cross-linked hyaluronic acid is in most perspectives identical to one component of the natural vitreous gel and has been found to be nontoxic to the retina [61–63]. The compound is used clinically for viscodissection of intraocular tissue or to maintain a filtering bleb in glaucoma surgery and has also been tested as temporary or partial retinal tamponade. This compound has a high degradation tendency and lasts for a short period of time, inducing elevated intraocular pressure while disintegrating [64–69]. By employing different cross-linking methods and techniques, the rapid disintegration and small molecule formation may be halted and tolerance enhanced [70] (Figure I.F-3).
Gels containing gelatin and collagen have been tested as vitreous substitutes but after a short duration, there was rapid disintegration. Polysaccharides, e.g., dextran, sodium alginate, and chondroitin sulfate did not elicit inflammation, but vitreous haze and high dissolving tendency were reported [71]. A natural crustacean product, chitosan, was tested with promising results regarding biocompatibility and gel stability [72].
B. Synthetic Hydrogels and “Smart Hydrogels”
Hydrogels are synthetic three-dimensional polymers that swell in aqueous solution and do not display any dissolution, resorption, or disintegration inside the eye. They are becoming popular due to their physical and chemical properties meaning that they stay stable, show biocompatibility, keep transparent, are highly hydrophilic, and do not shear when injected through small gauge cannulas [59, 73–76]. However, polyacrylamide and similar hydrogels are highly neurotoxic when in monomer form and only biocompatible after complete polymerization. To minimize toxicity and increase injectability, many investigators have used different methods such as cross-linking or chemical modifications making the (smart-) gel liquid during injection but spontaneously gelating inside the eye. This can occur due to a temperature-sensitive gel [77–80], or a gel responsive to the oxygen rich environment inside the eye [81]. Tai [82] has reported a polyethylene glycol gel, which is thermoresponsive and, after gelation, forms a covalently cross-linked hydrogel.
V. Developing an Artificial Vitreous
A. Experimental Models for Testing Artificial Vitreous In Vivo
Several animal models have been used to explore physical and biochemical properties of novel vitreous substitute candidates. The rabbit eye is one of the most commonly used since it is well suited for the vitrectomy procedure and is well characterized regarding retinal structure and function. We recently developed an in vivo rabbit model using vitrectomy and subsequent infusion of the candidate compound followed by morphological and electrophysiological retinal evaluation [72, 83].
1. Polyalkylimide
Polyalkylimide is a gel polymer comprised exclusively of networks of alkylimide groups (approximately 4 %) and non-pyrogenic water (approximately 96 %). Alkylimide belongs to the family of acryl derivatives, and its polymeric structure does not contain free monomers. The gel is commercially available and is used clinically in plastic surgery as nondegradable filler in esthetic lipoatrophic conditions and after post-traumatic or therapeutic atrophy of subcutaneous tissue [75, 84, 85]. When placed in the subcutaneous space, the gel forms a thin collagen capsule, and it is extractable even after several years without signs of degrading. This gel has been found to be nontoxic when applied on the human skin or on cultured fibroblast but did induce swelling and hyperemia after subcutaneous injection in the rat [86].
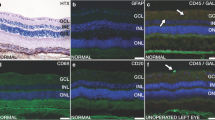
In a first experiment, we performed a regular 20G vitrectomy including posterior vitreous detachment, central vitrectomy, and infusion of approximately 1.0 ml of polyalkylimide (Bio-Alcamide®) hydrogel [59]. Hydrogel-filled eyes were compared with eyes filled with balanced salt solution. We found that the viscoelastic polyalkylimide hydrogel could be injected with ease into the vitreous cavity. The gel remained clear with retained viscosity for at least 28 days. In contrast to previous reports in reconstructive surgery, a surrounding capsule was not found, but the gel displayed uninterrupted apposition with the retinal surface. Pathological reactions were present early in the postoperative period in the form of neuroretinal swelling and posterior capsule opacification. In addition, ERG recordings showed a radical decrease in rod- and cone-derived b-wave amplitudes performed [87, 88] (Figure I.F-4). In histological sections, degenerative retinal changes were seen almost exclusively in central parts of the retina and not in the periphery, where the natural vitreous remained, suggesting that direct gel-to-retina contact was responsible for the adverse morphological effects on the retina (Figure I.F-5). In this setting, the rabbit retina may be especially vulnerable to biochemical changes due to its merangiotic (partly vascularized) nature, i.e., lack of retinal circulation, making it dependent on support from the vitreous and choroid [89] (Figure I.F-6). The conclusion of this experiment was that the intravitreal polyalkylimide gel displays excellent physical requirements of an ideal vitreous tamponade but that it induces severe retinal pathological reactions which limits its use as a potential artificial vitreous (Figure I.F-7). The reasons for the adverse intraocular reactions are not readily known, but recent evidence points to an inflammation-inducing capacity when used subcutaneously [90], so perhaps also in the eye.
Histology of experimental rabbit operations with polyalkylimide. (a–c) Hematoxylin and eosin staining, cryosections. There is choroidal edema and total neuroretinal destruction centrally (a, b) 6 days postoperatively. The peripheral neuroretina appears normal (c). (d) In another rabbit the border between severe neuroretinal degeneration and less affected tissue is seen. (e) In yet a third rabbit, invasion of inflammatory cells can be seen in the subretinal space. The RPE is disrupted, and degeneration of photoreceptors as well as inner retinal cells is evident
2. Polyethylene Glycol
For the next experiment [91] we used polyethylene glycol (PEG) [92], a synthetic water-soluble polymer approved by the FDA for use in a wide range of biomedical applications, including injectable hydrogels [93]. PEG has also been tested in formulations for intravitreal drug delivery, repair of scleral incisions, and sealing retinal detachments [54, 94, 95]. We used the same surgical protocol as in the polyalkylimide experiment but instead injected viscoelastic PEG sols of high molecular weight (>200 kDa) in phosphate-buffered saline (PBS). Molecular weights and concentration of PEG were chosen to approximate the mechanical properties of the natural vitreous. Sols of 5 wt.% PEG with a molecular weight of 400 kDa in PBS were shown to have mechanical and optical properties similar to the natural vitreous and were well tolerated by the retina, with minimal histological or electrophysiological changes, with the exception GFAP upregulation over a period of 41 days. However, the sols were not retained in vitreous throughout the postoperative period and were found to be completely dissolved. These results indicate that PEG displays excellent biocompatibility within the eye, but to extend its use to clinical application, further modification of the gel is needed.
B. Experimental Model for Testing Artificial Vitreous In Vitro
To provide an alternative to relatively costly and cumbersome in vivo testing, we developed a new model for primary in vitro assessment of novel artificial vitreous candidates [96]. For this experiment, the biological impact of three artificial vitreous candidates was explored in a retinal explant culture model: polyalkylimide (Bio-Alcamid®), a two-component hydrogel of 20 wt.% poly-(ethylene glycol) in phosphate-buffered saline (PEG), and a cross-linked sodium hyaluronic acid hydrogel (Healaflow®) (Figure I.F-3). The gels were applied to explanted adult rat retinas and then kept in culture for 5 days after which morphological evaluation using immunohistochemistry, TUNEL, and hematoxylin–eosin staining were performed. Explants kept under standard conditions as well as PEG-exposed explants displayed disruption of retinal layers with moderate pyknosis of first-, second-, and third-order neurons. They also showed moderate fragmentation of DNA (TUNEL). Polyalkylimide-exposed explants displayed severe thinning and disruption of retinal layers with massive cell death. In contrast, cross-linked sodium hyaluronic acid hydrogel-treated explants showed normal retinal lamination with significantly better preservation of retinal neurons compared with control specimens and almost no DNA fragmentation. We conclude that the explant culture system under standard conditions imposes reactions within the retina that can be used when evaluating artificial vitreous candidates. In our particular experiment, polyalkylimide adversely affected the in vitro retina, consistent with the results of prior in vivo trials. PEG gel imposed reactions similar to the control retinas, whereas Healaflow® showed protection from culture-induced trauma, indicating a favorable biocompatibility. The in vitro retinal explant model provides a method of biocompatibility testing prior to more costly and cumbersome in vivo experiments.
- AMD:
-
Age-related macular degeneration
- CS:
-
Chondroitin sulfate
- DNA:
-
Deoxyribonucleic acid
- ERG:
-
Electroretinography
- G:
-
Gauge
- GAG:
-
Glycosaminoglycans
- GFAP:
-
Glial fibrillar acid protein
- HA:
-
Hyaluronan
- HS:
-
Heparan sulfate
- ILM:
-
Inner limiting membrane
- kDa:
-
Kilodalton
- PBS:
-
Phosphate-buffered saline
- PEG:
-
Polyethylene glycol
- pg.ml:
-
Picograms/milliliter
- pO2 :
-
Partial pressure of oxygen
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- VEGF:
-
Vascular endothelial growth factor
References
Sebag J. Age-related changes in human vitreous structure. Graefes Arch Clin Exp Ophthalmol. 1987;225(2):89–93.
Sebag J. The vitreous: structure, function, and pathobiology. New York: Springer; 1989.
Chuo JY, Lee TY, Hollands H, Morris AH, Reyes RC, Rossiter JD, et al. Risk factors for posterior vitreous detachment: a case–control study. Am J Ophthalmol. 2006;142(6):931–7.
Los LI, van der Worp RJ, van Luyn MJ, Hooymans JM. Age-related liquefaction of the human vitreous body: LM and TEM evaluation of the role of proteoglycans and collagen. Invest Ophthalmol Vis Sci. 2003;44(7):2828–33.
Kleinberg TT, Tzekov RT, Stein L, Ravi N, Kaushal S. Vitreous substitutes: a comprehensive review. Surv Ophthalmol. 2011;56(4):300–23.
Pruett RC, Calabria GA, Schepens CL. Collagen vitreous substitute. I. Experimental study. Arch Ophthalmol. 1972;88(5):540–3.
Pruett RC, Schepens CL, Freeman HM. Collagen vitreous substitute. II. Preliminary clinical trials. Arch Ophthalmol. 1974;91(1):29–32.
Pruett RC, Schepens CL, Swann DA. Hyaluronic acid vitreous substitute. A six-year clinical evaluation. Arch Ophthalmol. 1979;97(12):2325–30.
Maruoka S, Matsuura T, Kawasaki K, Okamoto M, Yoshiaki H, Kodama M, et al. Biocompatibility of polyvinylalcohol gel as a vitreous substitute. Curr Eye Res. 2006;31(7–8):599–606.
Baino F. Towards an ideal biomaterial for vitreous replacement: historical overview and future trends. Acta Biomater. 2011;7(3):921–35.
Jacobson B. Degradation of glycosaminoglycans by extracts of calf vitreous hyalocytes. Exp Eye Res. 1984;39(3):373–85.
Toledo OM, Dietrich CP. Tissue specific distribution of sulfated mucopolysaccharides in mammals. Biochim Biophys Acta. 1977;498(1):114–22.
Meyer K, Palmer JW. The polysaccharide of vitreous humour. J Biol Chem. 1934;107:629–34.
Balazs EA, Sundblad L, Toth LXJ. In vitro formation of hyaluronic acid by cells in the vitreous body and by comb tissue. Abstr Fed Proc. 1958;17:184.
Balazs EA, Denlinger JL. The vitreous. In: Davson H, editor. The eye, vol. 1A. London: Academic; 1984. p. 533–89.
Meyer K. Chemical structure of hyaluronic acid. Fed Proc. 1958;17(4):1075–7.
Balazs EA. Molecular morphology of the vitreous body. In: Smelser GK, editor. Structure of the eye. New York: Academic; 1961. p. 293–310.
Breen M, Bizzell JW, Weinstein HG. A galactosamine containing proteoglycan in human vitreous. Exp Eye Res. 1977;24(4):409–12.
Allen WS, Otterbein EC, Wardi AH. Isolation and characterization of the sulfated glycosaminoglycans of the vitreous body. Biochim Biophys Acta. 1977;498(1):167–75.
Kamei A, Totani A. Isolation and characterization of minor glycosaminoglycans in the rabbit vitreous body. Biochem Biophys Res Commun. 1982;109(3):881–7.
Hultsch E, Freeman MI, Balazs EA. Transport and regeneration of hyaluronic acid in extracellular ocular compartments. Annual meeting of the Association for Research in Vision and Ophthalmology (ARVO). Invest Ophthalmol. 1974;11:97.
Borzacchiello A, Netti PA, Ambrosio L, Nicolais L. Hyaluronic acid derivatives mimic the rheological properties of vitreous body. In: Abatangelo G, Weigel PH, editors. New frontiers in medical sciences: redefining hyaluronan: proceedings of the symposium held in Padua, Italy, 17–19 June 1999. Amsterdam: Elsevier; 2000. p. 195–202.
Laurent UB, Fraser JR. Turnover of hyaluronate in the aqueous humour and vitreous body of the rabbit. Exp Eye Res. 1983;36(4):493–503.
Itakura H, Kishi S, Kotajima N, Murakami M. Decreased vitreal hyaluronan levels with aging. Ophthalmologica. 2009;223(1):32–5.
Laurent UB, Granath KA. The molecular weight of hyaluronate in the aqueous humour and vitreous body of rabbit and cattle eyes. Exp Eye Res. 1983;36(4):481–92.
Noulas AV, Theocharis AD, Feretis E, Papageorgakopoulou N, Karamanos NK, Theocharis DA. Pig vitreous gel: macromolecular composition with particular reference to hyaluronan-binding proteoglycans. Biochimie. 2002;84(4):295–302.
Comper WD, Laurent TC. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978;58(1):255–315.
Chakrabarti B, Park JW. Glycosaminoglycans: structure and interaction. CRC Crit Rev Biochem. 1980;8:255–313.
Reardon A, Heinegard D, McLeod D, Sheehan JK, Bishop PN. The large chondroitin sulphate proteoglycan versican in mammalian vitreous. Matrix Biol. 1998;17(5):325–33.
Kloeckener-Gruissem B, Bartholdi D, Abdou MT, Zimmermann DR, Berger W. Identification of the genetic defect in the original Wagner syndrome family. Mol Vis. 2006;12:350–5.
Goes RM, Nader HB, Porcionatto MA, Haddad A, Laicine EM. Chondroitin sulfate proteoglycans are structural renewable constituents of the rabbit vitreous body. Curr Eye Res. 2005;30(5):405–13.
Wong RW, Richa DC, Hahn P, Green WR, Dunaief JL. Iron toxicity as a potential factor in AMD. Retina. 2007;27(8):997–1003.
Ponsioen TL, van Luyn MJ, van der Worp RJ, Pas HH, Hooymans JM, Los LI. Human retinal Muller cells synthesize collagens of the vitreous and vitreoretinal interface in vitro. Mol Vis. 2008;14:652–60.
Junqueira LC, Montes GS. Biology of collagen-proteoglycan interaction. Arch Histol Jpn. 1983;46(5):589–629.
Jackson DS. Chondroitin sulphuric acid as a factor in the stability of tendon. Biochem J. 1953;54(4):638–41.
Gelman RA, Blackwell J. Collagen-mucopolysaccharide interactions at acid pH. Biochim Biophys Acta. 1974;342(2):254–61.
Gelman RA, Blackwell J, Kefalides NA, Tomichek E. Thermal stability of basement membrane collagen. Biochim Biophys Acta. 1976;427(2):492–6.
Tokita M, Fujiya Y, Hikichi K. Dynamic viscoelasticity of bovine vitreous body. Biorheology. 1984;21(6):751–6.
Swann DA, Constable IJ, Caulfield JB. Vitreous structure. IV. Chemical composition of the insoluble residual protein fraction from the rabbit vitreous. Invest Ophthalmol. 1975;14(8):613–6.
Scott JE. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992;6(9):2639–45.
Scott JE. The chemical morphology of the vitreous. Eye (Lond). 1992;6(Pt 6):553–5.
Rhodes RH, Mandelbaum SH, Minckler DS, Cleary PE. Tritiated fucose incorporation in the vitreous body, lens and zonules of the pigmented rabbit. Exp Eye Res. 1982;34(6):921–31.
Lundquist O, Osterlin S. Glucose concentration in the vitreous of nondiabetic and diabetic human eyes. Graefes Arch Clin Exp Ophthalmol. 1994;232(2):71–4.
Sebag J, Buckingham B, Charles MA, Reiser K. Biochemical abnormalities in vitreous of humans with proliferative diabetic retinopathy. Arch Ophthalmol. 1992;110(10):1472–6.
Sramek SJ, Wallow IH, Tewksbury DA, Brandt CR, Poulsen GL. An ocular renin-angiotensin system. Immunohistochemistry of angiotensinogen. Invest Ophthalmol Vis Sci. 1992;33(5):1627–32.
Berkowitz BA, Bansal N, Wilson CA. Non-invasive measurement of steady-state vitreous lactate concentration. NMR Biomed. 1994;7(6):263–8.
Worst JGF, Los LI. Functional anatomy of the vitreous. In: Worst JGF, Los LI, editors. Cisternal anatomy of the vitreous. Amsterdam: Kugler Publications; 1995. p. 33–50.
Hanashima C, Namiki H. Reduced viability of vascular endothelial cells by high concentration of ascorbic acid in vitreous humor. Cell Biol Int. 1999;23(4):287–98.
Sommer F, Brandl F, Weiser B, Tesmar J, Blunk T, Gopferich A. FACS as useful tool to study distinct hyalocyte populations. Exp Eye Res. 2009;88(5):995–9.
Shui YB, Fu JJ, Garcia C, Dattilo LK, Rajagopal R, McMillan S, et al. Oxygen distribution in the rabbit eye and oxygen consumption by the lens. Invest Ophthalmol Vis Sci. 2006;47(4):1571–80.
Reddy TS, Birkle DL, Packer AJ, Dobard P, Bazan NG. Fatty acid composition and arachidonic acid metabolism in vitreous lipids from canine and human eyes. Curr Eye Res. 1986;5(6):441–7.
Thomas MA, O’Grady GE, Swartz SL. Prostaglandin levels in human vitreous. Br J Ophthalmol. 1985;69(4):275–9.
Park D, Shah V, Rauck BM, Friberg TR, Wang Y. An anti-angiogenic reverse thermal gel as a drug-delivery system for age-related wet macular degeneration. Macromol Biosci. 2013;13(4):464–9.
Duvvuri S, Janoria KG, Pal D, Mitra AK. Controlled delivery of ganciclovir to the retina with drug-loaded Poly(d, L-lactide-co-glycolide) (PLGA) microspheres dispersed in PLGA-PEG-PLGA Gel: a novel intravitreal delivery system for the treatment of cytomegalovirus retinitis. J Ocul Pharmacol Ther. 2007;23(3):264–74.
Pritchard CD, O’Shea TM, Siegwart DJ, Calo E, Anderson DG, Reynolds FM, et al. An injectable thiol-acrylate poly(ethylene glycol) hydrogel for sustained release of methylprednisolone sodium succinate. Biomaterials. 2011;32(2):587–97.
Shui YB, Holekamp NM, Kramer BC, Crowley JR, Wilkins MA, Chu F, et al. The gel state of the vitreous and ascorbate-dependent oxygen consumption: relationship to the etiology of nuclear cataracts. Arch Ophthalmol. 2009;127(4):475–82.
Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139(2):302–10.
Stefansson E. Physiology of vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 2009;247(2):147–63.
Crafoord S, Andreasson S, Ghosh F. Experimental vitreous tamponade using polyalkylimide hydrogel. Graefes Arch Clin Exp Ophthalmol. 2011;249(8):1167–74.
Gao Q, Mou S, Ge J, To CH, Hui Y, Liu A, et al. A new strategy to replace the natural vitreous by a novel capsular artificial vitreous body with pressure-control valve. Eye (Lond). 2008;22(3):461–8.
Hruby K. Hyaluronic acid as vitreous body substitute in retinal detachment. Klin Monbl Augenheilkd Augenarztl Fortbild. 1961;138:484–96.
Balazs EA, Sweeney DB. The replacement of the vitreous body in the monkey by reconstituted vitreous and by hyaluronic acid. Bibl Ophthalmol. 1966;70:230–2.
Nakagawa M, Tanaka M, Miyata T. Evaluation of collagen gel and hyaluronic acid as vitreous substitutes. Ophthalmic Res. 1997;29(6):409–20.
Hong Y, Chirila TV, Vijayasekaran S, Shen W, Lou X, Dalton PD. Biodegradation in vitro and retention in the rabbit eye of crosslinked poly(1-vinyl-2-pyrrolidinone) hydrogel as a vitreous substitute. J Biomed Mater Res. 1998;39(4):650–9.
Hong Y, Chirila TV, Cuypers MJ, Constable IJ. Polymers of 1-vinyl-2-pyrrolidinone as potential vitreous substitutes: physical selection. J Biomater Appl. 1996;11(2):135–81.
Suri S, Banerjee R. In vitro evaluation of in situ gels as short term vitreous substitutes. J Biomed Mater Res A. 2006;79(3):650–64.
Denlinger JL, Balazs EA. Replacement of the liquid vitreus with sodium hyaluronate in monkeys. I. Short-term evaluation. Exp Eye Res. 1980;31(1):81–99.
Denlinger JL, El-Mofty AA, Balazs EA. Replacement of the liquid vitreus with sodium hyaluronate in monkeys. II. Long-term evaluation. Exp Eye Res. 1980;31(1):101–17.
Koster R, Stilma JS. Comparison of vitreous replacement with Healon and with HPMC in rabbits’ eyes. Doc Ophthalmol. 1986;61(3–4):247–53.
Schramm C, Spitzer MS, Henke-Fahle S, Steinmetz G, Januschowski K, Heiduschka P, et al. The cross-linked biopolymer hyaluronic acid as an artificial vitreous substitute. Invest Ophthalmol Vis Sci. 2012;53(2):613–21.
Gombos GM, Berman ER. Chemical and clinical observations on the fate of various vitreous substitutes. Acta Ophthalmol (Copenh). 1967;45(6):794–806.
Yang H, Wang R, Gu Q, Zhang X. Feasibility study of chitosan as intravitreous tamponade material. Graefes Arch Clin Exp Ophthalmol. 2008;246(8):1097–105.
Swindle KE, Ravi N. Recent advances in polymeric vitreous substitutes. Expert Rev Ophthalmol. 2007;2(2):255–65.
Soman N, Banerjee R. Artificial vitreous replacements. Biomed Mater Eng. 2003;13(1):59–74.
Claoue BL, Rabineau P. The polyalkylimide gel: experience with Bio-Alcamid. Semin Cutan Med Surg. 2004;23(4):236–40.
Baino F. The use of polymers in the treatment of retinal detachment: current trends and future perspectives. Polymers. 2010;2(3):286–322.
Katagiri Y, Iwasaki T, Ishikawa T, Yamakawa N, Suzuki H, Usui M. Application of thermo-setting gel as artificial vitreous. Jpn J Ophthalmol. 2005;49(6):491–6.
Cohn D, Sosnik A, Garty S. Smart hydrogels for in situ generated implants. Biomacromolecules. 2005;6(3):1168–75.
Dong Y, Saeed AO, Hassan W, Keigher C, Zheng Y, Tai H, et al. “One-step” preparation of thiol-ene clickable PEG-based thermoresponsive hyperbranched copolymer for in situ crosslinking hybrid hydrogel. Macromol Rapid Commun. 2012;33(2):101–71.
Dong Y, Hassan W, Zheng Y, Saeed AO, Cao H, Tai H, et al. Thermoresponsive hyperbranched copolymer with multi acrylate functionality for in situ cross-linkable hyaluronic acid composite semi-IPN hydrogel. J Mater Sci Mater Med. 2012;23(1):25–35.
Swindle-Reilly KE, Shah M, Hamilton PD, Eskin TA, Kaushal S, Ravi N. Rabbit study of an in situ forming hydrogel vitreous substitute. Invest Ophthalmol Vis Sci. 2009;50(10):4840–6.
Tai H, Howard D, Takae S, Wang W, Vermonden T, Hennink WE, et al. Photo-cross-linked hydrogels from thermoresponsive PEGMEMA-PPGMA-EGDMA copolymers containing multiple methacrylate groups: mechanical property, swelling, protein release, and cytotoxicity. Biomacromolecules. 2009;10(10):2895–903.
Imai K, Loewenstein A, de Juan Jr E. Translocation of the retina for management of subfoveal choroidal neovascularization I: experimental studies in the rabbit eye. Am J Ophthalmol. 1998;125(5):627–34.
Protopapa C, Sito G, Caporale D, Cammarota N. Bio-Alcamid in drug-induced lipodystrophy. J Cosmet Laser Ther. 2003;5(3–4):226–30.
Lahiri A, Waters R. Experience with Bio-Alcamid, a new soft tissue endoprosthesis. J Plast Reconstr Aesthet Surg. 2007;60(6):663–7.
Ramires PA, Miccoli MA, Panzarini E, Dini L, Protopapa C. In vitro and in vivo biocompatibility evaluation of a polyalkylimide hydrogel for soft tissue augmentation. J Biomed Mater Res B Appl Biomater. 2005;72(2):230–8.
Wallenten KG, Andreasson S, Ghosh F. Retinal function after vitrectomy. Retina. 2008;28(4):558–63.
Gjorloff K, Andreasson S, Ehinger B. Standardized full-field electroretinography in rabbits. Doc Ophthalmol. 2004;109(2):163–8.
De Schaepdrijver L, Simoens P, Lauwers H, De Geest JP. Retinal vascular patterns in domestic animals. Res Vet Sci. 1989;47(1):34–42.
Nelson L, Stewart KJ. Early and late complications of polyalkylimide gel (Bio-Alcamid)(R). J Plast Reconstr Aesthet Surg. 2011;64(3):401–4.
Pritchard CD, Crafoord S, Andreasson S, Arner KM, O’Shea TM, Langer R, et al. Evaluation of viscoelastic poly(ethylene glycol) sols as vitreous substitutes in an experimental vitrectomy model in rabbits. Acta Biomater. 2011;7(3):936–43.
Brandl F, Henke M, Rothschenk S, Gschwind R, Breunig M, Blunk T, et al. Poly(ethylene glycol) based hydrogels for intraocular applications. Adv Eng Mater. 2007;9(12):1141–9.
Sawhney AS, Pathak CP, Hubbell JA. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(.alpha.-hydroxy acid) diacrylate macromers. Macromolecules. 1993;26(4):581–7.
Wathier M, Johnson MS, Carnahan MA, Baer C, McCuen BW, Kim T, et al. In situ polymerized hydrogels for repairing scleral incisions used in pars plana vitrectomy procedures. ChemMedChem. 2006;1(8):821–5.
Ufret R, Yu S-Y, Christoforidis J, D’Amico DJ. Evaluation of a polyethylene glycol (PEG)-derived glue as a potential bioadhesive for vitreoretinal applications. Invest Ophthalmol Vis Sci. 2004;45(5):E-Abstract 2054.
Barth H, Crafoord SW, Vinchon C, O’Shea TM, Pritchard CD, Ghosh FK. A new model for in vitro testing of vitreous substitute candidates. ARVO meeting, May 6–10, 2012, Ft Lauderdale, USA.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Crafoord, S., Ghosh, F., Sebag, J. (2014). I.F. Vitreous Biochemistry and Artificial Vitreous. In: Sebag, J. (eds) Vitreous. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1086-1_6
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1086-1_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1085-4
Online ISBN: 978-1-4939-1086-1
eBook Packages: MedicineMedicine (R0)