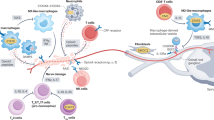
Abstract
Neuropathic pain often results from damage to peripheral nerves, which can mobilize the immune system, as in Guillain-Barré syndrome, postherpetic neuralgia, or trauma. Although most studies focused on detrimental effects of neuroinflammation, recent experimental data provide evidence on analgesic effects of leukocytes. Pain-ameliorating actions involve anti-inflammatory cytokines and immune cell-derived opioid peptides, which activate opioid receptors on peripheral terminals of sensory neurons in injured nerves. In addition, endocannabinoids are present in leukocytes, and mechanisms involved in the resolution of inflammation are mounted, but their significance to neuropathic pain modulation is yet to be examined. Clinical evidence is less compelling, although in some conditions the occurrence of pain seems to be associated with lowered numbers of macrophages or T lymphocytes. This chapter discusses studies addressing both unfavorable and beneficial actions of neuroinflammation in the regulation of painful neuropathies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Neuropathic pain can result from perturbations to the peripheral nervous system, which include diseases (e.g., diabetes), trauma (e.g., amputation, nerve compression, entrapment, stretch), or cancer-related chemotherapy. Debilitating consequences are ongoing/spontaneous pain and enhanced sensitivity to normally innocuous (allodynia) or noxious stimuli (hyperalgesia) (reviewed in [1, 2]). Maladaptive alterations in the nervous system include ectopic impulse generation (at the site of axonal damage and/or in cell bodies), sensory fiber degeneration, sensory and sympathetic nerve sprouting to areas they normally do not innervate, disinhibition (i.e., decreased activity or loss of inhibitory neurons), enhanced activity of descending facilitatory, or impaired activity of descending inhibitory transmission (reviewed in [3, 4]). Furthermore, increasing evidence shows that nerve damage mobilizes the immune system, which can occur in response to infection (e.g., by varicella zoster virus in postherpetic neuralgia), autoimmune disease (Guillain-Barré syndrome), nerve compression (e.g., by tumor), or amputation [5–8]. Neuroimmune mechanisms have been predominately examined in animal models, including spinal nerve ligation (SNL), in which lumbar L5 and/or L6 nerves are tightly ligated; chronic constriction injury (CCI), in which loose ligations are placed around the sciatic or saphenous nerves; and partial sciatic or saphenous nerve ligation (PSNL), in which the dorsal third to half of the nerve is tightly ligated (reviewed in [9, 10]). Traditionally, attention has been focused on the enhancement of pain by leukocytes in neuropathy (reviewed in [10–15]). Interestingly, recent research suggests that immune cells can also ameliorate pain associated with nerve lesion (reviewed in [9, 16]). This chapter provides an overview of bimodal actions of the immune response in the modulation of neuropathic pain: detrimental, which can be mediated by proinflammatory cytokines, and beneficial, which are mediated by anti-inflammatory cytokines and opioid peptides. Other possible mediators such as endocannabinoids and resolvins are also addressed.
2 Immune Responses in the Generation of Neuropathic Pain
Injury to peripheral nerves leads to activation of resident cells, such as fibroblasts, mast cells, and macrophages as well as Schwann cells, which secrete proinflammatory cytokines (e.g., tumor necrosis factor [TNF]-α, interleukin [IL]-1β, IL-6), chemokines, nitric oxide, reactive oxygen species, prostaglandins, growth factors, or metalloproteases. Additionally, damaged nerve fibers release vasoactive and algesic substances, including substance P and calcitonin gene-related peptide. Action of these mediators results in the blood-nerve barrier disruption, vasodilation, and enhanced blood vessel permeability and consequently in extravasation of blood-borne leukocytes (neutrophils, monocytes, and lymphocytes), which accumulate in lesioned nerves and dorsal root ganglia (DRG) (reviewed in [9–12, 14]).
Several studies have directly investigated the role of leukocytes in neuropathic pain. Systemic treatment with a cytotoxic neutrophil antibody decreased the number of neutrophils in the blood or injured nerves and diminished heat or mechanical hypersensitivity following PSNL [17, 18]. Stabilization of mast cells by injections of sodium cromoglycate increased the number of intact mast cells, reduced counts of neutrophils and macrophages at the site of nerve damage, and attenuated both forms of hypersensitivity after PSNL [19]. Treatments affecting macrophages were less consistent. Thus, mice with genetically delayed influx of macrophages had reduced heat hyperalgesia but enhanced mechanical hypersensitivity, compared to wild-type mice following CCI [20]. Treatment with liposome-encapsulated clodronate decreased the number of macrophages infiltrating injured nerves, reduced degeneration of nerve fibers, and attenuated thermal hyperalgesia following PSNL [21], but did not improve mechanical hypersensitivity after SNL [22]. Additionally, clodronate application in another study only slightly decreased sensitivity to noxious pressure and did not ameliorate sensitivity to innocuous mechanical stimulation in the PSNL model [23]. Furthermore, administration of macrophages to uninjured nerves did not induce mechanical hypersensitivity [22]. Together, these variable results suggest a limited contribution of macrophages to the generation of neuropathic pain. The role of T lymphocytes was assessed in athymic nude rats and mice, CD4 knockouts, recombination-activating gene-1 knockouts, and in mice with severe combined immunodeficiency. These animals developed less mechanical or thermal hypersensitivity compared to wild-type animals following CCI or transection of spinal nerves. However, the effects were usually moderate, often did not correlate with the temporal expression of T lymphocytes, and did not always appear to be solely attributed to their absence, but probably to the T lymphocyte genetic deficiency-related secondary alterations (e.g., decreased expression of astrocytes in the spinal cord) [24–27].
Enhancement of pain by leukocytes has been attributed to proinflammatory cytokines, of which TNF-α and IL-1β were most often examined. TNF-α mRNA or protein levels as well as TNF-α receptors were found at the site of nerve injury and in the DRG neurons following CCI [28, 29]. In animals without nerve damage, TNF-α and IL-1β applied on the sciatic nerves or into hind paws elicited discharges in peripheral sensory neurons [30] or induced mechanical and heat hypersensitivity [31]. Moreover, these electrophysiological and behavioral effects of TNF-α were enhanced following SNL [32]. Interestingly, however, the excitatory effects were produced by TNF-α and IL-1β in lower but not higher concentrations, which possibly resulted from activation of anti-inflammatory cytokines by higher doses of TNF-α and IL-1β [30–32]. Several other proinflammatory cytokines, IL-6, IL-15, IL-17, and IL-18, have also been implicated (reviewed in [14]). Consistently, strategies interfering with proinflammatory cytokine function ameliorated neuropathy-induced hypersensitivity. Thalidomide, which inhibits TNF-α synthesis, attenuated mechanical and heat hypersensitivity following CCI. These effects were associated with decreased endoneurial levels of TNF-α and enhanced amounts of the anti-inflammatory cytokine IL-10 in the injured nerves as well as of the opioid peptide Met-enkephalin in the spinal cord [33]. Both forms of hypersensitivity were also reduced by etanercept, which prevents TNF-α binding to its receptor [34], and by antibodies to TNF-α or IL-1β, following CCI [35, 36]. Additionally, approaches indirectly affecting proinflammatory cytokine actions have been applied. Hence, blocking adenosine triphosphate signaling by purinergic 2 receptor antagonist (pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid) decreased IL-1β mRNA in injured nerves, DRG, and spinal cord (where also IL-1β protein level was reduced) as well as IL-6 mRNA in nerves and diminished heat and mechanical hypersensitivity following CCI [37]. Similar effects were also found by antagonizing estrogen receptor β with the isoflavone genistein [38]. Interestingly, systemic treatment with neural stem cells resulted in their migration to the injured nerves (but not uninjured nerves, DRG, spinal cord, or brain), diminished mRNA and protein levels of IL-1β and IL-6, slightly enhanced anti-inflammatory cytokine IL-10 mRNA (but not protein), and improved heat and mechanical hypersensitivity after CCI [39]. Likewise, analgesic effects of systemically applied human adipose-derived stem cells were associated with lower levels of IL-1β and elevated levels of IL-10 in CCI nerves [40].
In summary, most animal studies show that dampening proinflammatory cytokine responses improves neuropathic pain. Depletion of immune cells produced less coherent results, particularly regarding T lymphocytes and macrophages, which might be related to their heterogeneity. For example, while in vitro polarized T helper-1 lymphocytes (producing proinflammatory cytokines) enhanced pain, the T helper-2 lymphocytes (producing anti-inflammatory cytokines) decreased mechanical and thermal hypersensitivity following CCI [24]. Macrophages are the key phagocytic cells for removing degenerating axons’ debris in a process of Wallerian degeneration, which facilitates the regrowth of injured axons (reviewed in [11]). Similar to lymphocytes, macrophages consist of M1 and M2 subpopulations [41], which might differentially modulate neuropathic pain. Additionally, immune cells are not the only source of cytokines, which are also present in Schwann, satellite glial, and neuronal cells (reviewed in [11, 13, 14]), and the relative contribution of each cell type has not been clarified. Moreover, as discussed below, immune cells produce opioid peptides, which serve as endogenous analgesics.
3 Analgesic Effects of Immune Responses in Neuropathy
3.1 Anti-inflammatory Cytokines
As described above, analgesic effects of various treatments aiming at inhibition of proinflammatory cytokines were associated with elevated levels of anti-inflammatory cytokines. For instance, amelioration of neuropathy-induced hypersensitivity by thalidomide and stem cells correlated with increased numbers of macrophages expressing IL-10 or elevated levels of IL-10 in CCI nerves [33, 39, 40], and splenocytes driven in vitro to produce IL-4, IL-10, and IL-13 attenuated CCI-induced hypersensitivity following in vivo transfer [24]. Additionally, an injection of IL-10 at the site of nerve damage diminished the number of endoneurial TNF-α-expressing cells and attenuated thermal hypersensitivity after CCI [42]. Additionally, a viral vector encoding IL-4 led to the expression of IL-4 protein in DRG neurons and reduced mechanical and heat hypersensitivity following SNL. These effects were associated with decreased levels of IL-1β, prostaglandin E2, and phosphorylated p38 mitogen-activated protein kinase in the spinal cord [43]. The beneficial effects of anti-inflammatory cytokines are predominantly mediated through suppression of proinflammatory cytokines. Additionally, IL-4 upregulates opioid receptors [44].
3.2 Opioid Peptides
Opioid peptides belong to three main groups represented by endorphins, enkephalins, and dynorphins, which derive from the respective precursors, pro-opiomelanocortin, proenkephalin, and prodynorphin. Two additional opioids, endomorphin-1 and endomorphin-2, are known, but their precursors have not been identified. Opioid peptides possess different selectivity for the three opioid receptors, μ (endomorphins, endorphins, enkephalins), δ (enkephalins, endorphins), and κ (dynorphins), which belong to the family of seven transmembrane domain Gαi/o protein-coupled receptors. In addition to the nervous system, opioid peptides are synthesized by leukocytes. The full-length pro-opiomelanocortin transcripts as well as enzymes required for processing of pro-opiomelanocortin and proenkephalin have been detected in rodent or human immune cells. Importantly, β-endorphin, Met-enkephalin, Leu-enkephalin, dynorphin A, and endomorphins were found in T lymphocytes, neutrophils, and monocytes/macrophages infiltrating injured tissues. Opioid receptors have also been found in immune cells; however, their significance in pain transmission has not been directly examined. Thus, in peripheral injured tissue, leukocyte-derived opioid peptides activate opioid receptors on peripheral sensory neurons to locally inhibit pain. Mechanisms of such peripheral opioid analgesia have been extensively examined in animal models of inflammatory pain and are addressed in recent review articles [16, 45, 46]. Briefly, opioid peptide-producing immune cells use adhesion molecules (selectins, intercellular adhesion molecule-1, integrins α4 and β2) and chemokine (C-X-C motif) ligands (CXCL1, CXCL2/3) to accumulate in peripheral inflamed tissues [47–49]. Upon stressful stimulation (e.g., experimental cold water swim) or in response to releasing agents, such as corticotropin-releasing factor (CRF), cytokines (TNF-α, IL-1β), chemokines (CXCL1, CXCL2/3), and formyl peptides, immune cells secrete opioid peptides. The release of opioids from leukocytes is CRF-, IL-1-, and formyl peptide-receptor specific. Depending on the cell type, opioid peptide secretion is mediated by extracellular Ca2+ or by inositol trisphosphate receptor-triggered release of Ca2+ from endoplasmic reticulum and is mimicked by potassium, consistent with vesicular secretion [50–52]. Moreover, blocking aminopeptidase N and neutral endopeptidase on leukocytes and peripheral terminals of sensory neurons prevented degradation of enkephalins and dynorphin A and locally ameliorated inflammatory pain [53].
The role of neuroimmune interactions involving opioids in the regulation of neuropathic pain has been recently investigated. β-Endorphin, Met-enkephalin, and dynorphin A proteins and their precursors’ mRNA were found in neutrophils, macrophages, and T lymphocytes accumulating at the injured nerves following CCI or PSNL [27, 54–56]. All three opioid receptors were expressed in sensory fibers, and μ- and δ-receptors were upregulated in injured nerves [54, 57, 58]. Consistent with the expression of CRF receptors on opioid peptide-containing leukocytes, application of CRF at the site of nerve damage reversed mechanical hypersensitivity following CCI. These analgesic effects were blocked by locally applied CRF receptor antagonist, antibodies to β-endorphin, Met-enkephalin or dynorphin A, and selective antagonists of μ-, δ-, and κ-opioid receptors, as well as by systemic injections of antibody to intercellular adhesion molecule-1 [54]. While opioid peptide-containing neutrophils and macrophages are involved at early (2–3 days) and later (14–15 days) neuropathy stages, β-endorphin-containing T lymphocytes mediated analgesia in advanced neuritis, as demonstrated in mice with severe combined immunodeficiency. Hence, attenuated CRF-induced analgesia in these mice was restored by transfer of wild-type mice-derived T lymphocytes in the CCI model [27]. Additionally, peripherally restricted opioid receptor antagonist (naloxone methiodide) applied at the nerve injury site enhanced heat hyperalgesia following PSNL, suggesting a tonic control of neuropathic pain by endogenous opioids [55]. Furthermore, systemic treatment with granulocyte colony-stimulating factor enhanced the number of granulocytes containing β-endorphin in injured nerves and attenuated heat and mechanical hypersensitivity in the naloxone methiodide-sensitive manner. Concomitantly, TNF-α and IL-6 were downregulated in the DRG, whereas microglial activation was attenuated in the spinal cord [56]. Clearly, immune cells can be protective against neuropathic pain by utilizing opioid peptides.
3.3 Other Mediators
Endocannabinoids N-arachidonoylethanolamine (anandamide) and 2-arachidonoyl-glycerol are synthesized from polyunsaturated fatty acid, and their levels are regulated by metabolizing enzymes. Anandamide is primarily inactivated by the fatty acid amide hydrolase, while 2-arachidonoyl-glycerol is metabolized by monoacylglycerol lipase. Endocannabinoids exert their actions via cannabinoid receptors 1 and 2, which belong to the family of seven transmembrane domain Gαi/o protein-coupled receptors. Both cannabinoid receptors and their ligands are expressed in the pain-modulating pathways of the central and peripheral nervous system. Additionally, anandamide and 2-arachidonoyl-glycerol were found to be produced and secreted by macrophage cell lines or native cultured macrophages. Similarly, cannabinoid receptor 2 and to a lesser extent cannabinoid receptor 1 are expressed on splenocytes, lymphocytes, natural killer cells, mast cells, monocytes, macrophages, and neutrophils in cell cultures and in vivo. Macrophage-derived anandamide and 2-arachidonoyl-glycerol interacting with vascular cannabinoid receptors were implied in endotoxin-induced hypotension, whereas activation of leukocytic cannabinoid receptors modulated leukocyte function (e.g., cytokine production and/or release, cell proliferation, migration, and apoptosis) (reviewed in [16, 59]). However, the significance of such immunomodulatory effects of cannabinoid receptors and of immune cell-derived endocannabinoids in pain transmission is yet to be established. For example, a peripherally restricted inhibitor of fatty acid amide hydrolase elevated levels of anandamide in peripheral tissue and suppressed hypersensitivity in inflammatory and neuropathic pain in a cannabinoid receptor 1-dependent manner, but the cellular source of anandamide was not identified [60].
Resolvins D and E are lipid mediators synthesized from polyunsaturated fatty acid by several enzymes, including cyclooxygenase-2, cytochrome P450, and 5- and 15-lipoxygenases, and are involved in the resolution of inflammation. Application of synthetic resolvin E1 into inflamed tissue reduced local neutrophil infiltration and expression of TNF-α, IL-1β, IL-6, and chemokine CCL2 and diminished heat hypersensitivity in an inflammatory pain model. Analgesic effects were also observed following injection of resolvin E1 on the spinal cord, in inflammatory and SNL pain models. These actions were mediated by Gαi-associated ChemR23 receptor. The receptor has been found on spinal microglia and central and peripheral terminals of DRG neurons as well as in macrophages accumulating in inflamed tissue (reviewed in [61]). Nevertheless, the relative contribution of each cell type as sources of ChemR23 receptors and resolvins to the modulation of neuropathic pain awaits clarification.
3.4 Clinical Evidence
Several clinical conditions associated with peripheral nerve damage involve immune reactions. In patients with neuropathies of various etiologies (including vasculitis, Guillain-Barré syndrome, alcohol abuse, and AIDS), the immunoreactivity of TNF-α in Schwann cells as well as of TNF-α, IL-1β, and IL-6 in macrophages and T lymphocytes in sural nerve biopsies was higher in patients with painful compared to those with nonpainful neuropathies. In addition, serum levels of TNF-α and IL-2 mRNA were higher in patients with painful neuropathy, while in those with painless neuropathy anti-inflammatory IL-4 and IL-10 mRNA levels were elevated. Proinflammatory IL-8 concentration in the cerebrospinal fluid was higher in postherpetic neuralgia patients compared to healthy controls, but there were no differences in serum levels of various other pro- and anti-inflammatory cytokines (reviewed in [62]). Interestingly, however, there were significantly fewer CD3+ and CD8+ T lymphocytes in biopsies of zoster skin lesions in these patients [5]. Furthermore, lowered plasma CD4+ T lymphocyte counts paralleled increased incidences of sensory neuropathies in HIV patients [63]. Additionally, patients who developed phantom pain had significantly lower number of macrophages in nerve biopsies compared to patients without phantom pain after leg amputation; there were no differences in the number of T and B lymphocytes, cells expressing TNF-α or TNF-α receptor 1 in nerves, as well as in the serum levels of TNF-α and IL-6 [8]. Together, it appears that in some conditions, decreased counts of macrophages or T lymphocytes were associated with the presence of pain, suggesting their beneficial role in neuropathy. It is more difficult to find clear relationships between neuropathic pain and the expression of cytokines in patients, which might be related to the variety of neuropathies, stages of the disease, and/or examined tissue.
The investigation of the role of opioid peptide-containing immune cells in neuropathic pain has just begun in preclinical studies. So far, the clinical relevance of peripheral endogenous opioid analgesia has been shown for somatic inflammatory pain. β-Endorphin and Met-enkephalin were detected in synovial granulocytes, monocytes/macrophages, lymphocytes, and plasma cells, while opioid receptors were found in synovial tissue sensory neurons in patients with acute knee trauma and chronic arthritis. Blockade of opioid receptors by the antagonist naloxone injected into such tissue exacerbated pain after knee surgery. Furthermore, in these patients, CRF receptors and β-endorphin were co-expressed in synovial leukocytes, and the injection of CRF into the knee joint resulted in a transient but significant reduction of postoperative pain. This strongly indicates that immune cells continuously release and can be stimulated to secret opioid peptides to counteract inflammatory pain (reviewed in [16]). It remains to be examined whether immune mechanisms involving opioids, cannabinoids, and resolvins contribute to the regulation of neuropathic pain in patients.
4 Conclusions
There is a compelling body of evidence on the association of neuropathy with activation of the immune system. Although a majority of studies concentrated on pain-generating properties of immune responses, the analgesic actions of opioid peptide-containing leukocytes in experimental neuropathy were recently reported, and the presence of pain in some clinical neuropathic conditions correlated with lowered numbers of macrophages or T lymphocytes. Thus, it will be interesting to investigate the opioid production/release in leukocytes in such patients. These findings suggest that immunosuppressive strategies for the treatment of inflammatory diseases carry a risk to exacerbate pain. Clinical therapy of neuropathic pain with immunomodulatory agents such as steroids, nonsteroidal anti-inflammatory drugs, or anti-TNF-α drugs showed limited efficacy and can be associated with serious side effects, such as gastrointestinal ulcers and bleeding, kidney and liver toxicity, infection, cardiovascular complications, and risk for tumor induction as well as neurological disorders, including demyelinating neuropathies (reviewed in [10, 64, 65]). Clearly, immune responses accompanying nerve injury are not exclusively maladaptive, and their favorable actions are not restricted to the removal of tissue debris and improvement of nerve regeneration. It appears that immune cells need to be stimulated to secrete opioids to produce adequate pain relief. Technology-oriented research [66] is needed to find novel ways to target opioid-containing cells, anti-inflammatory cytokines, and mediators involved in the resolution of inflammation in the relevant damaged tissues. This represents an attractive opportunity to use intrinsic beneficial effects of neuroinflammation as possible therapies of painful neuropathies. Importantly, since chronic pain is a complex biopsychosocial phenomenon, an interdisciplinary management, including psychological, physical, and occupational therapy, needs to be combined with pharmacological treatments [67].
Abbreviations
- CCI:
-
Chronic constriction injury
- CRF:
-
Corticotropin-releasing factor
- CXCL:
-
Chemokine (C-X-C motif) ligand
- DRG:
-
Dorsal root ganglion
- ICAM-1:
-
Intercellular adhesion molecule-1
- IL:
-
Interleukin
- PSNL:
-
Partial sciatic nerve ligation
- SNL:
-
Spinal nerve ligation
- TNF:
-
Tumor necrosis factor
References
Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9(8):807–19.
Bennett GJ. What is spontaneous pain and who has it? J Pain. 2012;13(10):921–9.
Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32.
Nickel FT, Seifert F, Lanz S, Maihöfner C. Mechanisms of neuropathic pain. Eur Neuropsychopharmacol. 2012;22(2):81–91.
Zak-Prelich M, McKenzie RC, Sysa-Jedrzejowska A, Norval M. Local immune responses and systemic cytokine responses in zoster: relationship to the development of postherpetic neuralgia. Clin Exp Immunol. 2003;131(2):318–23.
Nyland H, Matre R, Mørk S. Immunological characterization of sural nerve biopsies from patients with Guillain-Barré syndrome. Ann Neurol. 1981;9(suppl):80–6.
Benoliel R, Epstein J, Eliav E, Jurevic R, Elad S. Orofacial pain in cancer: part I-mechanisms. J Dent Res. 2007;86(6):491–505.
Stremmel C, Horn C, Eder S, Dimmler A, Lang W. The impact of immunological parameters on the development of phantom pain after major amputation. Eur J Vasc Endovasc Surg. 2005;30(1):79–82.
Machelska H. Dual peripheral actions of immune cells in neuropathic pain. Arch Immunol Ther Exp (Warsz). 2011;59(1):11–24.
Calvo M, Dawes JM, Bennett DL. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012;11(7):629–42.
Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82(4):981–1011.
Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10(11):1361–8.
Uçeyler N, Schäfers M, Sommer C. Mode of action of cytokines on nociceptive neurons. Exp Brain Res. 2009;196(1):67–78.
Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229(1–2):26–50.
Sacerdote P, Franchi S, Moretti S, Castelli M, Procacci P, Magnaghi V, Panerai AE. Cytokine modulation is necessary for efficacious treatment of experimental neuropathic pain. J Neuroimmune Pharmacol. 2013;8(1):202–11.
Stein C, Machelska H. Modulation of peripheral sensory neurons by the immune system: implications for pain therapy. Pharmacol Rev. 2011;63(4):860–81.
Perkins NM, Tracey DJ. Hyperalgesia due to nerve injury: role of neutrophils. Neuroscience. 2000;101(3):745–57.
Nadeau S, Filali M, Zhang J, Kerr BJ, Rivest S, Soulet D, Iwakura Y, de Rivero Vaccari JP, Keane RW, Lacroix S. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1β and TNF: implications for neuropathic pain. J Neurosci. 2011;31(35):12533–42.
Zuo Y, Perkins NM, Tracey DJ, Geczy CL. Inflammation and hyperalgesia induced by nerve injury in the rat: a key role of mast cells. Pain. 2003;105(3):467–79.
Sommer C, Schäfers M. Painful mononeuropathy in C57BL/Wld mice with delayed Wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 1998;784(1–2):154–62.
Liu T, van Rooijen N, Tracey DJ. Depletion of macrophages reduces axonal degeneration and hyperalgesia following nerve injury. Pain. 2000;86(1–2):25–32.
Rutkowski MD, Pahl JL, Sweitzer S, van Rooijen N, DeLeo JA. Limited role of macrophages in generation of nerve injury-induced mechanical allodynia. Physiol Behav. 2000;71(3–4): 225–35.
Barclay J, Clark AK, Ganju P, Gentry C, Patel S, Wotherspoon G, Buxton F, Song C, Ullah J, Winter J, Fox A, Bevan S, Malcangio M. Role of the cysteine protease cathepsin S in neuropathic hyperalgesia. Pain. 2007;130(3):225–34.
Moalem G, Xu K, Yu L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience. 2004;129(3):767–77.
Kleinschnitz C, Hofstetter HH, Meuth SG, Braeuninger S, Sommer C, Stoll G. T cell infiltration after chronic constriction injury of mouse sciatic nerve is associated with interleukin-17 expression. Exp Neurol. 2006;200(2):480–5.
Cao L, DeLeo JA. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur J Immunol. 2008;38(2):448–58.
Labuz D, Schreiter A, Schmidt Y, Brack A, Machelska H. T lymphocytes containing β-endorphin ameliorate mechanical hypersensitivity following nerve injury. Brain Behav Immun. 2010;24(7):1045–53.
Shubayev VI, Myers RR. Axonal transport of TNF-alpha in painful neuropathy: distribution of ligand tracer and TNF receptors. J Neuroimmunol. 2001;114(1–2):48–56.
Schäfers M, Geis C, Svensson CI, Luo ZD, Sommer C. Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur J Neurosci. 2003;17(4):791–804.
Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81(1):255–62.
Zelenka M, Schäfers M, Sommer C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain. 2005;116(3):257–63.
Schäfers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci. 2003;23(7):3028–38.
George A, Marziniak M, Schäfers M, Toyka KV, Sommer C. Thalidomide treatment in chronic constrictive neuropathy decreases endoneurial tumor necrosis factor-alpha, increases interleukin-10 and has long-term effects on spinal cord dorsal horn met-enkephalin. Pain. 2000;88(3):267–75.
Sommer C, Schäfers M, Marziniak M, Toyka KV. Etanercept reduces hyperalgesia in experimental painful neuropathy. J Peripher Nerv Syst. 2001;6(2):67–72.
Lindenlaub T, Teuteberg P, Hartung T, Sommer C. Effects of neutralizing antibodies to TNF-alpha on pain-related behavior and nerve regeneration in mice with chronic constriction injury. Brain Res. 2000;866(1–2):15–22.
Sommer C, Petrausch S, Lindenlaub T, Toyka KV. Neutralizing antibodies to interleukin 1-receptor reduce pain associated behavior in mice with experimental neuropathy. Neurosci Lett. 1999;270(1):25–8.
Martucci C, Trovato AE, Costa B, Borsani E, Franchi S, Magnaghi V, Panerai AE, Rodella LF, Valsecchi AE, Sacerdote P, Colleoni M. The purinergic antagonist PPADS reduces pain related behaviours and interleukin-1 beta, interleukin-6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain. 2008;137(1):81–95.
Valsecchi AE, Franchi S, Panerai AE, Sacerdote P, Trovato AE, Colleoni M. Genistein, a natural phytoestrogen from soy, relieves neuropathic pain following chronic constriction sciatic nerve injury in mice: anti-inflammatory and antioxidant activity. J Neurochem. 2008;107(1): 230–40.
Franchi S, Valsecchi AE, Borsani E, Procacci P, Ferrari D, Zalfa C, Sartori P, Rodella LF, Vescovi A, Maione S, Rossi F, Sacerdote P, Colleoni M, Panerai AE. Intravenous neural stem cells abolish nociceptive hypersensitivity and trigger nerve regeneration in experimental neuropathy. Pain. 2012;153(4):850–61.
Sacerdote P, Niada S, Franchi S, Arrigoni E, Rossi A, Yenagi V, de Girolamo L, Panerai AE, Brini AT. Systemic administration of human adipose-derived stem cells reverts nociceptive hypersensitivity in an experimental model of neuropathy. Stem Cells Dev. 2013;22(8):1252–63.
Lee S, Zhang J. Heterogeneity of macrophages in injured trigeminal nerves: cytokine/chemokine expressing vs phagocytic macrophages. Brain Behav Immun. 2012;26(6):891–903.
Wagner R, Janjigian M, Myers RR. Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment, and endoneurial TNF-alpha expression. Pain. 1998;74(1):35–42.
Hao S, Mata M, Glorioso JC, Fink DJ. HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain. 2006;2:6.
Kraus J. Regulation of mu-opioid receptors by cytokines. Front Biosci (Schol Ed). 2009;1:164–70.
Hua S, Cabot PJ. Mechanisms of peripheral immune-cell-mediated analgesia in inflammation: clinical and therapeutic implications. Trends Pharmacol Sci. 2010;31(9):427–33.
Bodnar RJ. Endogenous opiates and behavior: 2012. Peptides. 2013;50:55–95.
Machelska H, Mousa SA, Brack A, Schopohl JK, Rittner HL, Schäfer M, Stein C. Opioid control of inflammatory pain regulated by intercellular adhesion molecule-1. J Neurosci. 2002;22(13):5588–96.
Machelska H, Brack A, Mousa SA, Schopohl JK, Rittner HL, Schäfer M, Stein C. Selectins and integrins but not platelet-endothelial cell adhesion molecule-1 regulate opioid inhibition of inflammatory pain. Br J Pharmacol. 2004;142(4):772–80.
Brack A, Rittner HL, Machelska H, Leder K, Mousa SA, Schäfer M, Stein C. Control of inflammatory pain by chemokine-mediated recruitment of opioid-containing polymorphonuclear cells. Pain. 2004;112(3):229–38.
Cabot PJ, Carter L, Schäfer M, Stein C. Methionine-enkephalin-and Dynorphin A-release from immune cells and control of inflammatory pain. Pain. 2001;93(3):207–12.
Rittner HL, Labuz D, Schaefer M, Mousa SA, Schulz S, Schäfer M, Stein C, Brack A. Pain control by CXCR2 ligands through Ca2+-regulated release of opioid peptides from polymorphonuclear cells. FASEB J. 2006;20(14):2627–9.
Rittner HL, Hackel D, Voigt P, Mousa S, Stolz A, Labuz D, Schäfer M, Schaefer M, Stein C, Brack A. Mycobacteria attenuate nociceptive responses by formyl peptide receptor triggered opioid peptide release from neutrophils. PLoS Pathog. 2009;5(4):e1000362.
Schreiter A, Gore C, Labuz D, Fournie-Zaluski MC, Roques BP, Stein C, Machelska H. Pain inhibition by blocking leukocytic and neuronal opioid peptidases in peripheral inflamed tissue. FASEB J. 2012;26(12):5161–71.
Labuz D, Schmidt Y, Schreiter A, Rittner HL, Mousa SA, Machelska H. Immune cell-derived opioids protect against neuropathic pain in mice. J Clin Invest. 2009;119(2):278–86.
Liou JT, Liu FC, Mao CC, Lai YS, Day YJ. Inflammation confers dual effects on nociceptive processing in chronic neuropathic pain model. Anesthesiology. 2011;114(3):660–72.
Chao PK, Lu KT, Lee YL, Chen JC, Wang HL, Yang YL, Cheng MY, Liao MF, Ro LS. Early systemic granulocyte-colony stimulating factor treatment attenuates neuropathic pain after peripheral nerve injury. PLoS One. 2012;7(8):e43680.
Truong W, Cheng C, Xu QG, Li XQ, Zochodne DW. Mu opioid receptors and analgesia at the site of a peripheral nerve injury. Ann Neurol. 2003;53(3):366–75.
Kabli N, Cahill CM. Anti-allodynic effects of peripheral delta opioid receptors in neuropathic pain. Pain. 2007;127(1–2):84–93.
Tanasescu R, Constantinescu CS. Cannabinoids and the immune system: an overview. Immunobiology. 2010;215(8):588–97.
Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino NR, Spradley JM, Hohmann AG, Calignano A, Mor M, Tarzia G, Piomelli D. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010;13(10):1265–70.
Ji RR, Xu ZZ, Strichartz G, Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011;34(11):599–609.
Uçeyler N, Sommer C. Cytokine regulation in animal models of neuropathic pain and in human diseases. Neurosci Lett. 2008;437(3):194–8.
Childs EA, Lyles RH, Selnes OA, Chen B, Miller EN, Cohen BA, Becker JT, Mellors J, McArthur JC. Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology. 1999;52(3):607–13.
Fromont A, De Seze J, Fleury MC, Maillefert JF, Moreau T. Inflammatory demyelinating events following treatment with anti-tumor necrosis factor. Cytokine. 2009;45(2):55–7.
Woodcock J. A difficult balance–pain management, drug safety, and the FDA. N Engl J Med. 2009;361(22):2105–7.
Rosen H, Abribat T. The rise and rise of drug delivery. Nat Rev Drug Discov. 2005;4(5): 381–5.
Stein C. Opioids, sensory systems and chronic pain. Eur J Pharmacol. 2013;716(1–3):179–87.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Machelska, H. (2014). Peripheral Neuroimmune Interactions and Neuropathic Pain. In: Peterson, P., Toborek, M. (eds) Neuroinflammation and Neurodegeneration. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1071-7_6
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1071-7_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1070-0
Online ISBN: 978-1-4939-1071-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)