Abstract
β-Thymosins are a family of ubiquitous peptides with a molecular mass of about 5 kDa and with a sequence of 40–44 amino acid residues. The name thymosin derives from the first isolation of these peptides from calf thymus in 1966 by Goldstein et al. (Proc Natl Acad USA 56:1010–17) among other lymphocytopoietic factors. Thymosins are subdivided into three main groups according to their different isoelectric points: α-thymosins, β-thymosins, and γ-thymosins with a pH below 5.0, between 5.0 and 7.0, and above 7.0, respectively. Hannappel and coworkers first isolated Tβ4 from vertebrate’s and invertebrate’s cells through different schemes of purification. More than 15 β-thymosins were described but Tβ4 is known to be the most expressed peptide in mammalians including humans.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction: The β-Thymosin Family
β-Thymosins are a family of ubiquitous peptides with a molecular mass of about 5 kDa and with a sequence of 40–44 amino acid residues [1]. The name thymosin derives from the first isolation of these peptides from calf thymus in 1966 by Goldstein et al. [2] among other lymphocytopoietic factors. Thymosins are subdivided into three main groups according to their different isoelectric points: α-thymosins, β-thymosins, and γ-thymosins with a pH below 5.0, between 5.0 and 7.0, and above 7.0 respectively. Hannappel and coworkers first isolated Tβ4 from vertebrate’s and invertebrate’s cells through different schemes of purification [3, 4]. More than 15 β-thymosins were described but Tβ4 is known to be the most expressed peptide in mammalians including humans [4, 5]. In water solution, β-thymosins are destructured and N- and C- terminal helixs are generated after addition in alcohol or binding to G-actin. Moreover, thanks to a flexible structure, β-thymosins can interact with different intra and extra cellular proteins [5].
Thymosin β4
Tβ4 is an ubiquitous peptide with very interesting multiple functions. The complete amino acid sequence of Tβ4 was described in 1981: it contains 43 amino acids, with a high proportion of lysyl and glutamyl residues [6]. The human Tβ4 gene (hTβ4) is located on chromosome X and comprises three exons and two introns [7]. The translation product is modified by removal of the N-terminal methionine and acetylation. Tβ4 plays pivotal roles in the cytoskeletal system as G-actin sequestering peptide, activity that can explain Tβ4 effects on regulation and differentiation of T lymphocytes [8], and inhibition of macrophage migration [9]. Tβ4 is leaderless: as a consequence, the mechanism of its release is completely unknown [1]. Tβ4 is considered the most abundant among β-thymosin peptides in mammalian tissues: its activity has been mainly related to the regulation of actin polymerization in living cells [10, 11]. Tβ4 also exerts biological effects on hypothalamus and pituitary gland [12] and it is a potential precursor of seraspenide, the Ac-SPDK tetrapeptide corresponding to the N-terminal sequence of Tβ4 [13]. Seraspenide blocks hematopoietic pluripotent stem cells in the G0-phase, inhibiting their entry into the S-phase in vivo [14]. Tβ4 is also involved in many critical biological activities [15], including angiogenesis [16], wound healing [17], inflammatory response [18], and cell migration [19]. Furthermore, Tβ4 modifies the rate of spreading of endothelial cells on matrix components by inducing matrix metalloproteinases [20] and it is involved in the development and repair of heart [19] and brain damages [20] (Fig. 8.1). The presence of Tβ4 in human saliva and tears has been recently demonstrated by immunological techniques [21]: Tβ4 is highly expressed in saliva of human newborns, but not in saliva of adult subjects [22]. Tβ4 immunostaining was identified in acinar cells of the parotid, submandibular, and sublingual glands, as well as in minor salivary glands of fetuses, clearly indicating these cells as the source of Tβ4 in the saliva of human newborns [23].
Thymosin β10
Tβ10, a member of β-thymosins, was described for the first time in 1983 in mammalian tissues as a Tβ4 analogous [24, 25]. The Tβ10 gene is located on chromosome 2 and it consists in three exons and two introns. Tβ10 is a peptide with 43 amino acids and is located in the cytoplasm of different cell types. It plays a role in the modulation and organization of the cytoskeleton, by binding to G-actin. Thanks to this peculiarity, Tβ10 can interfere in cellular motility and proliferation [26]. There are many differences between Tβ4 and Tβ10: while Tβ4 promotes angiogenesis [27], Tβ10 inhibits it interfering with the Ras functions [28]. On the one hand, Tβ4 facilitates cellular migration through the production of metalloproteinases-2 and on the other hand Tβ10 inhibits endothelial cellular migration [29]. Tβ4 plays an important anti-apoptotic role preventing the apoptosis in cardiomyocytes [19] whereas hyperexpression of Tβ10 in cell lines of ovarian carcinoma enhances the process of apoptosis inhibiting tumoral growth [30]. Tβ10 has been reported to be over-expressed in human carcinogenesis [31], in carcinoma of the thyroid [32], in breast tumors [33], in lung carcinoma [34], in renal carcinoma [35], and in pancreatic tumors [36]. Tβ10 plays a critical role during human embryogenesis in multiple organs, including the central nervous system [22, 37, 38]. Interestingly, the high levels of Tβ10 found in human fetal brain were reported to drop rapidly after birth [39], suggesting a specific role for Tβ10 during human brain development [40, 41]. The role of Tβ10 during embryogenesis of neural cells was subsequently confirmed by studies showing its participation in neurite outgrowth [42]. Taken all together, these data suggest that Tβ10 is specifically implicated in the development of brain and nervous tissues. However, detailed expression patterns of Tβ10 in different cells and tissues of the human embryo and newborn, at the best of our knowledge, are not available.
Thymosin β10 in Fetal Salivary Glands
Tβ10 and Tβ4 are detectable in high concentration in whole saliva of human preterm newborns, while it disappeares in adults. On the basis of these data, it seemed of interest to study even the influence of Tβ10 during the development of the human salivary glands. To this end, we analyzed, using immunohistochemistry, the expression of Tβ10 in samples of the major and minor salivary glands obtained, at autopsy, from human fetuses and newborns, ranging from 13 up to 33 weeks of gestation. Tβ10 immunoreactivity was detected in all salivary glands examined, with marked differences from one gland to the next. The parotid glands showed the highest Tβ10 reactivity while the lowest reactivity was detected in the minor salivary glands. Marked changes were observed in Tβ10 expression and localization during embryogenesis. In particular, Tβ10 was mainly localized extracellularly in the youngest human fetuses (13 weeks), in the cytoplasm of immature duct cells at 20 weeks, in acinar cells, and in the duct lumen in 33 weeks old fetuses. For the first time we showed a strong expression of Tβ10 in the human salivary glands during the initial phases of the physiological development, Tβ10 being detected starting from the 13th week of gestation, and suggesting a role for the peptide in the salivary glands’ organogenesis (37) (Fig. 8.2).
The Role of β-Thymosins in Embryogenesis
First works on β-thymosins in human tissues focused on the role of Tβ4 in embryogenesis with the following aims: to identify whether the pattern of Tβ4 expression might change at different gestational ages during the intrauterine life, to control whether the Tβ4 expression pattern could change in neonates and adult subjects. To this end, we analyzed parotid, submandibular, sublingual, and minor salivary gland tissue samples obtained from human fetuses of different gestational ages. Immunohistochemical studies clearly demonstrated the presence of two main protein reactivity patterns: a granular pattern, observed in the cytoplasm of acinar cells, inside the ductal lumen, and in the connective tissues surrounding the epithelial structures; a diffuse pattern, characterized by the homogeneous staining of the entire cytoplasm, mainly detected in ductal cells [23]. We hypothesized that the granular immunoreactivity could be related to Tβ4 secretion in two ways: at the apical pole of acinar cells into saliva, in which the peptide is present in high quantities [18, 44] and at the basolateral pole into the connective tissues, in which the peptide could have autocrine or paracrine functions. The homogeneous cytoplasmic pattern, mainly found in the ductal cells of adult salivary glands, was interpreted as characteristic of the binding of Tβ4 to G-actin monomers [23]. Moreover, when we analyzed immunoreactivity for Tb4 in tumors originating from salivary glands we detected the peptide in the vast majority of neoplasias studied. In particular, a strong expression for the peptide was detected in mixed tumors of salivary glands, being found in the cytoplasm of myoepithelial tumor cells and in Warthin tumor cells. Our data collectively suggest that Tβ4 expression in human salivary glands may be summarized by: (1) a strong reactivity in fetal glands; (2) marked decrease in expression in adult glands and (3) re-expression in tumor progression. Moreover, in some salivary gland tumors, a great number of intra- and peritumoral mast cells were observed, all characterized by a strong immunostaining for the peptide [45]. These findings indicate a role for Tβ4 not restricted to the physiological development of salivary glands, but also in cancer development and progression, likely due to the utilization of fetal programs by salivary gland cancer cells. In line with this hypothesis, the observation of Tβ4-rich tumor-infiltrating mast cells in salivary gland tumors underscores the hypothesis that this peptide could serve as a local paracrine mediator, with a relevant role in cellular cross-talking within the tumor microenvironment [46]. Concerning the function of Tβ4 in neoplastic cells, this peptide has been shown to have antiinflammatory and cytoprotective functions by suppressing secretion of the proinflammatory cytokine IL8 and by protecting cells against TNF-induced apoptosis [47] (Fig. 8.1) and by inhibiting neutrophil infiltration and decreasing the expression of proinflammatory cytokines [48]. Because of its multifunctional roles in protecting cells against apoptosis [22, 48] and in stimulating neoangiogenesis [49], Tβ4 released by tumor cells and/or by mast cells in the tumor microenvironment could significantly contribute to cancer cell survival and diffusion. As a consequence, Tβ4 might represent a new molecular target to be considered for future antitumor strategies in different human tumors [50]. The finding of strong expression of Tβ4 in the cytoplasm of tumor-infiltrating mast cells [45] extends our knowledge regarding the immunophenotypic profile of mast cells and contributes to our understanding of immune cells/cancer cells cross talk. Tβ4 has been suggested as the ideal actin monomer sequestering protein [51]. Its function was first restricted to regulate actin polymerization of non-muscle cells, with multiple effects on cell surface remodeling and motility [52]. Further data suggesting a role of Tβ4 in modulating stem cell migration [49], activation [53], and inhibition [54], as well as in regulating integrin signaling [55] prompted some authors to speak of the “thymosin enigma.” [56]. The theory on the putative role of Tβ4 in the physiological development of embryos, as well as in vascularization and tissue recovery in acute and chronic ischemia, was reinforced by the discovery that Tβ4 is one of the most abundant factors secreted by embryonic endothelial progenitor cells [57]. Data suggesting a role for Tβ4 in the recruitment of stem cells in different organs, and in particular during the embryonic and fetal development, prompted us to investigate the expression of the peptide in human fetuses and embryos of different gestational ages, assessing a potential role of Tβ4 in the development of the different components of the gastrointestinal tract [58]. Moreover, we analyzed samples from gut, liver, and pancreas in order to study Tβ4 expression. Tβ4 was highly expressed in the epithelial cells during the early phases of the development, both in gut and pancreas, confirming previous studies indicating a possible relevant role of Tβ4 in the development of the gastrointestinal tract. For the first time, a marked heterogeneity of Tβ4 expression within the gastrointestinal tract was found, ranging from a diffuse immunoreactivity for the peptide in pancreas and gastrointestinal cells to the absence of the protein in the vast majority of fetal and newborn livers examined. Moreover, we detected marked differences in Tβ4 expression among different cell types within the single organs. The most striking differences were found in the fetal pancreas: Tβ4 immunoreactivity was strong in the endocrine cells of the Langerhans islets, in the absence of any significant reactivity in exocrine acinar and ductal cells. Interindividual differences were also reported regarding the intensity of the immunoreactivity for Tβ4 and its subcellular localization, primarily related to the different gestational age of the subjects studied. The strong positivity of Tβ4 in multiple cell types of the developing gastrointestinal tract in humans suggests a relevant role for the peptide in human physiological development. When Tβ4 expression pattern was analyzed in the adult gastrointestinal tract, we observed a marked decrease in immunoreactivity for the peptide. In particular, enterocytes of the ileum and colon did not show any significant reactivity for Tβ4. The pattern of immunostaining for Tβ4 in the adult pancreas appeared similar to that described in fetal pancreas. On the contrary, significant changes were detected in the adult human liver: Tβ4 was highly expressed in the vast majority of adult hepatocytes, with a preferential localization in the hepatocytes bordering the terminal veins (zone3 of the acinus) [59].
Thymosin β10 Expression in Human Nephrogenesis
The report that the Tβ10 is expressed at high levels in embryonic human tissues as well in human kidney induced us to study Tβ10 reactivity in the preterm kidney in order to verify the immunoexpression of this peptide during renal embryogenesis [37]. To this end, we analyzed by immunohistochemistry, the expression of Tβ10 in samples of human kidney obtained, at autopsy, from fetuses and preterm infants ranging from 25 to 36 weeks of gestation and at term newborns. Tβ10 immunoreactivity was detected in the majority of kidneys examined. In all kidneys, immunostaining for the peptide was mainly restricted to proximal and distal tubules. Tβ10-positive tubular cells showed a diffuse cytoplasmic immunoreactivity, in the absence of significant intraluminal reactivity. Occasionally, even nuclei of tubular cells showed a mild reactivity for the peptide. No significant immunoreactivity was observed in the collecting ducts. The glomerular compartment was mainly excluded by Tβ10 localization with the vast majority of glomeruli being completely negative. In half of kidneys examined we detected scattered reactive cells inside the glomerular tufts. Nevertheless, in all preterms older than 29 weeks of gestation, glomeruli were completely negative. The extent and the intensity of immunoreactivity for Tβ10 in proximal and distal tubular cells changed from one case to the next. Immunostaining for Tβ10 was also observed in the subcapsular regions, in areas of active glomerulogenesis in half of cases observed. In this area, the reactivity for the peptide was mainly granular, and localized in the cytoplasm of the comma- and S-shaped bodies. Even in the zones of active glomerulogenesis, developing collecting tubules did not show any reactivity for the peptide. The adult kidney, utilized as a control biopsy, showed reactivity for Tβ10 restricted to the cytoplasm of proximal and distal tubules. No reactivity was detected in the glomeruli. In that study we added some new data, showing that Tβ10 is highly expressed in the developing human kidney, being localized in the “comma-shaped bodies” and in the “S-shaped bodies” during the earliest phases of glomerulogenesis and in ductal cells in mature nephrons. Interestingly, reactivity for Tβ10 disappeared in the “S-shaped bodies” when glomerulogenesis started, with the generation of the primitive vascular tuft by vascular cells. Immunostaining for Tβ10 was more often absent in the glomeruli during their maturation, only scattered positive cells being found in half of cases. These data confirm even in the human kidney the selective localization of β-thymosins during development and the restriction of their immunoreactivity to specific peculiar structures and cells, with marked differences from one organ to the next. In the developing kidney, the marked preference of Tβ10 for the proximal and distal ductal structures, from their origin from the “S-shaped bodies” to the developed proximal and distal ducts, observed in this study is peculiar and does not parallel any previously reported reactivity for the peptide in other organs. The reason for this localization and the intimate function of Tβ10 during the different phases of kidney development remain, at the best of our knowledge, unknown. We showed, for the first time, a marked heterogeneity of Tβ10 expression among glomerular and tubular structures, ranging from a diffuse immunoreactivity for the peptide in the proximal and distal tubuli to the absence of Tβ10 immunostaining in the vast majority of glomeruli. Marked interindividual differences are also present in Tβ10 expression at tissue level, regarding the intensity of the immunoreactivity for Tβ10 and its localization, even in fetuses and newborn with the same gestational age, suggesting the presence of additional factors which might influence the expression of the peptide in the developing kidney.
Thymosin β4 Expression in Human Nephrogenesis
In order to verify if Tβ4 was involved in human nephrogenesis, immunoreactivity for this β-thymosin was performed in a series of fetal and newborn kidneys, ranging from 17 up to 38 weeks of gestation. The aim of our work was to verify if: (1) Tβ4 was expressed in the developing kidney; (2) Tβ4 was detectable in the same renal structures in which Tβ10 was previously observed; (3) the expression pattern of Tβ4 might change in the different phases of gestation. Here the preliminary results of our study are reported. These preliminary data show that, contrary to Tβ10, Tβ4 is not mainly expressed in the epithelial components of the developing kidney. In particular, in all kidney samples immunostained, Tβ4 reactivity was very weak or completely absent in all cell types of the nephrogenic zone in the subcapsular areas. Moreover, Tβ4 did not mark any cell component of developing glomeruli, of proximal tubules, and of collecting tubules (Fig. 8.3). Regarding the different segments of renal tubules, only in few cases anti-Tβ4 antibodies immunostained distal tubules (Fig. 8.4) and Henle loops (Fig. 8.5).
Contrasting with the absence of reactivity in the outer cortex, Tβ4 appeared strongly expressed at the renal hilum. In the perihilar regions the peptide appeared restricted to the mesenchymal/stromal cells, i.e., in the intersitium of the renal medulla (Fig. 8.6). Some peculiar zones appeared characterized by a stronger expression of Tβ4. The highest levels of Tβ4 immunoreactivity were frequently found in the stromal cells encircling the ureter (Fig. 8.7). In the ureteral wall, the negativity of the transitional epithelium contrasted with the high levels of Tβ4 expression in the majority of cells giving rise to the ureteral wall (Fig. 8.8). The second preferential location of Tβ4 reactivity was the arterial wall: in particular, Tβ4 was highly expressed in cells of the outer layer of arteries (Fig. 8.9). Undifferentiated mesenchymal stromal cells of the renal medulla often showed Tβ4 immunoreactivity, appearing as small cytoplasmic granules, contrasting with the absence of any reactivity for the peptide in the collecting tubules (Fig. 8.10). The expression of Tβ4 in the renal cortex was less evident at panoramic views. At higher power, Tβ4 expression appeared restricted to the cortical-stromal interstitial cells. The following compartments were mainly immunoreactive for Tβ4:
-
1.
The Bowman capsule cells were frequently encircled by a thin Tβ4-reactive line. Occasionally, Tβ4 was also expressed in the cytoplasm of capsular cells (Fig. 8.11).
-
2.
Tβ4 frequently marked the basal lamina of distal tubules, appearing as a thin line encircling epithelial tubular cells (Fig. 8.11).
-
3.
Interstitial cortical cells, located among glomeruli and tubuli, frequently showed immunostaining for the peptide, appearing as granular deposits in the cytoplasm of stromal cells (Fig. 8.12).
-
4.
A strong reactivity for Tβ4 was constantly detected in cells of the renal capsule.
Conclusions
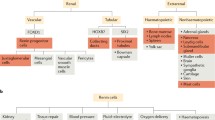
Tβ4 and Tβ10 are both involved in human nephrogenesis, being detected in fetal and neonatal kidney at different gestational ages. The most interesting finding emerging from our immunohistochemical studies is represented by the restriction of these two β-thymosins to different kidney compartments. Tβ10 appears to be mainly involved in the early phases of differentiation of the proximal nephron lineage, being expressed in the S-shaped bodies. Moreover, Tβ10 was also expressed in proximal tubular cells. Contrasting with the prevalent “epithelial immunoreactivity of Tβ10, Tβ4 was mainly expressed in cells of the non-nephron lineage and, in particular, in the stromal–interstitial cells located in the cortex and in renal medulla. According with these data, Tβ4 appears as an important factor involved in the differentiation of the multiple (and in part unknown) cell types of the stromal lineage during kidney development. From a practical point of view, given the scarcity of immunohistochemical markers useful for the identification of cortical and medullary stromal cells, we suggest that Tβ4 might be utilized in the study of the interstitial component of the fetal and the newborn kidney. Expression of Tβ4 by two epithelial components, the cells of the Henle loops and the cells of the Bowman capsule, adds new data to confirm the “Thymosin enigma” [56]. In conclusion, our data evidence that Tβ4 and Tβ10 are both involved in human nephrogenesis but that their expression is restricted to different cell compartments: Tβ4 to the stromal/interstitial cells, and Tβ410 to the nephron lineage [57–59]. Further studies are needed in order to better clarify the relationships between these two β-thymosins during the different phases of kidney development, with the purpose to better defining the role of these peptides during human kidney development.
References
Hannappel E. β-Thymosins. Ann N Y Acad Sci. 2007;1112:21–37.
Goldstein AL, Slater FD, White A. Preparation assay and partial purification of a thymic lymphocytopoietic factor (thymosin). Proc Natl Acad Sci U S A. 1966;56:1010–17.
Low TL, Goldstein AL. Chemical characterization of thymosin β4. J Biol Chem. 1982;257:1000–6.
Hannappel E, Huff T. The thymosins–prothymosin α, parathymosin, and β-thymosin: structure and function. In: Litwack G, editor. Vitamins and hormones, vol. 66. New York: Academic; 2003. p. 257–96.
Hannappel E, Huff T, Safer D. Intracellular β-thymosins. In: Lappalainen P, editor. Actin monomer binding proteins. Austin: Landes Bioscience; 2006. p. 61–70.
Low TL, Hu SK, Goldstein AL. Complete amino acid sequence of bovine thymosin beta 4: a thymic hormone that induces terminal deoxynucleotidyl transferase activity in thymocyte populations. Proc Natl Acad Sci U S A. 1981;78:1162–6.
Yang SP, Lee HJ, Su Y. Molecular cloning and structural characterization of the functional human thymosin beta4 gene. Mol Cell Biochem. 2005;272:97–105.
Low TL, Thrman GB, Chincarini C, McClure JE, Marshall GD, Hu SK, et al. Current status of thymosin research: evidence for the existence of a family of thymic factors that control T-cell maturation. Ann N Y Acad Sci. 2012;1269:131–46.
Weller FE, Mutchnick MG. Enzyme immunoassay measurement of thymosin b4. J Immunoassay. 1987;8:203–17.
Goldstein AL, Hannappel E, Sosne G, Kleinman HK. Thymosin beta4: a multifunctional regenerative peptide. Basic properties and clinical applications. Expert Opin Biol Ther. 2012;12:37–51.
Sanders MC, Goldstein AL, Wang YL. Thymosin beta 4 (Fx peptide) is a potent regulator of actin polymerization in living cells. Proc Natl Acad Sci U S A. 1992;89:4678–82.
Rebar RW, Miyake A, Low TL, Goldstein AL. Thymosin stimulates secretion of luteinizing hormone-releasing factor. Science. 1981;214:669–71.
Grillon C, Rieger K, Bakal J, Schott D, Morgat JL, Hannappel E, et al. Involvement of thymosin beta 4 and endoproteinase Asp-N in the biosynthesis of the tetrapeptide AcSerAspLysPro a regulator of the hematopoietic system. FEBS Lett. 1990;274:30–4.
Lenfant M, Wdzieczak-Bakala J, Guittet E, Prome JC, Sotty D, Frindel E. Inhibitor of hematopoietic pluripotent stem cell proliferation: purification and determination of its structure. Proc Natl Acad Sci U S A. 1989;86:779–82.
Sosne G, Qiu P, Goldstein AL, Wheater M. Biological activities of thymosin beta4 defined by active sites in short peptide sequences. FASEB J. 2010;24:2144–51.
Koutrafouri V, Leondiadis L, Avgoustakis K, Livaniou E, Czarnecki J, Ithakissios DS, et al. Effect of thymosin peptides on the chick chorioallantoic membrane angiogenesis model. Biochim Biophys Acta. 2001;1568:60–6.
Malinda KM, Sidhu GS, Mani H, Banaudha K, Maheshwari RK, et al. Thymosin beta4 accelerates wound healing. J Invest Dermatol. 1999;113:364–8.
Badamchian M, Fagarasan MO, Danner RL, Suffredini AF, Damavandy H, Goldstein AL. Thymosin beta(4) reduces lethality and down-regulates inflammatory mediators in endotoxin-induced septic shock. Int Immunopharmacol. 2003;3:1225–33.
Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–72.
Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, et al. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–82.
Badamchian M, Damavandy AA, Damavandy H, Wadhwa SD, Katz B, Goldstein AL. Identification and quantification of thymosin beta4 in human saliva and tears. Ann N Y Acad Sci. 2007;1112:458–65.
Inzitari R, Cabras T, Pisano E, Fanali C, Manconi B, Scarano E, et al. HPLC-ESI-MS analysis of oral human fluids reveals that gingival crevicular fluid is the main source of oral thymosins beta(4) and beta(10). J Sep Sci. 2009;32:57–63.
Nemolato S, Messana I, Cabras T, Manconi B, Inzitari R, Fanali C, et al. Thymosin beta(4) and beta(10) levels in pre-term newborn oral cavity and foetal salivary glands evidence a switch of secretion during foetal development. PLoS One. 2009;4:e5109.
Erickson-Viitanen S, Ruggieri S, Natalini P, Horecker BL. Thymosin beta 10, a new analog of thymosin beta 4 in mammalian tissues. Arch Biochem Biophys. 1983;225:407–13.
Yu FX, Lin SC, Morriosn-Bogoard M, Atkinson MA, Yin HL. Thymosin beta 10 and thymosin beta 4 both actin-sequestering proteins. J Biol Chem. 1993;268:502–9.
Golla R, Philip N, Safer D, Chintapalli J, Hoffman R, Collins L, et al. Coordinate regulation of the cytoskeleton in 3 T£ cells overexpressing thymosin beta-4. Cell Motil Cytoskeleton. 1997;38:187–200.
Philp D, Nguyen M, Scheremeta B, St-Surin S, Villa AM, Orgel A, et al. Thymosin β4 increases hair growth by activation of hair follicle stem cells. FASEB J. 2004;18:385–7.
Lee SH, Son MJ, Oh SH, Rho SB, Park K, Kim YJ, et al. Thymosin beta 10 inhibits angiogenesis and tumor growth by interfering with Ras function. Cancer Res. 2005;65:137–48.
Mu H, Ohashi R, Yang H, Wang X, Li M, Lin P, et al. Thymosin beta 10 inhibits cell migration and capillary-like tube formation of human coronary artery endothelial cells. Cell Motil Cytoskeleton. 2006;63:222–30.
Hall AK. Thymosin beta-10 accelerates apoptosis. Cell Mol Biol Res. 1995;41:167–80.
Santelli G, Califano D, Chiappetta G, Vento MT, Bartoli PC, Zullo F. Thymosin beta-10 gene overexpression is a general event in human carcinogenesis. Am J Pathol. 1999;155:799–804.
Chiappetta G, Pentimalli F, Monaco M, Fedele M, Pasquinelli R, Pierantoni GM, et al. Thymosin beta-10 gene expression as a possible tool in the diagnosis of thyroid neoplasias. Oncol Rep. 2004;12:239–43.
Verghese-Nikolakaki S, Apostolikas N, Livaniou E, Ithakissios DS, Evangelatos GP. Preliminary findings on the expression of thymosin beta-10 in human breast cancer. Br J Cancer. 1996;74:1441–4.
Gu Y, Wang C, Wang Y, Qiu X, Wang E. Expression of thymosin beta 10 and the role in non-small cell lung cancer. Hum Pathol. 2009;40:117–24.
Hall AK. Amplification-independent overexpression of thymosin beta-10 mRNA in human renal cell carcinoma. Ren Fail. 1994;16:243–54.
Li M, Zhang Y, Zhai Q, Feurino LW, Fisher WE, Chen C, et al. Thymosin beta-10 is aberrantly expressed in pancreatic cancer and induces JNK activation. Cancer Invest. 2009;27:251–6.
Gerosa C, Fanni D, Nemolato S, Locci A, Marinelli V, Cabras T, et al. Thymosin beta-10 expression in developing human kidney. J Matern Fetal Neonatal Med. 2010;23 Suppl 3:125–8.
Fanni D, Gerosa C, Nemolato S, Locci A, Marinelli V, Cabras T, et al. Thymosin beta 10 expression in developing human salivary glands. Early Hum Dev. 2011;87:779–83.
Huff T, Muller CS, Hannappel E. Thymosin beta4 is not always the main beta-thymosin in mammalian platelets. Ann N Y Acad Sci. 2007;1112:451–7.
Voisin PJ, Pardue S, Morrison-Bogorad M. Developmental characterization of thymosin b 4 and b 10 expression in enriched neuronal cultures from rat cerebella. J Neurochem. 1995;64:109–20.
Anadon R, Rodriguez Moldes I, Carpintero P, Evangelatos G, Livianou E, Leondiadis L, et al. Differential expression of thymosins b (4) and b (10) during rat cerebellum postnatal development. Brain Res. 2001;894:255–65.
van Kesteren RE, Carter C, Dissel HM, van Minnen J, Gouwenberg Y, Syed NI, et al. Local synthesis of actin-binding protein β-thymosin regulates neurite outgrowth. J Neurosci. 2006;26:152–7.
Fanni D, Gerosa C, Nemolato S, Locci A, Marinelli V, Cabras T. MUC1 in mesenchymal-to-epithelial transition during human nephrogenesis: changing the fate of renal progenitor/stem cells? J Matern Fetal Neonatal Med. 2011;2:63–6.
Badamchian M, Damavandy AA, Goldstein AL. Development of an analytical HPLC methodology to study the effects of thymosin β4 on actin in sputum of cystic fibrosis patients. Ann N Y Acad Sci. 2012;1270:86–92.
Nemolato S, Cabras T, Fanari MU, Cau F, Fraschini M, Manconi B, et al. Thymosin beta 4 expression in normal skin, colon mucosa and in tumor infiltrating mast cells. Eur J Histochem. 2010;54:e3.
Larsson LI, Holck S. Occurrence of thymosin beta4 in human breast cancer cells and in other cell types of the tumor microenvironment. Hum Pathol. 2007;38:114–9.
Reti R, Kwon E, Qiu P, Wheater M, Sosne G. Thymosin beta4 is cytoprotective in human gingival fibroblasts. Eur J Oral Sci. 2008;116:424–30.
Sosne G, Szliter EA, Barrett R, Kernacki KA, Kleinman H, Hazlett LD. Thymosin beta 4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury. Exp Eye Res. 2002;74:293–9.
Smart N, Rossdeutsch A, Riley PR. Thymosin beta4 and angiogenesis: modes of action and therapeutic potential. Angiogenesis. 2007;10:229–41.
Goldstein AL. Thymosin beta4: a new molecular target for antitumor strategies. J Natl Cancer Inst. 2003;95:1646–7.
Sun HQ, Kwiatkowska K, Yin HL. Beta-thymosins are not simple actin monomer buffering proteins. Insights from overexpression studies. J Biol Chem. 1996;271:9223–30.
Stossel TP, Fenteany G, Hartwig JH. Cell surface actin remodeling. J Cell Sci. 2006;119:3261–4.
Philp D, Goldstein AL, Kleinman HK. Thymosin beta4 promotes angiogenesis, wound healing, and hair follicle development. Mech Ageing Dev. 2004;125:113–5.
Bonnet D, Lemoine FM, Frobert Y, Bonnet ML, Baillou C, Najman A, et al. Thymosin beta4, inhibitor for normal hematopoietic progenitor cells. Exp Hematol. 1996;24:776–82.
Moon HS, Even-Ram S, Kleinman HK, Cha HJ. Zyxin is upregulated in the nucleus by thymosin beta4 in SiHa cells. Exp Cell Res. 2006;312:3425–31.
Sun HQ, Yin HL. The beta-thymosin enigma. Ann N Y Acad Sci. 2007;1112:45–55.
Kupatt C, Horstkotte J, Vlastos GA, Pfosser A, Lebherz C, Semisch M, et al. Embryonic endothelial progenitor cells expressing a broad range of proangiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005;19:1576–8.
Nemolato S, Cabras T, Cau F, Fanari MU, Fanni D, Manconi B, et al. Different thymosin beta 4 immunoreactivity in foetal and adult gastrointestinal tract. PLoS One. 2010;5:e9111.
Nemolato S, Van Eyken P, Cabras T, Cau F, Fanari MU, Locci A, et al. Expression pattern of thymosin beta 4 in the adult human liver. Eur J Histochem. 2011;55:e25.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Nemolato, S., Cabras, T., Messana, I., Gerosa, C., Faa, G., Castagnola, M. (2014). Do β-Thymosins Play a Role in Human Nephrogenesis?. In: Faa, G., Fanos, V. (eds) Kidney Development in Renal Pathology. Current Clinical Pathology. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-0947-6_8
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0947-6_8
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-0946-9
Online ISBN: 978-1-4939-0947-6
eBook Packages: MedicineMedicine (R0)