Abstract
Traumatic brain injury and its treatment or better still prevention has concerned man for centuries. While many of the pathways that result in neuronal damage are recognized, means to prevent or decrease impairment are less well understood. Many scores and more recently biomarkers have been used successfully to prognosticate survival. Early control of raised intracranial pressure is essential as is also normalization of systemic blood pressure and prevention of hypoxia and hypocarbia. Fluid resuscitation should be carefully balanced against output and sugar-containing solutions should be avoided.
The time has been that, when the brains were out the man would die, and there be an end; but now they rise again. (Macbeth. Act 3, Scene 4;78–80 (Macbeth on seeing the ghost of Banquo))
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Key words
- traumatic brain injury
- pathophysiology
- secondary insults
- geriatric brain injury
- fluid management
- coagulopathies
Historical Note
For millennia man has suffered and treated head injuries. The oldest known medical papyrus, the Edwin Smith Papyrus, dates from the seventeenth century bce and was written in black and red hieratic, the Egyptian cursive form of hieroglyphs [1]. It was named for an American Egyptologist, Edwin Smith (1822–1906) who was born in the year that Egyptian hieroglyphic was deciphered. The authorship of the manuscript is unknown but has been attributed to writings from an even earlier time, perhaps by a priest and physician of the Old Kingdom, Imhotep (3000–2500bce). The treatise describes 48 cases, 15 of which are head injuries, 12 facial wounds, and 7 vertebral fractures. Other cases refer to injuries of the upper thorax and shoulders. Diagnosis, management mainly by finger exploration, resulted in several treatment options that were classified as: (a) “An ailment which I will treat” (a gaping wound in the head penetrating to the bone); (b) “An ailment with which I will contend” (A gaping wound penetrating to the bone and splitting the skull); or (c) “An ailment not to be treated” (same as the previous cases but with the addition of fever and stiffness of the neck) [2]. Therapy for the most part included immobilization for head and spinal cord injuries. Surgical stitching of wounds of the lip, throat, and shoulder was described. Dressings included the application of fresh meat (to stop bleeding) and honey (honey is still used, especially in war zones, as a type of occlusive and antiseptic dressing).
Although trephination is not mentioned in the Edwin Smith papyrus, surgical incisions of the cranium were the most common treatment for patients with head wounds, especially if they were seizing. It may have been used to clean wounds after trauma in battle [3]. Trephination was a form of primitive emergency surgery after head wounds [4] to remove shattered fragments of bone from a fractured skull, and clean out hematoma. Such injuries were typical for primitive weaponry such as slingshot projectiles and war clubs. Hippocrates, who also described the systemic effects of head injury including cardiorespiratory changes, recommended trephination and diuresis for simple skull fractures and for contusions of the brain without fractures, especially to prevent complications [4, 5].
By the nineteenth century, treatment of head injuries may have taken a step backwards. Sir Astley Cooper, consulting surgeon to Guy’s Hospital in London, presented a series of lectures he had given in the operating theater at St Thomas’ Hospital on the principle and practice of surgery. He wrote “trephining in concussion is now so completely abandoned that in the last four years I do not know that I have [performed it once whilst 35 years ago I would have performed it five or six times a year]” [6]. He recommended frequent bleeding, calomel purges, and leeches to be applied to the temporal arteries. But then head injuries in the United Kingdom may have decreased with the passing of the slingshot, the axe, and good gun control (especially among the militia).
Hope was on the way. Neurosurgery became a specialty with such luminaries as Sir William Macewen in Scotland, Sir Victor Horsley in London, Professor Fedor Krause in Germany, and Dr. Harvey Cushing in the United States [7]. Greater understanding of intracranial dynamics developed. The Monro-Kellie doctrine, a synthesis of the works of eighteenth century Scottish anatomist (Alexander Monro) and nineteenth century American physiologist (George Kellie), stated that the cranial cavity is a closed rigid box, and that the quantity of intracranial blood must change through the displacement or replacement of cerebrospinal fluid [8, 9]. Walter Cannon, an American physiologist (who coined the phrase “fight or flight”) described intracranial pressure (ICP) monitoring in 1901, as an expansion of the work of Claude Bernard on homeostasis [10].
Now into the twenty-first century, we have taken from the past and refined our treatment with a better understanding of pathophysiology. While accurate diagnosis remains critical, addition of radiologic techniques has replaced manual palpation. Craniotomy, now under sterile conditions and with the benefit of anesthesia, is an improvement on trephination. The importance of controlling intracranial hypertension and cardiorespiratory perturbations, often for a prolonged period after the traumatic event, is underscored. Pharmacologic diuresis, rather than hit or miss herbal therapy, has replaced bloodletting to control intravascular volume and cerebral edema. Antibiotics appear to exert better results than honey in decreasing infection.
We do not have all the answers yet, in part because we still do not have many of the questions. But, slow as it is, given that the concern of head trauma is still with us, we have made many advances.
Scope of the Problem
Traumatic head injuries remain a major cause of death, and disability, despite the introduction of many guidelines for care. Traumatic brain injury (TBI) and head injury are often used interchangeably in the literature [11]. The classification is broad and includes neuronal, vascular, and cranial nerve injuries as well as intracranial hemorrhages, subdural hygromas among others. Further classification is made to open and close head injuries. At least 1.7 million people sustain a TBI in the United States annually and about 3 % are fatal [12, 13]. Of those individuals, about 52,000 die, 275,000 are hospitalized, and 1.365 million are treated and released from an emergency department. The number of people with TBI who are not seen in a hospital or emergency department or who receive no care is currently unknown. The current report from the CDC presents data on emergency department visits, hospitalizations, and deaths for the years 2002–2006 [12]. TBI is a contributing factor to a third of all injury-related deaths in this country. About 75 % of TBI’s are concussions or other forms of mild TBI [12, 13].
The CDC has further documented TBI by age [12]:
-
1.
Children 0–4 years, older adolescents, and adults >65 years are the most likely victims
-
2.
473,947 Emergency department visits for TBI are made annually by children aged 0–14 years
-
3.
Adults aged >75 years have the highest rates of TBI-related hospitalizations and death
-
4.
In all age groups, TBI rates are higher for males than for females
-
5.
Males aged 0–4 have the highest rates of TBI-related emergency department visits
Direct medical costs and indirect costs such as lost productivity totaled an estimated $76.5 billion in the United States in 2000, a number that may be higher in 2013 dollars [14].
Types of Injury
Many causes are related to the wide variety of injuries. Injuries in adults tend to be due to falls, motor vehicle accidents, and assault. Falls and being struck are the most common causes of head injury in children. Assault, child, and adult abuse are most common at the extremes of age. Major cerebral dysfunction can occur with little or no apparent external injury. Force applied to the head may cause the brain to be directly injured or shaken, impacting the inner wall of the skull. The trauma can potentially cause bleeding in the spaces surrounding the brain, contuse brain tissue, or damage the nerve connections within the brain.
Skull Fractures
The cranium is made up of many fused bones that form a solid box comprised of brain tissue (84 %), cerebrospinal fluid (11 %), and blood (5 %). Any increase in one component must be offset by a decrease in another to avoid an increase in ICP. Bony fractures may or may not damage the underlying brain, depending on their location. For the most part, skull fractures are described based on their location, the appearance of the fracture, and whether the fragment is depressed. Not all bones of the cranium have the same ability to withstand trauma, with some being thinner and more fragile than others. The temporal bone, which covers the meningeal artery, is relatively thin and more easily fractured than the occipital bone and can give rise to an epidural hematoma. A fracture may be linear or have a stellate-like pattern. Gunshot and stab wounds or impaled objects cause penetrating injury and usually imply damage to the brain substance. Depressed fractures, often in children, may require surgery to elevate the fragment. Brain injury may or may not be apparent. Open fractures, when the skin above the fracture is broken, carry a much higher rate of infection (Fig. 8.1).
Basilar fractures refer to fractures of the bones at the base of the skull. Signs include bruising around the eyes (raccoon eyes) and behind the ears (Battle sign). If the fracture line extends into the bones around the facial sinuses, there is increased risk of intracranial infection. Bacteria and other miscellaneous material may also be pushed into the brain by an inappropriately placed endotracheal, nasogastric tube, or temperature probe through the nose.
Diastases fractures occur in infants and young children in whom the suture lines have not yet fused and the fontanelles remain open, allowing for widening of these suture lines.
Intracranial Bleeding
Intracranial bleeding refers to any bleeding within the skull. Intracerebral bleeding describes bleeding within the brain (Fig. 8.2). Descriptions are based upon location. Intracranial bleeding may occur with an intact skull. Thus, a plain X-ray of the skull may fail to realize the extent of injury.
Subdural hematoma is caused by rupture of bridging veins within the subdural space as brain parenchyma moves during violent head motion. It may also occur due to arterial rupture. The clot may form at the site of injury or on the opposite side of the skull (contra coup injury) usually in a deceleration injury. This injury is the most common type of TBI, occurring in about 20–40 % [15]. A lucid interval is less likely. Chronic subdural hematoma may be the result of atrophy of brain tissue as may occur in the elderly or in some disease states. As the subdural space enlarges, bridging veins are stretched and may break. Often there are no symptoms or minimal behavioral changes that may be misdiagnosed as the extension of a dementia. Asymptomatic chronic subdural hematomas often resolve spontaneously.
Epidural hematoma collect in a small area outside the dura. As a clot forms, pressure can increase rapidly, impinging on the brain and causing significant injury. Incidence is about 1 % of all head trauma admissions, but it may also develop as a progressive lesion in up to 9 % of patients who have sustained a head injury [16]. As noted above, acute arterial epidural hematoma are most commonly caused by a blow to the temporal bones with rupture of the underlying middle meningeal artery (85 %). After a lucid interval, consciousness is often lost quickly and surgery to release the clot is urgent.
Subarachnoid hemorrhage refers to accumulation of blood within the space beneath the inner arachnoid layer of the meninges and is often associated with an intracerebral bleed. Cerebral spinal fluid (CSF) is also found in this space. Blood in this area causes significant meningeal irritation causing an almost immediate onset of severe headache, nausea, vomiting, and a stiff neck. Similar symptoms are displayed with leaking or ruptured cerebral aneurysms or arteriovenous malformation or meningitis. Treatment usually requires neuroradiologic intervention of surgery. However, days to weeks after TBI, traumatic aneurysms can form. Treatment then is more likely to be observational, especially if the aneurysm remains intact.
Intracerebral hemorrhage/cerebral contusion relate to bleeding within the brain caused by direct damage and also by resultant edema. Surgery is usually not a consideration unless the ICP is dangerously high when decompressive craniectomy may be the only choice [17].
Shear injury causes a diffuse axonal injury which is often fatal. The injury disrupts neuronal transmission. The patient is comatose with no evidence of intracerebral bleeding. MRI studies show a correlation between white matter lesions and impairment of consciousness after injury. The deeper the white matter lesions, the more profound and persistent is unconsciousness [18]. Postmortem evidence shows that about 30–40 % of individuals who die after TBI have diffuse axonal injury and ischemia [19]. The pathology is usually caused by deceleration-acceleration or lateral rotational injuries rather than direct contact. Treatment is mainly supportive.
Concussions are considered a milder form of this type of injury, although serious consequences may result in sports injuries (see below). Occasionally, concussion-type symptoms may be missed. Patients may experience difficulty concentrating, mood swings, lethargy or aggression, and altered sleep habits among other symptoms.
Pathophysiology
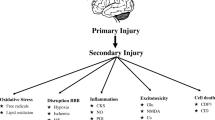
Head injuries are classified as primary or secondary. Primary damage results from the initial blow to the head. Histologic studies done immediately after injury may show no changes, indicating that the initial injury appears to be electrical transmission failure rather than mechanical injury. The common underlying physiologic processes that result in neuronal cell death are diminished cerebral blood flow (ischemia) and reduced blood oxygenation (hypoxemia) (Fig. 8.3). Global hypoxic-ischemic insults do not affect all brain structures uniformly. Rather, certain tissues in the brain are more likely to be injured and are injured earlier than others, a concept known as selective vulnerability. The observed patterns of injury reflect dysfunction of selected excitatory neuronal circuits, which causes a complex cascade of deleterious biochemical events and, ultimately, selective neuronal death [20]. Brain ischemia causes a change from oxidative phosphorylation to anaerobic metabolism, a highly inefficient means to produce energy. Adenosine triphosphate (ATP) is rapidly depleted and lactate accumulates within cells. Normal cellular membrane function is lost. As presynaptic neuronal cell membranes depolarize, excitatory neurotransmitters—in particular, glutamate—are released. Glutamate binds predominantly to N-methyl-d-aspartate (NMDA) receptor-mediated calcium (Ca2+) channels. Activation of NMDA receptors results in an influx of Ca2+ into postsynaptic neurons, and a corresponding extracellular increase in potassium. Several cytotoxic processes are triggered, including activation of membrane phospholipases and production of the oxygen-free radicals (such as nitric oxide) that damage cell membranes and constituents, especially the mitochondria. As the ATP-dependent glutamate reuptake pump fails, energy depletion is intensified resulting in cell death and/or apoptosis.
Cell death after TBI is the major cause of neurologic deficits and mortality [21]. Understanding the mechanisms of delayed posttraumatic cell loss should lead to new therapies and improved outcome. TBI induces changes in many cell types and recent work has emphasized the diversity of neuronal death phenotypes that have been defined as morphological or molecular changes [22]. The most effective neuroprotective strategies must, therefore, target multiple pathways to reflect regional and temporal changes underlying the different neuronal cell death phenotypes. Moreover, traditionally it was thought that adult neurons are in a permanent post-mitotic phase. Newer studies indicate that cell cycle constituents critically affect normal functions of the central nervous system ad also contribute to the pathophysiology of acute disorders. Cell cycle pathways are involved in mediating not only neuronal cell death, but also glial changes that play key roles in the pathophysiologic mechanisms underlying acute neurodegeneration. Thus, therapies that inhibit cell cycle may prove neuroprotective after acute insults by targeting multiple pathogenic mechanisms [23].
Rapid triage and decision-making in the treatment of TBI is challenging in “resource poor” environments such as the battlefield and developing areas of the world. Tests to guide treatment of TBI are needed and are means to differentiate between diffuse and focal brain injury and assess the potential for determining outcome, ICP management, and responses to therapy. Several biomarkers have been identified and shown promise as prognostic indicators.
CD40L or CD154 is a membrane glycoprotein and differentiation antigen expressed on the surface of T-cells. It is part of the tissue necrotizing factor superfamily of molecules and binds T cells and antigen-presenting cells. The CD40 ligand stimulates B-cell proliferation and secretion of all immunoglobulin isotopes in the presence of cytokines CD40 ligand has been shown to induce cytokine production and in peripheral blood monocytes. It also co-stimulates proliferation of activated T-cells and this is accompanied by the production of interferon (IFN-gamma), tumor necrosis factor (TNF-alpha), and interleukin 2 (IL). In fact, sCD40L represents a central event of immune adaptive response as it acts on so many cells. Johansson et al. studied 80 trauma patients admitted to a Level I Trauma Center. High circulating sCD40L was associated with enhanced tissue and endothelial damage (injury severity score, hcDNA, Annexin V, syndecan-1 and sTM), shock (pH, standard base excess), sympathoadrenal activation (adrenaline) and coagulopathy evidenced by reduced thrombin generation (PF1.2), hyperfibrinolysis (D-dimer), increased activated partial thromboplastin time (APTT), and inflammation (IL-6) (all P < 0.05) [23]. A higher ISS (P = 0.017), adrenaline (P = 0.049), and platelet count (P = 0.012) and lower pH (P = 0.002) were associated with higher sCD40L by multivariate linear regression analysis. High circulating sCD40L (odds ratio [OR] 1.84 [95 % CI 1.05–3.23], P = 0.034), high age (P = 0.002), and low Glasgow Coma Score (GCS) prehospital (P = 0.002) were independent predictors of increased mortality.
Glial fibrillary acidic protein (GFAP) is an intermediate filament protein found in the cytoskeleton of astroglia. Recent work has indicated that GFAP may serve as a serum marker of TBI that is released after central nervous system cell damage [24]. In a study of 39 patients with TBI, persistent elevation of GFAP on day 2 was predictive of increased mortality. Excellent specificity for CT-documented brain injury was found using a cutoff point of 1 pg/mL.
A systematic review and meta analysis of randomized controlled trials and observational studies investigating the ability and accuracy of the S-100b protein in predicting prognosis after moderate or severe TBI yielded 9,228 citations, 2 randomized control trials, and 39 cohort studies [25]. Serum S-100B protein concentrations were significantly associated with poor prognosis in short-, mid-, and long-term outcomes. However, optimal thresholds for discrimination remained unclear.
Other studies have suggested that postischemic release patterns of GFAB and also S-100B protein after acute stroke may allow insight into the underlying pathophysiology and could be used in clinical stroke treatment [26].
As noted above, cerebral contusion releases leukocytes and chemokines such as the interleukins (IL2, 6, 8), monocyte chemo attractant protein, and neuron-specific enolases. While increasing levels may not be predictors of expanding contusion, these inflammatory mediators may be predictive of a poor outcome in patients with TBI in which contusions are the predominant abnormality. However, they do not distinguish those patients who will deteriorate because of contusion enlargement [27]. Another animal study confirmed an early increase in IL-6 in brain, plasma, and cerebrospinal fluid protein levels [28]. In addition, secondary posttraumatic hypoxia led to prolonged elevations in plasma IL-6. A clinical study quantified IL-6 plasma levels in patients with closed head trauma and hemorrhagic contusions during the first 6–12 h after injury. A strong correlation between IL-6 levels, volume of traumatic hemorrhage, and in-hospital course was identified [29].
Other researchers have examined the role of mitochondrial damage. The mitochondrion is a major target of TBI, as seen by increased mitochondrial activity in activated and proliferating microglia (high energy requirements and/or calcium overload) as well as increased reactive oxygen species, changes in mitochondrial permeability transition, release of cytochrome c, caspase activation, reduced ATP levels, and neuronal death. Translocator protein (TSPO) is an 18-kDa outer mitochondrial membrane protein that interacts with the mitochondria permeability transition pore and binds to some drug ligands. TSPO levels in the brain are generally low but increase after brain injury. The use of TSPO expression as a marker of brain injury and repair has been suggested. TSPO drug ligands have been shown to participate in the control of mitochondrial respiration and function, mitochondrial steroid, and neurosteroid formation, as well as apoptosis [30].
It would seem that identification of the various biomarkers produced in the injured brain is a developing and promising tool in the management of the head-injured patients, not only for prognosis but also in treatment. Yokobori et al. have recently summarized the present status of plasma biomarkers in TBI. At present, there is insufficient evidence to support a role for diagnostic biomarkers in exactly distinguishing focal and diffuse injury or for accurate determination of raised ICP. Presently, neurofilament (NF), S100β, GFAP, and ubiquitin carboxyl terminal hydrolase-L1 (UCH-L1) seem to have the best potential as diagnostic biomarkers for distinguishing focal and diffuse injury, whereas C-tau, neuron-specific enolase (NSE), S100β, GFAP, and spectrin breakdown products (SBDPs) appear to be candidates for ICP reflective biomarkers. With the combinations of different pathophysiology related to each biomarker, a multibiomarker analysis seems to be indicated and would likely increase diagnostic accuracy. To date little research has focused on the differential diagnostic properties of biomarkers in TBI [31].
Secondary insult develops minutes to hours later due to hypotension, hypoxia, acidosis, edema, or other factors, probably accelerated by the production of free radicals. About 38 % of victims who die after head injury talked at some time following the initial insult [32]. In a review of 116 patients known to have talked at some point before dying, one or more avoidable factors were identified in 74 % and in 54 % an avoidable factor was judged certainly to have contributed to death. These secondary insults have been confirmed in many more recent studies [33] (Table 8.1). Most of the patients without identifiable factors died after rapid deterioration of an expanding intracranial bleed.
Diagnosis
As noted above, symptoms range from none to coma. Common findings are headache, vomiting, seizures, and visual disturbances. The American Academy of Neurology has devised a grading scale to categorize the degree of consciousness (Table 8.2).
The Glasgow Coma Scale was designed as a prognostic indicator of outcome after head injury, but is widely used as assessment of progress or deterioration (Table 8.3) [34]. Scores range from 3 to 15. Scores <8 are considered severe injury. Confounding factors include patients who are intubated or who have eye injuries. The scale is not as readily applicable to children who have greater cerebral plasticity.
Use of the GCS makes it possible for first responders and other less trained health care workers to quickly and reliably assess injured patients. It is part of the initial evaluation, but does not indicate the diagnosis as to the cause of coma. Since it “scores” the level of coma, the GCS can be used as a standard method for any healthcare practitioner to assess change in patient status. It is a component of the Acute Physiology and Chronic Health Evaluation (APACHE) II score, the (Revised) Trauma Score, the Trauma and Injury Severity Score (TRISS) and the Circulation, Respiration, Abdomen, Motor, Speech (CRAMS) Scale, demonstrating the widespread adoption of the scale. The Glasgow outcome score considers the end result and is scored 1–5 (Table 8.4)
Other descriptive terms include “decorticate” which refers to the cortex of the brain, which deals with movement, sensation, and thinking. A flexion response may be seen on stimulation.” Decerebrate” indicates that the cortex and the brain stem that unconsciously control basic functions like breathing and heart beat may not be functioning.
Risk Factors
Several risk factors have been associated with TBI (Table 8.5).
Extremes of age—Advancing age: Several factors point to an increasing concern regarding head trauma in the elderly. As of now, 13 % of the population is >65years, a percentage that is projected to exceed 21 % by 2050 [35]. Accidental trauma at present is the fifth most common cause of death and geriatric trauma will make up 395 of trauma by 2015. In older patients, GCS < 9 is associated with a mortality rate of 80 %. Poor outcome increases 40 %/decade of life. Elderly people are more likely to sustain low energy falls and high cervical fractures are common. Traffic accidents are related to poor vision, impaired hearing, and slower response times. Thermal injuries are related to decreased smell, vision, mobility, and reaction times. Abuse and neglect are also more common in older people. Also, younger patients are more likely to receive more and better care [35]. Deceased cardiac function; by as much as 50 % means that the aging myocardium is less responsive to the catecholamine surge that is one of the first responses after TBI. Elderly patients are often maintained on beta blockers, anticoagulants, or have diabetes. Renal function may be impaired so they are less able to deal with a fluid load during resuscitation. Respiratory function, including vital capacity, the ability to cough, and oxygen saturation are decreased. Rib fractures may compound the picture. The tissue response to thyroxin is decreased. Hypotension, defined as systolic blood pressure (SBP) less than 90 mmHg, is recognized as a sign of hemorrhagic shock and is a validated prognostic indicator. The definition of hypotension, particularly in the elderly population, deserves attention. Elderly patients are more likely to present to the emergency room hypotensive and hypothermic. A recent reevaluation of what constitutes hypotension in older people has been offered. The authors considered 24,438 trauma patients to identify the model that most accurately defined hypotension for three age groups. For patients 20–49, the optimal definition of hypotension was systolic pressure of 100 mmHg. Between the ages of 50 and 69, hypotension was defined if the SBP was <120 mmHg. By 70 years, the number rose to 140 mmHg. Studies indicate that 42 % of elderly victims have increased lactate and base deficit levels with vital signs that have been considered “normal” [35, 36]. Lactate levels >4 mmol/L and/or base deficit >6 are associated with a mortality rate of 40 %. In younger individuals, mortality rates at the same values approximate 12 % [36].
Recent studies have only confirmed what was published 20 years ago: the mortality rate for blunt hade trauma in elderly patients exceeds 30 % and is six times the average in the general population. Early intensive monitoring may help uncover resuscitative or anesthetic effects that may improve outcome [37]. A review of 18,856 patients who sustained motor vehicle accidents showed a mortality rate of 2.4 % with the first 24 h [38]. Head injury, multiple trauma, and advancing age (P = 0.0001) were significant risk factors. General guidelines for care of the geriatric head trauma victim are summarized in Table 8.6.
Pediatric trauma: At the other extreme of age, babies are also victims of head trauma but often due to different causes. TBI is the leading cause of death in children. Hypotension, hypoxia, hyperglycemia, and fever are particularly associated with poor outcomes. Carbon dioxide reactivity and autoregulation are altered and can result in devastating cerebral ischemia or hyperemia [39]. The mechanisms for these changes are not well elucidated. Understanding the effects of TBI on a child’s cerebral circulation is essential to develop protocols to improve outcome. Children who are reported to have fallen less than 4 ft are more likely to be victims of child abuse [40]. “Shaken baby” syndrome occurs mostly in children <1 year. The damage is caused by repetitive oscillations with rotational acceleration of the head. Injuries sustained include encephalopathy, retinal hemorrhages, and subdural hematomas. Fourteen to 38 % die and at least 30 % sustain neurologic sequelae [41].
Shock/Multiple trauma: The addition of major visceral or extremity injuries that cause shock significantly increases the risk of death (12–62 %), the need for rehabilitation (39–60 %), and the cost of disability [42]. These early findings have been shown again, especially concerning the number of rib fractures. Patients who had six or more rib fractures were three times more likely to die within 4 h of admission compared with patients with only one rib fracture [38]. However, especially when there is concomitant head injury, the value of SBP, heart rate (HR), and respiratory rate (RR) have been shown to be poor predictors of outcome. The shock index (SI) is a simple calculation of the relationship between HR and SBP (HR/SBP). Normal values are around 0.6 and as the number approaches 1 or higher, there is an increased risk of shock. Some newer markers including SI × age (SIA), SBP/age (BPA1), maximum HR (220-age)-HR (minpulse, MP), and HR/maximum HR (pulse max index, PMI) have been shown to be better predictors of 48 h mortality when compared with traditional vital signs [43]. The likelihood of death was 8.4 times higher if SIA was greater than 55.
Alcohol: Several studies have looked at the severity of injuries and outcome in intoxicated patients [44, 45]. In one study of motorcycle crashes, half of the victims died before reaching hospital and alcohol was a significant factor [45]. Another study of 106 males indicated that alcohol abuse was associated with a significantly higher incidence of injuries and increased postoperative morbidity and mortality [44]. Another review of 246 patients found that alcohol intoxication combined with age >60 was associated with a higher incidence of cerebral contusions that required surgery and had a poorer prognosis [46]. However, ethanol is a systemic immunomodulator and TBI initiates a neuroinflammatory response. Goodman et al. gavaged rats with ethanol or water prior to TBI [47]. Alcohol treatment prior to TBI decreased the local neuroinflammatory response to injury. Rats given alcohol all exhibited a faster posttraumantic righting response and neurologic recovery time. Pre-injury alcohol treatment was associated with reduced levels of proinflammatory cytokines, IL-6, MCP-1 among others. Transfer of this evidence to the human setting is still debated.
Gender/Male: Complicating the issue of alcohol abuse and multiple trauma from motor cycle accidents is the question of gender as there is a higher incidence alcohol abuse and bikers among males. A recent report from the US CDC showed that death from TBI was 3.4-times more common for males versus females [48]. Males were 2.3-times more likely to have sustained injury by motor vehicle crash, 2.5-times more likely to have a TBI secondary to falls and 6.0-times more likely to be injured via firearms. However, for the most part TBI research is not only from male subjects, but data has been lacking that separates gender in analyses. Thus, there remain gaps in understanding prevention, neuroprotection, secondary injury, rehabilitation timing and therapeutics, and specific outcome remain large [49].
Delay in transfer: Debate between “stay and play” or “swoop and scoop” has been ongoing in trauma transfer. Several studies over the past 30 years indicate that prompt transfer to an appropriate facility after the initial assessment and triage yields the best results. A short scene time is possible and strong medical control and excellent support systems are essential [50]. A review of 2,067 trauma victims admissions in Finland showed that 38 % were treated at a University hospital, 26 % in large non-teaching ICUs, 20 % in mid-size ICUs, and 15 % in small ICUs. Hospital mortality was 5.6 %, broken down as 4.7 % in university ICU and 6.6 % in mid-size ICU [51]. In two subgroup analyses of severely ill trauma patients with APACHE II points >25 or SOFA score >8 points, respectively, hospital mortality was significantly lower in university ICUs. A similar study of 2,875 trauma patients in Denmark found that around 50 % of all trauma deaths occurred at the scene [52]. Increased survival of severely injured patients may be achieved by early transfer to highly specialized care. Despite these analyses and the triage and transfer guidelines that are in place in most states in the United States, compliance is far from complete.
Guidelines published by the American College of Surgeons Committee on Trauma outline criteria for the immediate transfer of moderately to severely injured patients to Level I/II Trauma Centers. Acquisition of pretransfer computed tomography (CT) scans is not required. A retrospective review of 7,713 severely injured patients who met the criteria for transfer to a level one center found that 57 % had a pretransfer CT scan. Penetrating wounds, physiologic compromise, and Injury Severity scores ≥34 were associated with fewer pretransfer CT scans, while older age and female gender were associated with more. Pretransfer CT scans were not associated with in-hospital death or worsened secondary outcomes, but increased charges by $3,761,389 ($488/person transferred with severe injuries) [53]. The authors concluded that national guidelines for the transfer of severely injured patients are followed less than half the time and pretransfer CT scans do not improve outcomes yet increase costs. The potential for further delay in appropriate care arises.
Other prehospital guidelines established by the International Brain Trauma foundation are also not followed. These guidelines state that prehospital intubation is required for all patients with TBI and GCS < 8. A Dutch study of 127 patients who met these criteria found that only 56 % were intubated and in 27 cases, no emergency medical services were involved [54].
Management of Traumatic Brain Injury
Because only about 20 % of patients with TBI require operative intervention, most of the management revolved around resuscitation and intensive care.
Initial resuscitation: Immediate care of the head-injured patient is shown in Table 8.7.
The airway: Poor airway management is consistently identified as a cause of avoidable morbidity and mortality [55]. The idea of the “golden hour” when emergent care could improve survival was developed some 40 years ago. That intubation within 1 h could decrease mortality in head-injured patients from 38 to 22 % as was described by Goldenberg and Makela in 1981 (personal communication). Following head trauma the airway may be compromised by the central lesion of shock (loss of consciousness), by direct injury causing edema, hematoma, or maxilla-facial damage or can be drug-induced (self or otherwise). If two or more of the factors listed in Table 8.8 are present, urgent intubation is indicated. Immediately following TBI there is a period of apnea. Thus, patients frequently present hypoxic and/or hypercarbic.
The airway may be secured without any increase in ICP if intubation is performed after small doses of sedative agents. Appropriate agents include propofol (50–75 mg usually suffices), or etomidate or ketamine if the patient is hypotensive. The addition of succinylcholine (30–40 mg) is also often helpful. Initial respiratory care calls for supplemental oxygen, neutral head and neck position, clearing the mouth, inserting an airway, and reversing any narcotic depression. If pulse oximetry indicates saturation close to 95 %, controlled ventilation is not indicated and may promote aspiration. Also, it is important to auscultate the chest prior to application of positive pressure ventilation in an attempt to identify a pneumothorax which could develop a tension situation. The use of cricoid pressure is also controversial as it may obscure the view, requiring more neck extension and often does not occlude the esophagus, which often does not line up exactly behind the trachea. While application of cricoid pressure continues to be advocated, several studies conclude that although it may have a theoretical advantage during induction, there is little evidence of any benefit at this time [56, 57].
Association of neck fractures with head injury occurs in only about 7 % of patients [58]. Cervical injury is often not identified until hours or even weeks after injury. Nevertheless, trauma protocols often require that patients be transported with neck collars in place, which may do little more than engender pain in the anesthesiologist called to secure the airway. Several studies have emphasized that the need to secure the airway must take precedence. And indeed, subsequent neurologic damage related to the intubation is extremely rare [59]. Nasotracheal intubation and passage of nasogastric tubes are not recommended if there is a skull fracture, especially involving the base of the skull. Tin line stabilization from the feet to the top of the head will maintain neutral position of the head and neck. Also, use of a video laryngoscope allows for intubation with little or no neck motion. Cinefluroscopic studies of fresh cadaveric spine movement showed that cervical displacement during mask ventilation was at least twice as much as during oral or nasal intubation [60].
Cardiovascular sequelae: The importance of examining the cardiovascular system in TBI has long been recognized. Pulse examination was used as a prognostic indicator after head injury over 4,000 years ago in China [61]. As described in the Edwin Smith papyrus, the finding of a weak pulse “when the heart is too weary to speak” and a pale countenance had a poor prognosis but could still be treated, whereas a clammy appearance was “an ailment, not to be treated” [62].
The initial cardiovascular response to cerebral trauma is hypertension, tachycardia, and increased cardiac output, related to a catecholamine surge. As noted above, older patients maintained on cardiac medications who may also have decreased myocardial function may have a blunted response. Cervical spinal cord injury may also present with hypotension and bradycardia. Only in small children is the cranium relatively large enough to contain a hematoma that could result in hypotension based on the injury alone. Otherwise hypotension as the presenting feature in adults is related to systemic injuries with significant blood loss or catastrophic brain damage. SBP < 90 mmHg at the time of admission is associated with significantly increased morbidity and mortality [63]. Cushing described a combination of hypertension and bradycardia with raised ICP [64]. By the time this clinical picture presents, ICP equals diastolic pressure and the patient is usually brain dead.
Several dysrhythmic patterns have been observed. A correlation between outcome and QTc prolongation was noted. An interval of 0.44–0.49 corresponds to a mortality rate double that of patients with normal intervals and at intervals >0.5 the rate triples [65]. Myocardial damage may occur as shown by elevated creatinine phosphokinase (CPK) and myocardium brain (MB) levels. However, there does not appear to be any correlation between CPK and MB activity and electrocardiographic (EKG) changes and outcome.
Therapy is aimed at adrenergic blockade. As autoregulation is often impaired either globally or regionally, hypertension increases cerebral blood flow and thus ICP resulting in cerebral edema. Blood pressure elevations >30 % should be treated during monitoring of ICP. Maintenance of adequate cerebral perfusion pressure is essential. Beta adrenergic blockade with propranolol, esmolol, or labetalol, in increments or infusion, is indicated to reduce systemic blood pressure to <160 mmHg and diastolic pressure to <90 mmHg. Vasodilating agents such as sodium nitroprusside or nitroglycerine are not indicated. Hydralazine, 5–15 mg intravenously has also been used successfully.
Control of ICP: Uncontrolled rise in ICP is probably the most common cause of death in TBI. ICP may be increased after TBI due to brain edema, hyperemia (increased cerebral blood flow), a hematoma, or intracerebral bleed. Optimization of ICP within 24 h of injury has been shown to be the single best intervention to decrease mortality [66]. Even apparently trivial increases in ICP may result in cerebral ischemia, herniation, and pulmonary edema [67].
ICP may be measured by several means (Table 8.9). A CT scan will give a one-time estimation of brain edema.
Diffusion-weighted imaging has been used to study cerebral edema formation, but is difficult to use acutely. Radiological attenuation correlates linearly with estimated specific gravity in human tissue and thus the volume, weight, and specific gravity of any tissue can be measured by computed tomography. Lescot et al. have developed a software package (BrainView®) for Windows workstations, providing semi-automatic tools for brain analysis from DICOM images obtained from cerebral CT, The researchers found that the weight of the brain increased by an average of 82 g and the specific gravity of the contused brain also increased. They concluded that cytotoxic edema must contribute to brain edema rather than simply a breakdown in the blood brain barrier [68].
Therapy for intracranial hypertension includes diuretics (mannitol 0.25–1 g/kg), furosemide (0.25–0.5 mg/kg), head up position, improvement in oxygenation, drainage of CSF, release of intracranial hematomas, reduction of systemic hypertension, and sedation and paralysis if that is required to allow adequate ventilation. Hypertonic saline has also been shown to be as efficacious as mannitol in reducing ICP [69]. Cerebral perfusion is increased better than with normal saline and intravascular volume is stabilized more efficiently. 1-Arginine, a precursor of nitric oxide, may be added as a vasodilator. For many years hyperventilation was added. However, reduction of pCO2 causes vasoconstriction which puts cerebral tissue at risk of ischemia. Soukup et al. studied the effects of hyperventilation on regional cerebral blood flow (rCBF) [70]. A decrease in paCO2 of 20 % (that is 40–32 mmHG) resulted in a decrease in rCBF from 30 to 25 mL/100 g/min (P = 0.001). The partial pressure of oxygen within the brain tissue decreased from 20 to 15 mmHg (P = 0.001). These changes occurred without any changes in other vital signs. Thus, hyperventilation should be reserved only for those cases in which herniation is imminent. Modest positive end expiratory pressure may be added to improve oxygenation [71].
Opioids may release histamine and cause an increase in ICP without evidence of edema due to a direct vasodilatatory effect [72]. In cases where apparently adequate doses of diuretics have been given and ICP remains elevated, opiate infusions of boluses should be withheld.
In children especially altered autoregulation may result in hyperemia and in those circumstances a slight degree of hyperventilation might be indicated. Jugular venous bulb oxygen saturation may be used to assess whether ICP is raised due to hyperemia or edema according to the formula:
where >10 indicates cerebral edema and therapy is with diuretics.
(A−V DO2 is the difference in oxygen content between the radial artery and jugular bulb. CMRO2 = cerebral metabolic rate of oxygen consumption.)
Fluid management: The aims of fluid resuscitation for the head-injured patient are to maintain cardiovascular stability, ensure adequate cerebral perfusion pressure, allow good tissue oxygenation, promote satisfactory operating conditions and, hopefully, provide brain protection. A major problem in initial resuscitation in the emergency room may be an overzealous team that cannulates several veins with large bore catheters and then infuses several liters of crystalloids. Certainly, administration of fluids before operative control of an injury may be ineffective, causing more bleeding by volume expansion.
The question of which fluids to give in TBI has been much debated. Crystalloids have generally been used as the first line because of ready availability and less expense. Dose-related side effects include the risk of over volume expansion and cerebral edema. Colloidal expanders include albumin and hydroxyethylstarches (HES including Hespan®, Hextend®, Voluven®, and Volvulyte®). Albumin, 5 or 25 % supplied in 100 mL aliquots, is derived from pooled human venous plasma, heated to 60° for 10 h to inactivate hepatitis viruses. It contains no isoagglutinins and thus the risk of adverse reactions is very low. Preparation charges make it significantly more expensive. HES in 0.9 % sodium chloride is a synthetic polymer derived from a waxy starch composed of amylopetin. It is supplied in 500 mL bags. Dose-related side effects include coagulopathy, renal failure, and tissue storage. The newer HES 130/0.4 (Voluven®) is said to have a lower risk of side effects [73], although these claims may not have been sufficiently validated [74]. Several reports including the SAFE (saline versus albumin fluid evaluation) study compared the use of albumin alone and saline resuscitation in head-injured patients and found a tendency to increased 24 month mortality in severely injured patients who received only albumin (up to 2 L on the first day) [75]. Less severely compromised patients tended to do better with albumin. The same authors found that albumin resuscitation produced better survival rates in sepsis patients over saline [76]. The efficacy of colloids is being currently evaluated by Myburgh et al. in a 7,000 patient multicenter randomized controlled trial that compares the effects of 6 % hydroxyethyl starch (130/0.4) to normal saline for fluid resuscitation in intensive care patients (CHEST) [77]. Two Cochrane database reviews were unable to determine that albumin reduced mortality when compared to saline in the resuscitation of patients with trauma or postoperatively [78, 79]. In a review of 3,456 patients with sepsis, administration of hydroxyethyl starch increased the need for renal replacement therapy and blood transfusion [80]. Given that colloid is significantly more expensive than crystalloid, its use has been questioned. But in all these investigations, colloid alone in significantly higher doses was compared to crystalloids. Also, the study substance in many instances was albumin. The American Society of Anesthesiologists has advocated the combination of colloids and reduced crystalloids in the prevention of postoperative visual loss [81]. An animal study showed that isotonic crystalloids increased brain edema more than colloids at 3 and 24 h post-TBI, while blood volume was maintained by colloids but not by crystalloids [82, 83]. Oncotic pressure was reduced by crystalloids. The place of colloids may be as an adjuvant to crystalloid administration whereby the amounts infused of both may be reduced. But it is still not clear as to whether administration of colloids or a reduced volume of crystalloids results in improved patient outcome in all situations or only in certain subsets, or if the newer colloids are indeed harmful. The PRECISE RCT, now underway, may provide light on these issues [84].
The injured brain needs oxygen for survival and thus transfusion if frequently used, with residents often erring on the side of over transfusion. There is mounting evidence that blood transfusion carries many risks, not only of transmission of infection but also of antigen/antibody reactions among other consequences. Overall, adverse events from transfusions in the US account for about $17 billion adding more to the cost of each transfusion than acquisition and procedure costs combined [85]. While some complication risks depend on patient status or specific transfusion quantity involved, a baseline risk of complications simply increases in direct proportion to the frequency and volume of transfusion.
Perioperative hemoglobin determinations are far from reliable as an indicator of need to transfuse. Guidelines from the American Society of Anesthesiologists that note that transfusion is rarely needed if the Hb level is 7 g do not take the patient’s age, cardiovascular state, or other comorbidities into account or even the rate of blood loss. (Practice Guidelines for Perioperative Blood Transfusion and Adjuvant Therapies. Last amended October 25, 2005).
Hypertonic-hyperoncotic solutions restore plasma volumes rapidly, attenuate capillary endothelial swelling, increase cardiac output, restore peripheral blood flow, and release eiconosides (vasodilators) and certainly have a place in care of the patient with TBI.
Although the ideally fluid replacement has not been identified, administration of sugar containing solutions must be avoided. An injured brain cannot metabolize sugar through the usual aerobic pathways, but rather by anaerobic means which causes increase in the size of ischemic areas, especially in children [86].
A scheme for fluid management is shown in Table 8.10.
Coagulopathies: Coagulopathies are common soon after head injury and demonstration of worsening clotting studies indicates the need for repeat CT scanning [87]. Severe tissue damage coupled with systemic hypoperfusion results in a hemostatic disruption of coagulation, anticoagulation, fibrinolysis, platelets, and endothelium [88]. Routine tests for coagulation may be inadequate and viscoelastic modalities such as TEG and ROTEM may be required. Genet et al. compared patients with TBI and those with other severe injuries and measured biomarkers for sympathoadrenal activation, coagulation, fibrinolysis, endothelial/cell/glycocalyx damage, and vasculogenesis [89]. They found that acute coagulopathy of shock (ACS) related more to the severity rather than the localization of injury. However, the brain is a rich source of thromboplastin which is released immediately during ischemia causing an early disseminated coagulation and fibrinolysis syndrome. Coagulation profile should be obtained early and repeated and, if necessary, fresh frozen plasma should be given.
Anesthetic management: As noted previously only about 20 % of TBI patients require surgery. However, when it is indicated it is usually emergent and due to expanding hematoma. An acute arterial epidural hematoma is the most serious and raped release often results in an almost miraculous and swift recovery. Anesthetic care mat start with sedation and intubation in the emergency room. Most patients require CT or other radiologic procedures that also call for sedation. While it may be difficult for an anesthetic department to also provide coverage in these off site areas, it is essential that trained healthcare providers are in attendance to allow for adequate monitoring, appropriate sedation, and resuscitation if required. A summary of anesthetic management is shown in Table 8.11. Wide variation may be indicated according to the level of consciousness and stability of the patient. Nevertheless, some degree of sedation is necessary to avoid increases in ICP prior to the opening of the skull.
Not infrequently, patients can be safely extubated at the end of the case. Nevertheless, even in the best situation, careful postanesthetic observation is indicated.
Intensive care: Many patients require continued intubation and ventilation in an intensive care setting. Monitoring often includes cerebral oximetry as well as ICP and cardiorespiratory monitoring. Fluids and electrolytes must be balances, especially sodium. Cerebral salt-wasting syndrome (CSWS) is a rare condition manifested by hyponatremia and dehydration in response to trauma. It is due to excessive renal sodium excretion resulting from a centrally mediated process. It should be differentiated from the syndrome of inappropriate antidiuretic hormone (SIADH), which develops under similar circumstances and also presents with hyponatremia. The main clinical difference is that of total fluid status of the patient: CSWS leads to a relative or overt hypovolemia, whereas SIADH is consistent with a normal to hypervolemic range. Random urine sodium concentrations tend to be lower than 100 mEq/L in CSWS and greater in SIADH. If blood-sodium levels increase when fluids are restricted, SIADH is more likely. Posttraumatic hypopituitarism (PTHP) causing diabetes insipidus has been recognized for many years and is a rare occurrence. Changes in pituitary hormone secretion may be observed during the acute phase post-TBI, representing part of the acute adaptive response to the injury [90]. Moreover, diminished pituitary hormone secretion, caused by damage to the pituitary and/or hypothalamus, may occur at any time after TBI. Symptoms include extreme diuresis, which must be distinguished from the effects of overhydration or diuretic administration. It usually responds to desmopressin administration. However, diabetes insipidus related to TBI is frequently transient and can spontaneously disappear within a few days.
It is important to decrease invasive monitoring (central lines, urinary catheters, etc.) to decrease the risk of sepsis and multiple organ failure as quickly as possible. Excessive fluid administration may contribute to ventilator-associated pneumonia. All attempts should be made to discontinue supported respiration as soon as possible.
Directions for brain survival: Over the years many attempts have been made to improve survival after TBI. Barbiturate coma, while should theoretically be advantageous (it decreased ICP, decreases metabolism, and may be beneficial in regional ischemia), has not been shown to improve survival. Nevertheless, it is still used as a desperate measure. It suppresses neuronal activity but anaerobic metabolism continues as CSF levels of hypoxanthine and lactate remain elevated [91]. Barbiturate coma is associated with severe hypokalemia and abrupt discontinuation may cause hyperkalemia. It may also mask brain death. Target concentrations have not been established [92].
Hypothermia has also been used and also proved disappointing, both in adults and in children [93, 94]. However, its value may lie in the prevention of hyperthermia [93].
Decompressive craniectomy has recently been revived as a means to treat intractable intracranial hypertension [17]. Several studies in children have found fair survivals at 6 months and it appears to be a reasonable option for children with uncontrollable ICP [95]. Cranial bones are replaced at a later date with computer-designed flaps. While survival may be enhanced, older patients are still seven times more likely to have a poor functional outcome, resulting in an increased number of survivors with an unfavorable outcome [17].
Measurement of the serum biomarkers outlined earlier may help in guiding therapy such as control of cardiorespiratory parameter, fluid and electrolyte balance, and ICP.
Other recent studies have looked at on-going anticoagulant therapy in trauma victims. Unintentional discontinuation of statins may increase mortality after TBI [96]. An increasing body of evidence suggests that continuing aspirin and other antiplatelet therapy may significantly decrease the risk developing transfusion related lung dysfunction and multiple organ failure in severely injured patients [97]. Prospective clinical studies in giving trauma patients aspirin and antiplatelet therapy are now under consideration.
Guidelines
Several guidelines have been developed for the management of severe TBI by several organizations including the Brain Trauma Foundation (BTF), American Association of Neurological Surgeons, and the Congress of Neurological Surgeons [98–101]. Copies of the 3rd edn of these guidelines are available from Brain Trauma Foundation, 798 3rd Avenue, Suite 1810, New York, NY (btfinfo@braintrauma.org).
The most recent guidelines are from the Scandinavian Society and provide an evidence- and consensus-based algorithm to assist physicians in determining which patients are at higher risk for intracranial pathology, neuroimaging, and hospital admission [102]. Attention has also recently been drawn to sports-related head injuries [103, 104]. It is estimated that up to 3.8 million concussions are related to sports injuries annually in the US and some 50 % go unreported. Given that there can be serious long-term sequelae, prevention, recognition, and treatment must be reviewed, emphasizing a more individual approach with the realization that there may be no set timeline for return to play.
Conclusion
Much of the recent literature on TBI confirms what was determined years ago. There is no magic bullet yet to save the brain and basic principles must be applied. Although many guidelines have been formulated, adherence is low. Prompt and successful airway management and appropriate ventilation remain essential to good outcome after head injury. Rapid sequence induction/intubation is frequently indicated and some sedation is indicated. Normalization of cardiovascular dynamics and ICP is of equal importance. Application of cricoid pressure is probably not useful. Auscultation and chest X-ray help in the diagnosis of tension pneumothoraces, especially before positive pressure ventilation is provided. Small pneumothoraces can be managed conservatively. Identification of significant hemorrhage can be difficult in the head-injured patient when a catecholamine surge may give a falsely high blood pressure. While hypotension limits bleeding, it could worsen brain injury. The ideal initial resuscitation fluid remains controversial. Hypothermia can exacerbate bleeding and the benefit in TBI is uncertain.
References
Joost J, Sanchez GM, Burridge AL. The Edwin Smith Papyrus: a clinical reappraisal of the oldest known document on spinal injuries. Eur Spine J. 2010;19:1815–23.
Breasted JH. The Edwin Smith Surgical Papyrus: published in facsimile and hieroglyphic transliteration with translation and commentary in two volumes (University of Chicago Oriental Institute publications, v. 3-4. Chicago: University of Chicago Press, 1991) (See 1: pp. xvi, 6, 480-485, 487-489, 446-448, 451-454, 466; 2: pi. XVII, XVIIA).
Weber J, Czarnetzki A. Trepanationen im frühen Mittelalter im Südwesten von Deutschland—Indikationen, Komplikationen und Outcome (in German). Zentralbl Neurochir. 2001;62:10.
Rutkow IM. The origins of modern surgery, surgery-basic science and clinical evidence. New York: Springer; 2001. p. 2–19.
Adams F. The genuine works of Hippocrates translated from the Greek. London: The Sydenham Society; 1849. pp. 430, 431, 433, 455.
Cooper A. Lectures in the principles and practice of surgery. London: Westley; 1829. p. 119.
Frost EM. History of neuroanesthesia. In: Albin MS, editor. Textbook of neuroanesthesia. New York: McGraw-Hill; 1997. p. 1–20.
Monro A. Observations on the structure and function of the nervous system. Creech & Johnson: Edinburgh; 1783. p. 5.
Kelly G. Appearances observed in the dissection of two individuals; death from cold and congestion of the brain. Trans Med Chir Sci Edinb. 1824;1:84–169.
Cannon WB, Fraser J, Covell E. The preventive treatment of wound shock. JAMA. 1918;70:618–21.
McCaffrey, R. J. (Ed.) (1997). Special issues in the evaluation of mild traumatic brain injury. In The practice of forensic neuropsychology: Meeting challenges in the courtroom. New York: Plenum Press. pp. 71–75. ISBN 0-306-45256-1
Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010.
Centers for Disease Control and Prevention (CDC), National Center for Injury Prevention and Control. Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta: Centers for Disease Control and Prevention; 2003.
Finkelstein E, Corso P, Miller T, et al. The incidence and economic burden of injuries in the United States. New York: Oxford University Press; 2006.
Foulkes MA, Eisenberg HM, Jane JA, et al. The traumatic coma data bank; design, methods and baseline characteristics. J Neurosurg. 1991;75:S8–13.
Chen H, Guo Y, Chen SW, et al. Progressive epidural hematoma in patients with head trauma; incidence, outcome and risk factors. Emerg Med Int. 2012;2012:134905. doi:10.1155/2012/134905.
Honeybul S, Ho KM. The current role of decompressive craniectomy in the management of neurological emergencies. Brain Inj. 2013;27(9):979–91 [Epub ahead of print].
Jenkins A, Teasdale G, Hadley MD, et al. Brain lesions detected by magnetic resonance imaging in severe head injuries. Lancet. 1986;2(8504):445–6.
Miller JD, Sweet RC, Narayan R, et al. Early insults to the injured brain. JAMA. 1978;240(5):439–42.
Huang BY, Castillo M. Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics. 2008;28:417–39. doi:10.1148/rg.282075066.
Stoica BA, Faden AI. Cell death mechanisms and modulation in traumatic brain injury. Neurotherapeutics. 2010;7:3–12.
Stoica BA, Byrnes KR. Cell cycle activation and CNS injury. Neurotox Res. 2009;16:221–37.
Johansson PI, Sørensen AM, Perner A, et al. High sCD40L levels early after trauma are associated with enhanced shock, sympathoadrenal activation, tissue and endothelial damage, coagulopathy and mortality. J Thromb Haemost. 2012;10(2):207–16.
Lumpkins KM, Bochicchio GV, Keledjian K, et al. Glial fibrillary acidic protein is highly correlated with brain injury. J Trauma. 2008;65(4):778–84. doi:10.1097/TA.0b013e318185db2d.
Mercier E, Boutin A, Lauzier F, et al. Predictive value of S-100B protein for prognosis in patients with moderate and severe traumatic brain injury; a systematic review and meta-analysis. BMJ. 2013;346:f1757.
Herrmann M, Vos P, Wunderlich M, et al. Release of glial tissue-specific proteins after acute stroke. Stroke. 2000;31:2670–7.
Rhodes J, Sharkey J, Andrews P. Serum IL-8 and MCP-1 concentration do not identify patients with enlarging contusions after traumatic brain injury. J Trauma. 2009;66(6):1591–7.
Chatzipanteli K, Vitarbo E, Alonso OF, et al. Temporal profile of cerebrospinal fluid, plasma and brain interleukin -6 after normothermic fluid percussion brain injury; effect of secondary hypoxia. Ther Hypothermia Temp Manag. 2012;2(4):167–75.
Antunes AA, Sotomajor VS, Sakamoto KS, et al. Interleukin-6 plasma levels in patients with head trauma and intracerebral hemorrhage Asian. J Neurosurg. 2010;5(1):68–77.
Papadopoulos V, Lecanu L. Translocator protein (18 kDa) TSPO: an emerging therapeutic target in neurotrauma. Exp Neurol. 2009;219(1):53–7.
Yokobori S, Hosein K, Burks S, Sharma I, Gajavelli S. Biomarkers for the clinical differential diagnosis in traumatic brain injury—a systematic review. CNS Neurosci Ther. 2013;19(8):556–65. doi:10.1111/cns.12127 [Epub ahead of print].
Rose J, Valtonen S, Jennett B. Avoidable factors contributing to death after head injury. BMJ. 1977;2:615–8.
Rangel-Castilla L, Wyler AR Closed head trauma. Medscape Reference. http://emedicine.medscape.com/article/2518340overview. Accessed May 31, 2013.
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–4.
Banks SE, Lewis MC. Trauma in the elderly: considerations for anesthetic management. Anesthesiol Clin. 2013;31(1):127–39.
Edwards M, Lev E, Mirocha J, et al. Defining hypotension in moderate to severely injured trauma patients: raising the bar for the elderly. Am Surg. 2010;76(10):1035–8.
Stevens WC, Dhanaraj VJ. Characteristics of elderly patients affecting outcome from trauma. Anesthesiology. 1992;77(3A):1089.
Lien YC, Chen CH, Lin HC. Risk factors for 24 hour mortality after traumatic rib fractures owing to motor vehicle accidents: a nationwide population-based study. Ann Thorac Surg. 2009;88(4):1124–30.
Philip S, Udomphorn Y, Kirkham FJ, et al. Cerebrovascular pathophysiology in pediatric traumatic brain injury. J Trauma. 2009;67(2 Suppl):S128–34.
Chadwick DL, Chin S, Salerno C, et al. Deaths from falls in children: how far is fatal. J Trauma. 1991;31(10):1352–5.
Vitale A, Vicedomi ID, Vega GR, et al. Shaken baby syndrome: pathogenetic mechanism, clinical features and preventive aspects. Minerva Pediatr. 2012;64(6):641–7.
Siegel JH, Gens DR, Mamantov T, et al. Effect of associated injuries and blood volume replacement on death, rehabilitation and disability in blunt traumatic brain injury. Crit Care Med. 1991;19(10):1252–68.
Bruijns SR, Guly HR, Bouamra O, et al. The value of traditional vital signs, shock index, and age-based markers in predicting trauma mortality. J Trauma Acute Care Surg. 2013;74(6):1432–7.
Sonne NM, Tonneson H. The influence of alcoholism on outcome after evacuation of subdural hematoma. Br J Neurosurg. 1992;6(2):125030.
Carrasco CE, Godinho M, Berti de Azevedo Barros M, et al. Fatal motorcycle crashes: a serious public health problem in Brazil. World J Emerg Surg. 2012;7 Suppl 1:S1–5.
Chrastina J, Hrabovsky D, Riha I, et al. The effect of age, alcohol intoxication and type of brain injury on the prognosis of patients operated for craniocerebral trauma. Rozhi Chir. 2013;92(3):135–42.
Goodman MD, Makley AT, Campion EM, et al. Preinjury alcohol exposure attenuates the neuroinflammatory response to traumatic brain injury. J Surg Res. 2013;184(2):1053–8.
Adekoya D, Thurman DJ, White DD, Webb KW. Surveillance for traumatic brain injury deaths—United States, 1989-1998. MMWR Surveill Summ. 2000;51(10):1–14.
Hirschberg R, Weiss D, Zafonte R. Traumatic brain injury and gender: what is known and what is not. Future Neurology. 2008;3(4):483–9.
Spaite DW, Tse DJ, Valenzuela TD, et al. The impact of injury severity and prehospital procedures on scene time in victims of major trauma. Ann Emerg Med. 1991;20(12):1299–305.
Ala-Kokko TI, Ohtonen P, Koskenkari J, et al. Improved outcome after trauma care in university-level intensive care units. Acta Anaesthesiol Scand. 2009;53(10):1251–6.
Meisler R, Thomson AB, Abildstrom H, et al. Triage and mortality in 2875 consecutive trauma patients. Acta Anaesthesiol Scand. 2010;5492:218–23.
Mohan D, Barnato AE, Angus DC, et al. Determinants of compliance with transfer guidelines for trauma patients: a retrospective analysis of CT scans acquired prior to transfer to a level 1 trauma center. Ann Surg. 2010;251(5):946–51.
Franschman G, Peerdeman SM, Greuters S, et al. Prehospital endotracheal intubation in patients with severe traumatic brain injury: guidelines versus reality. Resuscitation. 2009;80(10):1147–51.
Harris T, Davenport R, Hurst T, et al. Improving outcome in severe trauma: trauma systems and initial management: intubation, ventilation and resuscitation. Postgrad Med J. 2012;88(1044):588–94.
Butler J, Sen A. Towards evidence-based emergency medicine; best BETs from the Manchester Royal Infirmary. BET 1: cricoids pressure in emergency rapid sequence induction. Emerg Med J. 2013;30(2):163–5.
Loganathan N, Liu EH. Cricoid pressure: ritual or effective measure? Singapore Med J. 2012;53(9):620–2.
Redan JA, Livingston DH, Bartholomew J, et al. The value of intubating and paralyzing patients in the emergency patient. J Trauma. 1991;31(3):371–5.
Grande CM, Barton CR, Stene JK. Appropriate techniques for airway management of emergency patients with suspected spinal cord injury. Anesth Analg. 1988;76:710–8.
Hauswald M, Sklar DP, Tandberg D, et al. Cervical spine movement during airway management0cinefluroscopic appraisal in human cadavers. Am J Emerg Med. 1991;9:535–8.
Veith I. The Yellow Emperor’s classic of internal medicine (trans). Berkeley: University of California; 1973. p. 159–60.
Breasted JH. The Edwin Smith Papyrus Chicago. Chicago: University of Chicago Press; 1930. p. 113–78.
Tolani K, Bendo AA, Sakabe T. Anesthetic management of head trauma. In: Newfield P, Cottrell JE, editors. Handbook of neuroanesthesia. Philadelphia: Wolters Kluwer; 2012. p. 100.
Cushing H. The blood-pressure reaction of acute cerebral compression, illustrated by cases of intracranial hemorrhage: a sequel to the Mutter lecture 1901. Am J Med Sci. 1903;125(6):1017–43.
Miner ME, Allen SJ. Cardiovascular effects of severe head injury. In: Frost E, editor. Clinical anesthesia in neurosurgery. Boston: Butterworth; 1984. p. 372–4.
Rubicsek S. Mortality after TBI ASA Annual Meeting 2008: A366. Eur J Anaesthesiol Suppl. 2008;42:110–4. doi:10.1017/S0265021507003304.
Nishikawa T. Risk management for neurosurgical anesthesia. Masui. 2009;58(5):545–51.
Lescot T, Degos V, Puybasset L. Does the brain become heavier or lighter after trauma? Eur J Anaesthesiol Suppl. 2008;42:110–4. doi:10.1017/S0265021507003304.
Sell SL, Avila MA, Yu G, Vergara L, et al. Hypertonic resuscitation improves neuronal and behavioral outcomes after traumatic brain injury plus hemorrhage. Anesthesiology. 2008;108(5):873–81. doi:10.1097/ALN.0b013e31816c8a15.
Soukup J, Bramsiepe I, Brucke M, et al. Evaluation of a bedside monitor of regional CBF as a measure of CO2 reactivity in neurosurgical intensive care patients. J Neurosurg Anesthesiol. 2008;20(4):249–55. doi:10.1097/ANA.0b013e31817ef487.
Young N, Rhodes JK, Mascia L, et al. Ventilatory strategies for patients with acute brain injury. Curr Opin Crit Care. 2010;16(1):45–52. doi:10.1097/MCC.0b013e32833546fa.
Hocker SE, Fogelson J, Rabinstein AA. Refractory intracranial hypertension due to fentanyl administration following closed head injury. Front Neurol. 2013;4:3. doi:10.3389/fneur.2013.00003.
James MF. The role of tetrastarches for volume replacement in the perioperative setting. Curr Opin Anaesthesiol. 2008;21(5):674–8.
Hartog CS, Bauer M, Reinhart K. The efficacy and safety of colloid resuscitation in the critically ill. Anes Analg. 2011;112(1):156–64.
Myburgh J, Cooper DJ, Finfer S, et al. (SAFE Study Investigators). Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357(9):874–84.
Finfer S, McEvoy S, Bellomo R, et al. (SAFE Study Investigators). Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Int Care Med. 2011;37(1): 86–96.
Myburgh J, Li Q, Heritier S, et al. Statistical analysis plan for the crystalloid versus hydroxyethyl starch trial (CHEST). Crit Care Resusc. 2012;14(1):44–52.
Alderson P, Bunn F, Lefebvre C, et al. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev. 2004;18(4):CD001208.
Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2013;2:CD000567. doi:10.1002/14651858.
Haase N, Perner A, Hennings LI, et al. Hydroxyethyl starch 130/0.38–0.45 versus crystalloid or albumin in patients with sepsis: a systematic review with meta-analysis and trial sequential analysis. BMJ. 2013;346:f839. doi: 10.1136/bmj.f839
American Society of Anesthesiologists Task Force on Perioperative Blindness. Practice advisory for perioperative visual loss associated with spine surgery. Anesthesiology. 2006;104:1319–28.
Jungner M, Grände PO, Mattiasson G, Bentzer P. Effects on brain edema of crystalloid and albumin fluid resuscitation after brain trauma and hemorrhage in the rat. Anesthesiology. 2010;112(5):1194–203. doi:10.1097/ALN.0b013e3181d94d6e.
Drummond JC. Colloid osmotic pressure and the formation of posttraumatic cerebral edema. Anesthesiology. 2010;112(5):1079–81. doi:10.1097/ALN.0b013e3181d94e53.
McIntyre L, Fergusson DA, Rowe B, et al. The PRECISE RCT: evolution of an early septic shock fluid resuscitation trial. Transfus Med Rev. 2012;26(4):333–41. doi:10.1016/j.tmrv.2011.11.003.
Shander A, Hofmann A, Gombotz H, Theusinger OM, Spahn DR. Estimating the cost of blood: past, present, and future directions. Best Pract Res Clin Anaesthesiol. 2007;21:271–89.
Michaud LJ, Rivera FP, Longstreth WT, et al. Elevated initial blood glucose levels and; poor outcome following severe brain injuries in children. J Trauma. 1991;31(10):1356–62.
Stein SC, Young GS, Taucci RC, et al. Delayed brain injury after head trauma: significance of coagulopathy. Neurosurgery. 1992;30(2):160–5.
Frith D, Brohi K. The pathophysiology of trauma-induced coagulopathy. Curr Opin Crit Care. 2012;18(6):631–6.
Genét GF, Johansson PI, Meyer MA, et al. Trauma-induced coagulopathy: standard coagulation tests, biomarkers of coagulopathy, and endothelial damage in patients with traumatic brain injury. J Neurotrauma. 2013;30(4):301–6. doi:10.1089/neu.2012.2612.
Bondanelli M, Ambrosio MR, Zatelli MC, et al. Hypopituitarism after traumatic brain injury. Eur J Endocrinol. 2005;152:679–91.
Stover JF, Pleines UE, Morganti-Kossmann MC, et al. Thiopental attenuates energetic impairment but fails to normalize cerebrospinal fluid glutamate in brain-injured patients. Crit Care Med. 1999;27(7):1351–7.
Neil MJ, Dale MC. Hypokalaemia with severe rebound hyperkalaemia after therapeutic barbiturate coma. Anesth Analg. 2009;108(6):1867–8. doi:10.1213/ane.0b013e3181a16418.
Cairns CJ, Andrews PJ. Management of hyperthermia in traumatic brain injury. Curr Opin Crit Care. 2002;8(2):106–10.
Adelson PD, Wisniewski SR, Beca J, et al. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): a phase3, randomized controlled trial. Lancet Neurol. 2013;12(6):546–53.
Shoja MM, Chern JJ. Decompressive craniectomy. J Neurosurg Pediatr. 2013;11(3):358. doi:10.3171/2012.7.PEDS12178. Epub 2013 Jan 11.
Orlando A, Bar-Or D, Salottolo K, Levy AS, et al. Unintentional discontinuation of statins may increase mortality after traumatic brain injury in elderly patients: a preliminary observation. J Clin Med Res. 2013;5(3):168–73. doi:10.4021/jocmr1333w.
Harr JN, Moore EE, Johnson J. Antiplatelet therapy is associated with decreased transfusion-associated risk of lung dysfunction, multiple organ failure, and mortality in trauma patients. Crit Care Med. 2013;41(2):399–404. doi:10.1097/CCM.0b013e31826ab38b.
Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. Neurotrauma. 2007;24 Suppl 1:S1–106.
Bullock MR, Chesnut R, Ghajar J, Surgical Management of Traumatic Brain Injury, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006;58(3 Suppl):S16–24.
Badjatia N, Carney N, Crocco TJ, et al. Guidelines for prehospital management of traumatic brain injury 2nd edition. Brain Trauma Foundation; BTF Center for Guidelines Management. Prehosp Emerg Care. 2008;12 Suppl 1:S1–52. doi:10.1080/10903120701732052.
Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 19. The role of anti-seizure prophylaxis following severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(3 Suppl):S72–5.
Unden J, Ingebrigtsen T, Romner B, et al. Scandinavian guidelines for initial management of minimal, mild, and moderate head injuries in adults: an evidence and consensus-based update. BMC Med. 2013;11:50. doi:10.1186/1741-7015-11-50. Pub on line Feb 2103.
Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47(1):15–26.
Mitka M. Guideline: tailor appraisal of concussion during sports. JAMA. 2013;309(15):1577.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Frost, E.A.M. (2014). Brain Injuries: Perianesthetic Management. In: Scher, C. (eds) Anesthesia for Trauma. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-0909-4_8
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0909-4_8
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-0908-7
Online ISBN: 978-1-4939-0909-4
eBook Packages: MedicineMedicine (R0)