Abstract
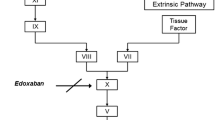
The blood coagulation pathway consists of a series of inactive and active enzymes (Fig. 1). In certain individuals, some of the elements, or factors, of this cascade may be inoperative or have reduced activity, and in some cases may be missing. Such cases give rise to the bleeding disorder hemophilia. There are three categories of patients with the deficiency: patients with normal amounts of factor that has reduced clotting activity; patients with factor and activity equally reduced; patients in whom the factor and its activity are undetectable. The existence of at least two forms of hemophilia was suggested by the results of experiments performed by Pavlovsky (1947) in which the mixing of the blood of two patients classified as hemophilics caused a correction of the clotting times of each blood sample. The more common hemophilia A, or classical hemophilia, and hemophilia B, or Christmas disease, occur as a result of factor VIII (FVIII) and factor IX (FIX) deficiency, respectively. These two diseases are X-linked recessive traits in which males are affected. Patients with severe hemophilia A or B have undetectable (less than 1% of normal) concentrations of FVIII or FIX and suffer from recurrent

Simplified scheme depicting the relationship of the intrinsic and extrinsic coagulation pathways and the fibrinolysis pathway. Roman numerals refer to individual coagulation factors, and tPA, UK, SK, and APSAC refer to tissue plasminogen activator, urokinase, streptokinase, and anisoylated plasminogen streptokinase activator complex, respectively.
spontaneous hemarthroses and retroperitoneal bleeding. A related disorder is von Willebrand’s disease which is caused by a lack of von Willebrand’s factor (and also FVIII). von Willebrand’s factor is essential for platelet aggregation. Other recognized hemophilias result from deficiencies in factors V, VII, X, XI, and XIII.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Aggeler, P. M., 1961, Physiological basis for transfusion therapy in hemorrhagic disorders, Transfusion 1:71–74.
Allain, J. P., 1984, Principles of in vivo recovery and survival studies, Scand. J. Haematol 33(Suppl. 40):161–165.
Astrup, T., and Permin, P. M., 1947, Fibrinolysis in animal organism, Nature 159:681–682.
Bakhit, C., Lewis, D., Billings, R., and Malfroy, B., 1987, Cellular catabolism of recombinant tissue-type plasminogen activator, J. Biol. Chem. 262:8716–8720.
Barrowcliffe, T. W., Stableforth, R., and Dormandy, K. M., 1973, Small scale preparation and clinical use of factor IX prothrombin complex, Vox Sang. 25:426–441.
Baughman R. A., 1987, Pharmacokinetics of tissue plasminogen activator, in: Tissue Plasminogen Activator in Thrombolytic Therapy (B. E. Sobel, D. Collen, and E. B. Grossbard, eds.), Dekker, New York, pp. 41–53.
Been, M., deBono, D. P., Muir, A. L., Boulton, F. E., Fears, R., Standring, R., and Ferres, H., 1986, Clinical effects and kinetic properties of intravenous anistre-plase-anisoylated plasminogen-streptokinase activator complex (BRL26921) in acute myocardial infarction, Int. J. Cardiol. 11:53–61.
Bertina, R. M., and Veltkamp, J. J., 1981, Physiology and biochemistry of factor IX, in: Haemostasis and Thrombosis (A. L. Bloom and D. P. Thomas, eds.), Churchill Livingstone, Edinburgh, pp. 98–110.
Bidwell, E., Booth, J. M., Dike, G. W. R., and Denson, K. W. E., 1967, The preparation for therapeutic use of a concentrate of factor IX containing also factors II, VII and X, Br. J. Haematol. 13:586–590.
Biggs, R., and Denson, K. W. E., 1963, The fate of prothrombin and factors VII, IX and X transfused to patients deficient in these factors, Br. J. Haematol 9:532–547.
Binder, B. R., Spragg, J., and Austen, K. F., 1979, Purification and characterization of human vascular plasminogen activator derived from blood vessel perfusates, J. Biol Chem. 254:1998–2003.
Bloom, A. L., 1990, Physiology of blood coagulation, Haemostasis 20(Suppl.): 14–29.
Brinkhous, K. M., Hedner, U., Garris, J. B., Diness, V., and Read, M. S., 1989, Effect of recombinant factor VIIa on the hemostatic defect in dogs with hemophilia A, hemophilia B, and von Willebrand disease, Proc. Natl Acad. Sci. USA 86:1382–1386.
Broze, G. J., and Majerus, P. W., 1980, Purification and properties of human coagulation factor VII, J. Biol. Chem. 255:1242–1247.
Broze, G. J., and Miletich, J. P., 1987, Characterization of the inhibition of tissue factor in serum, Blood 69:150–155.
Carson, S. D., 1987, Tissue factor (coagulation factor III) inhibition by apolipoprotein A-II, J. Biol Chem. 262:718–721.
Chavin, S. I., and Weidner, S. M., 1984, Blood clotting factor IX. Loss of activity after cleavage of sialic acid residues, J. Biol Chem. 259:3387–3390.
Christensen, L. R., and MacLeod, C. M., 1945, Proteolytic enzyme of serum: Characterization, activation, and reaction with inhibitors, J. Gen. Physiol. 28:559–583.
Col, J. J., Col-DeBeys, S. M., Renkin, J. P., LaVenne-Pardonge, E. M., Bachy, J. L., and Morian, M. H., 1989, Pharmacokinetics, thrombolytic efficacy and hemorrhagic risk of different streptokinase regimens in heparin-treated acute myocardial infarction, Am. J. Cardiol. 63:1185–1192.
Collen, D., Stassen, J. M., Marafino, B. J., Builder, S., DeCock, F., Ogez, J., Tajiri, D., Pennica, D., Bennett, W. F., Salwa, J., and Hoyng, C. F., 1984, Biological properties of human tissue-type plasminogen activator obtained by expression of recombinant DNA in mammalian cells, J. Pharmacol. Exp. Ther. 231:146–152.
Collen, D., Zamarron, C., Lijnen, H. R., and Hoylaerts, M., 1986, Activation of plasminogen pro-urokinase. II. Kinetics, J. Biol. Chem. 261:1259–1266.
Cossum, P., Littlewood, J., Ferraiolo, B., Green, J., and Bunting, S., 1990, Recombinant human tissue factor (rhTF) pharmacokinetics and effects in normal and hemophiliac dogs, Pharm. Res. 7(Suppl.):S-45 (abstract).
Daly, H. M., and Haddon, M. E., 1988, Clinical experience with a pasteurized human plasma concentrate in factor XIII deficiency, Thromb. Haemostas. 59:171–174.
Eaton, D., Rodriguez, H., and Vehar, G. A., 1986, Proteolytic processing of human factor VIII. Correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity, Biochemistry 25:505–512.
Esmail, A. F., Dupe, R. J., English, P. D., and Smith, R. A. G., 1984, Pharmacokinetic and pharmacodynamic comparison of acylated streptokinase plasminogen complexes with different deacylation rate constant, Haemostasis 14: 84.
Fear, J. D., Miloszewski, K. J. A., and Losowsky, M. S., 1983, The half-life of factor XIII in the management of inherited deficiency, Thromb. Haemostas. 49:102–105.
Fears, R., Ferres, H., and Standring, R., 1987, The protective effect of acylation on the stability of anisoylated plasminogen streptokinase activator complex in human plasma, Drugs 33(Suppl. 3):57–63.
Ferres, H., Hibbs, M., and Smith, R. A. G., 1987, Deacylation studies in vitro on anisoylated plasminogen streptokinase activator complex, Drugs 33(Suppl. 3):80–82.
Fisher, K. L., Gorman, C., Vehar, G., O’Brien, D. P., and Lawn, R. M., 1987, Cloning and expression of tissue factor cDNA, Thromb. Res. 48:89–99.
Fletcher, A. D., Alkjaersig, N., and Sherry, S., 1959, The clearance of heterologous protein from the circulation of normal and immunized man, J. Clin. Invest. 37:1306–1315.
Fong, K.-L., and Lynn, R. K., 1986, Disposition and metabolism of tissue-type plasminogen activator (tPA) in the isolate perfused rat liver, Pharmacologist 28: 117.
Fong, K.-L., Crysler, C. S., Mico, B. A., Boyle, K. E., Kopia, G. A., Kopaciewicz, L., and Lynn, R. K., 1988, Dose-dependent pharmacokinetics of recombinant tissue-type plasminogen activator in anesthetized dogs following intravenous infusion, Drug Metab. Dispos. 16:201–206.
Fuchs, H. E., Trapp, H. G., Griffith, M. J., Roberts, H. R., and Pizzo, S. V., 1984, Regulation of factor IXa in vitro in human and mouse plasma and in vivo in the mouse, J. Clin. Invest. 73:1696–1703.
Fuchs, H. E., Berger, H., and Pizzo, S. V., 1985, Catabolism of human tissue plasminogen activator in mice, Blood 65:539–544.
Garabedian, H. D., Gold, H. K., Leinbacj, R. C., Johns, J. A., Yasuda, T., Kanke, M., and Collen, D., 1987, Comparative properties of two clinical preparations of recombinant tissue-type plasminogen activator in patients with acute myocardial infarction, J. Am. Coll. Cardiol. 9:599–607.
Gemmill, J. D., Hogg, K. J., Burns, J. M., Rae, A. P., Dunn, F. G., Fears, R., Ferres, H., Standring, R., Greenwood, H., Pierce, D., and Hills, W. S., 1991, A comparison of the pharmacokinetic properties of streptokinase and anistreplase in acute myocardial infarction, Br. J. Clin. Pharmacol. 31:143–147.
Gill, F. M., 1984, The natural history of factor VIII inhibitors in patients with hemophilia A, Prog. Clin. Biol. Res. 150:19–24.
Girard, T. J., Warren, L. A., Novotny, W. F., Likert, K. M., Brown, S. G., Miletich, J. P., and Broze, G. J., 1989, Functional significance of the Kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature 338: 518–520.
Gonmori, H., and Takeda, Y., 1975, Properties of canine tissue thromboplastin from brain, lung, arteries and veins, Am. J. Physiol. 229:618–626.
Goodnight, S. H., Britell, C. W., Wuepper, K. D., and Osterud, B., 1979, Circulating factor IX antigen-inhibitor complexes in hemophilia B following infusion of a factor IX concentrate, Blood 53:93–103.
Grierson, D. S., and Bjornsson, T. D., 1987, Pharmacokinetics of streptokinase in patients based on amidolytic activator complex activity, Clin. Pharmacol. Ther. 41:304–313.
Gunzler, W. A., Steffens, G. J., Otting, F., Kim, S. M., Frankus, E., and Rohe, L., 1982, The primary structure of high molecular mass urokinase from human urine. The complete amino acid sequence of the A chain, Hoppe-Seylers Z. Physiol Chem. 363:1155–1165.
Hedner, U., and Kisiel, W., 1983, Use of human factor VIIa in the treatment of two hemophilia A patients with high-titer inhibitors, J Clin. Invest. 71:1836–1841.
Hedner, U., Glazer, S., Pingel, K., Alberts, K. A., Blomback, M., Schulman, S., and Johnsson, H., 1988, Successful use of recombinant factor VIIa in patients with severe haemophilia A during synovectomy, Lancet 2: 1193.
Hellstern, P., Miyashita, C., Kohler, M., von Blohn, G., Kiehl, R., Biro, G., Schwerdt, H., and Wenzel, E., 1987, Measurement of factor VIII procoagulant antigen in normal subjects and in hemophilia A patients by an immunoradiometric assay and by an enzyme-linked immunosorbent assay, Haemostasis 17:173–181.
Hoag, M. S., Aggeler, P. M., and Powell, A. H., 1960, Disappearance rate of concentrated proconvertin extracts in congenital and acquired hypoconvertinemia, J. Clin. Invest. 39:554–563.
Hoag, M. S., Johnson, F. F., Robinson, J. A., and Aggeler, P. M., 1969, Treatment of hemophilia B with a new clotting-factor concentrate, N. Engl J. Med. 280:581–583.
Holvoet, P., Cleemput, H., and Collen, D., 1985, Assay of human tissue-type plasminogen activator (t-PA) with an enzyme-linked immunosorbent assay (ELISA) based on three murine monoclonal antibodies to t-PA, Thromb. Haemostas. 54:684–687.
Hotchkiss, A., Refino, C. J., Leonard, C. K., O’Connor, J. V., Crowley, C., McCabe, J., Tate, K., Nakamura, G., Powers, D., Levinson, A., Mohler, M., and Spellman, M., 1988, The influence of carbohydrate structure on the clearance of recombinant tissue-type plasminogen activator, Thromb. Haemostas. 60: 255–261.
Ichinose, A., Fujikawa, K., and Suyama, T., 1986, The activation of prourokinase by plasma kallikrein and its inactivation by thrombin, J. Biol. Chem. 261: 3486–3489.
Jackson, K. W., and Tang, J., 1982, Complete amino acid sequence of streptokinase and its homology with serine proteases, Biochemistry 21:6620–6625.
Kadhom, N., Wolfrom, C., Gautier, M., Allain, J. P., and Frommel, D., 1988, Factor VIII procoagulant antigen in human tissues, Thromb. Haemostas. 59:289–294.
Kjellman, H., 1984, Calculations of factor VIII in vivo recovery and half-life, Scand. J. Haematol. 33(Suppl. 40): 165–174.
Kohler, M., Seifreid, E., Hellstern, P., Pindur, G., Miyashita, C., Morsdorf, S., Fasco, F., and Wenzel, E., 1988, In vivo recovery and half-life time of a steam-treated factor IX concentrate in hemophilia B patients, Blut 57:341–345.
Kohler, M., Hellstern, P., Pindur, G., Wenzel, E., and von Blohm, G., 1989, Factor VII half-life after transfusion of a steam-treated prothrombin complex concentrate in a patient with homozygous factor VII deficiency, Vox Sang. 56:200–201.
Kopia, G. A., Kopaciewicz, L. J., Fong, K.-L., Crysler, C. S., Boyle, K., and Ruffblo, R. R., 1988, Evaluation of the acute hemodynamic effects and pharmacokinetics of coronary thrombolysis produced by intravenous tissue-type plasminogen activator in the anesthetized dog, J. Cardiovasc. Pharmacol. 12:308–316.
Korninger, C., Stassen, J. M., and Collen, D., 1981, Turnover of human extrinsic (tissue-type) plasminogen activator in rabbits, Thromb. Haemostas. 46: 658–661.
Kuiper, J., Otter, M., Rijken, D. C., and van Berkel, T. J. C., 1988, Characterization of the interaction in vivo of tissue-type plasminogen activator with liver cells, J. Biol. Chem. 263:18220–18224.
Kuzel, T., Green, D., Stulberg, S. D., and Baron, J., 1988, Arthropathy and surgery in congenital factor VII deficiency, Am. J. Med. 84:771–774.
Lewis, J. H., Bontempo, F. A., Spero, J. A., Ragni, M. V., and Starzi, T. E., 1985, Liver transplantation in a hemophiliac, N. Engl. J. Med. 312:1189–1192.
Littlewood, J. D., and Barrowcliffe, T. W., 1987, The development and characterization of antibodies to human factor VIII in haemophilic dogs, Thromb. Haemostas. 57:314–321.
Loeliger, E. A., and Hensen, A., 1964, On the turnover of factors II, VII, IX, X under pathological conditions, Thromb. Diath. Haemorrh. 13(Suppl.):95.
Loewy, A. G., Dahlberg, A., Dunathan, D., Kriel, R., and Wolfinger, H. L., 1961, Fibrinases. II. Some physical properties, J Biol. Chem. 236:2634–2643.
Longo, G., Matucci, M., Messori, A., Morfini, M., and Rossi-Ferrini, P., 1986, Pharmacokinetics of a new heat-treated concentrate of factor VIII estimated by model-independent methods, Thromb. Res. 42:471–476.
Longo, G., Cinotti, S., Filimberti, E., Giustarini, G., Messori, A., Morfini, M., and Rossi-Ferrini, P., 1987, Single-dose pharmacokinetics of factor IX evaluated by model-independent methods, Eur. J. Haematol. 39:426–433.
Markus, G., Evers, J. L., Hobika, J. H., 1976, Activator activities of the transient forms of the human plasminogen-streptokinase complex during its proteolytic conversion to the stable activator complex, J. Biol. Chem. 251:6495–6504.
McLellan, D. S., Pelly, C., McLellan, H. G., Jones, P., and Aronstam, A., 1982, The in vivo survival characteristics of factor VIII procoagulant antigen (VIILCAg) in haemophilia A subjects, Thromb. Res. 25:33–39.
Mariani, G., Mannucci, P. M., Mazzucconi, M. G., and Capitanio, A., 1978, Treatment of congenital factor VII deficiency with a new concentrate, Thromb. Haemostas. 39:675–682.
Matucci, M., Messori, A., Donati-Cori, G., Longo, G., Vannini, S., Morfini, M., Tendi, E., and Rossi-Ferrini, P. L., 1985, Kinetic evaluation of four factor VIII concentrates by model-independent methods, Scand. J. Haematol. 34:22–28.
Messori, A., Longo, G., Matucci, M., Morfini, M., and Rossi-Ferrini, P. L., 1987, Clinical pharmacokinetics of factor VIII in patients with classic hemophilia, Clin. Pharmacokin. 13:365–380.
Messori, A., Longo, G., Morfini, M., Cinotti, S., Filimberti, E., Giustarini, G., and Rossi-Ferrini, P., 1988, Multi-variate analysis of factors governing the pharmacokinetics of exogenous factor VIII in haemophiliacs, Eur. J. Clin. Pharmacol 35:663–668.
Mohler, M. A., Refino, C. J., Chen, S. A., Chen, A. B., and Hotchkiss, A. J., 1986, D-Phe-Pro-Arg-chloromethylketone: its potential use in inhibiting the formation of in vitro artifacts in blood collected during tissue-type plasminogen activator thrombolytic therapy, Thromb. Haemostas. 56:160–164.
Morfini, M., Longo, G., Matucci, M., Vannini, S., Messori, A., Filimberti, E., Duminuco, M., Avanzi, G., and Rossi-Ferrini, P., 1984, Cryoprecipitate and factor VIII commercial concentrates: In vitro characteristics and in vivo compartmental analysis, Ric. Clin. Lab. 14:681–691.
Nilsson, I. M., Berntorp, E., and Zettervall, O., 1988, Induction of immune tolerance in patients with hemophilia and antibodies to factor VIII by combined treatment with intravenous IgG, cyclophosphamide and factor VIII, N. Engl. J. Med. 318:947–949.
Nilsson, I. M., Berntorp, E., Zettervall, O., and Dahlback, B., 1990, Noncoagulation inhibitory factor VIII antibodies after induction of tolerance to factor VIII in hemophilia A patients, Blood 75:378–383.
Nilsson, S., Wallen, P., and Mellbring, G., 1984, In vivo metabolism of human tissue-type plasminogen activator, Scand. J. Haematol 33:49–53.
Nilsson, S., Einarsson, M., Ekvarn, L., Haggroth, L., and Mattson, C., 1985, Turnover of tissue plasminogen activator in normal and hepatectomized rabbits, Thromb. Res. 39:511–521.
Noe, D. A., Bell, W. R., Ness, P. M., and Levin, J., 1986, Plasma clearance rates of coagulant factors VIII and IX in factor-deficient individuals, Blood 67:969–972.
Over, J., Sixma, J. J., Doucet-de Brune, M., Trieschnigg, M. M., Vlooswijk, R. A., Beeser-Visser, N. H., and Bouma, B. N., 1978, Survival of 125iodine-labelled factor VIII in normals and patients with classic hemophilia, J. Clin. Invest. 62:223–234.
Over, J., Sixma, J. J., Bouma, B. N., Bolhuis, P. A., Vlooswijk, R. A., and Beeser-Visser, N. H., 1981, Survival of iodine-125-labeled factor VIII in patients with von Willebrand’s disease, J Lab. Clin. Med. 97:332–344.
Owen, C. A., and Bowie, W. J., 1975, Infusion therapy in hemophilia A and B, in: Handbook of Hemophilia (K. M. Brinkhous and H. C. Hemker, eds.), Excerpta Medica, Amsterdam, pp. 449–463.
Pannell, R., and Gurewich, V., 1986, Pro-urokinase: A study of its stability in plasma and of a mechanism for its selective fibrinolytic effect, Blood 67:1215–1223.
Pavlovsky, A., 1947, Contribution to the pathogenesis of hemophilia, Blood 2:185–191.
Pennica, D., Holmes, W. E., Kohr, W. J., Harkins, R. N., Vehar, G. A., Ward, C. A., Bennett, W. F., Yelverton, E., Seeburg, H. L., Heyneker, H. L., Goeddel, D. V., and Collen, D., 1983, Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli, Nature 301:214–221.
Pohl, G., Kalstrom, M., Bergsdorf, N., Wallen, P., and Jornvall, H., 1984, Tissue plasminogen activator: peptide analyses confirm an indirectly derived amino acid sequence; identify the active site serine residue, establish glycosylation sites and localize variant differences, Biochemistry 23:3701–3707.
Ranby, M., Bergesdorf, N., and Nilsson, T., 1989a, Enzymatic properties of the one-and two-chain form of tissue plasminogen activator, Thromb. Res. 27: 175–183.
Ranby, M., Nguyen, G., Scarabin, P. Y., and Samama, M., 1989b, Immunoreactivity of tissue plasminogen activator and its inhibitor complexes: Biochemical and multicenter validation of a two-site immunosorbent assay, Thromb. Haemostas. 61: 409–414.
Rao, L. V. M., Rapaport, S. L., and Bajaj, S. P., 1986, Activation of human factor VII in the initiation of tissue factor-dependent coagulation, Blood 68:685–691.
Ratnoff, O. D., 1986, Factor VIII concentrates, J. Am. Med. Assoc. 255:325–326.
Reddy, K. N. N., 1976, Kinetics of active center formation in dog plasminogen by streptokinase and activity of a modified streptokinase, J. Biol. Chem. 251:3913–3920.
Reddy, K. N. N., 1988, Streptokinase—Biochemistry and clinical application, Enzyme 40:79–89.
Rick, M. E., Popovsky, M. A., and Krizek, D. M., 1985, Degradation of factor VIII coagulant antigen by proteolytic enzymes, Br. J. Haematol 61:477–486.
Rijken, D. C., and Collen, D., 1981, Purification and characterization of the plasminogen activator secreted by human melanoma cells in culture, J. Biol. Chem. 256:7035–7041.
Rijken, D. C., Wijngaards, G., Zaal-DeJong, M., and Welbergen, J., 1979, Purification and partial characterization of plasminogen activator from human uterine tissue, Biochim. Biophys. Acta 580: 140.
Rijken, D. C., Hoylaerts, M., and Collen, D., 1982, Fibrinolytic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator, J. Biol Chem. 257:2920–2925.
Rock, G. A., Cruickshank, W. H., Tackaberry, E. S., Ganz, P. R., and Palmer, D. S., 1983, Stability of VIII:C in plasma: The dependence on protease activity and calcium, Thromb. Res. 29:521–535.
Rodeghiero, F., Tosetto, A., DiBona, E., and Castaman, G., 1991, Clinical pharmaco-kinetics of a placenta-derived factor XIII concentrate in type I and type II factor XIII deficiency, Am. J. Hematol. 36:30–34.
Rousell, R. H., Kasper, C. K., and Schwartz, R. S., 1989, The pharmacology of a new pasteurized antihemophilic factor concentrate derived from human blood plasma, Transfusion 29:208–212.
Sandset, P. M., Warn-Cramer, B. J., Rao, L. V., Maki, S. L., and Rapaport, S. I., 1991, Depletion of extrinsic pathway inhibitor (EPI) sensitizes rabbits to disseminated intravascular coagulation induced with tissue factor: Evidence supporting a physiologic role for EPI as a natural anticoagulant, Proc. Natl. Acad. Sci. USA 88:708–712.
Schleef, R. R., Wagner, N. V., Sinha, M., and Loskutoff, D. J., 1986, A monoclonal antibody that does not recognize tissue-type plasminogen activator bound to its naturally occurring inhibitor, Thromb. Haemostas. 56:328–332.
Schneider, C. L., 1947, The active principle of placental toxin: thromboplastin; its inactivator in blood: antithromboplastin, Am. J. Physiol 149:123–129.
Schwartz, R. S., Abildgaard, C. F., Aledort, L. M., Arkin, S., Bloom, A. L., Brackmann, H. H., Brettler, D. R., Fukui, H., Hilgartner, M. W., Inwood, M. J., Kasper, C. K., Kernoff, P. B., Levine, P. H., Lusher, J. M., Mannucci, P. M., Scharrer, I., MacKenzie, M. A., Pancham, N., Kuo, H. S., and Allred, R. U., 1990, Human recombinant DNA-derived antihemophilic factor (factor VIII) in the treatment of hemophilia A, N. Engl. J. Med. 323:1800–1805.
Seligsohn, U., Kasper, C. K., Osterud, B., and Rapaport, S. L. 1979, Activated factor VII: Presence in factor IX concentrates and persistence in the circulation after infusion, Blood 53:828–837.
Siefried, E., and Tanswell, P., 1987, Comparison of specific antibody, D-Phe-Pro-Arg-chloromethylketone and aprotinin for prevention of in vitro effects of recombinant tissue-type plasminogen activator on haemostasis parameters, Thromb. Haemostas. 58:921–926.
Siefried, E., Tanswell, P., Rijken, D. C., Barrett-Bergshoeff, M. M., Su, C. A., and Kluft, C., 1988, Pharmacokinetics of antigen and activity of recombinant tissue-type plasminogen activator after infusion in healthy volunteers, Arzneim. Forsch. 38:418–422.
Siefried, E., Tanswell, P., Ellbruck, D., Haerer, W., and Schmidt, A., 1989, Pharmacokinetics and haemostatic status during consecutive infusion of recombinant tissue-type plasminogen activator in patients with acute myocardial infarction, Thromb. Haemostas. 61:497–501.
Siefring, G. E., and Castellino, F. J., 1976, Interaction of streptokinase with plasminogen: Isolation and characterization of a streptokinase degradation product, J. Biol. Chem. 257:3913–3920.
Smedsrod, B., Einarsson, M., and Pertoft, H., 1988, Tissue plasminogen activator is endocytosed by mannose and galactose receptors of rat liver cells, Thromb. Haemostas. 59:480–484.
Smith, K. J., and Thompson, A. R., 1981, Labeled factor IX kinetics in patients with hemophilia-B, Blood 58:625–629.
Standring, R., Fears, R., and Ferres, H., 1988, The protective effect of acylation on the stability of APSAC (Eminase) in human plasma, Fibrinolysis 2:157–163.
Staniforth, D. H., Smith, R. A. G., and Hibbs, M., 1983, Streptokinase and anisoylated streptokinase plasminogen complex—Their action on haemostasis in human volunteers, Eur. J. Clin. Pharmacol. 24:751–756.
Steffens, G. J., Gunzler, W. A., Otting, F., Frankus, E., and Flohe, L., 1982, The complete amino acid sequence of low molecular mass urokinase from human urine, Hoppe-Seylers Z. Physiol. Chem. 363:1043–1058.
Stern, D. M., Drillings, M., Nossel, H. L., Hurlet-Jensen, A., LaGamma, K., and Owen, J., 1983, Binding of factors IX and IXa to cultured vascular endothelial cells, Proc. Natl. Acad. Sci. USA 80:4119–4123.
Stern, D. M., Knitter, G., Kisiel, W., and Nawroth, P. P., 1987, In vivo evidence of intravascular binding sites for coagulation factor IX, Br. J. Haematol. 66:227–232.
Stump, D. C., Thienpont, M., and Collen, D., 1986a, Urokinase-related proteins in human urine. Isolation and characterization of single-chain urokinase (prourokinase) and urokinase-inhibitor complex, J. Biol. Chem. 261: 1267–1273.
Stump, D. C., Lijnen, H. R., and Collen, D., 1986b, Purification and characterization of single-chain urokinase-type plasminogen activator from human cell cultures, J. Biol. Chem. 261: 1274–1278.
Stump, D. C., Kieckens, L., De Cock, F., and Collen, D., 1987, Pharmacokinetics of single-chain forms of urokinase-type plasminogen activator, J. Pharmacol. Exp. Ther. 242:245–250.
Tanswell, P., Seifried, E., Su, P. C., Feuerer, W., and Rijken, D. C., 1989, Pharmacokinetics and systemic effects of tissue-type plasminogen activator in normal subjects, Clin. Pharmacol. Ther. 46:155–162.
Tanswell, P., Heinzel, G., Greischel, A., and Krause, J., 1990, Nonlinear pharmacokinetics of tissue-type plasminogen activator in three animal species and isolated perfused rat liver, J. Pharmacol. Exp. Ther. 255:318–324.
Tebbe, U., Tanswell, P., Seifried, E., Feuerer, W., Scholz, K. H., and Herrmann, K. S., 1989, Single-bolus injection of recombinant tissue-type plasminogen activator in acute myocardial infarction, Am. J. Cardiol. 64:448–453.
Thompson, A. R., Forrey, A. W., Gentry, P. A., Smith, K. J., and Harker, L. A., 1980, Human factor IX in animals: Kinetics from isolated, radiolabelled protein and platelet destruction following crude concentrate infusions, Br. J. Haematol. 45:329–342.
Toole, J. J., Knopf, J. L., Wozney, J. M., Sultzman, L. A., Buecker, J. L., Pittman, D. D., Kaufman, R. J., Brown, E., Shoemaker, C., Orr, E. C., Amphlett, G. W., Foster, W. B., Coe, M. L., Knutson, G. J., Foss, D. W., and Hewick, R. M., 1984, Molecular cloning of a cDNA encoding human antihemophilic factor, Nature 312:342–347.
Van der Werf, F., Vanhaecke, J., de Geest, H., Verstraete, M., and Collen, D., 1986, Coronary thrombolysis with recombinant single-chain urokinase-type plasminogen activator in patients with acute myocardial infarction, Circulation 74:1066–1070.
Van der Werf, F., Jang, I. K., and Collen, D., 1987, Thrombolysis with recombinant human single-chain urokinase-type plasminogen activator (rscu-PA): Dose-response in dogs with coronary artery thrombosis, J. Cardiovasc. Pharmacol. 9:91–93.
Verstraete, M., Bounameaux, H., de Cock, F., Van der Werf, F., and Collen, D., 1985, Pharmacokinetics and systemic fibrinolytic effects of recombinant human tissue-type plasminogen activator (rt-PA) in humans, J. Pharmacol. Exp. Ther. 235:506–512.
Wallen, P., Ranby, M., Bergsdorf, N., and Kok, P., 1981, Purification and characterization of tissue plasminogen activator: On the occurrence of two different forms and the enzymatic properties, in: Progress in Fibrinolysis, Volume 5 (J. P. Davidson, I. M. Nilsson, and B. Astedt, eds.), Churchill Livingstone, Edinburgh, pp. 16–23.
White, G. C., McMillan, C. W., Kingdon, H. S., and Shoemaker, C. B., 1989, Use of recombinant antihemophilic factor in the treatment of two patients with classic hemophilia, Lancet 320:166–170.
Williams, W. J., 1983, Life span of plasma coagulation factors, in: Hematology (W. J. Williams, ed.), McGraw-Hill, New York, pp. 1230–1237.
Wion, K. L., Kelly, D., Summerfield, J. A., Tuddenham, E. G., and Lawn, R. M., 1985, Distribution of factor VIII mRNA and antigen in human liver and other tissues, Nature 311:126–129.
Wood, W. I., Capon, D. J., Simonsen, C. C., Eaton, D. L., Gitschier, J., Keyt, B., Seeburg, P. H., Smith, D. H., Hollingshead, P., Wion, K. L., Delwart, E., Tuddenham, E. G., Vehar, G. A., and Lawn, R. M., 1984, Expression of active human factor VIII from recombinant DNA clones, Nature 312:330–337.
Working, P. K., and Cossum, P. A., 1991, Clinical and preclinical studies with recombinant human proteins: The effect of antibody production, in: Peptides, Peptoids and Proteins: Proceedings of the 5 th Pittsburgh Pharmacodynamics Conference (P. Gazonne, ed.), Harvey Wilkes Books, Cincinnati.
Zauber, N. P., and Levin, J., 1977, Factor IX levels in patients with hemophilia B (Christmas disease) following transfusion with concentrates of factor IX or fresh frozen plasma (FFP), Medicine 56:213–224.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1992 Springer Science+Business Media New York
About this chapter
Cite this chapter
Cossum, P.A., Baughman, R.A. (1992). Pharmacokinetics and Metabolism of Cardiovascular Therapeutic Proteins. In: Ferraiolo, B.L., Mohler, M.A., Gloff, C.A. (eds) Protein Pharmacokinetics and Metabolism. Pharmaceutical Biotechnology, vol 1. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-2329-5_6
Download citation
DOI: https://doi.org/10.1007/978-1-4899-2329-5_6
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4899-2331-8
Online ISBN: 978-1-4899-2329-5
eBook Packages: Springer Book Archive