Abstract
The overall goal of our research program is to design, synthesize and evaluate organic chelators for the specific binding/removal of actinides in a variety of environmentally relevant situations. Such chelators would be useful to selectively remove actinide ions such as plutonium from a variety of waste forms including soils and waste streams. We have identified a new class of polyhydroxamates as potential chelating agents for actinides based on computer modeling, solubility properties and other important features including ease of synthesis. Several members of this class of tetrahydroxamate chelators have been synthesized in our laboratory and evaluated for the binding of actinides and other metal ions in solution. Some of the hydroxamate chelators that we have developed have also been evaluated for their plutonium(IV) binding and the results are very encouraging. Detailed studies of the complexation behavior of this class of chelators are currently in progress and the goal of these experiments is to develop an understanding of the efficiency and nature of the metal complexation chemistry. This in turn should allow the further modification of this class of chelators to obtain agents with higher specificity for the actinide ions.
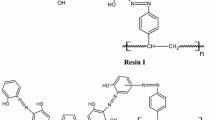
Another major goal of this program is to develop polymer supported, ion specific extraction systems for removing actinides and other hazardous metal ions from wastewaters. Selected ligands from our ongoing efforts are being incorporated into polymeric backbones to be evaluated for their abilities to selectively remove the target metal ions from process waste streams. The synthesis of some chelating polymers and results of their preliminary evaluation are described.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Berlin, R. E. and C. C. Stanton. “Radioactive Waste Management”, John Wiley: ew York, 1989.
U. S. Department of Energy Office of Environmental Restoration and Waste Management, DOE/EM-0109P, February 1994.
A. S. Gopalan, V. J. Huber, and H. K. Jacobs, in Waste Management: From Risk to Remediation. R. Bhada Ed., ECM, in press and references cited therein.
R.D. Hancock and A.E. Martell, Chem. Rev., 89, 1875, (1989).
K. N. Raymond, G. E. Freeman, and M. J. Kappel, Inorg. Chim. Acta, 94, 193, (1984).
M. Streater, P.D. Taylor, R.C. Hider, and J. Porter, J. Med. Chem., 33, 1749, (1990).
P.S. Dobbin, and R.C. Hider, Chemistry in Britain, 565, (1990).
G. Wilkinson, R. D. Gillard and J. A. McLeverty, Eds. Comprehensive Coordination Chemistry, Pergamon Pres: NY, 1987, Vol 1-6.
K. N. Raymond and P. W. Durbin, Proceedings of the First Hanford Separation Science Workshop, July 23–25, 1991, Richland Washington, II.15, (1993).
P. Yakirevitch; N. Rochel, A. M. Albrecht-Gary; J. Libman, and A. Shanzer, Inorg. Chem. 32, 1779, (1993).
K. N. Raymond and T. M. Garrett, Pure and Appl. Chem. 60, 1807, (1988).
M.J. Miller, Chem. Rev., 89, 1563, (1989).
W. L. Smith and K. N. Raymond, J. Am. Chem. Soc, 103, 3341, (1981).
R.J. Bergeron, S.J. Kline, J.D. Navratil, and C.M. Smith, Radiochimica Acta, 35, 47 (1984).
F.L. Weitl, K.N. Raymond, W.L. Smith, and J.R. Howard, J. Am. Chem. Soc, 100, 1170, (1978).
F.L. Weitl and K.N. Raymond, J. Am. Chem. Soc, 102, 2289, (1980).
M. J. Kappel, H. Nitsche, and K. N. Raymond, Inorg. Chem. 24, 605, (1985).
L. C. Uhlir, P. W. Durbin, N. Jeung, and K. N. Raymond, J. Med. Chem., 36, 504, (1993).
J. Xu, T. D. P. Stack, and K. N. Raymond, Inorg. Chem., 31, 4903, (1992).
A. Hou, D. W. Whisenhunt, Jr., J. Xu, and K. N. Raymond, J. Am. Chem. Soc. 116, 840, (1994).
A. Gopalan, O. Zincircioglu and P. Smith, Radioactive Waste Management and the Nuclear Fuel Cycle Journal, 17/3-4, 161, (1993).
W. R. Harris and K. N. Raymond, J. Am. Chem. Soc, 101, 6534, (1979).
A. E. Martell, R. M. Smith, and R. J. Motekaitis. NIST Critical Stabilitiy Constants of Metal Complexes Database. 1993.
A. Gopalan, V. Huber, O. Zincircioglu, and P. Smith, J. Chem. Soc, Chem. Commun., 1266, (1992).
J. M. Cleveland, The Chemistry of Plutonium, Gordon and Beach: New York, 1970.
J. J. Katz, G. T. Seaborg and L. R. Morss, Eds. The Chemistry of the Actinide Elements. 2nd Edition, Chapman and Hall: London, 1986.
The iron and plutonium hydrolysis constants (MOH) were not used in the determination of the metal-ligand binding constants or subsequent calculations. Spectrophotometric data did not indicate the presence of these species in detectable concentrations.
Y. Sun and A. E. Martell, Tetrahedron, 46, 2725, (1990).
S. Konetschny-Rapp, G. Jung, K. N. Raymond, J. Meiwes and H. Zähner, J. Am. Chem. Soc, 114, 2224, (1992).
C. Y. Ng, S. J. Rodgers and K. N. Raymond, Inorg. Chem. 28, 2062, (1989).
N. M. Koshti, H. K. Jacobs, P. A. Martin, P. H. Smith, and A. S. Gopalan, Tetrahedron Lett, 35, 5157, (1994).
N. Koshti, V. Huber, P. Smith, and A. S. Gopalan, Tetrahedron, 50, 2657, (1994).
D.C. Sherrington and D. Hodge, Eds. Synthesis and Separation Using Functional Polymers, John Wiley: NY, 1988.
A. Warshawsky, Ion Exchange and Sorption Processes in Hydrometallurgy, M. Streat and D. Naden, Eds., John Wiley: NY, 1987, pp 166–225.
C. Kantipuly, S. Katragadda, A. Chow, and H.D. Gesser, Talanta, 37, 491, (1990).
K. Geckler, G. Lange, H. Eberhardt, and E. Bayer, Pure and Appl. Chem., 52, 1883, (1980).
C. Calmon, J. Am. Water. Work Assc, 73, 652, (1981).
P. Hodge and D. C. Sherrington, Eds. Polymer-supported Reactions in Organic Synthesis, John Wiley: NY, 1980.
E. Marechal, Comprehensive Polymer Science, G. C. Eastmond, A. Ledwith, S. Russo, and P. Sigwalt, Eds., Pergamon Press: NY, 1989, Vol. 6, pp 1–47.
F. G. Thorpe, New Methods of Polymer Synthesis, J. R. Ebdon, Ed., Blackie & Son Ltd.: ondon, 1991, pp 139–161.
T. Hirotsu, S. Katoh, K. Sugasaka, M. Sakuragi, K. Ichimura, Y. Suda, M. Fujishima, Y. Abe and T. Misonoo, J. Polym. Sci. Part A: Polym. Chem. 24, 1953, (1986).
F. Vernon, Pure and Appl. Chem., 54, 2151, (1982).
C. Y. Liu, M. J. Chen, N. M. Lee, H. C. Hwang, S. T. Jou and J. C. Hsu, Polyhedron, 11, 551, (1992).
A. S. Gopalan, P. Smith, G. Jarvinen and D. Ford, unpublished results.
The effect of metal ion hydrolysis on the shapes of these titration curves has not yet been determined.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1995 Springer Science+Business Media New York
About this chapter
Cite this chapter
Gopalan, A. et al. (1995). Synthesis and Evaluation of Polyhydroxamate Chelators for Selective Actinide Ion Sequestration. In: Nash, K.L., Choppin, G.R. (eds) Separations of f Elements. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-1406-4_7
Download citation
DOI: https://doi.org/10.1007/978-1-4899-1406-4_7
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4899-1408-8
Online ISBN: 978-1-4899-1406-4
eBook Packages: Springer Book Archive