Abstract
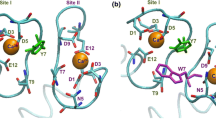
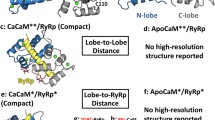
Several peptides exhibit calcium-dependent high affinity binding (dissociation constant ≃10-9M) to calmodulin (CaM), the ubiquitous calcium binding protein (Malencik and Anderson, 1983; Malencik & Anderson, 1984; Cox et al., 1985). These peptides, such as the three mastoparans, competitively displace enzymes (e. g. myosin light chain kinase) off the 1:1 CaM-enzyme complex to form the CaM-peptide complex (Malencik and Anderson, 1983). Consequently they are considered to be viable structural models for the CaM-interacting surfaces on the CaM-dependent enzymes. It was proposed independently by us and by others, that the ability to form a basic amphiphilic helix is probably the common driving force for these peptides to bind to CaM (Cox et al., 1985; McDowell et al., 1985). The purpose of this study was to probe the environment of the CaM-interacting region of some amphiphilic peptides by using the fluorescence signal from their single tryptophan (Trp) residues (CaM does not contain Trp). The single letter amino acid sequences of these peptides are shown in Table 1. With the exception of melittin the Trp in each peptide forms a part of the hydro-phobic face of the putative helical wheel (not shown, see McDowell et al., 1985). They all have large helical hydrophobic moments (Eisenberg et al., 1982) and difference circular dichroic (CD) spectra suggest that a high degree of helicity is induced in the peptides upon CaM binding (Maulet and Cox, 1983; McDowell et al., 1985).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Cox, J. A., Comte, M., Fitton, J. E., and Degrado, W. F., 1985, J. Biol. Chem., 260:2527
Eisenberg, D., Weiss, R. M., and Terwilliger, T. C., 1982, Nature (London), 299:371.
Gratton, E., and Barbieri, B., 1986, Spectroscopy, 1:28.
Gratton, E., Jameson, D. M., and Hall, R. D., 1984, Ann. Rev. Biophys. Bioeng., 13:105.
Lakowicz, J. R., and Weber, G., 1980, Biophys. J., 32:591.
Lakowicz, J. R., Maliwal, B. P., Cherek, H., and Baiter, A., 1983, Biochemistry, 22:1741.
Malencik, D. A., and Anderson, S. R., 1983, Biochem. Biophys. Res. Commun., 114:50.
Malencik, D. A., and Anderson, S. R., 1984, Biochemistry, 23:2420.
Maulet, Y., and Cox, J. A., 1983, Biochemistry, 22:5680.
McDowell, L., Sanyal., G., and Prendergrast, F. G., 1985, Biochemistry, 24:144.
Sanyal, G., Charlesworth, M. C., Ryan, R. J., and Prendergast, F. G., 1987, Biochemistry, 26:1860.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1989 Plenum Press, New York
About this paper
Cite this paper
Sanyal, G., Prendergast, F.G. (1989). Tryptophan Fluorescence as a Structural Probe of Interaction of Amphiphilic Peptides with Calmodulin. In: Jameson, D.M., Reinhart, G.D. (eds) Fluorescent Biomolecules. Springer, Boston, MA. https://doi.org/10.1007/978-1-4684-5619-6_35
Download citation
DOI: https://doi.org/10.1007/978-1-4684-5619-6_35
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4684-5621-9
Online ISBN: 978-1-4684-5619-6
eBook Packages: Springer Book Archive