Abstract
This paper summarizes three lines of experimental evidence showing that crossbridge interaction affects calcium sensitivity and probably also affects calcium binding. Evidence is presented that this is a true hysteresis, not just a slow approach to equilibrium.
-
1)
In barnacle single muscle fibers injected with aequorin to monitor Intracellular Ca, a long duration stimulus under voltage clamp conditions can produce a long duration calcium transient and force record which both approach steady levels. However, if the stimulus is briefly elevated to transiently produce a higher force early in the contraction, the same steady state Ca level can eventually maintain a higher steady force. Thus Ca sensitivity is modified.
-
2)
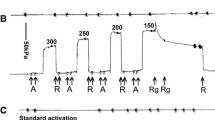
In “skinned” barnacle muscle activated by Ca in the presence of buffered Ca, MgATP, and pH, force was measured in split, detergent treated fiber while it was transferred consecutively between solutions which were relaxing, submaximal contracting, maximal contracting, the same submaximal contracting, and finally relaxing. The submaximal contracting solution produced more force when stepping down in Ca concentration (as in relaxation) than when stepping up. Extending the time in the initial submaximal contracting solution did not result in more force. Thus, the force-pCa relationship shows marked hysteresis. The same phenomenon was seen in frog and mammalian muscle fibers. These experiments confirm the findings that contraction modifies Ca sensitivity.
-
3)
In the barnacle single muscle fiber preparation (under both voltage clamp and controlled length conditions), phasic (400 msec) depolarization leads to a calcium transient and a twitch contraction. Releasing the muscle to allow it to shorten rapidly during the declining phase of the calcium transient causes the force to fall and leads to extra Ca in the sarcoplasm. Rapidly stretching the muscle produces the opposite effect. The extra Ca probably comes from a myofilament Ca activating site. Thus, a length change (force change) affects Ca binding.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Adelstein, R.S. and Eisenberg, E. (1980). Regulation and kinetics of the actin-myosin-ATP interaction. Ann. Rev. Biochem. 49: 921–956.
Allen, D.G. and Kurihara, S. (1982). The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J. Physiol. 27: 79–94.
Ashley, C.C. and Moisescu, D.G. (1977), Effect of changing the composition of the bathing solutions upon the isometric tension-pCa relationship in bundles of crustacean myofibrils. J. Physiol. 270: 827–652.
Brandt, P.W., Cox, R.N. and Kawai, M. (1980). Can the binding of Cat+ to two regulatory sites on troponin C determine the steep pCa/tension relationship of skeletal muscle? Proc. Natl. Acad. Sci. USA 77: 4717–4720.
Bremel, R.D. and Weber, A. (1972). Cooperation with actin filament in vertebrate skeletal muscle. Nature 238; 97–101.
Ebashi, S. and Endo, M. (1968). Calcium ion and muscle contraction. Prog. Biophys. Mol. Biol. 18: 123–183.
Fabiato, A. and Fabiato, F. (1978). Effects of pH on the myofilarnents and the sarcoplasmic reticulum of skinned cells from cardiac and skeletal muscles. J. Physiol. 276: 233–255.
Fuchs, F. (1977). The binding of calcium to glycerinated muscle fibers in rigor. The effect of filament overlap. Biochim. Biophys. Acta 491: 523–531.
Godt, R.E. (1974). Calcium-activated tension of skinned muscle fibers of the frog: dependence on magnesium adenosine triphosphate concentration. J. Gen. Physiol. 63: 722–739.
Gordon, A.M. and Ridgway, E.B. (1978). Calcium transients and relaxation in single muscle fibers. Eur. J. Cardiol. 7: 27–34.
Hellem, D.C. and Podolsky, R.J. (1969). Force measurements in skinned muscle fibres. J. Physiol. 200: 807–819.
Kerrick, W.G.L., Secrist, D., Coby, R. and Lucas, S. (1976). Development of difference between red and white muscles in sensitivity to Caz+ in rabbit from embryo to adult. Nature 260: 440–441.
Murray, J.M. and Weber, A. (1981). Competition between tropomyosin and myosin and cooperativìty of the tropomyosin-actin filament. In: The Regulation of Muscle Contraction. A.D. Grinnell and M.A.B. Brazier (Eds.), New York: Academic Press.
Ridgway, E.B. and Ashley, C.C. (1967). Calcium transients in single muscle fibers. Biochem. Biophys. Res. Comm. 29: 229–234.
Ridgway, E.B., Gordon, A.M. and Martyn, D.M. (1983) Hysteresis in the force-pCa relationship in muscle. Science, in press.
Robertson, S.P. and Kerrick, W.G.L. (1979). The effects of pH on Caz+ -activated force in frog skeletal muscle fibers. Pflügers Archiv 380: 41–45.
Taylor, E.W. (1979). Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit. Rev. Biochem. 6: 103–164.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1984 Plenum Press, New York
About this chapter
Cite this chapter
Gordon, A.M., Ridgway, E.B., Martyn, D.A. (1984). Calcium Sensitivity is Modified by Contraction. In: Pollack, G.H., Sugi, H. (eds) Contractile Mechanisms in Muscle. Advances in Experimental Medicine and Biology, vol 37. Springer, Boston, MA. https://doi.org/10.1007/978-1-4684-4703-3_49
Download citation
DOI: https://doi.org/10.1007/978-1-4684-4703-3_49
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4684-4705-7
Online ISBN: 978-1-4684-4703-3
eBook Packages: Springer Book Archive