Abstract
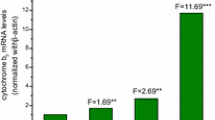
α-Naphthoflavone (7,8-benzoflavone [ANF])(see Figure 1) has been reported to elicit multiple effects with regard to the mixed-function oxidase enzymes that metabolize drugs, steroids, and xenobiotics (1). These effects -- enzyme induction, enzyme inhibition, and enzyme activation -- seem to occur by different mechanisms in the mammalian species studied. ANF has been reported to be a less effective inducer of cytochrome P-448-mediated hepatic mixed-function oxidases in rats than its isomer ß-rnaphthoflavone (BNF) (2). ANF is a potent inhibitor of 3-methylcholanthrene (3-MC)-induced or BNF-induced rat liver microsomes (cytochrome P-448) and was first described by Diamond and Gelboin (3) and Wiebel et al. (2). In addition, ANF is found to have no inhibitory effect on the mixed-function oxidases from phenobarbital (PB)-induced rat liver (cytochrome P-450) (2,4). In fact, ANF stimulates or activates these enzymes (2,4). This activation phenomenon is also observed in hepatic preparations from untreated rats, mice, rabbits, and humans (1). The mechanisms by which ANF activates or inhibits the mixed-function oxidases were of interest to us, and we began a series of investigations to try to elucidate them. This paper reviews these previous and present investigations and presents new findings that may help to explain the multiple effects of ANF observed in mammalian hepatic tissues.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Wiebel, F.J. 1980. Activation and inactivation of carcinogens by microsomal monooxygenase: Modification by benzoflavones and polycyclic aromatic hydrocarbons. In: Carcinogenesis, A Comprehensive Survey, Volume 5. T.J. Slaga, ed. Raven Press: New York. pp. 57–84.
Wiebel, F.J., J.C. Leutz, L. Diamond, and H.V. Gelboin. 1971. Aryl hydrocarbon benzo[a]pyrene hydroxylase in microsomes from rat tissues differential inhibition and stimulation by benzoflavones and organic solvents. Arch. Biochem. Biophys. 144: 78–86.
Diamond, L., and H.V. Gelboin. 1969. Alpha-naphthoflavone: An inhibitor of hydrocarbon cytoxicity and microsomal hydroxylase. Science 166: 1023–1025.
Nesnow, S. 1979. A preliminary structure activity study of the mixed-function oxidase inhibitor 7,8-benzoflavone. J. Med. Chem. 22: 1244–1247.
Mahal, H.S., and K. Venkataraman. 1934. Synthetical experiments in the chromone group part XIV. The action of sodamide on 1-acyloxy-2-acenaphthones. J. Chem. Soc. 1767–1769.
van der Hoeven, T.A., D.A. Haugen, and M.J. Coon. 1974. Preparation and properties of partially purified cytochrome P-450 and reduced nicotinamide adenine dinucleotide phosphate-cytochrome P-450 reductase from rabbit liver microsomes. J. Biol. Chem. 249: 6302–6310.
Lowry, 0.H., N.J. Rosebrough, A.L. Farr, and R.J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275.
Nesnow, S., and H. Bergman. 1981. Metabolism of a-naphthoflavone by rat liver microsomes. Cancer Res. 41: 2621–2626.
Nesnow, S., W.E. Fahl, and C.K. Jefcoate. 1977. An improved radiochemical assay for benzo[a]pyrene monooxygenase. Anal. Biochem. 80: 258–266.
Wiebel, F.J., and H.V. Gelboin. 1975. Aryl hydrocarbon (benzo[a]pyrene) hydroxylases in liver from rats of different age, sex, and nutritional status. Biochem. Pharmacol. 24: 1511–1515.
Omura, T., and R. Sato. 1964. The carbon monoxide binding pigment of liver microsomes 1. Evidence for its hemoprotein nature. J. Biol. Chem. 239: 2370–2378.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1983 Plenum Press, New York
About this chapter
Cite this chapter
Nesnow, S. (1983). Multiple Effects and Metabolism of α-Naphthoflavone in Induced and Uninduced Hepatic Microsomes. In: Langenbach, R., Nesnow, S., Rice, J.M. (eds) Organ and Species Specificity in Chemical Carcinogenesis. Basic Life Sciences. Springer, Boston, MA. https://doi.org/10.1007/978-1-4684-4400-1_17
Download citation
DOI: https://doi.org/10.1007/978-1-4684-4400-1_17
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4684-4402-5
Online ISBN: 978-1-4684-4400-1
eBook Packages: Springer Book Archive