Abstract
The excellent additive systems for acid copper sulfate bath developed in the 1960s successfully produce bright copper deposits with smooth surfaces and high ductility. Since then, many applications of copper plating were developed for electronic device and through-hole plating for PCBs as well as conventional decorative plating on steel, electroforming, etc.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Additive Chemistry
The excellent additive systems for acid copper sulfate bath developed in the 1960s successfully produce bright copper deposits with smooth surfaces and high ductility. Since then, many applications of copper plating were developed for electronic device and through-hole plating for PCBs as well as conventional decorative plating on steel, electroforming, etc. In 1997, the Copper Damascene Process was developed in improved interconnect material fabrication of ULSIs by IBM. With this turning point, the role of addition agents came under further study using advanced analytical techniques such as Enhanced Raman Spectrometry, QCM, AFM, TEM, EIS, in addition to conventional electrochemical plating research methods.
Addition agents in acid copper sulfate bath consist of suppressors, levelers, and accelerators. The former suppress copper deposition on convex regions, and the latter catalyze copper deposition on concave regions in the presence of a small amount of Cl− ions [1–4], resulting in via/trench copper. In the following sections, the roles of the addition agents will be described on the basis of knowledge accumulated by many researchers.
2 Role of Suppressors
PEG or PEG/PPG copolymers of nonionic surfactants and cationic dyes such as JGB containing nitrogen atoms are widely used as representative suppressors and levelers, respectively. Both of them exert a strong suppression effect on copper deposition reactions in the presence of Cl− ions. The suppressor acts in a relatively wide copper deposition current region, the leveler plays the role of a surface leveling agent in the low deposition current region, less than a few mA/cm2 [5]. For the suppression mechanism by surfactants of the PEG family, many researchers have studied it in detail since the mid-1980s, and it has basically become clear [6–29]. The action of levelers on copper deposition is under fundamental study for application to via/trench or through-hole copper filling [30–36].
2.1 The Role of Peg in Bright Copper Plating by the Hull-Cell Test
The Polyoxyethylene family of surface active agents, used as indispensable additives in various metal plating baths, play a decisive role in bright copper plating, through-hole plating, and on-chip wiring copper plating. Kardos [1] indicated in his patent drawing that the appropriate combination of three components, S containing compound, N containing compound, and ethylene oxide polymer give good bright copper plating in the presence of Cl− ions. Mirkova et al. [4] reported that PEG or PPG have a central function in leveling during bright copper plating. In addition, the authors [5] presented evidence that the suppression action of PEG on the copper deposition reaction emerges only in the presence of Cl−. Both PEG and Cl− are basic components of plating baths for bright copper plating as confirmed by Hull-Cell patterns and potential-current curve measurements. That is, bright copper plating can be obtained over the entire region of deposition current density by addition of appropriate amounts of sulfur compounds and N containing compounds only in the presence of both PEG and Cl−.
A Hull-Cell test is the practical method for control of plating baths, by which the surface appearance of plating over the wide current density region can be observed in a single plating operation with suitable cell current and plating time [37, 38]. A Hull-Cell is a trapezoidal cell in which a diagonal cathode substrate is placed opposite the anode as shown in Fig. 2.1. Plating conditions and addition agents used in the Hull-Cell experiments are noted in this figure.
Hull Cell for visual evaluation of copper plating over the wide range of deposition current. Cell volume; 267 mL, Temp; 30 °C, Electric quantity; 2A × 8 min. Bath composition; 0.4 MCuSO4 + 0.5 MH2SO4 Brightener components; PEG (M.W.: 3,300 suppressor); 1.6 μM ~ 1.0 mM 2-Mercaptobenzothiazole-S-Propanesulfonic acid (accelerator); ~2.0 mM Janus Green B (a derivative of safronic dye) (leveler); ~80 μM Cl− ; ~16 mM
Figure 2.2 shows the changes in Hull Cell patterns of copper plating with POE/POP (P) and JGB (J), in the presence of S and Cl− with constant concentrations. These patterns are found to have a tendency to widen the nodule (patchy) area with increasing J component, while having the tendency to narrow the nodule area into the high current density region with increasing P concentration. Finally, when P is 0.026 mM, and J is 0.004 mM, the total area of the Hull Cell panel came to have a bright copper surface. It is interesting to know that nodule formation behavior is controlled by the concentration ratio of P–J.
Figure 2.3 shows the changes in Hull Cell patterns with Changing Cl− concentration in baths which contain either P alone or P + S + J as additives in, respectively. In both baths, the nodule area in the current density region of 10–40 mA/cm2 at low Cl− concentration is narrowed into a high current density region with increasing Cl−. Although the patterns from the plating bath with only P are not brightened, the fundamental characteristics of changes in nodule formation patterns with Cl− concentration are very similar to the ones with three components, P + S + J. By observing the Hull Cell pattern changes carefully, it is found that P and Cl− are required to obtain bright copper plating, and S and J seem to contribute to brightening of the surface in the presence of P and Cl−.
Figure 2.4 shows SEM images of the surface morphology obtained at 20 mA/cm2 with varying Cl− concentration. Nodules as large as sub-mm meter size are observed at 0.5 mM Cl−. It is important to note that such nodules deposited at high current density can exist together with a relatively smooth surface at low current density on the same substrate. Similar surface morphologies were observed for the solution with PEG and nonionic surfactants of the PEG family such as PEG-PPG (polyoxyethylene-polyoxypropylene glycol), PNPE (Polyoxyethylen-nonylphenilether), POE (polyoxyethylene-oreilether), and so on [8]. In recent years, it has been reported that copper filling of via/trench in nanometer to micrometer size can be achieved by copper plating with a single PEG or Diallylamine-Type polymer Additive [30–35]. The filling is considered to be caused by the emergence of “two stable states” for copper deposition on the same surface [36]. It would be a closely linked phenomenon to nodule formation in Hull-Cells as described above.
2.2 Suppression Behavior of Copper Deposition and Dissolution by PEG
Many researchers have confirmed the suppression behavior of PEG in copper deposition and dissolution reactions by electrochemical methods [6–15]. To determine the effect of PEG on the cupper deposition and dissolution reactions in an acid copper sulfate bath, potential-current curves were measured for various PEG of different molecular weights in the absence or presence of Cl− ions [7]. Figure 2.5 shows a typical result for PEG of Mn = 20,000. For the solution without Cl−, shown as a dotted line, an abrupt increase in deposition current is observed at a potential of about 0.15 V versus SHE in the sweep direction from anodic to cathodic potential. The sudden decrease in the current was also found at ca. +0.17 V versus SHE in a reverse direction sweep. In the anodic polarization region no abrupt change appeared with a potential sweep, although the anodic current was suppressed.
While, for the Cl− containing solution, the reaction currents were suppressed over the entire potential range measured in this experiment. Even in a potential range more negative than ca. 0.15 V versus SHE, the suppression of copper deposition was maintained, and the Tafel relationship is observed in the polarization-current curves. The potential region where the reaction (B) Cu + ⇔Cuad is kept in a quasi reversible state, is also demonstrated in Fig. 2.5. Interestingly, it coincides nearly with the potential region where deposition current is suppressed considerably.
The characteristics of potential-current curves for the other PEGs were almost the same as the ones observed in Fig. 2.5 with the exception that lower molecular-weight PEG gave rise to weaker suppression. Also, similar polarization behavior was observed with solutions containing PPG or triblock copolymers of PEG-PPG [29].
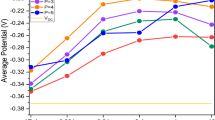
The rest of the potentials (Er) of the copper electrode, obtained immediately after cut-off of the potentiostatic electrolysis, were plotted against the set potentials as in Fig. 2.6. For the plating bath without Cl−, Er shifted gradually in the cathodic direction to give the most negative value of ca. +0.23 V versus SHE (−65 mV versus Cu2+/Cu) with variation of the set potentials in the cathodic direction from 0.36 V to 0.15 V versus SHE (+60 to −150 mV versus Cu2+/Cu), and abruptly shifted back to the potential of about 0.29 V, nearly equilibrium potential of Cu2+/Cu, at set potentials more negative than +0.15 V versus SHE. In the opposite direction, cathodic to anodic, a sudden change in Er was observed at ca. +0.18 V versus SHE. For the copper plating bath containing Cl−, no abrupt change in Er was observed.
Comparing the curves in Figs. 2.5 and 2.6, the change in currents representing copper deposition and dissolution suppression corresponds to the changes in Er with the electrode potential change. The larger the suppression rate, the more negative the Er was. The Er observed after cutoff of electrolysis reflects the situation of copper surface adsorbed by PEG under electrolysis.
To determine the PEG adsorption behavior on copper surface depending on electrode potential, double-step electrolysis was conducted with two consecutive plating baths. Figure 2.7 shows the current–time curves measured with the copper electrode at various set potentials of 0.14, 0.2, and 0.35 V versus SHE in the second CuSO4 + H2SO4 bath free of PEG, which had been electrolyzed in the first CuSO4 + H2SO4 bath containing PEG and Cl−. With the second bath containing Cl−, the reaction current at +0.14 V versus SCE increased slowly in the early stage of the electrolysis, reaching the value with a PEG free bath. At +0.2 V versus SHE, the current remained constant at a small value specific to the PEG containing bath. The current at +0.35 V was also maintained at the suppressed value, even though the current increased gradually with time.
With the Cl− free second bath, PEG has no suppression effect on the reaction current at +0.14 V versus SHE, while the current at +0.2 V and +0.35 V versus SHE increased drastically in the early stage of electrolysis and the suppression effect disappeared.
The changes in curves suggest that PEG molecules adsorb strongly on the copper surface in the potential range more positive than +0.15 V in the presence of Cl−, where the reaction (B) Cu + ⇔Cuad is kept in a quasi-reversible state. Cu+ ions of the reaction (B) are expected to play an important role in PEG adsorption on the copper surface.
Similar experiments were conducted in more detail by Kelly et al. [14], Willey et al. [27], Huerta et al. [28]. Strong suppression of copper deposition was observed in the presence of Cl− and Cu2+ together and they pointed out that adsorbed Cl− ions on copper surfaces have high freedom of desorption and adsorption.
2.3 Effect of PEG Concentration and Molecular Weight on Copper Deposition and Dissolution
Figure 2.8 shows the effect of PEG concentration on the copper deposition and dissolution currents at an electrode potential of ca 0.13 V versus SHE (η = −160 mV) and 0.35 V versus SHE (η = +60 mV) as a function of PEG molecular weight, which changed from 200 to 20,000. Regardless of the direction of the reaction current, currents were suppressed gradually by increasing the concentration, the higher the molecular weight, the greater the suppression.
Deposition and dissolution currents at a PEG concentration of 0.02 g/l were plotted against the PEG molecular weight, i.e., polymerization degree, as in Fig. 2.9. In both cases, the reaction currents measured at η = −160 mV, and η = +60 mV decreased drastically at the polymerization degree of 7–10, and to reach the critical minimum values with increasing polymerization degree.
Figure 2.10 shows the rest potentials (Er) of the copper electrode immediately after cutoff of electrolysis plotted against the polarization current, which were obtained with copper plating baths containing PEG of various molecular weight and in different concentrations. In the case of copper deposition at η = −160 mV, a linear relationship with a slope of 30 mV/decade between the Er and deposition current was obtained independent of the molecular weight of PEG. The results indicate that the polymerization degree and the concentration of PEG do not affect the copper deposition mechanism, but reduces the Cu2+ activity in the vicinity of the copper surface. From the most negative value of Er, the Cu2+ activity is expected to become as small as a hundredth part. In the case of copper dissolution, the changes in Er are very small comparing with those observed in copper deposition. This would be caused by the one-sided flow of reaction species and the loosely adsorbed PEG film on the copper surface.
Based on the experimental results, the authors developed a hypothesis for current suppression by PEG in terms of cation seizing by the PEG molecule [7]. That is, PEG having enough ether oxygen to form a pseudo crown would grasp the copper ions inside and adsorb on the copper surface electrostatically as a poly-cation, resulting in suppression of copper deposition and dissolution. Papke et al. [39] had reported that linear polyether has a helical conformation with a 0.26 nm inside diameter to react with alkali metals. The molecular model is one where the tunnel is lined with oxygen atoms with the proper orientation for coordination to cations inside the helix. Crown ethers had also been found to complex with alkaline cations [40].
2.4 Adsorption and Suppression Mechanism
Figure 2.11 shows a PEG adsorption model depending on electrode potential in the absence and presence of Cl− proposed by the authors. The PEG grasping Cu2+ or Cu+ ions with the formation of pseudo-crown moieties, positively charged, are attracted to the copper surface and/or coordinated to the negatively charged chloride ions adsorbed on the copper surface. In the potential region more positive than +150 mV versus SHE, where reaction (B) Cu + ⇔Cuad is in a reversible state, Cu+ ions existing in a large amount keep the PEG positively charged by being wrapped in it. While, in the potential region more negative than the definite potential values, the Cu+ in PEG is reduced to Cu in one direction to liberate the PEG into the electrolyte. In the presence of Cl−, Cu+ ions grasped in PEG moieties coordinate to Cl− ions on the copper surface, and keep the PEG in the adsorbed state. Cu2+ ions wrapped in PEG would be reduced to Cu+ by chloride bridging to the copper surface.
In this model, when the Cl− concentration is less than about 1 mM, too low to form a Cl− adsorbed monolayer, c (2 × 2) Cl, uneven suppression of copper deposition is expected [25] resulting in nodule formation [8].
Since then, many researchers have studied PEG adsorption on copper by various research methods, and presented experimental evidence for the chemical identity of the adsorbed species and for the PEG adsorption model.
Kang et al. [23] reported no inclusion of C and Cl− in deposits from PEG and Cl− bath by SIMS analysis. They also observed formation of big pyramidal features in deposits by AFM, and stated that deposition occurs under the organic layer which must float above the growing substrate.
Kelly et al. [14, 15], who studied the adsorption behavior of PEGs in the presence of Cl− by LSV, QCM, and EIS measurements, reported that adsorbed PEG forms nearly a monolayer in the presence of chloride ions, and competes for adsorption sites with Cu2+ ions on the copper surface. Further the introduction of PEG and C1− does not necessitate modifying kinetic parameters from values observed in the presence of Cl− alone.
For the hypothesis of “pseudo-crown ether complex” grasping Cu+ ions inside the ring, Stoychev et al. [9] studied the interaction of poly (ethylene glycol) Mn = 3000 with Cu+ and Cu2+ ions in aqueous-acidic media by measuring specific electrical conductivity, optical density, and the cyclic voltammetric curves in Cu+ and Cu2+ solutions, and suggested the formation of complexes of the {Cu + (–EO–)3·(x − 1)H2o} and {Cu2+(–EO–)4·(y − 1)(H2o)2} types. In view of the considerable differences in the conformation interactions of PEG units in water and nonwater media, they considered another scheme suggested by Suryanarayana et al. [40] According to which, the copper ion coordinates just a single oxygen atom of the PEG unit, while the remaining coordinations are occupied by already situated water molecules. The stoichiometry –EO–/Cu+ = 3 and –EO–/Cu2+ = 4 indicates the –EO– segment length including both the EO-unit coordinated with Cu ions, and its neighbors.
The form and the structure of the ethylene oxide units are strongly influenced by the nature of the solvent [39], and the pseudo-crown helix structures formed between PEG and alkaline metals are established in nonaqueous media and in the solid phase [41]. However, high coordination numbers are not typically found in aqueous Cu(I) chemistry. So, the complete pseudo-crown grasping Cu ions inside the ring proposed by the authors might be unlikely to exist in the plating solution.
Noma et al. [42] detected Cu+-PEG complexes with different chain lengths of n PEG segments from n = 4 to 43 in a copper plating solution in factory operation using Matrix-Assisted Laser Desorption/Ionization-Mass Spectroscopy (MALDI-MS) and showed that Cu+ ions exist in the solution as small complexes and large complexes with PEG. And the latter ones are more stable than the former ones to prevent chelating reactions of Cu+ with BCS (Bathocuroinedisulfonic acid disodium salt).
Further, Feng et al. [22] examined PEG complexes on copper surfaces in an acidic solution containing PEG and Cl− by Surface enhanced Raman spectroscopy (SERS) measurements, and confirmed the formation of a PEG–Cu–Cl species at the surface. On the basis of the spectroscopic data, two models of a three-coordinated Cu center associated with two oxygen atoms one chloride ligand are constructed with the help of calculations as shown in Fig. 2.12.
Structural Model of PEG-Cu+–Cl− complex with reference to the model by Feng et al. [22]. Cu+ ion is associated with two ether oxygen atoms and one Cl− ion adsorbed on copper surface
As stated above, the existence of PEG-Cu+ complexes in the solution and adsorbed PEG–Cu+–Cl− on copper surfaces are deduced from the electrochemical behavior of copper deposition and dissolution reactions, changes in electric conductivity and optical density of the solution, SERS, QCM measurements, and etc. Kondo et al. [18, 19] directly observed PEG adsorbed on copper electrodeposit by scanning electron microscopy (SEM) as shown in Fig. 2.13. The SEM images show the PEG molecules of about 30 nm in diameter absorbed preferentially at the edges of copper macrosteps and inhibiting the lateral growth of copper electrodeposits [16, 42]. The direct observation is a significant result, even though those images were not obtained in situ observation in the plating solution. Jin et al. [17] further investigated the dissolution/deposition of the intermediate complex of copper (PEG–Cu+) by detecting the simultaneous mass change of the electrode during cyclic voltammetric (CV) measurement by using EQCM. They observed two current peaks in the CV curves corresponding to the mass changes, illustrating the generation and consumption of intermediate complex PEG–Cu+–Cl− on the copper electrode. Mass changes depending on the electrode potential are shown in Fig. 2.14.
Field-emission SEM micrograph of the deposited copper surface. Additives are Cl− and PEG. (by Kondo et al. [18])
Measured and calculated (n = 2, reaction Cu/Cu2+) voltmassogram obtained at sweep rate 10 mV/s. in CuSO4 +H2SO4 + PEG + HCl electrolyte. (by Jin et al. [20])
2.5 PEG Adsorption on Coinage Metals
PEG form PEG-Cu+ complex and adsorb on copper surface in the presence of Cl−. However, PEG can adsorb on copper surfaces without Cu+ ions as long as Cl− ions are present in the solution.
Walker et al. [24] examined the adsorption of PEG and Cl− on polycrystalline Cu, Ag, and Au electrodes in the solution of 1.8 M H2SO4 + 88 μM PEG3400 + 1 mM Cl− by in-situ Ellipsometry. They found that PEG adsorption is clearly evident at potentials near or positive of the pzc for all metals only in the presence of Cl−. The thicknesses of the adsorbed PEGs are reported to be 1.30 nm at 0.35 V and 1.21 nm at 0.5 V for Au, 1.6 nm at 0.5 V for Ag, and 0.98 nm at 0 V and 0.85 nm at −0.2 V for Cu. The potential of zero charge (pzc) of Au, Ag, and Cu is 0.25 V, −0.68 V, and −0.35 V, respectively. All the potentials are referred to SHE. They concluded that the presence of Cl− is a necessary and sufficient condition for PEG adsorption, and univalent ions such as Cu+ and Ag+ are unnecessary.
Kelly et al. [14] also confirmed PEG adsorption on Au and Cu in 0.24 M Cu2+ +1.8 M H2SO4 solution by QCM measurement. Doblhofer et al. [21] further demonstrated CuCl formation and dissolution in acid copper sulfate solution with a Cl− concentration of more than 2 mM by EQCM measurement, and showed that the inhibiting layer forms by reaction between the adsorbate-covered copper electrode and PEG. Neither Cu+ nor Cu2+ from the electrolyte are required. Jin et al. [20], by using AFM, confirmed PEG adsorbed in a flat-cone shape with a bottom radius of 15–25 nm and a height of 2–4 nm on polished Cu surfaces after being dipped into the solution of H2SO4 + PEG + HCl at −0.01 V versus SHE for 2000s, as shown in Fig. 2.15.
AFM top view image and section analysis of polished Cu surface after being dipped into H2SO4 + PEG + HCl at −0.01 V versus SHE for 2000s. (by Jin et al. [20])
It has been shown that univalent metal ions, Cu+, are not necessary for PEG adsorption on copper surface, by QCM measurement, Ellipsometric study, and etc., as stated above. However, Huerta et al. [28] obtained interesting results about the character of the adsorption films formed on copper surfaces by conducting systematic double-step electrolysis with two consecutive plating baths. They found that PEG can adsorb in the presence of Cl− in a Cu2+-free solution, but does not strongly inhibit deposition when subsequently immersed in a CuSO4 solution. Only when Cl− and PEGs are present in the solution where Cu2+ is being reduced is strong inhibition observed.
The film adsorbed on copper surfaces in the solution without Cu2+ would be evidently different in structure from the film formed during copper deposition. PEG is expected to be positively charged by H+ ions in acid solution, and adsorbed on negatively charged copper surface by Cl− adsorption.
References
Kardos O, Durham HB, Tomson AJ, Arcilesi DA (1970) Electrodeposition of copper. USP 3542655
Stoychev DS, Vitanova I, Vitanov T Rashkov St (1978) Adsorption of substances acting as brighteners in the electrolytic deposition of copper. Surf Tech 7:427–432
Konishi S, Yokoi M, Goto S, Itabashi S (1978) Effects of bath composition and brightener on leveling of bright copper plating from copper sulfate baths. J Surf Finish Soc Jpn (in Japanese) 29:339
Mirkova L, Rashkov St, Nanev Chr (1982) The levelling mechanism during bright acid copper plating. Surf Tech 15:181–190
Yokoi M, Konishi S (1983) Interactions of Cl− and brightener-components in copper plating from an acid sulfate bath. J Surf Finish Soc Jpn (in Japanese) 34: 434–439
Hill MRH, Rogers GT (1978) Polyethylene glycol in copper electrodeposition on to a rotating disc electrode. J Electroanal Chem 86:179–188
Yokoi M, Konishi S, Hayashi T (1984) Adsorption behavior of polyoxyethyleneglycole on the copper surface in an acid copper sulfate bath. Denki Kagaku 52:218–223
Yokoi M, Konishi S (1984) Effect of polyoxyethylene surfactants on the hardness of copper electrodeposited from an acid copper sulfate bath, J Surf finish Soc Jpn (in Japanese) 35:421–427
White JR (1987) Reverse pulse plating of copper from acid electrolyte; a rotating ring disc electrode study. J Appl Electrochem 17:977–982
Reid JD, David AP (1987) Impedance behavior of a sulfuric acid-cupric sulfate/copper cathode interface. J Electrochem Soc 134:1389–1394
Healy JP, Pletcher D, Goodenough M (1992) The chemistry of the additives in an acid copper electroplating bath: part I. Polyethylene glycol and chloride ion. J Electroanal Chem 338:155–165
Stoychev DS , Tsvetanov C (1996) Behaviour of poly (ethylene glycol) during electrodeposition of bright copper coatings in sulfuric acid electrolytes. J Appl Electrochem 26:741–749
Stoychev DS (1998) On the role of poly (ethylene glycol) in deposition of galvanic copper coatings. Trans Inst Met Finish 76:73–80
Kelly JJ, West AC (1998) Copper deposition in the presence of polyethylene glycol; I. Quartz crystal microbalance study. J Electrochem Soc 145:3472–3476
Kelly JJ, West AC (1998) Copper deposition in the presence of polyethylene glycol; II. Electrochemical impedance specfroscopy. J Electrochem Soc 145:3477–3481
Kelly JJ, Tian C, West AC (1999) Leveling and microstructural effects of additives for copper electrodeposition. J Electrochem Soc 146:2540–2545
Kondo K, Yamakawa N, Hayashi K (2000) Role of damascene via filling additive-morphology evolution, ECS meeting abstracts of toronto meeting No. 358
Kondo K, Yamakawa N, Tanaka Z, Hayashi K (2003) Copper damascene electrodeposition and additives. J Electroanal Chem 559:137–142
Kondo K, Matsumoto T, Watanabe K (2004) Role of additives for copper damascene electrodeposition; experimental study on inhibition and acceleration effects. J Electrochem Soc 151:C250–C255
Jin Y, Kondo K, Suzuki Y, Matsumoto T, Barkey DP (2005) Surface adsorption of PEG and Cl additives for copper damascene electrodeposition. Electrochem Solid-State Lett 8:C6–C8
Doblhofer K, Wasle S, Soares DM, Weil KG, Ertl G (2003) An EQCM study of the electrochemical Copper(II)/Copper(I)/Copper System in the presence of PEG and Chloride ions. J Electrochem Soc 150:C657–C664
Feng ZV, Li X, Gewirth AA (2003) Inhibition due to the interaction of polyethylene glycol, chloride, and copper in plating baths: a surface-enhanced raman study. J Phys Chem B 107:9415–9423
Kang M, Gewirth AA (2003) Influence of additives on copper electrodeposition on physical vapor deposited (PVD) copper substrates. J Electrochem Soc 150:C426–C434
Walker ML, Richter LJ, Moffat TP (2005) In Situ ellipsometric study of PEG/Cl-Coadsorption on Cu, Ag, and Au. J Electrochem Soc 152:C403–C407
Hebert KR (2005) Role of chloride ions in suppression of copper electrodeposition by polyethyleneglycol. J Electrochem Soc 152(5):C283–C287
Ding R, Zhang X, Evans JW, Doyle FM (2006) EQCM study of the influence of copper ions on the adsorption of polyethyleneglycol and bis (sodiumsulfopropyl) disulfide at a copper cathode. ECS Trans 2(3):281–292
Willey MJ, West AC (2006) Microfluidic studies of adsorption and desorption of polyethylene glycol during copper electrodeposition. J Electrochem Soc 153(10):C728–C734
Huerta Garrido ME, Pritzker MD (2008) Voltammetric study of the inhibition effect of polyethylene glycol and chloride ions on copper deposition. J Electrochem Soc 155(4):D332–D339
Gallaway JW, West AC (2008) PEG, PPG, and their triblock copolymers as suppressors in copper electroplating. J Electrochem Soc 155(10):D632–D639
Hayase M, Taketani M, Aizawa K, Hatsuzawa T, Hayabusa K (2002) Copper bottom-up deposition by breakdown of PEG-Cl inhibition. Electrochem Solid-State Lett 5(10):C98–C101
Hayase M, Taketani M, Hatsuzawa T, Hayabusa K (2003) Trenches by consumption of halide ion preferential copper electrodeposition at submicrometer. Electrochem Solid-State Lett 6(6):C92–C95
Dow Wei-Ping, Liu De-Huei, Chun-Wei Lu, Chen Chien-Hung, Yan Jhih-Jyun, Huangb Su-Mei (2011) Through-hole filling by copper electroplating using a single organic additive. Electrochem Solid-State Lett 14:D13–D15
Takeuchi M, Kondo K, Kuri H, Bunya M, Okamoto N, Saito T (2012) Single Diallylamine-Type Copolymer Additive Which Perfectly Bottom-Up Fills Cu Electrodeposition. J Electrochem Soc 159:D230
Hayashi T, Kondo K, Saito T, Takeuchi M, Okamoto N (2011) High-speed through silicon via (TSV) filling using diallylamine additive II. Optimization of diallylamine concentration. J Electrochem Soc 158:D715–D718
Moffat TP, Josell D (2012) Extreme bottom-up superfilling of through-silicon-vias by damascene processing: suppressor disruption, positive feedback and turing patterns. J Electrochem Soc 159(4):D208–D216
Mac Intyre F, Hull RO (1943) Plating test control of plating baths. Proc Am Electroplater’s Soc 30:95
Terakado R, Nagasaka H, Finishing M (1976) A Study of The Electric Current Distribution in Hull Cell, J Surf finish Soc Jpn (in Japanese) 27:676–680
Papke BL, Ratner MA, Shriver DF (1982) Conformation and ion-transport models for the structure and ionic conductivity in complexes of polyethers with alkali metal salts. J Electrochem Soc 129(8):1694–1701
Yanagida S, Takahashi K, Okamura M (1977) Metal-ion complexation of noncyclic poly (oxyethylene) derivatives. I solvent extraction of alkali and alkaline earth metal thiocyanates and iodides. Bull Chem Soc Jpn 50:1386–1390
Suryanarayana D, Narayana PA, Kevan L (1983) Effect of molecular cage size on the motion and coordination of copper (2+) in cross-linked poly (vinyl alcohol) and poly (ethyleneoxide) gels: electron spin echo and electron spin resonance studies. Inorg Chem 2:474–478
Noma H, Koga T, Hirakawa C, Nonaka K, Kaibuki T, Moriyama S (2012) Analysis of Cu(I) in copper sulfate electroplating solution. J Surf Finish Soc Jpn (in Japanese) 63:124–128
Vogt MR, Lachenwitzer A, Magnussen OM, Behm RJ (1998) In-situ STM study of the initial stages of corrosion of Cu (100) electrodes in sulfuric and hydrochloric acid solution. Surf Sci 399:49–69
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Yokoi, M. (2014). Supression Effect and Additive Chemistry. In: Kondo, K., Akolkar, R., Barkey, D., Yokoi, M. (eds) Copper Electrodeposition for Nanofabrication of Electronics Devices. Nanostructure Science and Technology. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9176-7_2
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9176-7_2
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9175-0
Online ISBN: 978-1-4614-9176-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)