Abstract
My interest in the evolution of hearing systems in vertebrates manifested itself in several decades of comparative work on the anatomy and physiology of middle and inner ear hearing organs. Of especial interest were lepidosaurs, particularly the wide variety of lizards, archosaurs, especially song birds and owls, but also mammals. Physiologically, the main tools used were electrophysiological recordings from the auditory nerve and acoustical recordings of otoacoustic emissions. These data were compared to what is known about the ancestors of these groups to reach an overview of not only of how these organs arose, but also their current structure and function. Sensitive, frequency selective, and tonotopically organized hearing epithelia arose, largely independently, in all amniotes and each group shows characteristic structural configurations. They show extensive parallel evolution of features, including in the presence of active processes that led to a clear division of labor between groups of hair cells. Specializations are seen, such as large variations in tectorial membrane structure, extensions of frequency maps, and the presence of an auditory fovea.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
18.1 The Status of Comparative Research in Auditory Science
The book The Evolutionary Biology of Hearing (Webster et al., 1992), which was the publication of papers stemming from a conference, appeared at the same time that the Springer Handbook in Auditory Research series was being launched. The conference provided an excellent opportunity for the first major survey of and stocktaking in the field of comparative auditory physiology. It was very refreshing to confer with colleagues who did not question the validity of working on animals, some of which were quite unrelated to humans, colleagues who took for granted the usefulness of such studies. At that time, all comparative researchers had begun to feel more strongly the pressures of granting agencies and reviewers to work on something “relevant” to the human condition, something that would clearly justify spending public money on its research.
I have at that time, and would definitely still, insist that comparative auditory research should have a firmer place in the broad picture of auditory research, for several reasons. First, there is clear evidence that the results of comparative research support and help interpretations of research on mammals and humans. Given that many nonmammalian vertebrates and invertebrates are physiologically more robust animals than mammals and are frequently the animal of choice for long in vitro experiments, there is a substantial block of basic research—for example, on transduction channels and general hair cell function—that derives from nonmammals. Our current understanding of how hair cells work would be dramatically weaker, were it not for comparative studies. Were it also not for earlier research on auditory specialists such as bats and barn owls, our current understanding of how auditory processing in the brain works would be considerably poorer. Second, the evolutionary processes that have resulted in the extraordinary variety of vertebrate hearing organs and systems have provided us with an invaluable basis for comparisons of structure and function. To ignore this would be self-defeating, but using it, we can learn how complex functions are realized in the inner ear. Third, any physiological system is prone to failure and many experimental procedures have numerous elements that can “go wrong” during an experiment. The direct comparison of results from experiments using mammalian and nonmammalian subjects offers an invaluable control for systematic, hard-to-pin-down errors. Fourth and finally, humans are a powerfully cultural species and our approach to the world is enormously influenced by knowledge. We recognize that knowledge has intrinsic value and should only secondarily expect that this knowledge will, one day, have some sort of direct or indirect economic impact or be useful, for example, for medical procedures. Arguments against the use of animals in research are short-sighted, ignoring the true nature of “Nature” and are also one-sided, ignoring the great benefits to other species with which we share our lives (e.g., Heffner, 1999). Comparative auditory research benefits humans and domestic animals for all the above reasons and does not deserve to be relegated to a position of Cinderella among more “useful” fields.
The question of course arises: To what extent can nonmammals provide useful experimental objects for understanding mammalian and human ears? Any mammalian organ shows conservative features that arose early in evolution and others that arose later. Each of the modern lineages of vertebrates is a mosaic of ancestral and recent characters (Manley, 2010, 2012, 2013). Which features of mammals do we see in nonmammals? Here, we also need to differentiate between organizational levels—though, for example, hair cells themselves are definitely extremely old, a few features of mammalian hair cells are unique. This is seen, for example, in the differentiation into two unique populations that differ from the two populations seen in other groups (Fig. 18.1; Gleich et al., 2004). They also differ at the level of specific proteins and biochemical pathways. Thus, in general, the more detailed the question, the less likely it is that characteristics are the same in all vertebrate groups. Nonetheless, there are only a restricted number of evolutionary solutions to developmental and functional “problems” and the parallels between vertebrate lineages can be striking. One example is the tendency—discussed below—for hair cells to form separate populations differentiated to different functions and displaying analogous anatomical specializations, such as in their innervation. Comparative studies of hearing provide both a much broader foundation and a framework for understanding the principles of hearing. Specific cases of parallelism lead us to better-founded conclusions as to function.
Schematic comparison of the structure of the auditory papilla in different groups of amniotes. Each panel shows a papillar cross section and examples of hair cells. The single hair cell type with both afferent and efferent innervation in the turtle papilla is considered to be ancestral. In most lepidosaurs, such as lizards, there are specialized low- and high-frequency cells with a differentiated innervation. It is now known that in some species at least, there is some efferent innervation to high-frequency cells. Both birds and mammals have evolved two hair cell types that are present at all frequency locations and that have highly specialized innervation patterns. (Modified after Manley, 2000, Copyright (2000) National Academy of Sciences, U.S.A)
For me, 1992 also marked the beginning of the increased use of a then relatively new method for studying the auditory periphery, the measurement of otoacoustic emissions—a technique that was to lead to major advances in our understanding of hearing physiology in all vertebrates. Here, I offer a brief historical review of the development of my understanding of amniote hearing and some perspectives on the future of this field.
18.2 The Background
Prior to the 1992 conference, most of the research on the peripheral auditory physiology of lizards and birds had been summarized in my—at that time relatively new—book (Manley, 1990). That volume reviewed the huge efforts of earlier years to describe the anatomy of lizard (especially by Malcolm Miller and Glen Wever) and bird (Catherine Smith and others) inner ears. It also reviewed physiological studies that had shown that “reptile” ears show an enormous but systematic variety, whereas bird inner ears were more uniform but possessed two hair cell populations that have tantalizing resemblances to those of mammals (Fig. 18.1). (The term “reptile” is placed in quotation marks here because it is a polyphyletic group whose individual lineages are not closely related to each other). While other research groups (under Tom Weiss, working with alligator lizards, Rob Fettiplace, with turtles and Jim Hudspeth, with frog sacculi) had begun to use “reptile” and frog ears to ask fundamental questions regarding hair cell physiology, I was more interested in the evolutionary aspects. How was the huge structural variety, especially in lizard papillae, to be understood? What evolutionary pressures could have led to this variety and what were its functional correlates? What are the parallels and what the differences between the different kinds of auditory papillae? What functions do the independently evolved, different hair cell populations of mammals and birds play and do these functions differ (Manley & Köppl, 1998)?
In the two decades since 1992, I have been involved in many studies to better describe the anatomy and the physiology of lizard, bird, and mammal auditory papillae. Some principles of structure–function relationships became quickly obvious. For example, there is a general evolutionary trend in all amniote groups for the basilar papilla to become longer. This trend is weak in lizards, stronger in birds, and strongest in mammals, resulting in the mammals having the greatest frequency-space constants (length of papilla devoted to one octave; Manley, 1973). Although this presumably increased the number of afferent fibers to each octave, it did not result in mammals showing the sharpest frequency tuning. Curiously, that honor belongs to the birds, followed by geckos and with mammals last (in the same frequency ranges; Manley & Köppl, 1998). In continuing these studies, I built on previous experience, including earlier microelectrode studies of the spontaneous and sound-driven activity of lizard (European wall lizards [Podarcis], Tokay gecko [Gecko], monitor lizards [Varanus], Australian bobtail skinks [Tiliqua]) and bird (chicken [Gallus], starling [Sturnus]) auditory nerve fibers. Otoacoustic emissions, which had made it possible in lizards and birds to undertake very broadly based surveys of function without the necessity of carrying out terminal experiments, of course later strongly confirmed the importance of active processes.
18.3 An Interest in Paleontology
My training as a student at Cambridge included paleontology, and I have maintained a strong interest in studies of fossils. This not only greatly influenced my anatomical and physiological work, beginning with my doctoral thesis, but it has always also served to keep my interests broad. Thus, although I later almost exclusively worked on lizards and birds, some of my work has been on mammals—including early middle ear measurements to very high frequencies in guinea pigs and bats (carried out with Brian Johnstone in W. Australia). Later Eberhard Zwicker and I reported stimulus-frequency otoacoustic emissions (SFOAEs) from guinea pigs. Anges Kronester-Frei and I developed a new technique for direct in vivo observation of the exact position of an electrode in the organ of Corti. Using that technique, Geerd Runhaar and I reported data on the electrophysiological profile of the guinea pig organ of Corti—the only recordings to date carried out using a technique that made it possible to know exactly where the electrode tip was placed in relation to the cellular structures. We reported that there is no endocochlear potential in the inner sulcus, a finding that, since then, has been completely ignored.
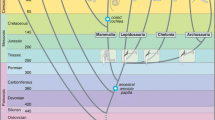
In recent years, I have returned to studying the middle and inner ears of fossil mammals by proxy, using published findings. Recent fossil finds in China and elsewhere, and especially the use of micro-CT scanning of fossils have dramatically increased our knowledge of the evolution of middle and inner ears. My main interest in these fossil data was to be a kind of mediator and collator, bringing the salient findings on the evolution of the structures underlying hearing to the attention of my colleagues in hearing research that lack a training in paleontology. This explains my recently published reviews of the evolution of mammalian middle ears and mammalian cochleae (Manley, 2010, 2012, 2013). It is remarkable to note, although not at all unusual in evolution, that both the mammalian middle and inner ears are partly the result of structural changes initially unrelated to hearing, the advantages of which for hearing only emerged much later in time. Cochlear coiling and high-frequency hearing in mammals emerged only after 100 million years of mammalian evolution and only in the therian lineage (and not, e.g., in the modern egg-laying monotreme mammals or other, now extinct, lineages, such as the Multituberculata; Fig. 18.2). Only in therians did the middle ear become light and suspended in space and did the cochlea become so elongated that coiling emerged as a means of ameliorating the problem of space. In therians also, bone merged into and stiffened cochlear soft tissue and changes in prestins occurred that were clear specializations for high frequency hearing. These three independent features gradually emerged in parallel over the last 100 million years of therian evolution and conferred some mammals—independently from birds and “reptiles”—with excellent hearing (Fig. 18.2; Manley, 2010, 2012, 2013).
Highly schematic diagram of the evolution of mammalian middle ears and cochleae from their common origin in the early Mesozoic, 230 million years ago, from mammal-like Synapsida. The gray shade indicates the developmental stage of the middle ear, light gray indicates a transitional mammalian middle ear (TMME), dark gray a definitive middle ear (DMME). The DMME was reached at different times in the three lineages of mammals. In addition, all lineages began with an approximately 2 mm long cochlea (outline drawing). In the extinct Multituberculata and the egg-laying Monotremate (Platypus and relatives), the cochlea remained more or less straight and never achieved a length of more than 7–8 mm (e.g., outline drawing top left). Only in the therian lineage, which gave rise to the Placentalia and Marsupialia, did the cochlea fully coil after approximately 50 million years of evolution and then continue to elongate independently in these two lineages. (From Manley, 2013, with permission)
18.4 A Huge Resource of Limited Usefulness
Glen Wever—as noted earlier—not only examined inner ear anatomy in a huge range of lizards and related species. He also carried out parallel physiological experiments on most of these species in the form of cochlear microphonic (CM) measurements. This resulted in hundreds of CM audiograms that were purported to indicate auditory thresholds in each species. These measurements would have provided a huge resource of information but for one fatal flaw, an error in thought that makes Wever’s lizard CM data essentially uninterpretable (Manley, 1990). Wever did not take into account the fact that—as he and others had described—in the higher-frequency regions of all lizard species, there are two hair cell populations whose stereovillar bundles are oppositely oriented. This means that at every frequency of sound stimulation, the cm produced by the two cell populations would be out-of-phase over each cycle of a sound wave and thus electrically cancel within the inner ear. Only if one population is larger would there be a residual CM at the stimulus frequency. Thus measurements from frequency ranges processed by two populations of hair cells (that is, above 1 kHz) cannot be compared to measurements at low frequencies in lizards, where the hair cell bundles are—in most but not all species—all oriented in the same direction. Not only are low- and high-frequency CM thus not comparable, but they also cannot be compared across frequencies within one ear or across species, as the patterns of hair cell orientations also differ, both along a given papilla and also strongly between the papillae of different lizard families. An example of the cancellation effect can be seen in a comparison of Wever’s CM data on the one hand and threshold measurements for single auditory nerve fibers in Gecko gecko as collected in my lab on the other. In Wever’s data, the cm response above 1 kHz steadily loses sensitivity toward higher frequencies. By contrast, the nerve-fiber responses are sensitive and sharply tuned above 1 kHz, with a clear second sensitivity optimum near 2 kHz. The conclusion can only be drawn that very unfortunately, Wever’s CM data for lizards—the only group with such orientation patterns among amniote vertebrates (Manley, 2004; Manley & Köppl, 2008)—simply cannot be used. There is, however, one exception. The exception is that in a number of species, Wever (generally while working with Yehudah Werner), compared CM measurements before and after severing the middle ear connection. This within-ear control is of course free of hair cell problems and provides very useful and interesting data on the effectiveness of lizard one-ossicle middle ears (e.g., sensitivity improvements of up to 65 dB).
18.5 Remote Sensing: Otoacoustic Emissions
By 1992, our understanding of lizard ears was best in Tiliqua rugosa and in the alligator lizard Gerrhonotus. I and Christine Köppl had recently published a comprehensive study of spontaneous and driven auditory nerve activity in Tiliqua rugosa and, also in cooperation with Brian Johnstone’s auditory lab at the University of Western Australia, followed this up with a look at distortion-product otoacoustic emissions (DPOAEs). DPOAE suppression characteristics in bobtail skinks revealed clear resemblances to the characteristic tuning patterns in the auditory nerve, and this was one of the first indications that OAE can be directly attributed to hair cell activity (Manley & Köppl, 2008). These, and our later DPOAE studies in birds, thus clearly also supported the diagnostic use of these emissions in the medical clinic.
During DPOAE recordings, we also noticed very small peaks in the spectra that were unrelated to our stimuli. These turned out to be very stable spontaneous otoacoustic emissions (SOAE) originating in the higher-frequency area of the bobtail papilla. These SOAE provided the basis of extensive follow-up studies that I carried out with Christine Köppl and that revealed detailed parallels between the characteristics of SOAE, DPOAE, and auditory nerve activity, including the frequency selectivity of emission suppression behavior in the presence of external tones (Fig. 18.3). To a remarkable degree, SOAE could, in spite of being a “remote sensing” technique, reveal details of peripheral sensitivity and tuning selectivity—and I later used this technique to study the auditory periphery in lizard species of a variety of families. In concert with auditory nerve studies, these SOAE data made it possible to understand differences in activity patterns in lizard inner ears on the basis of their anatomy, in particular the papillar size and type of tectorial structures. These in turn made it possible to understand the functional consequences of the wide structural variety of the papilla that had been achieved during lizard evolution (Manley, 2011).
A comparison of different measures of cochlear frequency selectivity or tuning in the bobtail skink (Tiliqua, a–c) and the barn owl (d). In (a), frequency tuning curves are shown for the suppression of four spontaneous otoacoustic emissions by 2 dB. In (b), threshold tuning curves for four single primary afferents are shown. (c) The lowest sound pressure levels at which distortion-product otoacoustic emissions can be detected for, in blue, the product 2f1–f2 and in brown for 2f2–f1. In (d), green curves are tuning curves for single primary auditory afferents, red curves are levels at which individual spontaneous otoacoustic emissions were suppressed by 2 dB. (Partially after Taschenberger and Manley, 1997, Manley and Köppl, 2008; barn owl data kindly provided by Christine Köppl)
18.6 Simple and Complex Lizard Papillae
Basically, it can be assumed that in most lizards, the selective pressures on hearing were not great. As long as a reasonable hearing ability up to a few kilohertz was maintained, in most lizard families it was not very important how the auditory periphery achieved this. Thus even very tiny papillae, with, for example, only approximately 60 hair cells (in, e.g., some iguanid and agamid lizards) were maintained over long evolutionary time periods. All the very small papillae have lost their tectorial membrane (independently in different families; Fig. 18.4c, d). This loss reduced the coupling between hair cells arranged along the papilla and made it possible to code for several octaves of sound frequencies with a very small frequency space constant—at the cost, however, of sensitivity and frequency selectivity (Fig. 18.4e–h; Manley, 2011). The most complex lizard papillae, which presumably result from stronger selection pressures, are found in geckos, the only really vocal lizard family. In geckos, as we now know from studies in Jim Hudspeth’s and Christine Köppl’s laboratories, there is one population of hair cells that completely lacks an afferent innervation (in this case it is the hair cells on the inner or neural side of the papilla). This was suggested to be a parallel evolutionary development to the two hair cell populations of mammals and birds (Chiappe et al., 2007).
Schematic illustration of the influence of the tectorial membrane on threshold and tuning of primary afferent fibers for high-frequency regions of lizard papillae. The hair cells of the bobtail skink (Tiliqua) are covered by a tectorial membrane, shown as a gray block in (a) and (b) for low- and high-frequency fibers, respectively. In the alligator lizard Gerrhonotus (now called Elgaria), there is no tectorial membrane over hair cells with best frequencies above 1 kHz and (c) and (d) illustrate hair cell structures for the 1 kHz and 4 kHz regions, respectively. Panels (e) and (f) show best thresholds and Q10dB sharpness coefficients, respectively, for Gerrhonotus primary afferents, (g) and (h) the same for Tiliqua. The possession of a tectorial membrane improves threshold and raises Q10dB values. (After Manley, 2000, Copyright (2000) National Academy of Sciences, U.S.A.; Manley and Köppl, 2008 and used with permission)
My recent studies with Hanna Kraus of Australian pygopod geckos produced tantalizing suggestions that there might be interactions between hair cell populations, but this is far from being understood (Manley and Kraus, 2010; Manley, 2011). In field studies of the auditory sensitivity of pygopods of the genus Delma, we measured compound action potentials (CAP) forward-masked by narrow-band noise. In these species, as in other geckos, Christine Köppl has shown that all auditory afferents only innervate the outer, postaxial hair cell population. CAP suppression curves derived for tones above about 3 kHz showed two sensitivity maxima, one below 8 kHz (as expected, near the probe frequency) and one above 8 kHz. It is tempting, but as yet without a mechanistic explanation, to assume that the two hair cell populations somehow interact and the noninnervated population induces the sensitivity at high frequencies. In general, geckos show the sharpest frequency tuning of all lizards and are the only lizards—indeed the only amniotes—that have a reversed tonotopic organization. As I have explained in reviews of the evolution of lizard papillae, this is a logical and thus not unexpected result of the derivation of all lizard papillae from an ancestral, tripartite papilla having two (redundant) higher-frequency areas, one at each end (Manley, 2011). A few modern lizard families maintain this organization, but in most lizard families either one or the other of the high-frequency areas was lost.
18.7 Active Processes and Hair Cell Specialization
Jim Hudspeth and his coworkers have suggested that in geckos, the noninnervated hair cells should be regarded as an analogue of mammalian outer hair cells, forming a subunit that has primarily a motor function (Chiappe et al., 2007). We had suggested the same for avian short hair cells (Manley and Köppl, 1998; Manley, 2000). In fact, the tendency to hair cell functional specialization—and therefore modification of their innervation patterns—is more extreme in geckos and in birds, where one hair cell population (the preaxial cells in geckos and the short hair cells in birds) completely lacks an afferent innervation. In mammals, the afferent innervation of outer hair cells is merely strongly reduced, perhaps vestigial. We can assume that ancient hair cells were all both afferently and efferently innervated. If some hair cell populations lost their afferents, it was presumably because their most important function was within the papilla. Mammalian hair cell evolution has thus not proceeded quite as far as evolution has in other lineages (Fig. 18.1). As I first pointed out in 1986, we can thus recognize an evolutionary trend towards receptor cell specialization that occurred independently in various amniote groups (Manley, 2000). This began early in the evolution of the different lineages, and certainly began in the mammal lineage before the evolution of tympanic middle ears and before the extreme specialization of prestins that we see in eutherians (Manley, 2012). Thus hair cell specialization into motor and receptor groups occurred initially at least only on the basis of the stereovillar bundle active process (Manley, 2001).
18.8 The Definitive Localization of an Active Process to the Hair Cell Bundle
In 1992, one of the most discussed issues in auditory research was the question of the mechanisms underlying active processes (Manley, 2001). Whereas on the one hand those studying mammalian ears were understandably excited by somatic motility, some much earlier studies of the fundamental properties of hair cells (Fettiplace’s group in turtle papillae and Hudspeth’s group in frog sacculus) had clearly shown in vitro that hair cell stereovillar bundles spontaneously oscillate, and had localized the motor activity to the transduction complex. The underlying active process is a phylogenetically very old mechanism that almost certainly existed in hair cells of vestibular systems that were the forerunners of auditory hair cells (Manley, 2001). Our own later studies, with Andy Forge, of hair cell membranes in geckos and barn owls, two species that we had shown to produce SOAEs, found no evidence for the presence of dense concentrations of membrane-bound particles (prestin tetrads) that are characteristic of the lateral membranes of outer hair cells and are part of the somatic motor in mammals.
Two active processes had been implicated in 1992, but could it be shown in vivo which one was really operating? It could—and one of our most important results from emission studies was made possible by the fact that, uniquely, in all lizard papillae, hair cell areas exist that, have their stereovillar bundles oppositely oriented. Christine Köppl and I, working together with the late Des Kirk and Graeme Yates in W. Australia, predicted that, were we able to electrically stimulate active processes in lizard hair cells and influence these with low-frequency sound, there would be two possible and clearly predictable patterns in the electrically evoked emissions (EEOAEs; Manley et al., 2001). These patterns would make it clear as to whether the active process itself was to be found in the hair cell bundle or in the cells’ lateral membranes. In the first (bundle) case, EEOAEs generated by the opposite orientations of two sets of hair cell bundles would be out-of-phase and thus essentially cancel each other out within the ear. In the second case (somatic), they would add. In the second case, also, stimulation with low-frequency sound should show no sound-phase–dependent amplitude and phase fluctuations in the EEOAEs. The results of such experiments using Tiliqua were unambiguous (Manley et al., 2001): in the absence of an added, low-frequency sound, high-frequency electrical stimulation produced only tiny EEOAEs, far smaller than predicted based on mammalian studies. In the presence of low-frequency sound, however, EEOAE size increased for each half-cycle of the sound, suggesting that the emissions were emerging from a cancellation process between the hair cell groups. The high-frequency EEOAE components were out-of-phase during the half-cycles of the sound. This was exactly as predicted for the case of an active process that resided in the hair cell stereovillar bundles (Fig. 18.5; review in Manley & Köppl, 2008). Later studies by Hudspeth’s group revealed, of course, that in mammals, both active processes are to be found.
Model predictions for the results of high-frequency current injections into the cochlea of the Bobtail skink Tiliqua. Panels (a) and (b) illustrate the predicted effects of putative cellular motors. In (a), the left figure illustrates a cellular shortening or elongation as the result of a putative motor in the lateral cell membranes. The right panel in (a) shows the putative motor in the stereovillar bundles and, for the same current, opposed in their effects. In (b), waveforms of electrically evoked otoacoustic emissions induced by current injection into scala media are shown for the anatomical situations in (a). Red and green curves (that are in phase and therefore almost completely overlap in the left panel but almost cancel in the left panel of b) are the contributions of hair cells on each side of the papilla. The black curve illustrates the resultant emission, which is large in the left panel and very small in the right panel. (c) Predicted emission curves (blue resultant curve) for the two motor systems, somatic membrane motor on the left and stereovillar motor on the right, when adding a very-low-frequency sound bias of increasing level from top to bottom traces (red curves) during current injection. Whereas the left panels show almost no modulation due to sound, the stereovillar motor emissions shown on the right are very sensitive to sound and, at high sound levels, change their phase 180° for every half-wave of the sound stimulus. (d) A sample of data from an experiment. The results are remarkably similar to the predicted results for the stereovillar motor, including the phase shift (arrow). (Partially after Manley et al., 2001, Copyright (2001) National Academy of Sciences, U.S.A)
In other studies in collaboration with Pim van Dijk, we also showed that the statistical properties of lizard and bird SOAEs indicated that they were, indeed, derived from active processes and were not simply filtered noise. In Anolis, species that have a very small papilla, my group also demonstrated that small SOAE spectral peaks could result from the activity of only two or three hair cells (reviewed in Manley & van Dijk, 2008).
18.9 Calcium and the Evolutionary Consequences of the Loss of the Lagena Macula
Spontaneous hair-bundle activity in lizards was also examined by altering the calcium concentration in vivo in the endolymph of Tiliqua, work carried out in W. Australia with Des Kirk, Christine Köppl, and Ulrike Sienknecht. We either lowered calcium level using BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, a calcium-binding molecule) or lowered or raised it by injecting various concentrations of calcium dissolved in artificial endolymph. These procedures showed that raising calcium levels above about 1 mM led to an increase in the frequency of spontaneous hair cell bundle oscillation, inducing calcium levels below 1 mM led to a fall in oscillation frequency. This was consistent with earlier in vitro results obtained on hair cells of the bullfrog sacculus by Pascal Martin and Jim Hudspeth. Our data also clearly suggested that in bobtail lizards, the endolymph calcium concentration is about 1 mM. Such a high concentration is presumably necessary to maintain the integrity of the otoliths of the lagena macula that lies adjacent to the auditory papilla. In birds and frogs, the concentration is lower, around 250 μM, but still at least 10 times higher than in mammalian endolymph. This interesting fact suggests that when therian mammals lost their lagena macula, it was no longer necessary to maintain such high levels of endolymphatic calcium. It still needs to be studied what—perhaps decisive—consequences this huge drop in calcium concentrations had, for example, on the tectorial membranes, the prestin active mechanism or on the transduction channel micromechanics of therian mammals (Manley, 2012).
18.10 Frequency Maps of the Papilla and the Functions of the Tectorial Membrane
Our earlier mathematical models of the frequency map and micromechanics of the papillae of Tiliqua (with Graeme Yates) and Gecko (with Stephan Authier) were based on detailed anatomical data collected by Christine Köppl. The model predictions not only correlated well with physiological results (e.g., predicting the reversed tonotopic map of geckos), but also emphasized the great importance of the tectorial membrane. Auditory papillae lacking a tectorial membrane showed poorer sensitivity and poorer frequency selectivity than those that had either a continuous tectorial structure or a chain of tectorial sallets (Fig. 18.4). Coupling hair cells via a tectorial membrane both sharpened tuning and improved sensitivity. On the other hand, the lack of hair cell coupling because of the loss of a tectorial membrane enabled species with very small papillae (e.g., <200 μm) and very few hair cells (<100) to have tuned afferents over a broad range of frequencies, but at the cost of poorer selectivity and sensitivity (Manley & Köppl, 2008; Manley, 2011). We also showed that the frequency selectivity in geckos could not be modeled using normal values for endolymph viscosity. Only the—impossible—assumption that the viscosity was about one-tenth of normal produced matching frequency selectivity. This was, of course, equivalent to assuming that an active process driving the stereovillar bundle reduced the effective viscosity, and was thus a proxy for the active process that we had helped identify in lizards. These conclusions regarding tuning and tectorial membranes have been recently confirmed by Chris Bergevin’s studies of SFOAEs in various lizard and mammal species (e.g., Bergevin, 2011).
18.11 “High-Frequency” Hearing in Lizards
OAE studies in lizards from my own and from Bergevin’s lab suggested that some species, including, remarkably, Anolis, with its tiny papilla, have a higher upper frequency limit than expected from earlier data, for example from Gerrhonotus, the alligator lizard. Although frequency tuning in lizards is, of course, temperature sensitive, comparisons at the same temperatures put some upper hearing limits nearer 7 or 8 kHz than the previously known rough limit near 5 kHz. This was supported by the results of my studies with Jakob Christensen-Dalsgaard of the directionality of sound-induced eardrum vibrations of lizards (review in Manley, 2011). Not only did these data indicate that lizards have (species-varying) an extremely effective pressure-gradient middle ear system that provides a strong directionality prior to the sound being detected by the inner ear. The data also showed that small gecko eardrums, for example, still show responses in the 8 kHz range. My recent detailed field study with Hanna Kraus of hearing in a group of legless geckos, the Australian pygopods, indicated that, remarkably, species of the genus Delma show auditory nerve activity up to 13 kHz, which is higher than the upper limit even of birds.
18.12 Hearing in Birds
After I developed the technique of recording avian auditory nerve fiber activity in the cochlear ganglion of the starling in 1977 (review in Manley, 1990), my lab continued to study physiologically avian hearing by using the chicken, especially to examine the development of the frequency map during ontogeny. Even before 1992, Jutta Brix, Alex Kaiser, and I had established that—contrary to published reports—the frequency map of hatchling chickens did not change with age. This was confirmed and extended by Jones and Jones’ remarkable study of embryonic chickens and Dick Salvi’s group’s data on the adult chicken frequency map (Gleich et al., 2004).
In 1994, one other important fact about bird ears was established by anatomical studies carried out in my group by the late Franz Peter Fischer. Fischer demonstrated that, contrary to expectations, in all avian species, a certain—sometimes quite large—population of abneurally located hair cells totally lacked an afferent innervation (Fig. 18.1). Obviously, these hair cells must have a function restricted to the papilla, and this strengthened our concept that these “short” hair cells were in fact motor cells. Fischer proposed that these short hair cells—previously defined arbitrarily on the basis of their shape—could now be specifically defined anatomically through their lack of afferents. Their massive stereovillar bundles presumably pass energy via the tectorial membrane to the “tall” hair cells. Kuni Isawa and Christine Köppl’s recent study of avian hair cell bundles supports this idea, as does recent work from Robert Fettiplace’s lab. Otto Gleich had previously shown in the starling that the most sensitive afferent fibers connect to hair cells close to the neural edge of the papilla (this part of the papilla is not atop the free basilar membrane) and that the sensitivity reduced by 6 dB/ hair cell across the papilla to the middle. Modeling by Charles Steele suggested that in birds, the tectorial membrane could indeed transport motor activity from the short to the tall hair cell region (Gleich et al., 2004).
One small but interesting aspect of the activity of avian auditory nerve fibers that was later also seen in lizard data was the presence of preferred intervals in the spontaneous activity, which—although then controversial—I now see as one of the earliest signs of active processes in the hair cells of nonmammals. It was claimed that these peaks were the result of inadvertent noise stimuli. However, quite apart from our careful checks of the sound system, there were two other good reasons why this could not have been the case. First, the characteristic frequencies of the cells when driven by tonal stimuli did not always correspond to the best frequency as calculated from the preferred intervals, and second, the thresholds of some cells showing this phenomenon were too high to be even contemplated as responding to inadvertent noise. In songbird and chicken data, there is always a wide spread of thresholds (>50 dB) in ears in good condition, the result of different thresholds of the hair cells across the wide papilla. Had there been so much noise artifact, then cells with better thresholds should all have shown even stronger preferred intervals, which was not the case. Thus these data suggested a spontaneous activity at the hair cell level that is at least partly driven by an active process. The coupling of the avian tectorial membrane is strong, however, perhaps making it difficult for the activity of local hair cell groups to be transported into fluid movements, and so far SOAEs in birds have been detected only in the barn owl (Gleich et al., 2004).
18.13 Barn Owls, the Hearing Specialists
Following a research visit with Christine Köppl to Mark Konishi’s lab in 1988, where we had worked on the barn owl (Tyto alba pratincola, now known as Tyto furcata) auditory brain stem, we established our own colony of European barn owls in order to carry out research on their auditory periphery. Much earlier studies in Johann Schwarzkopf’s lab had shown that owl cochleae in general are large and the cochlea of the barn owl is about 11 mm long, far longer than in song birds (3–5 mm) and chickens (5 mm). This was confirmed and detailed anatomical data derived by Christine Köppl and Franz Peter Fischer of my lab, who showed that the barn owl papilla showed very interesting features when compared to those of other birds. Christine Köppl, Otto Gleich, and I also mapped frequency in the auditory papilla and showed that the owl hearing organ possesses a clear fovea, an area of expanded frequency representation (Fig. 18.6a). Here, the fovea extends over one octave, from 5 to 10 kHz, and occupies the entire basal half of the papilla (>5 mm). In this region, the most neural hair cells have an afferent innervation denser than yet seen in other avian species. Christine’s later auditory nerve data of the barn owl showed that, remarkably, nerve fibers from this foveal region were not especially sharply tuned (Fig. 18.6b). Instead, the fovea seems to be a mechanism for producing massive parallel processing in a frequency range that is vital for the owl in sound localization and thus prey capture (Köppl, 2009).
Diagrammatic representation of the characteristics of auditory-nerve afferent fibers in the barn owl. In (a), the peripheral origin of characterized and stained afferents (blue diamonds) are shown as the response frequency as a function of the distance of the stain from the cochlear apex. The green curve is a fourth-order polynomial fit to the data. The dashed lines show that in the region of the auditory fovea one octave (~6 kHz–10 kHz) occupies the basal half of the auditory papilla (a length of 5.5 mm). (b) A sample of threshold tuning curves of auditory primary afferents of different characteristic frequency in the barn owl, illustrating that there is no increase in frequency selectivity in the foveal region of the papilla. (c) Data illustrating the extraordinary ability of barn owl primary auditory afferents to phase lock to high frequencies. The blue dots show vector strength of phase locking in a large number of auditory primary afferents over the hearing range. The green curve is a moving window average of the data. In comparison, the orange curve is equivalent average data from the cat auditory nerve; above about 4 kHz, barn owl afferents show equivalent phase locking an octave higher than the cat. (All data kindly supplied by C. Köppl)
One feature in which barn owl afferent fibers excelled was their ability to phase lock to very high frequencies. In contrast to other birds and to mammals, in which the highest phase-locking frequency is generally 3–5 kHz, higher-frequency barn owl afferents showed useful phase locking at least an octave higher, to 9 kHz (Köppl, 1997b; Fig. 18.6c). This feature is now known to be essential for the extreme ability of barn owls to compare binaural inputs and localize sound in the horizontal plane. Grit Taschenberger in my lab then found SOAEs in the barn owl, which is still to date the only bird species showing this phenomenon. Almost all SOAEs were found above 7.5 kHz, at frequencies of the foveal region. This suggested that the expanded space constant (~5 mm per octave) in the fovea coupled so many active hair cells of the same best frequency together that they were able to synchronize and drive the tectorial membrane and surrounding fluids. Suppression of these SOAEs using pure tones showed that their thresholds and their tuning sharpness was the same as that seen in single auditory nerve afferents of this species by Christine Köppl (Köppl, 1997a; Taschenberger & Manley, 1997; Fig. 18.3d). Thus within the limited frequency range of their occurrence, barn owl SOAEs reflect in detail the function of the auditory papilla (Manley & van Dijk, 2008).
Using contralateral sound stimuli to suppress SOAEs and DPOAEs via the efferent system, Grit Taschenberger, Horst Oeckinghaus and I also showed that in the barn owl, efferent effects can be large and are not attributable to reflex middle ear responses to the (sometimes loud) contralateral sound. As shown by Alex Kaiser in my lab, tuning of efferents in the chicken brain stem is usually relatively poor. Unlike in mammals, also, chicken efferent cells of the brain stem could show excitation or inhibition during tonal stimulation. Similarly, in the barn owl, the effects of contralateral sound stimulation on DPOAEs could be either facilitation or suppression but with a frequency tuning on average sharper than for efferent activation in the chicken.
18.14 Avian Diversity and a Unique Feature
Over the years, we studied a variety of birds. One interesting species was the emu (Dromaius novaehollandiae), a representative of the very basal avian group, the paleognaths. For obvious reasons, we used emu chicks (among other things, adult emus weigh more than 50 kg and can be very dangerous; one does not like to imagine the effect a loose adult emu could have on a lab full of equipment). Not unexpectedly for such a large bird, even animals just a few weeks old heard very well at low frequencies. A basal status for the ear was confirmed by the large percentage of tall hair cells and the almost perfectly logarithmic frequency map we measured for the auditory papilla. Interestingly, a recent study by Christine Köppl and Andrew Affleck of another basal bird, the New Zealand kiwi (Apteryx) showed clear indications of a cochlear fovea, at a position consistent with its possible use for individual call recognition in this nocturnal species.
Christine Köppl and I, in cooperation with Graeme Yates in Australia, studied the rate-intensity (RI) functions of auditory nerve fibers of emus and barn owls. Graeme had earlier made important contributions to research in mammalian hearing by providing a consistent explanation for the existence of three basic forms of RI functions in mammals. His idea was based on their thresholds in relation to the saturating rate level function of the organ of Corti–basilar membrane complex. Although bird afferents did show the same pattern of RI types, the relationship to one another differed; the data suggested that each hair cell afferent response unit in birds has its own individual threshold-response relationship and is not governed by a global response pattern as in mammals. In birds, Otto Gleich’s data indicated that the most sensitive hair cells were supported not by the basilar membrane but by the solid neural limbus. Thus, unlike in mammals, hair cell activity cannot be fully integrated into a global oscillation of basilar membrane and hearing organ together (Manley & Köppl, 1998). As shown by Rainer Klinke’s group in the pigeon, any reflections of hair cell activity in a traveling wave of the basilar membrane in birds are poor, at least compared to those in mammals.
One of the most useful discoveries in nonmammals in recent decades was that of Doug Cotanche, that birds are capable of quickly regenerating hair cells. Otto Gleich in my lab cooperated with Bob Dooling and others to show that in the “Waterslager” race of canaries, a genetic defect leads to continuous hair cell degeneration, but that the hair cells also continuously regenerate. On average at any one time, enough hair cells are defective to confer birds of this race with higher auditory thresholds, which presumably explains why they sing so loudly. Such examples illustrate that—contrary to the opinion of some grant reviewers—birds can be extremely useful organisms for studying the mechanisms of hair cell regeneration.
18.15 Projecting from Birds Back to Dinosaurs
One later idea that emerged partly from data collected in my lab and collated by Otto Gleich in Regensburg was the possibility of estimating the hearing abilities of bird ancestors—the dinosaurs—from comparative studies of modern species. Gleich, Dooling, and I, and again later in cooperation with paleontologists coordinated by Stig Walsh, were able to show that in birds, hearing frequency limits correlate sufficiently well with animal size that extrapolations to extinct organisms are possible and reasonable. Using this, the upper hearing limits of early birds, of immense quadropedal dinosaurs, and of very large bipedal dinosaurs could be estimated from measurements from endocasts of fossil cochleae. Thus the largest dinosaurs were estimated to have had a best frequency response below 2 kHz. Archaeopteryx, an ancient bird, probably had a best hearing frequency of 3 kHz and an upper frequency limit below 7 kHz. Against this background, we can say that all the squeaks, honks, groans, and bellows of television and film animations of these animals are reasonably accurate, although vocalizations were unlikely to have been emitted as frequently as has been portrayed and probably not by all species.
18.16 What Have We Learned?
-
1.
Evolutionary processes acted in parallel on the various lineages of amniotes and produced sensitive, frequency selective auditory papillae in all groups. Over the eons, selective pressures induced convergent and parallel effects, such as the evolution of specialized hair cell populations in concert with the utilization of active processes (Manley &Köppl, 1998; Manley, 2000, 2001).
-
2.
Some structural changes during evolution clearly had important consequences for function. In particular, it has proven possible to understand the tectorial membrane better through the effects of its loss on sensitivity and frequency selectivity (Manley & Köppl, 2008). In addition, the frequency maps of basilar papillae can now be better understood and modeled.
-
3.
The various hearing organs of the different lineages of amniotes show strong resemblances. Each type of papilla does, however, have unique features, and these resemblances and differences can be understood only in the context of comparative studies. The main functional difference in mammal hearing, as compared to other amniotes, is the extension of the high-frequency range in most mammals, and this was the result of unrelated and fortuitous events in their early evolutionary history (Manley, 2010, 2012, 2013).
-
4.
Comparative studies of hearing have provided, and still provide, a powerful and flexible tool to widen our knowledge and to understand in detail the complex mechanisms underlying the hearing of vertebrate organisms, including humans.
18.17 Perspectives for the Future
The research fields covered above are so diverse that it is difficult to select areas for special attention in the future. Here, I touch briefly on four potentially fruitful fields.
-
1.
Obviously, the interactions between hair cell populations are of huge general interest and here, the birds and the geckos certainly deserve more attention. Very recent data from the chicken from Fettiplace’s lab is already providing fascinating insights into bird hearing.
-
2.
The remarkable ability of barn owl auditory afferents to phase lock one octave higher than any other species needs an explanation at the cellular and biochemical levels.
-
3.
The huge fall in the calcium concentration in the endolymph of therian mammals during evolution likely had profound consequences for various biochemical processes. It presumably led to the changed constitution of the tectorial membrane (which is highly sensitive to the ionic medium) and affected the further evolution of prestins and the transduction machinery. But what were these effects and how did they influence hearing in mammals?
-
4.
Both lizards and birds can show obvious effects of anesthesia, up to a total loss of responses in the ear. We showed, for example, that DPOAE amplitudes in barn owls drift over time during anesthesia. It is possible that this sensitivity to anesthetics, which has not been reported in mammals, is related to another effect not obvious in mammals, the effects of temperature on frequency responses. The latter can be quite large in lizards and birds. What biochemical mechanisms underlie these differences from mammals?
References
Bergevin, C. (2011). Comparison of otoacoustic emissions within gecko subfamilies: Morphological implications for auditory function in lizards. JARO, 12, 203–217.
Chiappe, M. E., Kozlov, A. S., & Hudspeth, A. J. (2007). The structural and functional differentiation of hair cells in a lizard’s basilar papilla suggests an operational principle of amniote cochleas. Journal of Neuroscience, 27, 11978–11985.
Gleich, O., Fischer, F. P., Köppl, C., & Manley, G. A. (2004). Hearing organ evolution and specialization: Archosaurs. In G. A. Manley, A. Popper, and R. R. Fay (Eds.), Evolution of the vertebrate auditory system (pp. 224–255). New York: Springer Science+Business Media.
Heffner, H. E. (1999). The symbiotic nature of animal research. Perspectives in Biology and Medicine, 43, 128–139.
Köppl, C. (1997a). Frequency tuning and spontaneous activity in the auditory nerve and cochlear nucleus magnocellularis of the barn owl Tyto alba. Journal of Neurophysiology, 77, 364–377.
Köppl, C. (1997b). Phase locking to high frequencies in the auditory nerve and cochlear nucleus magnocellularis of the barn owl, Tyto alba. Journal of Neuroscience, 17, 3312–3321.
Köppl, C. (2009). Evolution of sound localization in land vertebrates. Current Biology, 19, R635–R639.
Manley, G. A. (1973). A review of some current concepts of the functional evolution of the ear in terrestrial vertebrates. Evolution, 26, 608–621.
Manley, G. A. (1990). Peripheral hearing mechanisms in reptiles and birds. New York: Springer-Verlag.
Manley, G. A. (2000). Cochlear mechanisms from a phylogenetic viewpoint. Proceedings of the National Academy of Sciences of the USA, 97, 11736–11743.
Manley, G. A. (2001). Evidence for an active process and a cochlear amplifier in non-mammals. Journal of Neurophysiology, 86, 541–549.
Manley, G. A. (2004). The lizard basilar papilla and its evolution. In G. A. Manley, A. Popper, & R. R. Fay (Eds.), Evolution of the vertebrate auditory system (pp. 200–223). New York: Springer Science+Business Media.
Manley, G. A. (2010). An evolutionary perspective on middle ears. Hearing Research, 263, 3–8.
Manley, G. A. (2011). Lizard auditory papillae: An evolutionary kaleidoscope. Hearing Research, 273, 5–64.
Manley, G. A. (2012). Evolutionary paths to mammalian cochleae. JARO, 13, 733–743.
Manley, G.A. (2013). Mosaic evolution of the mammalian auditory periphery. Advances in Experimental Medicine and Biology. 01/2013; 787, 3–9.
Manley, G. A., & Köppl, C. (1998). Phylogenetic development of the cochlea and its innervation. Current Opinion in Neurobiology, 8, 468–474.
Manley, G. A., & Köppl, C. (2008). What have lizard ears taught us about auditory physiology? Hearing Research, 238, 3–11.
Manley, G. A., & van Dijk, P. (2008). Otoacoustic emissions in amphibians, lepidosaurs and archosaurs. In G. A. Manley, R. R. Fay, & A. Popper (Eds.), Active processes and otoacoustic emissions in hearing (pp. 211–260). New York: Springer Science+Business Media.
Manley, G. A., & Kraus, J. E. M. (2010). Exceptional high-frequency hearing and matched vocalizations in Australian pygopod geckos. Journal of Experimental Biology, 213, 1876–1885.
Manley, G. A., Kirk, D., Köppl, C., & Yates, G. K. (2001). In-vivo evidence for a cochlear amplifier in the hair-cell bundle of lizards. Proceedings of the National Academy of Sciences of the USA, 98, 2826–2831.
Taschenberger, G., & Manley, G. A. (1997). Spontaneous otoacoustic emissions in the barn owl. Hearing Research, 110, 61–76.
Webster, D. B., Fay, R. R., & Popper, A. N. (1992). The evolutionary biology of hearing. New York: Springer-Verlag.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Manley, G.A. (2014). Fundamentals of Hearing in Amniote Vertebrates. In: Popper, A., Fay, R. (eds) Perspectives on Auditory Research. Springer Handbook of Auditory Research, vol 50. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9102-6_18
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9102-6_18
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9101-9
Online ISBN: 978-1-4614-9102-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)