Abstract
The treatment of pediatric facial fractures can be challenging, stimulating, and occasionally frustrating, especially for those practitioners who only rarely manage these injuries. Difficulties arise from the fact that unlike their adult counterparts, no standardized protocols exist for the management of pediatric facial fractures; there are only broad guidelines and rare case reports. When evaluating a pediatric facial trauma patient, it should be remembered that the same fracture pattern will rarely be seen in a 5-month-old, a 5-year-old, and a 15-year-old due to the inherent differences in craniofacial anatomy, craniofacial proportions, and mechanism of injury. Even if a similar fracture pattern were observed, the treatment for patients of different ages would vary. The differences in craniofacial anatomy, patterns of injury, and capacity for remodeling and future growth must all be considered when determining a specific treatment option. It is paramount to remember that the pediatric patient is not just a small adult. Even with similarly aged patients and fracture patterns, the treatment plan must take into account patient and parent compliance and stage of dentition in that particular child.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The treatment of pediatric facial fractures can be challenging, stimulating, and occasionally frustrating, especially for those practitioners who only rarely manage these injuries. Difficulties arise from the fact that unlike their adult counterparts, no standardized protocols exist for the management of pediatric facial fractures; there are only broad guidelines and rare case reports. When evaluating a pediatric facial trauma patient, it should be remembered that the same fracture pattern will rarely be seen in a 5-month-old, a 5-year-old, and a 15-year-old due to the inherent differences in craniofacial anatomy, craniofacial proportions, and mechanism of injury. Even if a similar fracture pattern were observed, the treatment for patients of different ages would vary. The differences in craniofacial anatomy, patterns of injury, and capacity for remodeling and future growth must all be considered when determining a specific treatment option. It is paramount to remember that the pediatric patient is not just a small adult. Even with similarly aged patients and fracture patterns, the treatment plan must take into account patient and parent compliance and stage of dentition in that particular child.
Demographics
Pediatric facial fractures are an uncommon entity, comprising less than 15 % of all facial fractures. Many studies of pediatric craniofacial injuries have had a significant bias as they were based on inpatient admissions, separated by treating specialty, or restricted to patients undergoing operative intervention. In the authors’ series, comprised of all pediatric emergency room visits to a Level 1 pediatric hospital over a 5-year period, a more complete picture of the incidence and demographics of pediatric facial fractures was obtained. In general, these patients were more likely to be older and male (see Fig. 23.1). Fracture pattern, causation, and level of care varied with age, with a general evolution of injury pattern from cranial to caudal (see Fig. 23.2). As age increased, injuries were more often the result of adult behaviors that occurred outside of the home, whereas younger children were more often injured as the result of falls and motor vehicle accidents (see Fig. 23.3).
Although pediatric facial fractures are a rare cause of pediatric trauma presentations (less than 10 %), these patients tend to have higher injury severity scores, prolonged hospitalizations, and more significant morbidity and mortality. These findings are likely due to the high energy required to overcome the resilience of the pediatric craniofacial skeleton, and it follows that the most common cause of injury in these patients is motor vehicle accidents. Pediatric trauma patients, especially the youngest children, are more likely to require admission to the intensive care unit. Pediatric patients with facial fractures have serious associated trauma in 25–75 % of cases. Brain injury is twice as common in patients with facial fractures as in those without facial fractures. In the authors’ series, nearly half of the patients had an associated neurologic injury and 48.6 % suffered a concussion in keeping with the reported literature (see Fig. 23.4). These children require a high index of suspicion for an associated neurologic injury; if a concussion is diagnosed, they require rest and serial testing to chart resolution and prevent long-term impairment. Unlike their adult counterparts, pediatric facial trauma patients are unlikely to suffer cervical spine injuries. In the authors’ series, only 2.3 % of patients had an associated cervical spine injury. The most common related facial fracture was an orbital fracture, and all of the patients who had a cervical spine injury were either involved in a motor vehicle collision or a fall.
The incidence of facial fractures increases with age, with the mean age in the author’s series being 10.7 years. The greater likelihood of an older child to suffer a facial fracture is due to both the changing anatomy of the child’s craniofacial anatomy and the changing environment of the growing child. Small children are more likely to spend the majority of their life in a closely supervised situation, while the teenaged child spends more time unsupervised and partakes in more adult behaviors, such as sports and recreational activities that put them at risk for injury. This pattern was seen in the authors’ series as the cause of injury changed from predominantly falls and motor vehicle accidents in the young child to violence, sports, and use of all-terrain vehicles in the older children (see Fig. 23.5). In general, motor vehicle collisions are the most common cause of facial fracture in children across all age groups, and children who are improperly restrained are more likely to suffer facial fractures. Older male patients from lower socioeconomic areas were more likely to have suffered a facial fracture as the result of violence (see Fig. 23.6). Non-accidental trauma is a not uncommon cause of pediatric facial trauma, with an incidence from 4 to 12 % in the literature.
Anatomic Considerations
The relatively low incidence of facial fractures observed in the pediatric population is due to both environmental and anatomic factors. The pediatric craniofacial skeleton is unique from the adult craniofacial skeleton in several important facets that impact the patterns of fractures observed as well as the management employed. The infant has a large cranium-to-face ratio, 8:1, at birth that decreases to 4:1 by 5 years of age and progresses to 2.5:1 by adulthood. The growth of the cranium is 75 % complete by age 7 and 95 % complete by age 10. The upper face grows secondary to brain and ocular development and orbital growth is completed by 6–8 years of age. Midface growth follows the development of the nasal capsule and dentition. The palate and maxilla are two-thirds of adult size at age 6 and are largely fully grown by age 12–14, as is nasal growth. The sinuses of the infant are largely undeveloped and poorly pneumatized. The sphenoid sinus begins to develop at age 2 and matures through adulthood. The maxillary sinus develops in parallel with dental eruption, and the frontal sinus does not develop until full facial skeletal maturity is reached. The ethmoid sinus is the first to fully develop and is completed by age 12. The infant mandible is joined in the midline by a cartilaginous symphysis, which ossifies in the first year of life. Unerupted tooth buds form the majority of mandibular volume in early childhood. As the teeth erupt, cortical bone becomes the primary component of the mandible as full growth is achieved. The condyles are a major growth center of the mandible, and development of the mandible is the result of addition of bone at these centers as well as along the posterior border of the ramus. In addition, the overall increase in size of the mandible is the result of surface apposition; the mandible is continuously undergoing changes related to remodeling until growth is complete. In females, adult mandibular size is achieved by age 14–16 and in males by 18–20.
Injury Patterns
Pediatric facial fractures differ from adult facial fractures in incidence, related injuries, and injury patterns. The unique aspects of the pediatric craniofacial skeleton can explain these observed relationships. The pediatric craniofacial skeleton not only differs in the relative proportion of the face to cranium but also in the amount of sinus aeration, buccomaxillary fat pad volume, and cancellous-to-cortical bone ratio. The decreased bone mineral content of the pediatric craniofacial skeleton results in increased tolerance to force without fracture; fractures that do occur are more likely to be unicortical, or greenstick, fractures. Although mandible fractures are widely reported as the most common pediatric facial fractures, cited as anywhere from 20 to 50 % of all pediatric facial fractures, several series have reported that children younger than 5 years of age are more likely to sustain cranial, orbital, and nasal fractures. In one series, cranial vault fractures comprised 54 % of all craniofacial fractures with the highest incidence observed in the youngest patients. In fact, in the authors’ series, orbital fracture was the most common fracture observed across all age groups. In children under 6 years of age, cranial and orbital fractures were the most common, followed by mandibular, nasal, and maxillary fractures. Zygomaticomaxillary (ZMC) and nasoorbitoethmoid (NOE) fractures were rare. In children aged 6–11, orbital fractures were again the most common, followed by nasal, mandibular, skull, and maxillary fractures. Again, ZMC and NOE fractures were rare but more frequent with age. In the oldest group, aged 12–18, orbital fractures were the most common, followed by nasal, mandibular, maxillary, and skull fractures. Again, ZMC and NOE fractures were uncommon but more likely in this age group than in either of the two younger age groups (see Fig. 23.2). Mandible fractures demonstrate an age-related distribution; condylar head and subcondylar fractures are the most common (48 %) but decrease with age, while body and angle fractures increase.
Lack of mineralization, increased cancellous-to-cortical bone ratio, incomplete development of sinuses, and dental eruption all lead to different patterns of fracture in the pediatric craniofacial skeleton. Le Fort fracture patterns are rarely observed in the skeletally immature child and, in one series, only seen after 10 years of age. Until 10 years of age, the lack of an aerated maxillary sinus allows forces to be transmitted directly to the alveolus, resulting in alveolar fractures rather than Le Fort I fractures in this age group. Unilateral NOE fractures occur rather than the Le Fort II fractures observed in the mature craniofacial skeleton; oblique craniofacial fractures are the pediatric equivalent of Le Fort III fractures. Oblique craniofacial fractures are observed in the pediatric population as the non-aerated sinuses allow forces to be transmitted easily from the site of impact to the cranium and skull base (see Figs. 23.7 and 23.8).
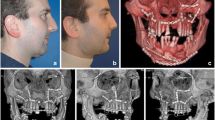
Another entity to be aware of in the care and treatment of the pediatric patient is the growing skull fracture. Orbital-cranial fractures can develop into growing skull fractures as the brain pulsations are transmitted through dural disruptions and impede healing, leading to a growing bony diastasis. These lesions have been documented in 0.6–2 % of pediatric skull fractures. Children at risk for developing these lesions must be followed long term with a high index of suspicion and a low threshold for repeat imaging to demonstrate complete bony healing (see Figs. 23.9, 23.10, 23.11, and 23.12).
Coronal CT corresponding to clinical photo in Fig. 23.9. The yellow circle indicates the growing skull fracture
Evaluation
A systematic approach to the evaluation of facial injuries cannot be overemphasized in the pediatric population. Examining an uncooperative child is difficult in itself; if a systematic examination is not undertaken, important findings may be missed. A maxillofacial CT scan with fine cuts and 3D reconstructions is invaluable in the full evaluation of a pediatric patient with a suspected facial fracture. Depending upon the fracture pattern present, the examiner should have a low threshold to involve their neurosurgery, ophthalmology, and dental colleagues.
Superior orbital fissure syndrome (internal and external ophthalmoplegia (CN II, IV, VI paralysis), proptosis, and CN V paresthesia) and orbital apex syndrome (superior orbital fissure syndrome with blindness secondary to CN II involvement) must be emergently addressed. Periorbital ecchymosis, hemotympanum, and cranial nerve palsy may indicate a skull base fracture. Ptosis may be secondary to levator paralysis. Exophthalmos and inferior globe displacement point to an orbital roof fracture. Extraocular muscle restriction or globe displacement will cause diplopia. Forced ductions should be performed on the obtunded patient to rule out muscle entrapment. Limitations of gaze, with relatively minimal associated findings, may represent entrapment in the “white-eyed blowout fracture.” A bowstring test (palpation of the bony medial canthal attachment with distraction on the lower eyelid) should be performed to assess the integrity of the medial canthal tendon in NOE fractures.
Maxillary mobility and malocclusion may indicate a midface fracture just as in an adult patient, but it should be remembered that true Le Fort fractures are rare and the younger patient is much more likely to have sustained an alveolar fracture. ZMC fractures can be indicated by an upper buccal sulcus hematoma, a preauricular depression, cheek flattening, or lateral canthal dystopia. Impingement of a depressed zygomatic arch on the coronoid will give the patient trismus. Nasal fractures in children are not uncommon and can be associated with nasal deviation, compressibility of the dorsum, as well as septal hematoma, which must be ruled out.
Evaluation of the occlusion in a pediatric patient can be difficult, especially in mixed dentition. Attention must be paid to the wear facets; preinjury dental records and parent input can be helpful as well. Physical exam findings consistent with a mandible fracture include drooling, trismus, decreased maximal incisive opening, discomfort on excursion, and dental step-offs. Evidence of an anterior open bite is indicative of bilateral condylar fractures. A unilateral condylar fracture will result in a contralateral posterior open bite.
Indications for Treatment
In regard to operative intervention for pediatric facial fractures, the decision to intervene is essentially a judgment call with the practitioner weighing the benefits of precise reduction and fixation against the risk of future growth disturbance. It is the senior author’s opinion that the younger the patient, the higher the threshold should be for operative intervention. The literature reports rates from 25 to 78 % operative intervention for pediatric facial fractures. In the authors’ series, 36 % of all patients went on to undergo operative intervention (see Fig. 23.13). Older children, and those with multiple facial fractures, were more likely to undergo surgery, a finding consistent with the published literature. When the decision is made for operative intervention, surgery should be performed in a more acute fashion than in the adult population as long as the degree of swelling is not prohibitive and the overall patient’s status allows. Converse advocated for prompt repair in the 1960s, and others note the propensity of the pediatric patient for rapid adherence of loose fragments within 3–4 days of injury. When operative intervention is undertaken, periosteal stripping should be minimized in order to have minimal impact on growth and development in keeping with Moss and Salentijn’s “functional matrix” principle. While the care of pediatric facial fractures can be considered “treating a moving target,” the following is a fracture-specific discussion of anatomic and developmental factors to guide the clinician in making informed decisions on a patient-by-patient basis.
Cranial Base and Skull Fractures
Indications for operative intervention for cranial base and skull fractures include a cerebrospinal fluid (CSF) leak that persists despite conservative management, significant displacement, deformation of facial contour, and growing skull fractures. The goals of cranial base and skull fracture repair include protection of the cranial contents, dural reconstruction, control of CSF leaks, prevention of infection, and restoration of craniofacial contour. In older patients with a developed frontal sinus, Rodriguez et al. have presented an algorithmic approach. With obstruction of the nasofrontal duct, obliteration or cranialization of the frontal sinus is indicated. Cranialization allows for single-stage elimination of the sinus as a potential site of infection. With a traumatized but patent duct, nondisplaced fractures of the anterior and posterior tables can be managed conservatively with close follow-up. Significantly displaced isolated anterior table fractures, with resulting contour deformities, can be reconstructed. However, in the child with an immature and underdeveloped frontal sinus, self-correction, with continued growth and development of the sinus, may correct contour deformities. The authors have reported a particularly illustrative case involving an 11-year-old struck in the forehead with a hockey stick. CT scan demonstrated a comminuted, depressed fracture through the left superior orbital rim extending cephalad through the anterior wall of the frontal sinus, as well as caudally into the ethmoids. No radiographic evidence of injury to the nasofrontal duct or extension of the fracture through the posterior table of the frontal sinus was present. Although there was palpable irregularity of the forehead on exam, no visible deformity was present. Therefore, the fracture was managed conservatively with close radiographic follow-up to ensure maintenance of a “safe sinus” where mucus from the frontal sinus would be able to drain into the nose. A repeat CT scan 6 weeks post-trauma demonstrated significant fracture healing and sinus remodeling. On a 1 year follow-up scan, the frontal sinus had completely remodeled with normal aeration, and there was in fact no evidence of prior fracture.
Alternatively, if a CSF leak is present, this finding will direct management. Observation and strict bed rest, with or without lumbar drain, may be pursued for 4–7 days. If the leak is persistent after this course, cranialization may be performed. If the leak resolves with conservative management and the nasofrontal duct is not obstructed, the sinus can be preserved. However, if the leak resolves but the duct is obstructed, then the sinus may be treated with “partial obliteration” (obliteration of the ducts and base of the sinus) and complete removal of the sinus mucosa.
Bone loss is a difficult problem in those patients too old to heal large calvarial defects spontaneously and too young to yield high-quality split calvarial grafts. Autogenous bone grafts are associated with potential donor site morbidity including pain, hemorrhage, nerve injury, and infection in up to 8 % of patients. Artificial bone substitutes are not biocompatible and are susceptible to infection. For these reasons, in donor sites of the calvarium, the authors prefer a bilaminate construct composed of intra- and extracranially placed bioresorbable mesh with interposed demineralized bone matrix mixed with the patient’s own bone shavings harvested with a Hudson brace (Medicon, Germany) from the inner cortex of the graft.
Orbital Fractures
In the management of adult patients with orbital fractures, there exist relatively succinct criteria for operative intervention (fracture area greater than 1 cm2 or involvement of more than 50 % of an orbital wall). Other indications include immediate enophthalmos, vertical ocular dystopia (VOD), superior orbital fissure syndrome, and frontal-temporal-orbital fractures with resulting exophthalmos. In the pediatric population, these indications are less straightforward. The more robust orbital periosteum and supporting ligaments in the pediatric orbit are believed to make enophthalmos and VOD less likely. These sturdier supporting structures may make open reduction and internal fixation (ORIF) less necessary. The authors evaluated operative necessity in a three-group orbital fracture classification system (n = 81): type 1, pure orbital fractures; type 2, craniofacial fractures (oblique fractures extending from the skull into the orbital roof); and type 3, orbital fractures associated with classically described patterns (blowout, ZMC, NOE). On retrospective analysis, type 1 fractures were treated nonoperatively (88 %) unless there was acute enophthalmos, VOD, or muscle entrapment. Type 2 fractures were managed conservatively with serial scans until an absolute operative indication developed; 17 % were ultimately treated operatively. Type 3 fractures were more likely to undergo operative intervention (72 %). Overall, 23 of 81 (28.3 %) of orbital fractures underwent operative intervention. Based on this data, the authors recommend that all isolated (type 1) pediatric orbital fractures be treated conservatively, despite the findings on CT scan, unless there are clinical indications for perusing an operative approach, such as acute enophthalmos, acute VOD, or muscle entrapment.
For those fractures requiring operative intervention, either to restore globe position, to correct diplopia, or to relieve an entrapment, the transconjunctival approach is preferred due to a generally perceived lower risk for ectropion. A transcaruncular approach to the medial wall can be used as well. All tissues are cleared from the fracture site, and care is taken so that the entire circumference of the defect is visualized. In the case of a “trap-door” fracture with entrapment of the muscle and/or periorbita, the fracture fragment may require further displacement into the maxillary sinus in order to fully mobilize all soft tissues back into the orbit. The residual defect is repaired with resorbable mesh or split calvarial graft depending on the surgeon’s preference.
Nasal Fractures
Nasal fractures, with associated septal hematomas, warrant immediate intervention. Closed reduction of nasal fractures often fails to completely correct the deformity secondary to inadequate release; however, aggressive open treatment in children has the potential to affect facial and nasal growth adversely. Hence, the authors recommend that, for all children presenting with a nasal fracture resulting in an obvious deformity, a closed reduction be offered, with the intention of at least improving the deformity. Patients and families should be informed that the option of pursuing definitive open management at skeletal maturity to address any residual deformity or airway obstruction is a possibility. The maneuvers for closed reduction in children are similar to those in adults: an elevator or knife handle may be passed endonasally to outfracture depressed nasal bones, or digital manipulation can be utilized to infracture laterally displaced nasal bones. The septum can be reduced/relocated with Asch forceps. If necessary, internal splints are used in addition to external splints. Septal hematomas, if present, should be addressed with incision, drainage, and then elimination of the dead space with either quilting sutures or internal splints.
NOE fractures are uncommon in the pediatric patient but become more common with advancing age. The age-specific norms for intercanthal distance must be kept in mind when evaluating and treating these patients: newborn = 10–15 mm, 2-year-old = 20 mm, 12-year-old = 25 mm, and adult = 35 mm. Several classification schemes for NOE fractures have been used; however, the one developed by Markowitz et al. is probably the most widely employed as it defines pathology as well as treatment plan. This classification system is more relevant to the adult craniofacial skeleton and does not take into account the higher likelihood of pediatric NOE fractures to be associated with skull base fractures. A separate classification system, developed by Burstein et al., incorporates these differences and can be useful to the pediatric maxillofacial surgeon. The literature is sparse in regard to treatment and long-term follow-up of pediatric NOE fractures. One review of 20 patients who had midface fractures with an NOE component found a rate of 40 % requiring revisional surgery, with younger children being more likely to require additional procedures when compared with older children.
Midface Fractures
Nonoperative management is advocated for minimally displaced or greenstick midface fractures, particularly in the younger child. Dentoalveolar fractures are typically nonoperative and treated with splinting by the pediatric dentists. Palatal fractures may require ORIF or splinting with mandibulomaxillary fixation (MMF). The pediatric patient in primary or mixed dentition presents a challenge to the operating surgeon to achieve appropriate MMF; classically, circummandibular wiring and piriform suspension wiring have been very useful options (see Fig. 23.14). The authors have found, however, that − despite dogma advising against the use of arch bars in mixed and primary dentition for fear of disrupting the development of permanent dentition − arch bars in this population are quite efficacious and benign. Specifically, in 21 such patients with 34 fractures, no adverse effects on permanent dentition were seen after rigorous dental and radiographic assessment. Regardless of the method of MMF employed, the younger the patient, the shorter the course of MMF required; some authors advocate 1 week or less, followed by dental elastics. The unerupted tooth buds must always be kept in mind when performing ORIF in these young patients.
The goals in operative treatment of zygomaticomaxillary complex (ZMC) fractures include correction of VOD or enophthalmos, restoration of occlusion, and preservation of facial appearance. Flattening of the malar eminence can occur secondary to a ZMC fracture, as well as inferior displacement of the lateral canthal tendon due to its attachment to Whitnall’s tubercle. This constellation can cause significant cosmetic deformity if left untreated or if treated inadequately. The zygoma can be approached via an upper buccal sulcus and eyelid incision; isolated arch fractures may be approached with a Gillies incision. The zygomaticofrontal (ZF) suture can be accessed through a subciliary incision and a subconjunctival incision with a lateral cantholysis or through the lateral portion of an upper lid blepharoplasty incision. Adequate reduction at the lateral wall of the orbit, or the greater wing of the sphenoid, is essential to proper reconstruction. Reduction must also be achieved at the ZF suture, the inferior orbital rim, and the zygomaticomaxillary (ZM) buttress. The surgeon must ascertain that orbital volume is not altered by the reduction, and reconstruction of the orbital floor may be required.
Mandible Fractures
The pediatric mandible fracture may represent the most challenging pediatric facial fracture to treat. In an attempt to direct therapy, the authors have organized the treatment of pediatric mandible fractures into 4 levels of intervention as follows (see Table 23.1). Level 1: conservative management such as soft diet to avoid fracture displacement, rest, and physical therapy. Level 1 management is indicated for mandibular fractures that are isolated and minimally displaced and that do not disrupt occlusion or mandibular function such as a nondisplaced and nonmobile parasymphyseal fractures (see Fig. 23.15). Level 2: nonsurgical stabilization with a C-collar or ACE wrap. Level 2 management is employed when a short course of immobilization is desired (condylar head or neck fractures) or when some degree of mobility is appreciated in a displaced fracture that does not significantly disrupt occlusion or mandibular function (e.g., isolated parasymphyseal fractures). Nonoperative stabilization is appropriate even for the multiply fractured mandible if stability can be maintained with ACE wrap or C-collar and occlusion is preserved. Level 3: operative CREF with arch bars and elastics or MMF. Level 3 management is indicated if fracture displacement or significant mobility interferes with occlusion or mandibular function such as a significantly displaced condylar neck fracture affecting occlusion. Level 4: ORIF ± arch bars and elastics or MMF. Level 4 management is performed most frequently for displaced mobile fractures of the non-condylar region that affect function or occlusion. Level 4 management is also indicated for the common presentation of a condylar head/neck fracture treated best with physical therapy, combined with a parasymphyseal fracture requiring rigid fixation.
The pediatric condyle warrants special mention as it is an important growth center of the mandible, sensitive to disruptions in blood supply which can result in ankylosis and altered development. Pure intracapsular injuries should be provided Level 1 conservative management to minimize growth disturbance and TMJ ankylosis. Some advocate for more aggressive treatment of dislocated condylar neck fractures in older patients as the condyles are less likely to regenerate in children over 7 years of age. These injuries, in very rare circumstances, may progress to require osteotomy and cartilage grafting for TMJ function and restoration of occlusion. In patients with unilateral condylar neck fractures affecting occlusion, Level 3 treatment with arch bars and contralateral elastics is preferable (see Figs. 23.16, 23.17, and 23.18). The elastics can overcome the patient’s trismus and close the open bite while allowing early motion in order to avoid ankylosis. During healing, the occlusion can be fine-tuned by adjusting the vector of the elastics. In the case of bilateral condylar neck fractures, particularly in the older child with a resultant anterior open bite, Level 4 management with ORIF of one side, along with a short course of intermaxillary fixation, is reasonable. The alternative approach in the immature skeleton would be to perform Level 3 management with IMF, with the understanding that elective orthognathic surgery may be required secondarily if an anterior open bite results. A condylar head fracture, in the presence of another mandible fracture, is an indication for Level 4 management with ORIF of the non-condylar fracture, along with elastic therapy allowing for early TMJ range of motion. Further indications for open treatment include a foreign body in the TMJ, inability to normalize occlusion with closed management, and displacement of a condyle into the middle cranial fossa. Open treatment should be avoided in intracapsular fractures, high condylar neck fractures, coronoid fractures, and any injury where occlusion is preserved and the patient is able to return to early range of motion.
Again, it should be noted that creativity may be required in placing the pediatric patient in primary or mixed dentition into adequate MMF. After 11 years of age (8 years for symphysis fractures), transosseous wiring and bicortical screws can be used. In managing postreduction malocclusion, it should be remembered that the primary teeth will exfoliate and that subsequent orthodontics can improve residual occlusal discrepancies. It is likely prudent to accept a slight malocclusion rather than risk injuring a permanent tooth bud with an attempt at ORIF.
Growth Concerns, Outcomes, and Complications
Our understanding and management of pediatric facial fractures are ever-evolving. Imaging modalities and anatomical understanding continue to improve, while reporting of treatment strategies, complications, and adverse outcomes continues to progress. The literature varies widely in the reported rates of complications associated with pediatric facial fractures (anywhere from 7.4 to 27.8 %). This fact underscores the variability both in definition and follow-up of pediatric fractures.
In an effort to clarify the discussion of adverse outcomes in pediatric facial fractures, our group developed and reported the following classification system of adverse outcomes: type 1, adverse outcomes related to the fracture itself (i.e., the loss of a permanent tooth with a mandible fracture); type 2, adverse outcomes secondary to intervention and surgical management (i.e., nerve injury after open reduction and internal fixation of a fracture); and type 3, adverse outcomes that may result from a combination of the fracture, its management, and subsequent growth and development (i.e., asymmetric mandibular growth). In the authors’ series of 177 pediatric patients with facial fractures, 32.2 % had an adverse outcome (see Fig. 23.19).
In general, the complications associated with pediatric facial fractures follow a different pattern of distribution than that in adult facial fractures. The more common complications seen in adults (infection, nonunion, and malunion) are rarely seen in the pediatric population. Here, the main concern is for long-term growth disturbances. The actual potential for this adverse outcome is incompletely understood. The relative contributions of open reduction (and associated periosteal stripping), placement of hardware, and injury to growth centers are all still undergoing investigation.
In cranial- and skull-based fractures, complications can include meningitis, sinusitis, mucoceles, mucopyoceles, brain abscesses, and CSF leaks. Often, a CSF leak will resolve with conservative management within 1 week, and persistent leaks can be treated with lumbar drain prior to surgical intervention. A growing skull fracture can occur in 0.03–1 % of skull base fractures associated with a dural disruption, especially if they occur during the period of rapid skull growth, i.e., less than 3 years of age. These growing skull fractures can lead to further complications. In the authors’ series, 40 % of skull fractures had an adverse outcome. Of these adverse outcomes, 1.5 % were type 1, 20 % were type 2, and 35 % were type 3. The complications included growing skull fractures, CSF leaks, enophthalmos, vertical orbital dystopia, ptosis, amblyopia, and exophthalmos.
Orbital fractures can be complicated by diplopia and enophthalmos, both of which may require further reconstruction. In the authors’ series, 10.7 % of isolated orbital fractures had an adverse outcome. Of those, 3.6 % were type 1, 3.6 % were type 2, and 3.6 % were type 3. Three patients had enophthalmos, which was not clinically significant (less than 2 mm). While no persistent diplopia was reported in this series, it has been reported in as many as 36 % of cases in other series.
When considering the complications related to nasal deformity, these can be functional, aesthetic, or both. Nasal deviation can result from inadequate reduction and cartilaginous changes. Callous formation and bony deposition can lead to a dorsal hump. The untreated septal hematoma can lead to cartilage destruction and ultimately to a saddle nose deformity. When considering NOE fractures, damage to the lacrimal system can lead to persistent obstruction, necessitating dacryocystorhinostomy. In the authors’ series, 21.7 % of nasal fractures exhibited adverse outcomes; 8.7 % of those were type 1 and 17.4 % were type 3, related to persistent deformity and airway obstruction. Corrective rhinoplasty is typically delayed until skeletal maturity in these instances, unless nasal airway obstruction is severe.
In reviewing a series of 215 mandible fractures in 120 patients under 18 years of age, the authors have found that adherence to the treatment guidelines described above results in largely uncompromised mandibular function and growth. Adverse outcomes assessed in this series include dental trauma, restricted maximal incisive opening, mental nerve paresthesia, hardware problems, TMJ deviation, TMJ ankylosis, TMJ pain, TMJ click, and growth disturbances. Of the 56 patients in this series with at least 1 year of follow-up, 14.3 % had a type 1 adverse outcome, 8.9 % had a type 2, and 25.0 % had a type 3. None of these adverse outcomes were clinically significant, where “clinical significance” was defined as a functional or aesthetic concern for the patient, family, or treating practitioner. All type 1 outcomes encountered were dental injuries. The most commonly reported type 2 adverse outcome was hardware failure. Type 3 adverse outcomes included primarily TMJ symptoms and disturbances in mandibular morphology. The chances of an adverse outcome increased significantly with having either multiple mandible fractures or mandible fractures requiring operative intervention (p < 0.05). While there was a trend towards increasing adverse outcome rates with increasing age (0–5-year-olds had a 35.0 % chance of an adverse outcome, 6–12-year-olds had a 40.0 % chance of an adverse outcome, and 12–18-year-olds had a 45.5 % chance of an adverse outcome), these values were not statistically significant (p > 0.05, chi-square). With respect to postoperative growth and development, there were no significant differences between lateral cephalometric measurements (SNB, ANB, SN-GoGn, Ar-Go-Gn, Go-Pg, Ar-Go) in our cohort of pediatric mandible fractures when compared to age-/sex-matched Bolton norms (see Fig. 23.20). As well, no significant differences were found on posterior-anterior cephalometric measurements (Ar-Go, Go-Me, Ar-Me) (see Fig. 23.21) of injured and uninjured hemi-mandibles. These findings held when the data were stratified by age at injury and by fracture type. In another report, patients who sustained mandible fractures between the ages of 4 and 7 were most likely to have growth disturbances and facial asymmetry, and mandible fractures occurring prior to 4 years of age were least likely to have a growth disturbance.
Conclusions
The pediatric craniofacial skeleton is structurally unique from its adult counterpart. This distinct structure yields specific injury patterns in response to trauma, and these diagnoses must be astutely recognized to facilitate appropriate management. One must exercise special caution in managing pediatric facial fractures such that growth potential is not unnecessarily sacrificed in efforts to achieve perfect reduction and rigid fixation. This growth potential, if protected, lends a powerful plasticity to the pediatric craniofacial skeleton allowing for inherent compensation to the traumatic insult. An increasing recognition of this resilience and an expanding appreciation for the role post-traumatic orthodontics can play in rehabilitating these injuries are coalescing to favor less invasive management of many pediatric craniofacial fractures.
Bibliography
Anderson FM, Geiger L. Craniosynostosis: a survey of 204 cases. J Neurosurg. 1965;22:229–40.
Bite U, Jackson IT, Forbes GS, Gehring DG. Orbital volume measurements in enophthalmos using three-dimensional Ct imaging. Plast Reconstr Surg. 1985;75:502–8.
Burstein F, Cohen S, Hudgins R, Boydston W. Frontal basilar trauma: classification and treatment. Plast Reconstr Surg. 1997;99:1314–21; discussion 1322–3.
Chao MT, Losee JE. Complications in pediatric facial fractures. Craniomaxillofac Trauma Reconstr. 2009;2:103.
Chao MT, Jiang S, Smith D, DeCesare GE, Cooper GM, Pollack IF, Girotto J, Losee JE. Demineralized bone matrix and resorbable mesh bilaminate cranioplasty: a novel method for reconstruction of large-scale defects in the pediatric calvaria. Plast Reconstr Surg. 2009;123:976–82.
Chen KT, Chen CT, Mardini S, Tsay PK, Chen YR. Frontal sinus fractures: a treatment algorithm and assessment of outcomes based on 78 clinical cases. Plast Reconstr Surg. 2006;118:457–68.
Cho YR, Gosain AK. Biomaterials in craniofacial reconstruction. Clin Plast Surg. 2004;31:377–85, v.
Cole P, Boyd V, Banerji S, Hollier Jr LH. Comprehensive management of orbital fractures. Plast Reconstr Surg. 2007;120:57S–63.
Converse JM, Smith B, Obear MF, Wood-Smith D. Orbital blowout fractures: a ten-year survey. Plast Reconstr Surg. 1967;39:20–36.
Cope MR, Moos KF, Speculand B. Does diplopia persist after blow-out fractures of the orbital floor in children? Br J Oral Maxillofac Surg. 1999;37:46–51.
David L, Argenta L, Fisher D. Hydroxyapatite cement in pediatric craniofacial reconstruction. J Craniofac Surg. 2005;16:129–33.
Demianczuk AN, Verchere C, Phillips JH. The effect on facial growth of pediatric mandibular fractures. J Craniofac Surg. 1999;10:323.
Dufresne CR, Manson P. Pediatric facial injuries. In: Mathes SJ, editor. The head and neck, part 2, vol. 3. Philadelphia: Elsevier; 2006.
Eggensperger Wymann NM, Holzle A, Zachariou Z, Iizuka T. Pediatric craniofacial trauma. J Oral Maxillofac Surg. 2008;66:58–64.
Farkas LG, Posnick JC, Hreczko TM. Growth patterns of the face: a morphometric study. Cleft Palate Craniofac J. 1992;29:308–15.
Ferreira PC, Amarante JM, Silva PN, Rodrigues JM, Choupina MP, Silva AC, Barbosa RF, Cardoso MA, Reis JC. Retrospective study of 1251 maxillofacial fractures in children and adolescents. Plast Reconstr Surg. 2005;115:1500–8.
Gassner R, Tuli T, Hachl O, Moreira R, Ulmer H. Craniomaxillofacial trauma in children: a review of 3,385 cases with 6,060 injuries in 10 years. J Oral Maxillofac Surg. 2004;62:399–407.
Grunwaldt L, Smith DM, Zuckerbraun NS, Naran S, Rottgers SA, Bykowski M, Kinsella C, Cray J, Vecchione L, Saladino RA, Losee JE. Pediatric facial fractures: demographics, injury patterns, and associated injuries in 772 consecutive patients. Plast Reconstr Surg. 2011;128:1263–71.
Hatef DA, Cole PD, Hollier Jr LH. Contemporary management of pediatric facial trauma. Curr Opin Otolaryngol Head Neck Surg. 2009;17:308–14.
Havlik RJ, Sutton LN, Bartlett SP. Growing skull fractures and their craniofacial equivalents. J Craniofac Surg. 1995;6:103–10; discussion 111–2.
Holland AJ, Broome C, Steinberg A, Cass DT. Facial fractures in children. Pediatr Emerg Care. 2001;17:157–60.
Imahara SD, Hopper RA, Wang J, Rivara FP, Klein MB. Patterns and outcomes of pediatric facial fractures in the United States: a survey of the national trauma data bank. J Am Coll Surg. 2008;207:710–6.
Jordan DR, Allen LH, White J, Harvey J, Pashby R, Esmaeli B. Intervention within days for some orbital floor fractures: the white-eyed blowout. Ophthal Plast Reconstr Surg. 1998;14:379–90.
Kaban LB, Mulliken JB, Murray JE. Facial fractures in children: an analysis of 122 fractures in 109 patients. Plast Reconstr Surg. 1977;59:15–20.
Lehman Jr JA, Saddawi ND. Fractures of the mandible in children. J Trauma. 1976;16:773–7.
Losee JE, Jiang S. Pediatric facial trauma. In: Chung K, Disa J, Gosain AK, et al., editors. Plastic surgery. New York: Saunders Elsevier; 2009. p. 645–55.
Losee JE, Smith DM. Pediatric facial fractures. In: Neligan P, editor. Plastic surgery, vol. 3. 3rd ed. London: Elsevier; 2012. p. 671–85.
Losee JE, Afifi A, Jiang S, Smith D, Chao MT, Vecchione L, Hertle R, Davis J, Naran S, Hughes J, Paviglianiti J, Deleyiannis FW. Pediatric orbital fractures: classification, management, and early follow-up. Plast Reconstr Surg. 2008a;122:886–97.
Losee JE, Jiang S, Deleyiannis FW. Craniofacial fractures. In: Bentz M, Bauer B, Zuker R, editors. Principles and practice of pediatric plastic surgery. St. Louis: Quality Medical Publishing; 2008b. p. 1047–72.
Manson PN, Iliff N. Management of blow-out fractures of the orbital floor. II. Early repair for selected injuries. Surv Ophthalmol. 1991;35:280–92.
Manson PN, Grivas A, Rosenbaum A, Vannier M, Zinreich J, Iliff N. Studies on enophthalmos: II. The measurement of orbital injuries and their treatment by quantitative computed tomography. Plast Reconstr Surg. 1986;77:203–14.
Markowitz BL, Manson PN, Sargent L, Vander Kolk CA, Yaremchuk M, Glassman D, Crawley WA. Management of the medial canthal tendon in nasoethmoid orbital fractures: the importance of the central fragment in classification and treatment. Plast Reconstr Surg. 1991;87:843–53.
Mathes SJ, editor. Plastic surgery, vol. 3. 2nd ed. Philadelphia: Elsevier; 2006.
Mathog RH, editor. Maxillofacial trauma. Baltimore: Williams & Wilkins; 1983.
McCoy FJ, Chandler RA, Crow ML. Facial fractures in children. Plast Reconstr Surg. 1966;37:209–15.
Mericli AF, DeCesare GE, Zuckerbraun NS, Kurland KS, Grunwaldt L, Vecchione L, Losee JE. Pediatric craniofacial fractures due to violence: comparing violent and nonviolent mechanisms of injury. J Craniofac Surg. 2011;22:1342–7.
Messinger A, Radkowski MA, Greenwald MJ, Pensler JM. Orbital roof fractures in the pediatric population. Plast Reconstr Surg. 1989;84:213–6; discussion 217–8.
Moss ML, Salentijn L. The primary role of functional matrices in facial growth. Am J Orthod. 1969;55:566–77.
Naran S, Afrooz PN, Bykowski MR, Smith DM, Rottgers SA, Losee JE. Arch bar use in primary and mixed dentition: feasibility, safety and outcomes. Plast Reconstr Surg. 130:100. Abstract 34p.
Pappachan B, Alexander M. Correlating facial fractures and cranial injuries. J Oral Maxillofac Surg. 2006;64:1023–9.
Parsons GS, Mathog RH. Orbital wall and volume relationships. Arch Otolaryngol Head Neck Surg. 1988;114:743–7.
Perheentupa U, Kinnunen I, Grenman R, Aitasalo K, Makitie AA. Management and outcome of pediatric skull base fractures. Int J Pediatr Otorhinolaryngol. 2010;74:1245–50.
Roaten JB, Partrick DA, Nydam TL, Bensard DD, Hendrickson RJ, Sirotnak AP, Karrer FM. Nonaccidental trauma is a major cause of morbidity and mortality among patients at a regional level 1 pediatric trauma center. J Pediatr Surg. 2006;41:2013–5.
Rodriguez ED, Stanwix MG, Nam AJ, St Hilaire H, Simmons OP, Christy MR, Grant MP, Manson PN. Twenty-six-year experience treating frontal sinus fractures: a novel algorithm based on anatomical fracture pattern and failure of conventional techniques. Plast Reconstr Surg. 2008;122:1850–66.
Rottgers SA, DeCesare G, Chao M, Smith DM, Cray JJ, Naran S, Vecchione L, Grunwaldt L, Losee JE. Outcomes in pediatric facial fractures: early follow-up in 177 children and classification scheme. J Craniofac Surg. 2011;22(4):1260–5.
Sherick DG, Buchman SR, Patel PP. Pediatric facial fractures: a demographic analysis outside an urban environment. Ann Plast Surg. 1997;38:578–84; discussion 584–5.
Singh DJ, Bartlett SP. Pediatric craniofacial fractures: long-term consequences. Clin Plast Surg. 2004;31:499–518, vii.
Smartt Jr JM, Low DW, Bartlett SP. The pediatric mandible: II. Management of traumatic injury or fracture. Plast Reconstr Surg. 2005;116:28e–41.
Smith DM. Mandible fractures in 96 children: demographics, treatment, early outcomes and a prospective analysis of growth and development. Oxford: Society of Craniofacial Surgery XIII Biennial International Congress, 2009; 2009.
Smith DM, Bykowski MR, Cray JJ, Naran SS, Rottgers A, Shakir S, Vecchione L, Schuster L, Losee JE. 215 mandible fractures in 120 children: demographics, treatment, outcomes, and early growth data. Plast Reconstr Surg. 2013;131(6):1348–58.
Thaller SR, Huang V. Midfacial fractures in the pediatric population. Ann Plast Surg. 1992;29:348–52.
Thoren H, Iso-Kungas P, Iizuka T, Lindqvist C, Tornwall J. Changing trends in causes and patterns of facial fractures in children. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:318–24.
Vyas RM, Dickinson BP, Wasson KL, Roostaeian J, Bradley JP. Pediatric facial fractures: current national incidence, distribution, and health care resource use. J Craniofac Surg. 2008;19:339–49; discussion 350.
Ziccardi VB, Ochs MW, Braun TW, Malave DA. Management of condylar fractures in children: review of the literature and case presentations. Compend Contin Educ Dent. 1995;16:874, 876, 878–80 passim; quiz 888.
Zimmermann CE, Troulis MJ, Kaban LB. Pediatric facial fractures: recent advances in prevention. Diagnosis and management. Int J Oral Maxillofac Surg. 2006;35:2–13.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Monson, L.A., Smith, D.M., Losee, J.E. (2015). Pediatric Facial Fractures. In: Taub, P., Patel, P., Buchman, S., Cohen, M. (eds) Ferraro's Fundamentals of Maxillofacial Surgery. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8341-0_23
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8341-0_23
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8340-3
Online ISBN: 978-1-4614-8341-0
eBook Packages: MedicineMedicine (R0)