Abstract
Agro-industrial wastes are unavoidable waste materials continuously generated in bulk quantity. Most of these materials can be used as nutrient source for industrial fermentation. However, commercial fermentation of low-value high-volume products generally suffer financial crisis. Alternatively, sustainable biotransformation of agro-industrial waste into fine biochemical, such as aroma compounds and fragrances, has been widely investigated. Significant variation of substrate quality imparts great variations in the production methodology of these processes. Further, a range of microorganisms are known to be used and different genetic engineering strategies have been applied for improved bioconversion. Moreover, novel strategies for detection, identification, and purification of the final products have been developed, and in some particular cases, successful commercialization has also been achieved. To have, however, further benefit from this potential strategy, a systematic study of the type and nature of the feedstock and their abundance should be evaluated. Similarly, presently used processes and their scale-up potential should be determined and different options for their economic competitiveness should be identified. The goal of this chapter, therefore, is to improve the basic understanding of the interesting strategy and to summarize the recent advancements in production of aroma compounds and fragrances.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Aroma compounds or fragrances are the chemical compounds having pleasant smell. In general, they are volatile compounds which can easily reach the olfactory system. An aroma compound could also be a flavor which has a smell as well as a taste (e.g., diacetyl, 2-phenylethanol, acetoin, and vanillin). Aroma compounds are the integral parts of foods and beverages as well as they are also found in cosmetics and personal care products. Presently, flavors represent around 25 % of global food additive market (Longo and Sanromán 2006). Aroma/flavor compounds may naturally occur in foods and beverages or it could be externally included to enhance their taste/quality. Aroma/flavor compounds of foods and beverages may come from the raw materials or they may be produced during processing (e.g., fermentation) of such materials. Likewise, artificially added aroma/flavor compounds may be of natural origin, such as plant-derived materials, compounds produced by fermentation, or could be synthetic materials produced by chemical process. Most flavoring compounds presently available in the market are either synthetic compounds or products obtained by extraction from natural sources (Longo and Sanromán 2006). The foods and beverages containing aroma/flavor compounds of natural origin are preferred by the customers and it could be considered as a marketing advantage (Krings and Berger 1998; Longo and Sanromán 2006). Actually, the value of naturally produced aroma/flavor compounds is significantly higher than their chemically synthesized counterparts. For example, vanillin produced by conventional chemical process has a market value of US & 12/kg; however, at the same time natural vanillin could have a market value as high as US & 1,200–4,000/kg (Sindhwani et al. 2012). Meanwhile, flavor compounds extracted from natural sources, such as botanical sources, are also considered natural and an environment/health friendly alternative to chemically synthesized products. However, the extraction methods may be time consuming and laborious and require more space and chemicals/organic solvents for feedstock pretreatment and product extraction. For example, prior to extraction of natural vanillin, vanilla plants should be cultivated in large scale and the flower should be manually pollinated. Therefore, it is a labor-intensive process and it is difficult to encourage the farmers for large-scale plantation of vanilla plants (Ramachandra Rao and Ravishankar 2000). Alternatively, production of natural aroma/flavor compounds by microorganisms through fermentation has great potential and this option has been largely investigated for these products (Feron et al. 1996). In fact, considerable advancements have been made in genetic engineering, enzyme, or bioprocess technology for flavor/aroma production. However, owing to economic constrains only a small fraction of commercially available natural flavor compounds are produced by microbial technology (Feron and Waché 2006). Requirement of expensive synthetic media components could be considered as the most important factor to limit the economic feasibility of such processes. Alternatively, utilization of agro-industrial wastes as the feedstock for biotechnological production of flavor/aroma compounds could bring economic feasibility for such commercial processes (Bicas et al. 2010). Thus, the present chapter discusses about a few commercially important aroma/flavor compounds for which biotechnological production has been widely explored. Furthermore, possible application of agro-industrial waste for the production of such compounds has been analyzed as well as recent advances on aroma compounds production using agro-industrial wastes has been summarized.
2 Types and Few Industrially Important Aroma Compounds
Based on physicochemical as well as sensorial properties, aroma compounds could be of different types. If only chemical properties are considered, they may be volatile fatty acids or esters, aldehydes, ketones, alcohols, and lactones. The detailed classification of different aroma compounds has been reviewed by Dastager (2009). Similarly, a detailed list of biotechnologically produced aroma/flavor compounds, their precursors, and market potential could be found in Feron and Waché (2006). Among these compounds some particular aroma compounds have been widely investigated for possible commercial production by bioprocess technology. In this section, few aroma compounds have been discussed to have a better understanding about their substrate specificity, microorganism involved in their production, productivity, present scale of production, and possible metabolic/genetic engineering strategy for improved product yield. Similarly, this discussion will be helpful to determine the suitability and nature/type of agro-industrial waste which could be possibly used for such processes.
2.1 Diacetyl
Diacetyl is a flavor compound having butter-like flavor which may be naturally present or could be artificially added as a flavoring agent to certain foods, such as dairy products (Duboff et al. 1996; Hugenholtz et al. 2000; Alvandi and Azar 2008; Quach et al. 2012). Diacetyl is a by-product resulting during beer and wine production process (Garc 1994; Fornachon and Lloyd 2006). The well-known chemical synthesis method for diacetyl production is through oxidative decarboxylation of α-acetolactate (Hugenholtz et al. 2000). In the case of fermentative diacetyl production, pyruvate is first converted to α-acetolactate which is further broken down to diacetyl by enzymatic reaction (Benson et al. 1996; Swindell et al. 1996). Actually, two molecules of pyruvate can produce one molecule of α-acetolactate by enzymatic condensation reaction (Hugenholtz et al. 2000). Lactic acid bacteria, such as Lactococcus lactis and Lactobacillus casei, are mostly investigated for fermentative diacetyl production (Benson et al. 1996; Swindell et al. 1996; Boumerdassi et al. 1997; Nadal et al. 2009). Alvandi and Azar (2008) have optimized different process parameters (temperature, agitation, and media components) for diacetyl production by lactic acid bacteria of Lactococcus and Leuconostoc genus. Based on the optimized parameters, a scale-up study using 10 L fermenter resulted in maximum diacetyl concentration of 945 mg/L (Alvandi and Azar 2008). Similarly, a patented study by Duboff et al. (1996) have demonstrated that diacetyl yield of above 500 ppm could be achieved by fermentation of a pectin substrate using lactic acid bacteria (Duboff et al. 1996). Meanwhile, in different investigation it was observed that in the case of citrate-positive Lactococcus lactis diacetyl production could be enhanced by the addition of Cu2+ and Fe3+ and simultaneously, citrate uptake activity could be inhibited probably due to the accumulation of diacetyl (Kaneko et al. 1990a ). Redox potential of the culture media can play an important role in diacetyl production. According to Monnet et al. (1994), the redox potential of the culture drops towards the end of the process and for diacetyl production a high redox potential is preferable. Further, by constant agitation a relatively higher redox potential could be maintained as compared to an unagitated system and a higher diacetyl production could be obtained by a process with continuous agitation (Monnet et al. 1994). Likewise, initial oxygen concentration is another important factor in diacetyl production. Bassit et al. (1993) showed that diacetyl production could be improved by a factor of 18 by increasing the initial oxygen concentration from 0 to 100 % (Bassit et al. 1993). Furthermore, different genetic engineering strategies for improved diacetyl production are also known. According to Benson et al. (1996), the production of α-acetolactate, the intermediate of diacetyl production by pyruvate bioconversion, is catalyzed by two α-acetolactate synthases produced by Lactococcus lacti. The authors have used a plasmid-based gene expression strategy and 3.6-folds improvement in product formation has been reported (Benson et al. 1996). Boumerdassi et al. (1997) have used chemical mutagen N-methyl-N′-nitro-N-nitrosoguanidine to develop three mutant strains of Lactococcus lactis. The isolated mutants capable of diacetyl production were reported to have improved glucose utilization ability (Boumerdassi et al. 1997). Similarly, improved diacetyl production by genetic manipulation of Lactococcus lactis MG1363 has been reported by Swindell et al. (1996). According to the report, ilvBN – the genes encoding α-acetolactate synthase – was overexpressed in a mutant of Lactococcus lactis MG1363 and the resulting strain was able to produced higher level of the aroma compounds, such as acetoin and diacetyl (Swindell et al. 1996). Likewise, improved diacetyl and acetoin production by genetic manipulation of Lactobacillus casei has also been reported. Recently, Nadal et al. (2009) have mutated a L. casei strain having acetohydroxy acid synthase encoding ilvBN genes of Lactococcus lactis. According to the authors, the mutant was capable of improved production of diacetyl using whey permeate (Nadal et al. 2009). Similarly, application of yeast, such as Debaryomyces hansenii, is also known for diacetyl production (Deiana et al. 1990). From cheese, two strains of Debaryomyces hansenii have been isolated by Deiana et al. (1990). The production of the aroma compound by the isolated strains could vary depending upon the substrate as well as strain used (Deiana et al. 1990). Thus, from this discussion it could be concluded that considerable investigations on possible biotechnological production of diacetyl have been made and significant success have been achieved. Hence, diacetyl could be an ideal candidate for possible industrial production by using agro-industrial waste feed stocks. However, at the same time recent report on health hazards associated with vaporized diacetyl and its possible effect on diacetyl market should also be considered (Quach et al. 2012).
2.2 2-Phenylethanol
2-Phenylethanol is an aroma compound with rose-like fragrance which is widely used in cosmetics, food, and pharmaceutical industries (Hua and Xu 2011). Relatively small amount of it is also used in soft drinks and cookies for improving the flavor (Fabre et al. 1998; Etschmann et al. 2003). Biotransformation of l-phenylalanine by suitable yeast strain is mostly used for biotechnological production of 2-phenylethanol (Etschmann et al. 2003; Hua and Xu 2011; Achmon et al. 2011; Rong et al. 2011). Likewise, 2-phenylethanol can be extracted from the distillation residues of alcohol production (Savina et al. 1999). However, presently 2-phenylethanol is mostly produced by chemical synthesis. Benzene and styrene are the two raw materials mainly used for chemical synthesis of 2-phenylethanol; however, both of them are known as health/environmental hazards (Etschmann et al. 2003; Hua and Xu 2011). Flavors produced by such chemical processes are not considered as safe products and even their uses in food, beverages, and cosmetics have been restricted by European legislation (Xu et al. 2007). Similarly, botanical production of 2-phenylethanol is also known where the product is extracted from rose or essential oils; however, such processes are expensive (Etschmann et al. 2003). Thus, biotechnological production of food grade 2-phenylethanol has well prospective.
As shown in Table 5.1, Saccharomyces cerevisiae and Kluyveromyces marxianus are the two commonly used yeast strains for this purpose. Ehrlich pathway present in yeast, such as S. cerevisiae, can convert a range of amino acids to a variety of alcohols which are commonly known as fusel alcohols. Production of 2-phenylethanol by biotransformation of the amino acid l-phenylalanine is one common example of Ehrlich pathway. Moreover, recently a metabolically engineered Escherichia coli strain has been developed which can produce 2-phenylethanol along with five other aroma compounds (Koma et al. 2012). Further, from Table 5.1 it can be easily assumed that biotransformation of l-phenylalanine for 2-phenylethanol production has been extensively investigated by different researchers. Etschmann et al. (2003) have screened 14 different yeast strains for 2-phenylethanol production and according to the author, Kluyveromyces marxianus CBS 600 and CBS 397 were the two most productive strains (Etschmann et al. 2003). Similarly, Rong et al. (2011) have reported fed-batch production of 2-phenylethanol by using active dry yeast (S. cerevisiae). The authors also have investigated the catalytic effect of ethanol on the process and different parameters have been optimized (Rong et al. 2011). Similarly, Mameeva et al. (2010) have reported high (1.21 g/L) 2-phenylethanol production by S. cerevisiae UCM Y-514 and UCM Y-524 (Mameeva et al. 2010). In an interesting study by Wittmann et al. (2002), 13C labeled l-phenylalanine has been used as the substrate for 2-phenylethanol production by Kluyveromyces marxianus. Finally, the authors have concluded that during the process, 73.3 % of the labeled l-phenylalanine was converted to the major products (2-phenylethanol and 2-phenylethylacetate), 22.4 % was catabolized via the cinnamate pathway, and 4.3 % was utilized for protein synthesis (Wittmann et al. 2002). Further, Eshkol et al. (2009) have screened different thermotolerant and multi-stress resistant S. cerevisiae strains for improved 2-phenylethanol production. Growth rate, biomass dry weight, and product yields were considered as the basic criteria for the selection and according to the authors high biomass production could be correlated with high product concentration. Moreover, based on the investigation thermotolerant strain, Ye9-612 has been identified as the most efficient strain for 2-phenylethanol production among different strains considered (Eshkol et al. 2009). Thus, a number of successful investigations could be found for 2-phenylethanol production; however, most of the investigations were carried out in relatively small scale and pilot scale efficiency of the process needs to be evaluated. Similarly, from Table 5.1 it could also be observed that apart from l-phenylalanine, the process should be supplemented with relatively high amount of carbon source, such as glucose, sucrose, or molasses. Likewise, in situ product recovery (ISPR) is a commonly used technique for biotechnological production of 2-phenylethanol. Actually, accumulation of 2-phenylethanol in the culture media is inhibitory for further product formation and hence, it needs to be continuously removed during fermentation. Liquid-liquid two-phase system, application of hydrophobic microsphere, and application of resin for preferential simultaneous adsorption/separation of the product from the culture broth are different techniques presently used for in situ recovery of 2-phenylethanol. In a two-phase system developed by Etschmann et al. (2003), oleyl alcohol was used as the second liquid phase for in situ product recovery. According to the author the strategy was successful to improve 2-phenylethanol production and product yield as high as 3 g/L has been reported (Etschmann et al. 2003). In a recent investigation, Wang et al. (2011) have used resin FD0816 (by weight 10 % of the medium) to obtain the overall 2-phenylethanol concentration as high as 13.7 g/L (Wang et al. 2011). Similarly, Hua et al. (2010) have screened a range of macroporous adsorbent resins and resin HZ818 has been selected for in situ recovery of 2-phenylethanol. The authors also have indicated that resin HZ818 can preferably adsorbed 2-phenylethanol with minimum adsorption of l-phenylalanine, the parent amino acid. Further, by adding the selected resin 2-phenylethanol concentration was found to be improved by 66.2 % (Hua et al. 2010). Similarly, application of hydrophobic polymethylmethacrylate microspheres for in situ recovery of 2-phenylethanol has been reported by Achmon et al. (2011). Microspheres of 1.53 ± 0.10 μm diameter were used for continuous removal of the product by the mechanism of swelling. The authors have reported tenfolds improvement in 2-phenylethanol productivity for an in situ product recovery system containing nearly 10 % (w/v) of the microspheres (Achmon et al. 2011). Likewise, improved production of 2-phenylethanol using ISPR technology has also been reported by Rong et al. (2011). Sendovski et al. (2010) have used immiscible ionic liquids as the second phase in a two-phase system used for in situ recovery of 2-phenylethanol. Nine different liquids were screened based on their biocompatibility with respect to yeast and anions [Tf2N] were reported to be the most biocompatible nonaqueous phase among them. Further, the authors have also reported that 3–5-folds improvement could be achieved by the application of ISPR technique using a biocompatible immiscible ionic liquid (Sendovski et al. 2010). Serp et al. (2003) have successfully developed a highly absorbent and mechanically stable resin to facilitate ISPR and to increase the volumetric productivity of 2-phenylethanol by a factor of 2. Moreover, according to the authors, the product could be easily back extracted from the resin and unlike a two-liquid phase system, no stable emulsion would be formed during the process. Therefore, the authors have concluded that easier downstream processing and possible commercial application of the proposed system could be expected (Serp et al. 2003). In order to study ISPR of 2-phenylethanol, Etschmann et al. (2005) have demonstrated improved 2-phenylethanol production by introducing an organophilic pervaporation unit (having a polyoctylmethylsiloxane membrane) for ISPR of 2-phenylethanol from the bioreactor (Etschmann et al. 2005). Thus, it can be concluded that 2-phenylethanol has significant prospect as biotechnological product and it has been widely explored by different researchers mostly in last decade. Further, possible application of agro-industrial waste as a substrate for 2-phenylethanol production could be considered. Most importantly, as ISPR is widely used for product recovery and the downstream processing is relatively simple, application of a complex feedstock, such as agro-industrial waste should not be a concern. Biotechnological production of 2-phenylethanol has been reviewed in detail by Etschmann et al. (2002) and Hua and Xu (2011) and potential readers can consult those work for further detail.
2.3 Acetoin
Acetoin is another flavor compound with typical butter-like flavor (Xu et al. 2011 b). It can be found in wine, honey, coffee, and dairy products, such as butter, cheese, and fruits, such as strawberry and currants (Xu et al. 2011 b; Liu et al. 2011). Owing to its flavor, it is mostly used as artificial flavor enhancer in food products. Moreover, it is also a precursor/intermediate for the synthesis of valuable fine chemicals and widely used as platform chemical (Xu et al. 2011 b; Liu et al. 2011). Acetoin could be produced by traditional chemical processes; however, being a food additive its natural production has good commercial prospect. In recent years, biotechnological production of acetoin has been investigated by different researchers and considerable success has been achieved (Table 5.2).
In an interesting report, Kaneko et al. (1990b ) have reported that hemin (an iron-containing porphyrin) or Cu2+ can stimulate the activity of certain enzymes (e.g., diacetyl synthase) of acetoin production pathway and can improve acetoin production from glucose by Lactococci sp. (Kaneko et al. 1990 b). Similarly, Teixeira et al. (2002) have identified initial glucose concentration and temperature as the main parameters that can affect acetoin production by Hanseniaspora guilliermondii. According to the authors, 63 g/L initial glucose concentration and 28 °C incubation temperature were found to be optimum for acetoin production (Teixeira et al. 2002). Recently, the acetoin production by Bacillus licheniformis MEL09 has been investigated by Liu et al. (2011) and maximum acetoin concentration of 41.26 g/L was obtained (Liu et al. 2011).
Similarly, Sun et al. (2012) have used sucrose and corn steep-based media and concluded that the concentration of these two components has significant effect on acetoin production. Further, the authors have proposed a two-stage agitation strategy for improved acetoin production and maximum concentration of 60.5 g/L has been reported (Sun et al. 2012). Two-stage agitation strategy has also been tested for acetoin production by Bacillus amyloliquefaciens FMME044 (Zhang et al. 2012a). The authors showed that upon depletion of glucose level in the media, 2,3-butanediol (produced during the process) could be transformed to acetoin. Interestingly, lower agitation is suitable for 2,3-butanediol production whereas, higher agitation speed could be attributed to further transformation of 2,3-butanediol to acetoin. Therefore, the authors have used 350 rpm in first 24 h and it was increased to 500 rpm for rest of the fermentation to record improved acetoin production (Zhang et al. 2012a). Likewise, Zhang et al. have investigated acetoin production by newly isolated Paenibacillus polymyxa CS107. According to the authors, fed-batch fermentation using a 5 L fermenter was capable of achieving a maximum concentration of 55.3 g/L of acetoin (Zhang et al. 2012b). Considering possible industrial production of acetoin by fermentation technology, feasibility of pilot scale production of acetoin has also been investigated. Xu et al. (2011a ) have developed a mutant strain of Bacillus subtilis (TH-49) and investigated it for acetoin production by using a 100 L fermenter. The author have reported that Bacillus subtilis TH-49 was capable of producing acetoin as high as 56.9 g/L by using glucose as the main feedstock (Xu et al. 2011a ). Thus, based on above discussion it could be concluded that acetoin is an industrially important aroma compound and considerable success has been achieved for its large-scale production by fermentation technology. However, techno-economic feasibility of the process should be evaluated and the possibility of reducing the process cost should be explored.
2.4 Vanillin
It is one of the industrially produced aroma compounds, which is a crystalline powder in its isolated form (Priefert et al. 2001). Major application of vanillin is as flavoring agent in foods and beverages; however, a significant portion of vanillin produced in the world is also used in cosmetics and pharmaceutical products (Priefert et al. 2001). Vanilla planifolia, a vanillin-containing orchid is the major natural source of vanillin (Sindhwani et al. 2012). Similarly, other natural sources of vanillin are Peru balsam, essential oil of Java citronella and clove bud oil; however, it is mostly produced from guaiacol or lignin by using chemical processes (Priefert et al. 2001; Sindhwani et al. 2012). Alternatively, fermentative production of vanillin by biotransformation of ferulic acid, eugenol, and isoeugenol has been extensively investigated (Hua et al. 2007). Similarly, microbiological de novo synthesis of vanillin is also known (Hua et al. 2007). Due to high consumer perception, naturally prepared vanillin is considered more suitable as a food additive and hence, it has higher market value as compared to its synthetic version (Priefert et al. 2001; Hua et al. 2007; Sindhwani et al. 2012). A summary of the recent investigations on biotechnological production of vanillin is provided in Table 5.3.
For improved vanillin production by ferulic acid bioconversion, a genetically engineered E. coli strain (JM109) with genes from Pseudomonas fluorescens BF13 has been developed by Barghini et al. (2007). The recombinant strain was found to be capable of producing vanillin without accumulation of undesirable metabolites (Barghini et al. 2007).
In silico metabolic engineering strategy could be another interesting strategy for improved vanillin production. Recently, Brochado et al. (2010) have recently developed a metabolically engineered Saccharomyces cerevisiae strain and according to the authors, fivefold improvement in vanillin production has been achieved (Brochado et al. 2010). As already mentioned, similar to ferulic acid eugenol could also be used as a substrate for fermentative vanillin production. Sindhwani et al. (2012) have screened a range of eugenol-degrading bacteria for vanillin production. Based on their investigation the authors have identified Bacillus species strain BR as a novel eugenol-degrading bacterium capable of vanillin production (Sindhwani et al. 2012). Although, vanillin could be produced from more than one feedstock, all vanillin producing microorganisms are not capable of producing vanillin from different substrates. Bloem et al. (2007) have investigated vanillin production from different substrates by lactic acid bacteria. The authors have reported that tested lactic acid bacteria were not able to produce vanillin from the substrates, such as eugenol, isoeugenol, and vanillic acid. However, Lactobacillus sp. tested was capable of producing vanillin by using ferulic acid as the substrate (Bloem et al. 2007). Similarly, vanillin production by ferulic acid biotransformation has also been described by Sarangi et al. (2010). According to the authors, vanillin production by Staphylococcus aureus could be improved by nearly fourfold by the addition of glucose as the supplementary carbon source (Sarangi et al. 2010). Recently, Yiyong and Hong (2011) have demonstrated that final vanillin concentration of 15 g/L could be achieved by biotransformation of ferulic acid in a 50 L fermenter (Yiyong and Hong 2011).
Product inhibition is one common problem associated with fermentative production of vanillin. According to Hua et al. (2007), fed-batch production of vanillin by bioconversion of high concentration of ferulic acid could be problematic owing to substantial product inhibition (Hua et al. 2007). In situ product recovery could be considered as a potential technology to mitigate such problem for improved product yield. Hua et al. (2007) have screened a range of macroporous adsorbent resins for in situ recovery of vanillin produced during bioconversion of ferulic acid by Streptomyces sp. strain V-1. The results indicated that resin DM11 was the most efficient among tested adsorbents and was able to improve the productivity (Hua et al. 2007).
The compound isoeugenol could also be used as a substrate for vanillin production. Li et al. (2005) have investigated enzymatic conversion of isoeugenol into vanillin. According to the authors, the efficiency of the process could be improved by addition of an adsorbent to the process. Further, the authors have reported that a maximum vanillin concentration of 2.46 g/L could be achieved in 36 h using 10 g/L of powdered activated carbon as an adsorbent (Li et al. 2005). Less expensive downstream processing could be considered as a prerequisite for successful commercialization of biotechnological products. Vanillin is also not an exception and considerable success has been achieved in this regard. In a recent report, Badcock (2011) has described an organophilic pervaporation method for vanillin recovery from fermentation media. This method is a single step, sustainable, and solvent/adsorbent-free technique for efficient vanillin recovery (Badcock 2011).
Thus, it could be concluded that considerable success has been obtained for biotechnological vanillin production. However, possible application of agro-industrial waste as substrate could be considered as an option to alleviate the overall production cost.
3 Possible Application of Agro-Industrial Wastes for Aroma Compound Production
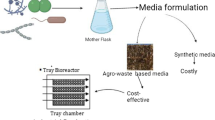
From above discussion it is evident that different investigations on aroma/flavor production by fermentation technology are remarkably successful. Further, it should be noted that in almost all such studies carbon sources, such as glucose, other synthetic supplementary media components, and precursor of the particular aroma compound, were unavoidably used (Tables 5.2 and 5.3). However, application of such synthetic media components can increase the process cost of flavor production. At the same time, it should be noted that aroma/flavor compounds produced by chemical processes are less expensive. Thus, biotechnologically produced products (aroma/flavor compounds) should at least have a reasonable price, if not lesser than the products produced by chemical processes. In this context, application of agro-industrial waste materials as the substrate for the production of aroma/flavor compounds could be considered. These are generally plant-based materials containing polysaccharides, proteins, and minerals (Gonçalves et al. 2011), and hence, they have great potential as the feedstock to be used for fermentation. Moreover, utilization of these materials may solve inherent environmental pollution and disposal problem associated with agro-industrial wastes (Chapla et al. 2010). Successful application of various agro-industrial wastes, such as sugarcane bagasse, sugar beet pulp, wheat stillage, apple pomace, and cassava bagasse for high-volume low-value products, such as ethanol, single cell protein, and mushrooms, could be found in the literature (Sasaki et al. 1991; Pandey et al. 2000; Davis et al. 2005; Ruanglek et al. 2006; Philippoussis 2009). However, relatively fewer reports on aroma/flavor compounds production using agro-industrial waste could be found in the literature and some of such reports are summarized in Table 5.4.
Fruity flavor production by Ceratocystis fimbriata has been studied by Soares et al. (2000). The authors have investigated possible application of coffee husk as the substrate for fruity aroma production using solid-state fermentation. Further, the report has also described the addition of glucose as additional carbon source as well as the effect of precursor (e.g., leucine, soya bean oil) and saline supplements on aroma production (Soares et al. 2000). According to the authors, acetaldehyde, ethanol, isopropanol, and ethyl acetate were the major volatile compounds identified in the headspace of the system. Similarly, production of other volatile compounds, such as ethyl isobutyrate, isobutyl acetate, isoamyl acetate, and ethyl-3-hexanoate, were also detected albeit at lower concentrations (Soares et al. 2000). Similarly, Christen et al. (1997) evaluated wheat bran, sugarcane bagasse, and cassava bagasse as possible substrates for aroma/flavor production by Ceratocystis fimbriata. According to the authors, sensory characteristics of the final product could vary with respect to the synthetic supplement added to the process and the aroma production process was found to be growth associated. Nearly 20 compounds including alcohols, aldehyde, esters, and ketones were detected in the headspace gas mixture of the process (Christen et al. 1997). In yet another report of aroma compound production by Ceratocystis fimbriata Rossi et al. (2009) have demonstrated that citric pulp, a waste generated during citric juice production could be successfully utilized for aroma compound production. The solid-state fermentation was carried out in 250 mL Erlenmeyer flasks and the results showed that application of supplementary carbon and nitrogen sources have a great role in improved aroma production using the waste feedstock. Soy molasses, soy bran, sugarcane molasses, and urea were used as supplementary nutrients, and it has been demonstrated that enrichment of the citric pulp with soy bran (50 %), sugarcane molasses (25 %), and mineral saline solution was found to have positive effect on the aroma production (Rossi et al. 2009). Likewise, a two-step process for vanillin production from waste residue of rice bran oil derived ferulic acid has been demonstrated by Zheng et al. (2007). According to the process, first vanillic acid was produced by Aspergillus niger CGMCC0774 and it was further bioconverted into vanillin by Pycnoporus cinnabarinus CGMCC1115 (Zheng et al. 2007). Edible fungus Rhizopus oryzae is known to produce volatile aroma compounds, such as acetaldehyde using solid agro-industrial waste as substrates. Bramorski et al. (1998) have demonstrated the production of volatile metabolites by R. oryzae using solid-state fermentation of various tropical agro-industrial waste substrates, such as amaranth grain and cassava bagasse (Bramorski et al. 1998).
The application of yeast strains for the production of aroma production from agro-industrial waste have been also attempted by different researchers. Medeiros et al. (2000) have studied aroma compound production from different agro-industrial wastes by the yeast Kluyveromyces marxianus and cassava bagasse and giant palm bran were found to be the potential substrates. The authors also have mentioned that addition of glucose as an additional carbon source was found to have significant effect on aroma compound production. Based on headspace gas analysis, the authors have reported that alcohols, aldehyde, and esters were present in the produced gas mixture and esters were responsible for its fruity aroma (Medeiros et al. 2000). From above discussion it could be concluded that fungi are the most investigated organism for biotechnological production of aroma compounds using agro-industrial wastes (Table 5.4). However, yeast strains could also be potentially used for this purpose. The application of bacteria for aroma compound production using agro-industrial by-products has also been researched. Xiao et al. (2007) have investigated agro-industrial by-products, such as molasses and soybean meal hydrolysate, as the feedstock for acetoin production by Bacillus subtilis CICC 10025. The authors have optimized the nutritional requirement by statistical techniques and concluded that molasses and soybean meal hydrolysate has remarkable influence on acetoin production with 99 % significant level (Xiao et al. 2007).
4 Concluding Remarks
Agro-industrial waste materials, such as cassava bagasse, giant palm bran, citric pulp, soya bran, and sugarcane molasses, have been successfully utilized for the production of a range of natural aroma/flavor compounds. Considering the nature of the feedstock, solid-state fermentation was the mostly used process for this purpose. Produced compounds were generally volatile; hence, complex nature of agro-industrial waste-based feedstock is not going to be a problem for downstream processing. Different fungal and bacteria as well as yeast strains are known to be used for aroma compound production using agro-industrial waste; however, fungi are the mostly used microorganisms. The fact that fungi can easily grow on solid feed stocks as they resemble their natural habitats could be a possible reason for such observation. Similarly, it is also observed that so far investigations were being carried out in relatively lab scale, using Erlenmeyer flasks. Therefore, scaling up of the process and an evaluation of its techno-economic feasibility are necessary. A range of volatile compounds may produce during the process and some of these compounds may be unwanted as an aroma compound. However, downstream processing of produced gas mixture is hardly dealt with in any reports and it will be interesting to explore it. Meanwhile, in the case of mostly studied aroma compounds such as, 2-phenylethanol and vanillin, product inhibition has been observed and therefore, in situ product recovery (ISPR) techniques are widely applied. Therefore, efficiency of such techniques should be demonstrated on industrial scale prior to their commercial application. Further, along with the precursor glucose is exclusively used as the supplementary carbon source for the production of the aroma compounds, such as 2-phenylethanol and vanillin. Hence, considering possible industrial production of such compounds by bioprocess technology, less expensive agro-industrial wastes could be evaluated as a replacement of glucose.
References
Achmon Y, Goldshtein J, Margel S, Fishman A (2011) Hydrophobic microspheres for in situ removal of 2-phenylethanol from yeast fermentation. J Microencapsul 28(7):628–638. doi:10.3109/02652048.2011.599443
Alvandi H, Azar M (2008) Diacetyl production in batch fermentation process by lactic starter culture. Iran J Food Sci Technol 5(2):27–39
Badcock M (2011) Sustainable recovery of pure natural vanillin from fermentation media in one step. http://blogs.rsc.org/gc/2011/08/01/sustainable-recovery-ofpure-natural-vanillin-from-fermentation-media-in-one-step/. Accessed on 30 Oct 2012
Barghini P, Di Gioia D, Fava F, Ruzzi M (2007) Vanillin production using metabolically engineered Escherichia coli under non-growing conditions. Microb Cell Fact 6(1):13
Bassit N, Boquien C-Y, Picque D, Corrieu G (1993) Effect of initial oxygen concentration on diacetyl and acetoin production by Lactococcus lactis subsp. lactis biovar diacetylactis. Appl Environ Microbiol 59(6):1893–1897
Benson K, Godon J, Renault P, Griffin H, Gasson M (1996) Effect of < i > ilvBN -encoded α-acetolactate synthase expression on diacetyl production in Lactococcus lactis. Appl Microbiol Biotechnol 45(1):107–111. doi:10.1007/s00253005065
Bicas JL, Silva JC, Dionísio AP, Pastore GM (2010) Biotechnological production of bioflavors and functional sugars. Ciência e Tecnologia de Alimentos 30(1):07–18
Bloem A, Bertrand A, Lonvaud‐Funel A, De Revel G (2007) Vanillin production from simple phenols by wine‐associated lactic acid bacteria. Lett Appl Microbiol 44(1):62–67
Boumerdassi H, Monnet C, Desmazeaud M, Corrieu G (1997) Isolation and properties of Lactococcus lactis subsp. lactis biovar diacetylactis CNRZ 483 mutants producing diacetyl and acetoin from glucose. Appl Environ Microbiol 63(6):2293–2299
Bramorski A, Christen P, Ramirez M, Soccol CR, Revah S (1998) Production of volatile compounds by the edible fungus Rhizopus oryzae during solid state cultivation on tropical agro-industrial substrates. Biotechnol Lett 20(4):359–362
Brochado AR, Matos C, Møller BL, Hansen J, Mortensen UH, Patil KR (2010) Improved vanillin production in baker’s yeast through in silico design. Microb Cell Fact 9(1):84
Chapla D, Divecha J, Madamwar D, Shah A (2010) Utilization of agro-industrial waste for xylanase production by Aspergillus foetidus MTCC 4898 under solid state fermentation and its application in saccharification. Biochem Eng J 49(3):361–369. doi:10.1016/j.bej.2010.01.012
Christen P, Meza J, Revah S (1997) Fruity aroma production in solid state fermentation by Ceratocystis fimbriata: influence of the substrate type and the presence of precursors. Mycol Res 101(8):911–919
Davis L, Jeon YJ, Svenson C, Rogers P, Pearce J, Peiris P (2005) Evaluation of wheat stillage for ethanol production by recombinant Zymomonas mobilis. Biomass Bioenergy 29(1):49–59
Deiana P, Cecchi L, Lodi R, Berardi E, Farris G, Fatichenti F (1990) Some aspects of diacetyl and acetoin production by Debaryomyces hansenii. Ital J Food Sci 2(1):35–42
Dastager SG (2009) Aroma compounds, biotechnology for agro-industrial residues utilisation. In: Pandey A, Nigam P (eds) Utilisation of Agro-Residues. Springer, Netherlands, pp 105–127. doi:10.1007/978-1-4020-9942-7_6
Duboff SA, Kwon SS, Vadehra DV (1996) Diacetyl production. EP Patent 0,564,770
Eshkol N, Sendovski M, Bahalul M, Katz‐Ezov T, Kashi Y, Fishman A (2009) Production of 2‐phenylethanol from L‐phenylalanine by a stress tolerant Saccharomyces cerevisiae strain. J Appl Microbiol 106(2):534–542
Etschmann M, Bluemke W, Sell D, Schrader J (2002) Biotechnological production of 2-phenylethanol. Appl Microbiol Biotechnol 59(1):1–8
Etschmann MMW, Sell D, Schrader J (2003) Screening of yeasts for the production of the aroma compound 2-phenylethanol in a molasses-based medium. Biotechnol Lett 25(7):531–536. doi:10.1023/a:1022890119847
Etschmann MMW, Sell D, Schrader J (2005) Production of 2-phenylethanol and 2-phenylethylacetate from L-phenylalanine by coupling whole-cell biocatalysis with organophilic pervaporation. Biotechnol Bioeng 92(5):624–634. doi:10.1002/bit.20655
Feron G, Bonnarme P, Durand A (1996) Prospects for the microbial production of food flavours. Trend Food Sci Technol 7(9):285–293. doi:10.1016/0924-2244(96)10032-7
Feron G, Waché Y (2006) Microbial biotechnology of food flavor production. Food Sci Technol 148:407, Marcel Dekker, New York
Fornachon J, Lloyd B (2006) Bacterial production of diacetyl and actoin in wine. J Sci Food Agric 16(12):710–716
Fabre C, Blanc P, Goma G (1998) 2-Phenylethyl alcohol: an aroma profile. Perfumer flavorist 23(3):43–45
Garc AI (1994) Modelling of diacetyl production during beer fermentation. J Inst Brew 100:179–183
Gonçalves FA, Sanjinez-Argandoña EJ, Fonseca GG (2011) Utilization of agro-industrial residues and municipal waste of plant origin for cellulosic ethanol production. J Environ Protect 2(10):1303–1309
Hua D, Ma C, Song L, Lin S, Zhang Z, Deng Z, Xu P (2007) Enhanced vanillin production from ferulic acid using adsorbent resin. Appl Microbiol Biotechnol 74(4):783–790. doi:10.1007/s00253-006-0735-5
Hua D, Lin S, Li Y, Chen H, Zhang Z, Du Y, Zhang X, Xu P (2010) Enhanced 2-phenylethanol production from L-phenylalanine via in situ product adsorption. Biocatal Biotransform 28(4):259–266. doi:10.3109/10242422.2010.500724
Hua D, Xu P (2011) Recent advances in biotechnological production of 2-phenylethanol. Biotechnol Adv 29(6):654–660. doi:10.1016/j.biotechadv.2011.05.001
Hugenholtz J, Kleerebezem M, Starrenburg M, Delcour J, De Vos W, Hols P (2000) Lactococcus lactis as a cell factory for high-level diacetyl production. Appl Environ Microbiol 66(9):4112–4114
Kaneko T, Watanabe Y, Suzuki H (1990a) Enhancement of diacetyl production by a diacetyl-resistant mutant of citrate-positive Lactococcus lactis ssp. lactis 3022 and by aerobic conditions of growth. J Dairy Sci 73(2):291–298. doi:10.3168/jds.S0022-0302(90)78672-9
Kaneko T, Takahashi M, Suzuki H (1990b) Acetoin fermentation by citrate-positive Lactococcus lactis subsp. lactis 3022 grown aerobically in the presence of hemin or Cu2+. Appl Environ Microbiol 56(9):2644–2649
Koma D, Yamanaka H, Moriyoshi K, Ohmoto T, Sakai K (2012) Production of aromatic compounds by metabolically engineered Escherichia coli with shikimate pathway expansion. Appl Environ Microbiol 78:6203–6216. doi:10.1128/aem.01148-12
Krings U, Berger R (1998) Biotechnological production of flavours and fragrances. Appl Microbiol Biotechnol 49:1–8
Li YH, Sun ZH, Zhao LQ, Xu Y (2005) Bioconversion of isoeugenol into vanillin by crude enzyme extracted from soybean. Appl Biochem Biotechnol 125(1):1–10
Liu Y, Zhang S, Yong YC, Ji Z, Ma X, Xu Z, Chen S (2011) Efficient production of acetoin by the newly isolated Bacillus licheniformis strain MEL09. Process Biochem 46(1):390–394
Longo MA, Sanromán MA (2006) Production of food aroma compounds: microbial and enzymatic methodologies. Food Technol Biotechnol 44(3):335–353
Mameeva O, Ostapchuk A, Podgorsky V (2010) The 2-phenylethanol and ethanol production by yeast Saccharomyces cerevisiae. http://www.nbuv.gov.ua/Portal/Chem_Biol/Mib/2010_1/2.pdf. Accessed on 17 Nov 2012
Medeiros ABP, Pandey A, Freitas RJS, Christen P, Soccol CR (2000) Optimization of the production of aroma compounds by Kluyveromyces marxianus in solid-state fermentation using factorial design and response surface methodology. Biochem Eng J 6(1):33–39. doi:10.1016/s1369-703x(00)00065-6
Monnet C, Schmilt P, Divies C (1994) Diacetyl production in milk by an α-acetolactic acid accumulating strain of Lactococcus lactis ssp. lactis biovar. diacetylactis. J Dairy Sci 77(10):2916–2924. doi:10.3168/jds.S0022-0302(94)77232-5
Nadal I, Rico J, Pérez-Martínez G, Yebra M, Monedero V (2009) Diacetyl and acetoin production from whey permeate using engineered Lactobacillus casei. J Ind Microbiol Biotechnol 36(9):1233–1237
Pandey A, Soccol CR, Nigam P, Soccol VT, Vandenberghe LPS, Mohan R (2000) Biotechnological potential of agro-industrial residues. II: cassava bagasse. Bioresour Technol 74(1):81–87
Philippoussis AN (2009) Production of mushrooms using agro-industrial residues as substrates. Biotechnology for agro-industrial residues utilisation. Springer, Netherlands, pp 163–196
Priefert H, Rabenhorst J, Steinbüchel A (2001) Biotechnological production of vanillin. Appl Microbiol Biotechnol 56(3):296–314. doi:10.1007/s002530100687
Quach AT, Liu C, Davies IY, Elston LD (2012) Toxicology report for Diacetyl. http://www.jakeheng.com/lulu.pdf. Accessed on 17 Nov 2012
Ramachandra Rao S, Ravishankar G (2000) Vanilla flavour: production by conventional and biotechnological routes. J Sci Food Agric 80:289–304
Rong S, Ding B, Zhang X, Zheng X, Wang Y (2011) Enhanced biotransformation of 2-phenylethanol with ethanol oxidation in a solid–liquid two-phase system by active dry yeast. Curr Microbiol 63(5):503–509. doi:10.1007/s00284-011-0008-0
Rossi S, Vandenberghe L, Pereira B, Gago F, Rizzolo J, Pandey A, Soccol C, Medeiros A (2009) Improving fruity aroma production by fungi in SSF using citric pulp. Food Res Int 42(4):484–486
Ruanglek V, Maneewatthana D, Tripetchkul S (2006) Evaluation of Thai agro-industrial wastes for bio-ethanol production by Zymomonas mobilis. Process Biochem 41(6):1432–1437
Sarangi PK, Nanda S, Sahoo H (2010) Maximization of vanillin production by standardizing different cultural conditions for ferulic acid degradation. NY Sci J 3(7):77–79
Sasaki K, Noparatnaraporn N, Nagai S, Martin A (1991) Use of photosynthetic bacteria for the production of SCP and chemicals from agroindustrial wastes. Bioconversion of waste materials to industrial products. Elsevier, New York, NY, pp 225–264
Savina JP, Kohler D, Brunerie P (1999) Method for extracting 2-phenylethanol. Google Patents
Sendovski M, Nir N, Fishman A (2010) Bioproduction of 2-phenylethanol in a biphasic ionic liquid aqueous system. J Agric Food Chem 58(4):2260–2265. doi:10.1021/jf903879x
Serp D, von Stockar U, Marison IW (2003) Enhancement of 2-phenylethanol productivity by Saccharomyces cerevisiae in two-phase fed-batch fermentations using solvent immobilization. Biotechnol Bioeng 82(1):103–110. doi:10.1002/bit.1054
Sindhwani G, Ilyas U, Aeri V (2012) Microbial transformation of eugenol to vanillin. J Microbiol Biotechnol Res 2(2):313–318
Soares M, Christen P, Pandey A, Soccol CR (2000) Fruity flavour production by Ceratocystis fimbriata grown on coffee husk in solid-state fermentation. Process Biochem 35(8):857–861
Sun J, Zhang L, Rao B, Han Y, Chu J, Zhu J, Shen Y, Wei D (2012) Enhanced acetoin production by Serratia marcescens H32 using statistical optimization and a two-stage agitation speed control strategy. Biotechnol Bioprocess Eng 17(3):598–605
Swindell SR, Benson KH, Griffin HG, Renault P, Ehrlich S, Gasson MJ (1996) Genetic manipulation of the pathway for diacetyl metabolism in Lactococcus lactis. Appl Environ Microbiol 62(7):2641–2643
Teixeira R, Cavalheiro D, Ninow J, Furigo A Jr (2002) Optimization of acetoin production by Hanseniaspora guilliermondii using experimental design. Braz J Chem Eng 19(2):181–186
Wang H, Dong Q, Guan A, Meng C, Xa S, Guo Y (2011) Synergistic inhibition effect of 2-phenylethanol and ethanol on bioproduction of natural 2-phenylethanol by Saccharomyces cerevisiae and process enhancement. J Biosci Bioeng 112(1):26–31. doi:10.1016/j.jbiosc.2011.03.006
Wittmann C, Hans M, Bluemke W (2002) Metabolic physiology of aroma‐producing Kluyveromyces marxianus. Yeast 19(15):1351–1363
Xiao Z, Liu P, Qin JY, Xu P (2007) Statistical optimization of medium components for enhanced acetoin production from molasses and soybean meal hydrolysate. Appl Microbiol Biotechnol 74(1):61–68
Xu P, Hua D, Ma C (2007) Microbial transformation of propenylbenzenes for natural flavour production. Trends Biotechnol 25(12):571–576
Xu H, Jia S, Liu J (2011a) Production of acetoin by Bacillus subtilis TH-49. In: Consumer Electronics, Communications and Networks (CECNet), International Conference, 2011, IEEE, pp 1524–1527
Xu H, Jia S, Liu J (2011b) Development of a mutant strain of Bacillus subtilis showing enhanced production of acetoin. Afr J Biotechnol 10(5):779–788
Yiyong DUYZZZ, Hong C (2011) A new bioprocess to produce natural vanillin by microbial fermentation. Flavour Frag Cosmet 3:003
Zhang Y, Li S, Liu L, Wu J (2012a) Acetoin production enhanced by manipulating carbon flux in a newly isolated Bacillus amyloliquefaciens. Bioresource Technol 130:256–260. http://dx.doi.org/10.1016/j.biortech.2012.10.036
Zhang L, Chen S, Xie H, Tian Y, Hu K (2012b) Efficient acetoin production by optimization of medium components and oxygen supply control using a newly isolated Paenibacillus polymyxa CS107. J Chem Technol Biotechnol 87(11):1551–1557
Zheng L, Zheng P, Sun Z, Bai Y, Wang J, Guo X (2007) Production of vanillin from waste residue of rice bran oil by Aspergillus niger and Pycnoporus cinnabarinus. Bioresour Technol 98(5):1115–1119
Acknowledgements
The authors are sincerely thankful to the Natural Sciences and Engineering Research Council of Canada (Discovery Grants 355254) and INRS-ETE for financial support. The views or opinions expressed in this article are those of the authors.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Sarma, S.J., Dhillon, G.S., Hegde, K., Brar, S.K., Verma, M. (2014). Utilization of Agro-industrial Waste for the Production of Aroma Compounds and Fragrances. In: Brar, S., Dhillon, G., Soccol, C. (eds) Biotransformation of Waste Biomass into High Value Biochemicals. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8005-1_5
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8005-1_5
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8004-4
Online ISBN: 978-1-4614-8005-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)