Abstract
Enzymes are important in all living cells because they act as biological catalysts that accelerate chemical reactions without being consumed in the process. Enzymes are crucial elements of every living entity and also address the dominant underlying causes of several health problems. Pharmaceutically important enzymes are an important component of the pharmaceutical market. They are broadly defined as prodrugs that target a specific biological reversible or irreversible reaction to treat a particular disease. Microorganisms are major source of pharmaceutically important enzymes, but several enzymes are also obtained from animal and renewable plant sources. Enzymes which are used for pharmaceutical applications include cysteine proteinases, asparaginase, streptokinase, urokinase, deoxyribonuclease I, hyaluronidase, pegademase, and glucocerebrosidase. Immobilized enzymes are also used in pharmaceutical industry. In pharmaceutical industry, the major applications of immobilized enzymes are the production of 6-aminopenecillinic acid using immobilized penicillin amidase which helps in the deacylation of the side chain of either penicillin G or penicillin V. There are several benefits of enzymes immobilization such as cost-effectiveness, protection from degradation and deactivation, retention of enzyme, enhanced stability, recycling, and repetitive use. The industrially important enzymes, such as α-amylase, protease, and alkaline lipase, are required in large volumes, but have an inherently low unit value so that they demand significantly lower manufacturing cost. On the other hand, pharmaceutical enzymes are produced in lower volumes and have inherently higher manufacturing cost.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Enzymes are biomolecules that catalyze and accelerate chemical reactions. Almost all processes in a biological cell require enzymes in order to occur at desired place. Enzymes are extremely selective for their substrates, and their activity is regulated by factors like substrate concentration, pH, and temperature. Enzyme activity is determined by the quantity of substrate transformed or product formed per unit time. The reaction rate depends on a number of experimental conditions, such as temperature, pH, ionic strength, and the presence or absence of inhibitors or activators. All these attributes make the enzymes important therapeutic tools offering a diversification platform to pharmaceutical industry. For example, adenosine deaminase is highly specific towards its substrate and can be potentially used to treat severe combined immunodeficiency disease (SCID) (Aiuti 2002). Nowadays, the application of enzyme technologies to pharmaceutical research, development, and manufacturing is a growing field. Unlike common medicinal products which can temporarily solve the particular health problems, pharmaceutical enzymes address the underlying cause of health problem and the patient can achieve permanent relief.
Although pancreatic enzymes and pepsin and papain were vogue for therapeutic use even before 1940, the concept of the therapeutic enzymes is only about 40 years old. A therapeutic enzyme was described as part of replacement therapies for genetic deficiencies in the 1960s by de Duve (de Duve 1996). The industrial enzymes are required in bulk and absolute purity is not essential, whereas pharmaceutical enzymes are required in small quantities but in absolutely pure form. Enzymes and enzyme-generated products are administered to patients in very small dose to avoid possible side effects (Vellard 2003). The recombinant enzyme drug, Activase (recombinant human tissue plasminogen activator), used for removal of blockage of a coronary artery by a clot, was approved by the Food and Drug Administration (FDA), USA, in 1987 (Vellard 2003). After insulin in 1982, this was the second recombinant protein drug to be marketed. Adagen (pegademase bovine) was the first therapeutic enzyme considered as orphan drug approved by the FDA, USA, in 1990 under the Orphan Drug Act (Vellard 2003). The drug was particularly used for the treatment of severe combined immunodeficiency disease (SCID), which is caused by the chronic deficiency of ADA (adenosine deaminase) (Hershfield et al. 1993). The Orphan Drug Act was passed in 1983 in the United States to encourage pharmaceutical companies to develop treatments for rare medical diseases affecting only small number of people (<2,00,000). Under this act, companies that develop such a drug may sell it without competition for 7 years and may get clinical trial tax incentives. The orphan drugs can be defined as those drugs intended to treat either a rare disease or a more common disease where manufacturer cannot expect to make profits. The orphan diseases are often so rare that a physician may observe only one case a year or less (Sharma et al. 2010).
2 Structure of Enzyme
Enzymes have a complex globular three-dimensional structure and their activity depends upon their three-dimensional structure. A part of enzyme comprising 3–4 amino acids is called active site which is involved in substrate binding and catalysis. The shape and chemical environment of the active site facilitates the enzyme reaction. The enzymes are very specific to substrates because both enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another. Some enzymes do not need any additional components to show full activity. However, others require nonprotein molecules called cofactors for their activity. Cofactors may be inorganic ions such as Fe2+, Mg2+, Mn2+, and Zn2+ and organic or metallo-organic molecules. Cofactors are classified depending upon how tightly they bind to an enzyme. Dissociable (loosely bound) cofactors are called as coenzymes and non-dissociable (tightly bound) cofactors as prosthetic groups. The inactive enzyme, without the cofactor, is called an apoenzyme, while the complete enzyme with cofactor is called holoenzyme.
3 Nomenclature and Classification of Enzymes
Enzymes are generally classified into six groups on the basis of the type of reactions they catalyze. Since the nomenclature was determined by the Enzyme Commission in 1961 updated in 1992, all enzymes have been assigned an “EC” number:
-
(a)
EC 1 Oxidoreductases: catalyze oxidation/reduction reactions.
-
(b)
EC 2 Transferases: transfer a functional group (e.g., a methyl or phosphate group).
-
(c)
EC 3 Hydrolases: catalyze the hydrolysis of various bonds.
-
(d)
EC 4 Lyases: cleave various bonds by means other than hydrolysis and oxidation.
-
(e)
EC 5 Isomerases: catalyze isomerization changes within a single molecule.
-
(f)
EC 6 Ligases: join two molecules with covalent bonds.
More than 3,000 human enzymes have been identified and named. Except for some of the originally studied enzymes like pepsin, rennin, and trypsin, most enzymes have a suffix “ase” which is attached to the name of substrate on which it acts like lactase as the enzyme breakdown disaccharide lactose to monosaccharide glucose. In some cases the type of reaction is also added to the name like DNA polymerases synthesizes the assembly of the DNA. Most of the pharmaceutical enzymes belong to the EC 3 hydrolase group, such as galsulfase. However, some pharmaceutical enzymes, such as rasburicase, belong to the oxidoreductase group.
4 Biopharmaceutical Enzymes
The enzymes used as drugs have two important features which distinguish them from the conventional drugs, such as:
-
1.
Unlike drugs they bind and act on their targets with great affinity.
-
2.
They are highly specific and act as catalyst to convert multiple target molecules to the desired products.
These two features make enzymes specific and potent drugs that can accomplish therapeutic biochemistry in the body that small molecule (synthetic active ingredient) cannot. The catalytic activity of enzymes is exploited in industrial manufacturing of drugs. Enzymes are also used as digestive aid where they are used to supplement digestive enzymes like amylase, lipase, and protease. Almost all enzyme therapies developed till date deal with the genetic disorders. Also the enzyme replacement therapy is used for relatively rare, inborn error of metabolism (Germain 2002; Chan et al. 2005). Several enzymes are also used to prevent and treat common diseases like heart attack and stroke (Longstaff et al. 2008). The enzyme collagenase has been reported for healing burn wound in children and enzyme chondroitinase ABC in the treatment of spinal cord injury (Ozcan et al. 2002; Bradbury et al. 2002). The enzymes used in pharmaceutical industry are listed in Table 15.1. Several enzymes are also used as prodrug, a drug that is administered in an inactive or significantly less active form, but once administered it is metabolized in vivo into an active metabolite through bioactivation process. The prodrug is generally used for absorption, distribution, optimize excretion, and complete metabolism of drug (Stella et al. 1985). There are several advantages associated with the prodrugs:
-
1.
Increased absorption and elimination of unpleasant taste.
-
2.
Decreased toxicity and metabolic inactivation.
-
3.
Increased chemical stability and prolonged or short-lived action.
The prodrugs can be classified in two groups:
-
(a)
Carrier-linked prodrugs: It contains a group that can be easily removed enzymatically (such as an ester) to reveal the true drug. For example, dipivefrine is prodrug of epinephrine used to treat glaucoma (Fig. 15.1a). The dipivaloyl esters allow for greater corneal permeability which is hydrolyzed by corneal and aqueous humor esterases. It is available as ophthalmic solution. It causes vasoconstriction and converted to epinephrine upon penetration of the cornea (Nakamura et al. 1993).
Fig. 15.1 (a) Structure of dipivefrine. (b) Structure of sulfasalazine. Available from http://www.drugbank.ca/drug
-
(b)
Bioprecursor prodrugs: These are metabolized into a new compound which may itself be active or is further metabolized into an active metabolite (e.g., amine to aldehyde to carboxylic acid). Sulfasalazine (Fig. 15.1b) is used in the treatment of ulcerative colitis, an inflammatory bowel disease, and rheumatoid arthritis (McGirt et al. 2006). Anaerobic bacteria in the lower bowel metabolically reduce sulfasalazine to the therapeutic agent 5-aminosalicylic acid. It is often well tolerated, but sometimes may cause severe depression in young males.
5 Application of Enzymes as Pharmaceuticals
5.1 Galsulfase
Galsulfase is a recombinant human N-acetylgalactosamine 4-sulfatase and was used in enzyme replacement therapy for the treatment of mucopolysaccharidosis VI (MPS VI) (Harmatz et al. 2004). Mucopolysaccharidosis VI (MPS VI) or Maroteaux-Lamy syndrome is a rare recessive lysosomal storage disease (LSD) resulting from a deficiency in the enzyme N-acetylgalactosamine 4-sulfatase. N-acetylgalactosamine 4-sulfatase is a soluble monomeric protein with a molecular weight of 56 kDa. The glycosylated product has an apparent molecular weight of 66 kDa on SDS-PAGE. The predicted amino acid sequence and the nucleotide sequence of the recombinant enzyme are identical to native human N-acetylgalactosamine 4-sulfatase (also known as arylsulfatase B, EC 3.1.6.12) (Bond et al. 1997). The enzyme is responsible for hydrolysis of the sulfate moiety of the glycosaminoglycan (GAG) dermatan sulfate during its stepwise degradation. Deficiency of N-acetylgalactosamine-4-sulfatase leads to the accumulation of its substrate, dermatan sulfate, in the lysosomes of many cell types (Neufeld and Muenzer 2001). The accumulation causes a progressive disorder with multiple organ and tissue involvement.
Recombinant-engineered galsulfase supplies N-acetylgalactosamine 4-sulfatase and catalyzes the cleavage of the sulfate ester from terminal N-acetylgalactosamine 4-sulfate residues of GAG chondroitin 4-sulfate and dermatan sulfate. Increased catabolism of GAG in turn reduces systemic dermatan sulfate accumulation, thereby reducing the primary symptoms of MPS VI. This drug was approved by the FDA, USA, in May 2005 for the treatment of patients with mucopolysaccharidosis type VI. It is the first approved product for the treatment of mucopolysaccharidosis type VI and has been granted orphan drug status (Hopwood et al. 2006). Galsulfase treatment is well tolerated although some patients developed antibodies against the enzymes and also some less serious side effects such as headache, joint pain, and eye redness were observed. Naglazyme is a formulation of galsulfase by BioMarin Pharmaceuticals Inc., USA, which is a purified human enzyme that is produced by recombinant DNA technology in a Chinese hamster ovary cell line (White et al. 2008).
5.2 Asparaginase
Asparaginase (E.C.5.1.1) is widely used in the treatment of childhood acute lymphoblastic leukemia (ALL). Asparaginase hydrolyzes l-asparagine to l-aspartic acid and ammonia in leukemic cells, resulting in the depletion of asparagine, inhibition of protein synthesis, cell cycle arrest in the G1 phase, and apoptosis in susceptible leukemic cell populations (McCredie et al. 2008). The asparaginases isolated from Escherichia coli (EcA) and Erwinia chrysanthemi (EcA) are useful antileukemic agents (Hill et al. 1967). In a significant number of patients with acute leukemia, particularly lymphocytic, the malignant cells depend on an exogenous source of asparagine for survival. Normal cells, however, are able to synthesize asparagine and thus are least affected by the rapid depletion produced by treatment with the enzyme asparaginase. l-asparaginase, intravenously injected into patients, is primarily distributed in the plasma (Ho et al. 1970). A study revealed that, in patients with metastatic cancer and leukemia, initial plasma levels of l-asparaginase following intravenous administration were correlated to dose. Daily administration resulted in a cumulative increase in plasma levels (Ho et al. 1970). Elspar (asparaginase) contains the enzyme l-asparaginase amidohydrolase derived from Escherichia coli and marketed by Merck & Co., Inc., USA. Asparaginase is also marketed by other two companies, Lundbeck Inc., USA, and Prescript Pharmaceuticals, USA.
Since asparaginase has relatively short half-life (10–0 h), PEGylated asparaginase (pegaspargase) with 10–15 times longer half-life was developed during 1970–1980. Indeed the asparaginase also has other disadvantages, like the need for frequent intramuscular injection and a very high rate of allergic reaction (Graham 2003). To overcome this problem, l-asparaginase is modified by covalently conjugating units of monomethoxy polyethylene glycol (PEG), molecular weight of 5,000, to the enzyme, forming the active ingredient PEG-l-asparaginase. Pegaspargase is more effective than asparaginase and facilitates production of oxaloacetate which is needed for general cellular metabolism (Wetzler et al. 2007). This particular drug is marketed by Ben Venue Laboratories Inc., USA, and Enzon Inc., USA.
5.3 Dornase Alfa
Dornase alfa is a highly purified recombinant human deoxyribonuclease I (rhDNase) used in the treatment of cystic fibrosis, a most common lethal recessive disorder in white population, and is caused by a defective cystic fibrosis transmembrane conductance regulator and a chloride channel protein, leading to improper salt balance and thick tenacious secretions (Collins 1992). Dornase alfa is produced by genetically engineered Chinese hamster ovary cells containing DNA encoding for the native human deoxyribonuclease I (DNase) (EC 3.1.21.1), an enzyme which selectively cleaves DNA. The enzyme contains 260 amino acids with an approximate molecular weight of 37 kDa (Shak et al. 1990). The primary amino acid sequence is identical to that of the native human deoxyribonuclease I. Dornase alfa hydrolyzes the DNA present in sputum/mucus of cystic fibrosis patients and reduces viscosity in the lungs, promoting improved clearance of secretions (Shah et al. 1996). The enzyme does not appear to affect sputum in the absence of an inflammatory response to infection nor does it affect the sputum of healthy individuals. Clinical trials and observation studies on the efficacy of dornase alfa (commonly known as Pulmozyme) inhalatory therapy have shown improvement in lung function and a decrease of respiratory exacerbations in patients with cystic fibrosis and moderate lung disease (Suri et al. 2001). This drug is well tolerated but some side effects such as chest pain and skin rashes have been reported (Davies et al. 1997).
5.4 Agalsidase
Agalsidase is used in enzyme replacement therapy for Anderson-Fabry disease, which is an X-linked defect of glycosphingolipid metabolism (El Dib and Pastores 2010). The primary driver of the disease is the accumulation of glycolipids (globotriaosylceramide [GL-3]) in a variety of cell types, including vascular endothelial cells, a range of renal cell types, cardiomyocytes, and neurons, which is caused by deficient activity of the lysosomal enzyme, alpha-galactosidase (Schaefer et al. 2009). Clinical manifestations of Fabry disease include renal failure, cardiomyopathy, and cerebrovascular accidents.
Fabrazyme (agalsidase beta) is a recombinant human α-galactosidase A enzyme (EC 3.2.1.22), responsible for the breakdown of alpha-galactosides in the lysosome (Guce et al. 2010) with the same amino acid sequence as the native enzyme. Fabrazyme (agalsidase beta) is produced by recombinant DNA technology in a Chinese hamster ovary mammalian cell expression system (Schaefer et al. 2009) and marketed by Genzyme Inc., USA. Fabrazyme is intended to provide an exogenous source of alpha-galactosidase A and to limit the accumulation of the glycolipids in the tissues. This drug also has side effects such as difficulty in breathing, choking of throat, hives, rashes, itching, and fever.
5.5 Therapeutic Protein Inhibitors of Elastase
Human neutrophil elastase is a trypsin-type serine protease (EC 3.4.21.37). The key physiological role of neutrophil elastase is in innate host defense. It can also participate in tissue remodeling and possesses secretagogue actions that are now recognized as important to local inflammatory responses (Chughtai and O’Riordan 2004). A number of potent reversible and irreversible inhibitors of neutrophil elastase have been developed for its potential therapeutic use:
-
1.
Pre-Elafin: The drug pre-elafin, also known as trappin-2, is an elastase-specific inhibitor that could be an ideal candidate for the treatment of neutrophil elastase-driven lung diseases. Neutrophil elastase (EC 3.4.21.37) is a potent serine protease involved in host’s defense. The inhibitory activity of pre-elafin resides in its COOH-terminal region that can be released as mature elafin (Tremblay et al. 2002).
-
2.
Prolastin: Prolastin is an alpha-1 proteinase inhibitor marketed by Talecris Biotherapeutics C (Bayer, Germany). Its primary mechanism is inhibiting the action of the serine protease called elastase in the lungs, and its deficiency is associated with progressive ultimately fatal emphysema (Karnaukhova et al. 2006).
5.6 Pancrelipase
Exocrine pancreatic insufficiency is a serious condition which occurs in several diseases including chronic pancreatitis, cystic fibrosis, pancreatic cancer, and post-pancreatic surgery (Sikkens et al. 2010). Pancreatic enzymes are essential for digestion of food as it can be absorbed by the body. In the case of chronic pancreatitis or pancreatic cancer, the pancreas may not produce enough enzymes for complete digestion of food. As a result the food was not completely absorbed by the body. This phenomenon is called malabsorption (Fieker et al. 2011). The major malabsorption problems arise from incomplete fat digestion. Pancrelipase or pancreatic enzyme replacement therapy was used to treat malabsorption. It is a protein mixture isolated from porcine or bovine pancreas, sometimes called pancreatin, and contains three enzymes, amylase (to digest starchy carbohydrate), lipase (to digest fat), and protease (to digest protein). Exogenous pancrelipase reduces the amount of nitrogen and fat excreted in the stool, but the overdose of pancrelipase causes diarrhea or stomach upset (Fieker et al. 2011). The drug is marketed by the brand names of Creon, Nutrizym, Pancrease HL, or Pancrex.
5.7 Imiglucerase
Imiglucerase is used for the treatment of type 1 Gaucher disease, characterized by a functional deficiency in human β-glucocerebrosidase enzymatic activity (Weinreb et al. 2005). β-Glucocerebrosidase (β-d-glucosyl-N-acylsphingosine glucohydrolase, EC 3.2.1.45) is a lysosomal glycoprotein enzyme which catalyzes the hydrolysis of the glycolipid glucocerebroside to glucose and ceramide. Absence of the enzyme, β-glucocerebrosidase, causes accumulation of lipid glucocerebroside in tissue macrophages which become engorged which are termed as Gaucher cells. Injection of imiglucerase into Gaucher disease patients leads to elevated levels of the enzyme in serum and reduction in the accumulation of glucocerebroside which reduces anemia, thrombocytopenia, spleen and liver size, and decreased cachexia (Pastores et al. 2004).
Imiglucerase is marketed as Cerezyme by Genzyme Inc., USA, and produced by recombinant DNA technology using Chinese hamster ovary. Purified imiglucerase is a monomeric glycoprotein of 497 amino acids, containing four N-linked glycosylation sites. Cerezyme differs from placental glucocerebrosidase by one amino acid at position 495, where histidine is substituted for arginine. This drug was approved by the FDA in 1994 and more than 5,600 patients in 90 countries have been treated with Cerezyme (Weinreb et al. 2005). Cerezyme is given intravenously, and its application leads to a marked improvement in the clinical manifestation of the Gaucher disease (Pastores et al. 2004). General side effects, such as fatigue, headache, abdominal pain, fever, dizziness, chills, and backache, have been reported but only in 1.5 % patients (Ali et al. 2011).
5.8 Pegademase Bovine
The drug, pegademase, is bovine adenosine deaminase (EC 3.5.4.4) enzyme derived from bovine intestine. It is used for enzyme replacement therapy for treating severe combined immunodeficiency disease (SCID) associated with the deficiency of adenosine deaminase (ADA) (Polmar et al. 1975). The enzyme has been extensively PEGylated for extended serum half-life. It is a conjugate of numerous strands of monomethoxy polyethylene glycol (molecular weight 5,000), covalently attached to the enzyme adenosine deaminase (Hershfield et al. 1987). The enzyme adenosine deaminase is responsible for converting adenosine to inosine. In the absence of adenosine deaminase, the purine substrate adenosine, 2′-deoxyadenosine and its metabolites are actually toxic to lymphocytes thereby leading to diminished immune function. Pegademase converts 2′-deoxyadenosine to 2′-deoxyinosine via deamination (Pesu et al. 2005). Severe combined immunodeficiency disease (SCID) is a primary immune deficiency caused by several genetic defects in the immune system (Hershfield and Mitchell 1995). Adagen (pegademase bovine) injection was the first successful application of enzyme replacement therapy for an inherited disease (Hershfield et al. 1987) and approved in 1990 by the FDA, USA. Adagen has been used to treat nearly 150 patients with ADA-deficient SCID worldwide. The possible side effects of this drug are allergic reactions like difficulty in breathing, choking of throat, swelling of the lips, tongue, face, hives, and signs of infection such as sore throat, fever, or congestion.
5.9 Tissue Plasminogen Activators
Tissue plasminogen activator (tPA) (EC 3.4.21.68) is a serine protease found on endothelial cells and catalyzes the conversion of plasminogen to biologically active plasmin. It is the main enzyme for breaking down the blood clots (fibrin clots) and allows blood flow to the affected areas, thus preventing brain damage (Tsurupa and Medved 2001; Imming et al. 2006). As a protease, tPA plays a crucial role in regulating blood fibrinolysis, maintaining the homeostasis of extracellular matrix and modulating the posttranslational activation of growth factors. tPA is found not only in the blood, where its primary function is as a thrombolytic enzyme, but also in the central nervous system (CNS). It participates in a number of physiological and pathological events in the CNS, as well as the role of neuroserpin as the natural regulator of tPA’s activity in these processes.
Tissue plasminogen activator (tPA) is used in clinical medicine as a thrombolytic agent to treat embolic or thrombotic diseases (Longstaff et al. 2008), like pulmonary embolism (blockage of the main artery of the lung or one of its branches by a substance that has travelled from elsewhere in the body through the bloodstream), myocardial infarction, heart attack (results from the interruption of blood supply to a part of the heart), and stroke or cerebrovascular accident (rapid loss of brain function(s) due to disturbance in the blood supply to the brain) (Del Zoppo et al. 2009). Some of the examples of tissue plasminogen activators used clinically and also approved by the FDA, USA, are mentioned below. About one third of the patients treated with intravenous thrombolytic therapy exhibited an improvement poststroke (Saver 2004).
5.9.1 Alteplase
Alteplase binds to fibrin-rich clots via the fibronectin finger-like domain and the kringle-2 domain. The protease domain then cleaves the Arg/Val bond in plasminogen to form plasmin. Plasmin in turn degrades the fibrin matrix of the thrombus, thereby exerting its thrombolytic action. It also produces limited conversion of plasminogen in the absence of fibrin. This drug is marketed by the name of Activase and used in all thrombolytic diseases such as acute myocardial infarction, acute ischemic stroke, and lysis of acute pulmonary emboli (Hacke et al. 2008). It is synthesized using the complementary DNA (cDNA) for natural human tissue-type plasminogen activator obtained from a human melanoma cell line. The manufacturing process involves secretion of alteplase into the culture medium by an established Chinese hamster ovary cell lines into which the cDNA for alteplase had been genetically inserted.
5.9.2 Reteplase
Reteplase is a recombinant non-glycosylated form of human tissue plasminogen activator, which has been modified to contain 357 of the 527 amino acids of the native human tPA (amino acids 1–3 and 176–527) and retained the activity-related kringle-2 and serine protease domains of human tPA. Retavase is considered a “third-generation” thrombolytic agent, genetically engineered to retain and delete certain portions of human tPA. Reteplase is similar to alteplase but the modifications give reteplase a longer half-life of 13–16 min. It is produced by recombinant DNA technology in E. coli and was marketed by the name of Retavase. The protein is isolated as inactive inclusion bodies from E. coli, converted into active form by an in vitro folding process and purified by chromatographic separation. Reteplase also binds fibrin with lower affinity than alteplase, improving its ability to penetrate into clots. It works best to help people with strokes caused by clots (ischemic strokes) when it is given right away after the stroke symptoms begin (Hilleman et al. 2007).
5.9.3 Tenecteplase
Tenecteplase is a 527 amino acid glycoprotein developed by introducing the following modifications to the complementary DNA (cDNA) for natural human tPA: a substitution of threonine 103 with asparagine and a substitution of asparagine 117 with glutamine, both within the kringle-1 domain and a tetra-alanine substitution at amino acids 296–299 in the protease domain (Ohman et al. 2005). The brand name of this drug is TNKase and is used for the treatment of myocardial infarction and lysis of intracoronary emboli. It binds to fibrin-rich clots and cleaves the Arg/Val bond in plasminogen to form plasmin (Gurbel et al. 2005). Plasmin in turn degrades the fibrin matrix of the thrombus, thereby exerting its thrombolytic action. This helps to remove blood clots and arterial blockages that cause myocardial infarction. Tenecteplase is known to have a long half-life; therefore, a single bolus injection is sufficient for the treatment (Davydov and Cheng 2001).
5.9.4 Anistreplase
Anistreplase is another human tissue plasminogen activator and is used to eliminate blood clots or arterial blockages that cause myocardial infarction. It cleaves the Arg/Val bond in plasminogen to form plasmin. Plasmin in turn degrades the fibrin matrix of the thrombus, thereby exerting its thrombolytic action. It was marketed by the name of Eminase by Wulfing Pharma GmbH, Germany. Eminase is a complex of Lys-plasminogen (p-anisoyl derivative of the primary Lys-plasminogen) and streptokinase (Lee et al. 2004). A p-anisoyl group is chemically conjugated to a complex of bacteria-derived streptokinase and human plasma-derived Lys-plasminogen proteins.
5.10 Urokinase
Urokinase (EC 3.4.21.73) is also a serine protease, which specifically cleaves the Arg-Val bond in inactive plasminogen to form active plasmin. Urokinase was originally isolated from human urine, but is present at several physiological locations, such as blood stream and the extracellular matrix. Activation of plasmin triggers a proteolysis cascade that, depending on the physiological environment, participates in thrombolysis and extracellular matrix degradation. Urokinase is used for the treatment of pulmonary embolisms (Overington et al. 2006). It is available by the name of Abbokinase and Kinlytic and administered by intravenous infusion. It has some side effects such as epistaxis, bleeding gums, and bloody or tarry stools (http://www.rxlist.com/cgi/generic2/tenecteplase.htm).
5.11 Streptokinase
This is another thrombolytic agent and was also used in the treatment of acute evolving transmural myocardial infarction, pulmonary embolism, deep vein thrombosis, arterial thrombosis or embolism, and occlusion of arteriovenous cannulae (Meneveau et al. 1997). Streptokinase (EC 3.4.99.0) creates an active complex to form the proteolytic enzyme plasmin. Streptokinase forms a highly specific 1:1 active enzymatic complex with plasminogen which also cleaves the Arg/Val bond in plasminogen (Mundada and Prorok 2003) and converts inactive plasminogen molecules into active plasmin (Sikri and Bardia 2007). Plasmin degrades fibrin clots as well as fibrinogen and other plasma proteins. Streptokinase is marketed under the name of Streptase. It is a purified preparation of a bacterial protein elaborated by group C (beta)-hemolytic streptococci. Streptokinase in some cases has been shown to be more effective than tissue plasminogen activator alteplase (Capstick and Henry 2005).
5.12 Bromelain
Bromelain is a proteolytic enzyme found in pineapple juice and stems that offers variety of health benefits. Bromelain can be taken to support healthy digestion and for treating inflammatory, cardiovascular, and skin disorders. The two main enzymes are stem bromelain (EC 3.4.22.32) and fruit bromelain (EC 3.4.22.33). Bromelain is used for treating osteoarthritis (Brien et al. 2004) and is also a promising anti-inflammatory agent (Fitzhugh et al. 2008).
5.13 Hyaluronidase
Hyaluronidase (EC 3.2.1.35) is used in conjunction with other drugs to speed their dispersion and delivery of medicines. Hyaluronidase hydrolyzes hyaluronic acid by splitting the glucosaminidic bond between C1 of the glucosamine moiety and C4 of glucuronic acid. This temporarily decreases the viscosity of the cellular cement and increases diffusion of injected fluids and localized transudates and exudates, facilitating their absorption (Csoka et al. 1999). It also increases the absorption rate of parenteral fluids given by hypodermoclysis and is an adjunct in subcutaneous urography for improving resorption of radiopaque agents. The drug has been branded as Hydase™ (animal-derived hyaluronidase), Vitrase (marketed by ISTA Pharmaceuticals, USA), and Amphadase (marketed by Amphastar Pharmaceuticals, USA).
5.14 Rasburicase
Rasburicase is derived from a cDNA code from a modified Aspergillus flavus strain and expressed in a modified yeast strain of Saccharomyces cerevisiae (Collings et al. 2010). It is recombinant urate oxidase enzyme (EC 1.7.3.3) and is used to treat lymphoid leukemia, non-Hodgkin’s lymphoma, and acute myelogenous leukemia. Rasburicase converts existing uric acid to allantoin, which is 5–10 times more soluble in urine than uric acid. The oxidation of uric acid to allantoin by rasburicase produces hydrogen peroxide and carbon dioxide (Ribeiro and Pui 2003). The injection of rasburicase reduces levels of uric acid and mitigates the toxic effects of chemotherapy induced tumor lysis. This drug is marketed by the name of Elitek by Sanofi-Aventis Inc., USA. The possible side effects of this drug are vomiting, fever, nausea, abdominal pain, and diarrhea (Ho et al. 2006).
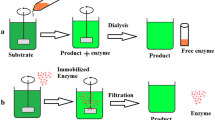
6 Application of Immobilized Enzymes as Pharmaceutical
Chemical immobilization of proteins and enzymes was first attempted in 1960s, and it is an emerging approach to new drug therapies. Immobilization means the enzymes with restricted mobility or rendered less motile by chemical or physical treatment. It was first prepared by loading to polymeric matrices or binding onto carrier materials. Industrial use of enzymes is greatly limited because they are relatively unstable, have a very high cost of purification, and have cumbersome process of recovery of active enzyme from reaction mixture after the completion of catalytic process. Immobilized enzymes are more stable to pH and temperature stress and less susceptible to the denaturing agents. In addition, an immobilized enzyme should have long-term stability and unaltered sensitivity and biological activity after attachment to the matrix than free enzyme when used as therapeutic purpose (Klein and Langer 1986). Immobilization has been successfully utilized for studies with such enzymes, as cytochrome P-450, UDP-glucuronosyltransferases, glutathione S-transferases, S-methyltransferases, and N-acetyltransferases (Dulik and Fenselaut 1998).
One of the major applications of immobilized enzymes in pharmaceutical industry is the production of 6-aminopenicillanic acid (6-APA) by deacylation of the side chain in either penicillin G or V, using penicillin acylase (penicillin amidase). Today more than 50 % of 6-APA is enzymatically produced using the immobilized route which is core of penicillin antibiotic. Penicillin amidase from E. coli is immobilized on cellulose triacetate fibers for producing 6-APA from penicillin G (Alvaro et al. 1990). Similarly for producing 6-APA from penicillin V, penicillin amidase is immobilized by covalent binding to Amberlite XAD-7 with glutaraldehyde through physical adsorption to bentonite or by ionic binding to DEAE-Sephadex and also by covalent binding to a copolymer of acrylamide and maleic anhydride (Arshad et al. 2007). The major reasons for its success is in obtaining a pure product, thereby minimizing the purification costs (Giordano et al. 2006). This process of the immobilized enzyme technology was also approved in India. The first industrial process for the production of 6-APA was started in 1970s by Astra, Sweden, and Riga Biochemical Plant (former USSR).
Immobilization has also been used for the production of 7-aminodeacetoxy-cephalosporanic acid, an intermediate in the production of semisynthetic cephalosporins. Conversion of 7-amino-3-deacetoxy-cephalosporanic acid (7-ADCA) to cephalexin by immobilized penicillin G acylase (IMPGA) has been investigated. It was observed that under optimized conditions, IMPGA can attain 85 % conversion of 7-ADCA to cephalexin. Furthermore, IMPGA can be reused for about ten cycles (Maladkar 1994). Production of cefazolin by immobilized cefazolin synthetase from E. coli as a biocatalyst has been possible. The complex of the physicochemical studies makes it possible to design a highly efficient technological process for production of cefazolin (Kurochkina and Nys 1999). Macrolide antibiotics tylosin and nikkomycin also can be produced by Streptomycin spp., immobilized with calcium alginate.
7 Production of Biopharmaceuticals at Industrial Scale
The main focus of the pharmaceutical industry is to develop such processes for producing recombinant therapeutic and diagnostic proteins of high value at large scale that utilize agricultural residue or waste as main component of the medium. Most of the enzymes are produced by submerged fermentation or by solid state fermentation at industrial scale owing to the inherent advantages such as higher yield, less energy requirement, and less cumbersome in downstream processing. The α-amylase was produced from agricultural by-products such as wheat bran, rye straw, wheat straw, and rice bran by Bacillus cereus MTCC 1305 by solid state fermentation (Singh et al. 2010). α-Amylases are used as a digestive aid by hydrolyzing α-(1 → 4) glycosidic linkages of polysaccharides to yield dextrin, oligosaccharides, maltose, and d-glucose. In recent years, the biopharmazymes are produced and overexpressed in E. coli and in mammalian cells by recombinant technology.
The pharmaceutical manufacturing of recombinant proteins most frequently employs single-cell suspension cultures in stirred-tank bioreactors of variable sizes up to 20,000 L (Wurm 2004). Advances in media and process optimization for mammalian cell culture system have already resulted in more than 100-fold improvement in yield. Monoclonal antibodies (mAb) for treating breast cancer and an immunoglobulin-TNF (tumor necrosis factor) receptor fusion protein for treating rheumatoid arthritis, are produced in suspension cell lines including suspension-adapted Chinese hamster ovary or murine myelomas in stirred-tank reactors (Chu and Robinson 2001). Further insights and subsequent targeted modifications with respect to genome-scale technologies including genomics, transcriptomics, and proteomics are expected to contribute to the development of mammalian cell-based production systems in bioreactors and are hoped to further improve protein yields and quality in the near future (Matasci et al. 2008).
8 Future Prospects
New strategies are continuously emerging for synthesizing and immobilization of new enzymes to enhance their role and efficiency to treat variety of diseases. In response to the need in the pharmaceutical industry for more complex, chiral molecules, fine chemical companies are adopting new manufacturing technologies to produce more efficient compounds with wide spectrum of activity. In particular, recent developments in biocatalysis combined with novel process engineering have provided improved methods for the production of valuable chemical intermediates (Huisman and Gray 2002). Enzymes with antioxidative property are still an area of intense research within the pharmaceutical industry. Superoxide dismutase which transforms the highly toxic superoxide anion to moderately toxic hydrogen peroxide has been of interest to the pharmaceutical industry for quite some time and is still under research (Veronese et al. 2002).
Several researchers are also working in the area of inhibition of human neutrophil elastase (EC 3.4.21.37) having implications in the treatment of chronic obstructive pulmonary disease (COPD) consortium, such as emphysema and chronic bronchitis. Human butyrylcholinesterase, a naturally occurring serum detoxification enzyme, acts to break down acetylcholine. It could be useful for the treatment of cocaine overdose, as demonstrated by recent results (Melov et al. 2000). Several other lysosomal storage diseases are also being investigated with enzyme replacement therapy, including Hurler’s disease and Maroteaux-Lamy syndrome (Harmatz et al. 2004). The enzymes which are designed and designated as orphan drugs under investigation in the USA are listed in Table 15.2.
9 Conclusions
The total number of pharmaceutical enzymes in use around the world probably exceeds 3,000 not including the tens of thousands of formulations containing different combinations of these ingredients. Enzymes are important in therapeutic and in commercial processes because they accelerate specific chemical reaction to produce a useful effect or product. From the perspective of a supplier of pharmaceutically important enzymes, the success of business lies in identifying the particular technological niche for improved product. Advancements in biotechnology over the past 10 years have allowed pharmaceutical companies to produce safer and cheaper enzymes with enhanced potency and specificity. More recently, identifying pharmacological activity based upon an understanding of how enzymes work at the molecular level has enabled the industry to discover many new groups of successful drugs. Along with these advances, changes in orphan drug laws and new initiatives taken by the FDA, USA, have been effective in facilitating efforts to develop enzyme drugs. India’s pharmaceutical fine chemical industry is undergoing readjustment to the structural changes evolving patent regime.
References
Aiuti A (2002) Advances in gene therapy for ADA-deficient SCID. Curr Opin Mol Ther 4:515–522
Ali MA, Saleh FM, Das K et al (2011) Gaucher disease. Mymensingh Med J 20:490–492
Alvaro G, Fernandez LR, Blanco RM et al (1990) Immobilization-stabilization of penicillin G acylase from Escherichia coli. Appl Biochem Biotechnol 26:181–195
Arshad R, Farooq S, Ali SS (2007) 6-Aminopenicillanic acid production by intact cells of E. coli containing penicillin G acylase (PGA). Pak J Biol Sci 10:3190–3194
Bond CS, Clements PR, Ashby SJ et al (1997) Structure of a human lysosomal sulfatase. Structure 5:277–289
Bradbury E, Moon L, Popat R et al (2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416:636–640
Brien S, Lewith G, Walker A (2004) Bromelain as a treatment for osteoarthritis: a review of clinical studies. eCAM 1:251–257
Capstick T, Henry MT (2005) Efficacy of thrombolytic agents in the treatment of pulmonary embolism. Eur Respir J 26:864–874
Chan B, Wara D, Bastian J et al (2005) Long-term efficacy of enzyme replacement therapy for adenosine deaminase (ADA)-deficient severe combined immunodeficiency (SCID). Clin Immunol 117:133–143
Chu L, Robinson DK (2001) Industrial choices for protein production by large-scale cell culture. Curr Opin Biotechnol 12:180–187
Chughtai B, O'Riordan TG (2004) Potential role of inhibitors of neutrophil elastase in treating diseases of the airway. J Aerosol Med 17:289–298
Collings I, Watier Y, Giffard M et al (2010) Polymorphism of microcrystalline urate oxidase from Aspergillus flavus. Acta Crystallogr D Biol Crystallogr 66:539–548
Collins FS (1992) Cystic fibrosis: molecular biology and therapeutic implications. Science 256:774–779
Csoka AB, Scherer SW, Stern R (1999) Expression analysis of six paralogous human hyaluronidase genes clustered on chromosomes 3p21 and 7q31. Genomics 60:356–361
Davies J, Trindade MT, Wallis C et al (1997) Retrospective review of the effects of rhDNase in children with cystic fibrosis. Pediatr Pulmonol 23:243–248
Davydov L, Cheng JWM (2001) Tenecteplase: a review. Clin Ther 23:982–997
de Duve C (1996) The significance of lysosome in pathology and medicine. Proc Inst Med Chic 26:73–76
Del Zoppo GJ, Saver JL, Jauch EC et al (2009) Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke 40:2945–2948
Dulik DM, Fenselaut C (1998) Use of immobilized enzymes in drug metabolism studies. FASEB J 2:2235–2240
El Dib RP, Pastores GM (2010) Enzyme replacement therapy for Anderson-Fabry disease. Cochrane Database Syst Rev doi: 10.1002/14651858
Fieker A, Philpott J, Armand M (2011) Enzyme replacement therapy for pancreatic insufficiency: present and future. Clin Exp Gastroenterol 4:55–73
Fitzhugh DJ, Shan S, Dewhirst MW et al (2008) Bromelain treatment decreases neutrophil migration to sites of inflammation. Clin Immunol 128:66–74
Germain DP (2002) Fabry disease: recent advances in enzyme replacement therapy. Expert Opin Investig Drugs 11:1467–1476
Giordano RC, Ribeiro MPA, Giordano RLC (2006) Kinetics of β-lactam antibiotics synthesis by penicillin G acylase (PGA) from the viewpoint of the industrial enzymatic reactor optimization. Biotechnol Adv 24(1):27–41
Graham ML (2003) Pegaspargase: a review of clinical studies. Adv Drug Deliv Rev 55:1293–1302
Guce AI, Clark NE, Salgado EN et al (2010) Catalytic mechanism of human alpha-galactosidase. J Biol Chem 285:3625–3632
Gurbel PA, Hayes K, Bliden KP et al (2005) The platelet-related effects of tenecteplase versus alteplase versus reteplase. Blood Coagul Fibrinolysis 16:1–7
Hacke W, Kaste M, Bluhmki E et al (2008) Thrombolysis with alteplase 3–4.5 h after acute ischemic stroke. N Engl J Med 359:1317–1329
Harmatz P, Whitley CB, Waber L et al (2004) Enzyme replacement therapy in mucopolysaccharidosis type VI (Maroteaux–Lamy syndrome). J Pediatr 144:574–580
Hershfield MS, Buckley RH, Greenberg ML et al (1987) Treatment of adenosine deaminase deficiency with polyethylene glycol-modified adenosine deaminase. N Engl J Med 316:589–596
Hershfield MS, Chaffee S, Sorensen RU (1993) Enzyme replacement therapy with polyethylene glycol-adenosine deaminase in adenosine deaminase deficiency: overview and case reports of three patients, including two now receiving gene therapy. Pediatr Res 33:S42–S48
Hershfield MS, Mitchell BS (1995) Immunodeficiency diseases caused by adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, 7th edn. McGraw-Hill, New York, pp 1725–1768
Hill JM, Roberts J, Loeb E et al (1967) l-Asparaginase therapy for leukemia and other malignant neoplasms, remission in human leukemia. J Am Med Assoc 202:882–888
Hilleman DE, Tsikouris JP, Seals AA et al (2007) Fibrinolytic agents for the management of ST-segment elevation myocardial infarction. Pharmacotherapy 27:1558–1570
Ho DHW, Thetford BS, Carter CJK et al (1970) Clinical pharmacologic studies of l-asparaginase. Clin Pharmacol Ther 7:408–417
Ho VQ, Wetzstein GA, Patterson SG et al (2006) Abbreviated rasburicase dosing for the prevention and treatment of hyperuricemia in adults at risk for tumor lysis syndrome. Support Cancer Ther 3:178–182
Hopwood JJ, Bate G, Kirkpatrick P (2006) Galsulfase. Nat Rev Drug Discov 5:101–102
Huisman G, Gray D (2002) Towards novel processes for the fine chemical and pharmaceutical industries. Curr Opin Biotechnol 13:352–358
Imming P, Sinning C, Meyer A (2006) Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov 5:821–834
Karnaukhova E, Ophir Y, Golding B (2006) Recombinant human alpha-1 proteinase inhibitor: towards therapeutic use. Amino Acids 30:317–332
Klein MD, Langer R (1986) Immobilized enzymes in clinical medicine: an emerging approach to new drug therapies. Trends Biotechnol 4:179–186
Kurochkina VB, Nys PS (1999) Enzymatic synthesis of beta-lactam antibiotics. I. Cefazolin. Antibiot Khimioter 44:12–16
Lee KY, Kim DI, Kim SH et al (2004) Sequential combination of intravenous recombinant tissue plasminogen activator and intra-arterial urokinase in acute ischemic stroke. Am J Neuroradiol 25:1470–1475
Longstaff C, Williams S, Thelwell C (2008) Fibrin binding and the regulation of plasminogen activators during thrombolytic therapy. Cardiovasc Hematol Agents Med Chem 6:212–223
Maladkar NK (1994) Enzymatic production of cephalexin. Enzyme Microb Technol 16:715–718
Matasci M, David L, Hacker DL, Lucia Baldi L et al (2008) Recombinant therapeutic protein production in cultivated mammalian cells: current status and future prospects. Drug Discov Today Tech 5:37–42
McCredie KB, Ho DHW, Freireich EJ (2008) l-Asparaginase for the treatment of cancer. CA Cancer J Clin 23:220–227
McGirt LY, Vasagar K, Gober LM et al (2006) Successful treatment of recalcitrant chronic idiopathic urticaria with sulfasalazine. Arch Dermatol 142:1337–1342
Melov S, Ravenscroft J, Malik S et al (2000) Extension of life-span with superoxide dismutase/catalase mimetics. Science 289:1567–1569
Meneveau N, Schiele F, Vuillemenot A et al (1997) Streptokinase vs. alteplase in massive pulmonary embolism. A randomized trial assessing right heart haemodynamics and pulmonary vascular obstruction. Eur Heart J 18:1141–1148
Mundada L, Prorok M (2003) Structure–function analysis of streptokinase amino terminus. J Biol Chem 278:24421–24427
Nakamura M, Shirasawa E, Hikida M (1993) Characterization of esterases involved in the hydrolysis of dipivefrin hydrochloride. Ophthalmic Res 25:46–51
Neufeld EF, Muenzer J (2001) The mucopolysaccharidoses. In: Scriver CR et al (eds) The metabolic and molecular bases of inherited disease, 8th edn. McGraw-Hill, New York, pp 3421–3452
Ohman EM, Van de Werf F, Antman EM et al (2005) Tenecteplase and tirofiban in ST-segment elevation acute myocardial infarction: results of a randomized trial. Am Heart J 150:79–88
Overington JP, Al-Lazikani B, Hopkins AL (2006) How many drug targets are there? Nat Rev Drug Discov 5:993–996
Ozcan C, Ergun O, Celik A et al (2002) Enzymatic debridement of burn wound with collagenase in children with partial-thickness burns. Burns 28:791–794
Pastores GM, Weinreb NJ, Aerts H et al (2004) Therapeutic goals in the treatment Gaucher disease. Semin Hematol 41:4–14
Pesu M, Candotti F, Husa M et al (2005) Jak3, severe combined immunodeficiency, and a new class of immunosuppressive drugs. Immunol Rev 203:127–142
Polmar SH, Wetzler EM, Stern RC et al (1975) Restoration of in-vitro lymphocyte responses with exogenous adenosine deaminase in a patient with severe combined immunodeficiency. Lancet 2:743–746
Ribeiro RC, Pui CH (2003) Recombinant urate oxidase for prevention of hyperuricemia and tumor lysis syndrome in lymphoid malignancies. Clin Lymphoma 3:225–232
RXList-Theinternet drug index (2013) http://www.rxlist.com/cgi/generic2/tenecteplase.html. Accessed 21 Jan 2013
Saver JL (2004) Number needed to treat estimates incorporating effects over the entire range of clinical outcomes: novel derivation method and application to thrombolytic therapy for acute stroke. Arch Neurol 61:1066–1070
Schaefer RM, Tylki-Szymanska A, Hilz MJ (2009) Enzyme replacement therapy for Fabry disease: a systematic review of available evidence. Drugs 69:2179–2205
Shah PL, Scott SF, Knight RA et al (1996) In vivo effects of recombinant human Dnase I on sputum in patients with cystic fibrosis. Thorax 51:119–125
Shak S, Capon DJ, Hellmiss R et al (1990) Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. PNAS 87:9188–9192
Sharma A, Jacob A, Tandon M et al (2010) Orphan drug: Development trends and strategies. J Pharm Bioallied Sci 2:290–299
Sikkens EC, Cahen DL, Kuipers EJ et al (2010) Pancreatic enzyme replacement therapy in chronic pancreatitis. Best Pract Res Clin Gastroenterol 24:337–347
Sikri N, Bardia A (2007) A history of streptokinase use in acute myocardial infarction. Tex Heart Inst J 34:318–327
Singh RK, Mishra SK, Kumar N (2010) Optimization of α-amylase production on agriculture byproduct by Bacillus cereus MTCC 1305 using solid state fermentation. RJPBCS 1:867–876
Stella VJ, Charman WN, Naringrekar VH (1985) Prodrugs. Do they have advantages in clinical practice? Drugs 29:455–473
Suri R, Metcalfe C, Lees B et al (2001) Comparison of hypertonic saline and alternate day or daily recombinant human deoxyribonuclease in children with cystic fibrosis: a randomised trial. Lancet 358:1316–1321
Tremblay GM, Vachon E, Larouche C et al (2002) Inhibition of human neutrophil elastase-induced acute lung injury in hamsters by recombinant human pre-elafin (trappin-2). Chest 121:582–588
Tsurupa G, Medved L (2001) Identification and characterization of novel tPA- and plasminogen-binding sites within fibrinogen alpha C-domains. Biochemistry 40:801–808
Vellard M (2003) The enzyme as drug: application of enzymes as pharmaceuticals. Curr Opin Biotechnol 14:444–450
Veronese F, Calceti P, Schiavon O et al (2002) Polyethylene glycol-superoxide dismutase, a conjugate in search of exploitation. Adv Drug Deliv Rev 54:587–606
Weinreb NJ, Barranger JA, Charrow J et al (2005) Guidance on the use of miglustat for treating patients with type 1 Gaucher disease. Am J Hematol 80:223–229
Wetzler M, Sanford BL, Kurtzberg J et al (2007) Effective asparagine depletion with pegylated asparaginase results in improved outcomes in adult acute lymphoblastic leukemia: Cancer and Leukemia Group B Study 9511. Blood 109:4164–4167
White JT, Argento ML, Prince WS et al (2008) Comparison of neutralizing antibody assays for receptor binding and enzyme activity of the enzyme replacement therapeutic Naglazyme (galsulfase). AAPS J 10:439–449
Wurm FM (2004) Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 22:1393–1398
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Das, D., Goyal, A. (2014). Pharmaceutical Enzymes. In: Brar, S., Dhillon, G., Soccol, C. (eds) Biotransformation of Waste Biomass into High Value Biochemicals. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8005-1_15
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8005-1_15
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8004-4
Online ISBN: 978-1-4614-8005-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)