Abstract
Antimicrobial agents are substances of chemical or biological origin, which help in either inhibiting the growth or killing of the microorganisms, such as bacteria, viruses, fungi, algae, or other parasites. The demand for antimicrobial compounds (products) throughout the world has been continuously increasing as the population is more and more concerned about the health and hygiene. Global change in the lifestyle pattern and livelihood has resulted in generation of increasing quantities of solid wastes, which include agro-industrial residues. These residues are usually organic in nature which makes them potential candidates as feedstock for developing the bioprocesses through biotechnological interventions. The usage of these residues as substrates not only opens a new avenue in their utilization but also reduces the pollution concerns, which their disposal in the environment would have caused otherwise.
Numerous scientific investigations have reported the production of antimicrobial products from a variety of agro-industrial residues, such as vegetable peels, seeds, cereal, and fruits waste, and essential oils from the peel of various fruits, which have shown effective properties to be used as preservatives or food additives and in pharmaceuticals because of their antimicrobial, anti-inflammatory, and antioxidant characteristics. Antimicrobial products are also isolated from the plants as these are present in all parts of the plant, viz., bark, stalks, leaves, fruits, roots, flowers, pods, seeds, stems, latex, hull, and fruit rind, and are usually derivatives of phenolic acids and flavonoids. The hydroxyl groups of polyphenols are very reactive in neutralizing the free radicals by donating a hydrogen atom or an electron, chelating metal ions, inactivating lipid-free radical chains, and preventing hydroperoxide conversions into reactive oxyradicals. These are thus effective against various deadly diseases and for processed food preservation, pharmaceuticals, alternative medicine, and natural therapies.
A newer application on the usage of the antimicrobial has emerged in recent times with the aid of nanotechnology to fight against the disease-causing organisms, replacing heavy metals and toxins and may attain effective application in future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Dating back to human history, the microbial infections have been one of leading cause of diseases. Over the year, a large number of antimicrobial compounds have been discovered which has made this world a much better place to live. Antibiotics, such as penicillin, have metamorphed the way diseases are tackled worldwide. However, indiscriminate use of antimicrobial agents, particularly antibiotics, is leading to the development of multiple drug resistance in human pathogenic microorganisms. The antibiotic-resistant bacterial strains travel very fast, making existing antibiotics ineffective. Therefore, it becomes essential to continuously look for new substances from the sources with proven antimicrobial activity.
Plant origin is the most important field for discovery of more effective and useful antimicrobial agents. Various parts of plants are used as source and template for synthesis of antimicrobial agents (Pretorius et al. 2003). In fact, plants and other natural sources provide unlimited scope for discovery of new antimicrobial agents due to chemical diversity. They are rich in secondary metabolites having antimicrobial properties, such as tannins, terpenoids, alkaloids, flavonoids, and other phenolic compounds. Plant extracts and phytochemicals as antimicrobial compounds in use for ages in crude form have been of great significance in therapeutic treatments (Kubo et al. 1993; Artizzu et al. 1995; Izzo et al. 1995; Nascimento et al. 2000). Although 250,000–500,000 species of plants are estimated to be available on the earth (Borris 1996), only less than 10 % of these are thought to be used as food and even less for medicinal purposes (Moerman 1996). In fact, major portion of plants, fruits, vegetables, and other food items are being either used as low-value items or thrown as waste.
As a result, huge amount of waste is being generated by almost every field, i.e., agriculture, food, and pharmaceuticals, among others. The European Union alone produced about 2 billion tonnes of wastes in the year 2006. By the year 2020, the production of waste was estimated to be 45 % higher than it was in 1995. About 30 % of the total waste was reported to be agrowaste (Piccirillo et al. 2010). There exists a huge scope for utilizing plant and agrowaste for various purposes including production of antimicrobial products. Fruit and vegetable wastes, such as pomace, skins, seeds, and other agrowaste, are rich sources of antimicrobials compounds. Therapeutic and other compounds from these natural wastes are not only eco-friendly but their recovery would also be economically attractive.
In addition to the therapeutic use, bioactive properties of plant and agrowastes have immense applications in food industry. Natural preservatives in food items have become very attractive as globalization has necessitated the transportation of high-quality food items from one corner of the world to the other (Davidson and Branen 2005). Various bioactive compounds having valuable application in food industry have been obtained from different types of agrowastes. Natural antimicrobial compounds from plants and agrowastes provide a viable and effective alternative to synthetic preservatives in the food industry.
2 Antimicrobial Chemistry
Antimicrobial agents are substance of chemical or biological or biochemical origin which inhibits the growth of microorganisms, such as bacteria, viruses, fungus, algae, or other parasites. Mechanism of inhibition of bacterial growth by antibacterial compounds depends on the target bacteria. Inhibition of nucleic acid synthesis, interference with cell wall synthesis, cell membrane interference, inhibition of protein synthesis, and disruption to metabolic pathway (Tenover 2006) are the common ways through which antimicrobial agents act against infectious microbes.
2.1 Inhibition of Nucleic Acid Synthesis
The metabolism of nucleic acids can be altered either at the DNA-dependent RNA polymerase or in the process of DNA coiling. DNA gyrase and DNA topoisomerase IV are two essential enzymes for cell growth and division. DNA gyrase controls DNA supercoiling. The enzyme binds to DNA and introduces double-strand breaks that allow the DNA to unwind. Some compounds, such as nitroimidazoles and nitrofurans, directly affect DNA (Calvo and Martinez 2009).
Antimicrobial agents, such as fluoroquinolones and rifampin, inhibit synthesis of nucleic acid. Some antimicrobials like fluoroquinolones interfere with DNA synthesis causing double-strand DNA breaks during DNA replication. Antimicrobial agents, such as rifampin, bind to DNA-dependent RNA polymerase, which blocks the synthesis of RNA and results in cell death.
2.2 Cell Wall and Cell Membrane Interference
The bacterial cell wall can be affected by antimicrobial compounds at different stages of either synthesis (fosfomycin, cycloserine) or transport (bacitracin, mureidomycins) of its metabolic precursors or by direct action on its structural organization (β-lactams, glycopeptides) (Calvo and Martinez 2009). Bacterial cell walls have a single layer of peptidoglycan which is a macromolecule with repeating units of N-acetylglucosamine acid (NAM) and N-acetylmuramic acid (NAG) cross-linked with amino acids between the NAM subunits. Synthesis of the peptidoglycan layer is inhibited by compounds, such as β-lactams, thus interfering with the synthesis of the bacterial cell (Tenover 2006). Antimicrobial compounds, such as vancomycin and teicoplanin, prevent the cross-linking steps required for stable cell wall synthesis by binding to the terminal d-alanine residues of the nascent peptidoglycan chain (McManus 1997). β-lactams (e.g., penicillins, cephalosporins), carbapenems, monobactams, glycopeptides, vancomycin, and teicoplanin are some of the common antibacterial compounds which inhibit bacterial cell wall synthesis.
Some antimicrobial compounds, such as polymyxins, bind to the plasma membrane of the cell and disrupt it. Polymyxin molecules diffuse through the outer membrane and cell wall of susceptible cells to the cytoplasmic membrane. They bind to the cytoplasmic membrane and disrupt and destabilize it. This causes increase in the bacterial cytoplasmic membrane permeability leading to leakage of bacterial cell contents and subsequent death of the cell. Antimicrobial compounds, such as daptomycin, irreversibly binds to the cell membrane of Gram-positive bacteria and causes rapid depolarization of the antibacterial membrane potential by directly binding to the bacterial membrane, which very often lead to death of the bacterium (Carpenter and Chambers 2004).
2.3 Inhibition of Protein Synthesis
A number of antimicrobial compounds, such as tetracyclines, aminoglycosides, chloramphenicol, and macrolides, inhibit protein synthesis by interfering at any of the phases of protein synthesis process, i.e., activation, initiation, binding of the transfer RNA (tRNA) amino acid complex to ribosomes, and elongation (Calvo and Martinez 2009). Protein synthesis begins with the transcription, i.e., formation of messenger RNA (mRNA) from DNA. mRNA carrying the codes for amino acids moves to the cytoplasm where these codes are translated. The ribosome moves one codon further along mRNA, releasing empty tRNA. The A site is free for the next charged tRNA, and the cycle is repeated as the ribosome moves to form polypeptide chain. Translation is terminated by stop codons recognized by release factors which help release the fully synthesized polypeptide chain from ribosomes. Translation ends with dissociation of ribosomal subunits.
Antimicrobial compounds, such as aminoglycosides, bind to the 30S subunit of bacterial ribosomes and blocks the initiation of translation and causes the misreading of mRNA. They can attach to the 30S subunit of the ribosome and prevent the 30S subunit from attaching to mRNA or presence of the aminoglycoside on the ribosome may cause misreading of the mRNA. This may either cause the insertion of the wrong amino acid into the protein or inhibit the ability of amino acids to connect with one another.
2.4 Inhibition of Metabolic Pathway
Certain compounds, such as trimethoprim and sulfonamides, inhibit the bacterial metabolic pathways. Both these agents act on the folic acid synthesis pathway, which is an important precursor to the synthesis of nucleic acids. Para-aminobenzoic acid (PABA) is an essential metabolite in the folic acid synthesis. Sulfonamides are structural analogs of PABA; therefore, they act as competitive inhibitors. Trimethoprim is structural analog for DHF and acts on the folic acid synthesis pathway at latter stage than sulfonamides in the pathway. Trimethoprim and sulfonamides are often used together. When used together, they produce a sequential blocking of the folic acid synthesis pathway and have a synergistic effect.
3 Biochemistry of Antibiotic Resistance
The site of the World Health Organization describes antimicrobial resistance as “Antimicrobial resistance (AMR) is resistance of a microorganism to an antimicrobial medicine to which it was previously sensitive. Resistant organisms (they include bacteria, viruses and some parasites) are able to withstand attack by antimicrobial medicines, such as antibiotics, antivirals, and antimalarials, so that standard treatments become ineffective and infections persist and may spread to others. AMR is a consequence of the use, particularly the misuse, of antimicrobial medicines and develops when a microorganism mutates or acquires a resistance gene.” About 440,000 new cases of multidrug-resistant tuberculosis (MDR-TB) emerge annually, causing at least 150,000 deaths worldwide. Drug-resistant tuberculosis (XDR-TB) has been reported in 64 countries till mid of year 2012 (WHO 2012).
Bacterial resistance is a serious concern in the treatment of infectious disease. Effectiveness of antibacterial agent remains very good in the beginning of its introduction. However, as the use of antibacterial compound increases, pathogens develop resistance to it making the compound ineffective. In fact, pathogens acquire resistance to most of the antibacterial compounds very easily, thus creating need for new sources and more antimicrobial agents.
Bacteria develop resistance to antibacterial agents either due to their inherent characteristics, genetically encoded in its DNA, or acquire it from mutation in the host DNA or through encoded extra chromosomal materials in a horizontal transfer process. Several mechanisms have evolved which confer microbes with antimicrobial resistance. Four main mechanisms which confer antimicrobial resistance are (1) enzymatic inactivation of the antimicrobial molecules by the enzymes produced by bacteria, (2) reducing the affinity of the antimicrobial compound to the target by modifications, (3) efflux of the antibiotic from the cell through membrane-associated pumping proteins, and (4) resistance acquired through mutation or horizontal transfer.
3.1 Inactivation of Antimicrobial Agent
Antimicrobial molecules may be inactivated by either direct destruction or modification by enzymes synthesized by bacteria that selectively target and destroy the activity of the compound. There are three main mechanisms through which antimicrobial molecules may get deactivated: enzymatic hydrolysis, group transfer, or redox process. Many antimicrobial molecules have hydrolytically susceptible chemical bonds which are essential for their antimicrobial activity. Certain enzymes target and cleave these bonds and destroy antimicrobial activity of the compound. Bacteria produce such enzymes which require only water as a co-substrate which leads to inactivation of the antimicrobial molecules (Wright 2005). Some enzymes can induce resistance by modifying the antimicrobial molecules by chemical substitution or addition of acetyl groups to the periphery of the antibiotic molecule. This modifies the structure of the antimicrobial molecule causing poor antibiotic–bacteria interaction. Some bacteria metabolize antibiotics by oxidation and reduction mechanisms to detoxify and resist antibiotic effects.
3.2 Alteration of the Target
Antibacterial compounds bind to a target which is necessary to exhibit the desired antibiotic effect. However, it binds with the target only if it has affinity for it. Affinity between a target and an antimicrobial compound depends on the complementarity of the antimicrobial compound and the target. Any alteration made on the structure of the target molecules will reduce its affinity with the antibacterial compound. Such changes may occur in the target by mutation that reduces its susceptibility to antimicrobial agent (Spratt 1994). In fact, such alteration can lead to total disappearance of the affinity between the target molecule and the antibiotic compound leading to failure of antibiotic compound to bind with target, thereby causing total inhibition of antibiotic effect. Certain bacteria develop resistance to antibiotics by altering the structure of the target molecule.
3.3 Efflux Pumps and Reduced Outer Membrane Permeability
Certain antibiotics, such as macrolides and tetracyclines, act in the cytosol. Therefore, they have to be transported across the cell membrane before they reach their target. A minimum concentration of antimicrobial agent in the cytosol is essential to have desired antibacterial effect. In some bacteria, membrane proteins, called efflux pumps, export antibiotic molecules from the cytoplasm to the extracellular space, thus reducing the concentration of the antibiotic in the cytoplasm making the antibacterial compound less effective. Efflux pumps are drug specific but many efflux systems are multidrug transporters that are capable of expelling a wide spectrum of structurally unrelated drugs, making the bacteria multidrug resistant (Nikaido and Zgurskaya 1999; Webber and Piddock 2003).
Similarly, reduced outer membrane permeability will lead to reduced antibiotic uptake, thus reducing the effectiveness of antimicrobial compound.
3.4 Acquired Resistance
Above-explained mechanisms of resistance to antibacterial compound are intrinsic characteristics of the organism, but resistance to antibacterial compounds as well can be “acquired” by organism by mutation of cellular genes or through acquisition of foreign resistance genes or through combination of these two mechanisms (Dzidic et al. 2008). Two mechanisms of acquiring resistance are mutational adaptation and horizontal transfer by plasmids and the transposons (Roberts 2003).
It is now proven fact that microorganism tends to mutate in order to adapt to the environment of the antibiotic if it is exposed to inadequate doses of antibiotic for longer periods. New strain of the microorganism produced under such conditions becomes resistant to the antibiotic which was earlier effective against the microorganism in adequate doses. Antibiotic resistance occurs by nucleotide point mutations which are also able to produce a resistance phenotype (Woodford and Ellington 2007).
Horizontal transfer of resistance gene by microorganism is also a common mechanism which spreads antibiotic resistance. Microbes can acquire resistance gene from mobile genetic element, such as plasmids, integrons, and transposons. Horizontal transfer of resistance genes can take place into the recipient chromosome by recombination. Mobile genetic elements can be transmitted through transformation, transduction, or conjugation.
4 Common Antimicrobial Compounds from Plant and Agrowaste
Most of the isolated antimicrobial compounds from plants are secondary metabolites. These substances are normally synthesized by plants as plant defense mechanisms against microorganisms, insects, and herbivores, among others. They give odors, pigments, and flavors to plants. More than 300 natural metabolites with antimicrobial activities have been reported in the period 2000–2008 (Saleem et al. 2010). Many of these are very useful for humans as medicines and food preservatives, among others. Common antimicrobial compounds from plant and agro sources are phenolics or simple phenols, flavonoids, essential oils, coumarins, quinones, tannins, alkaloids, lignans, peptides, iridoids, and xanthones.
Polyphenols are the most common secondary metabolites of plants. These compounds are building blocks for cell wall structures and serve as defense against pathogens. Phenolic compounds from plants are well known for high antioxidant, anti-inflammatory, antiallergic, antithrombotic, and antimicrobial properties (Jayaprakasha et al. 2003; Baydar et al. 2004; Shoji et al. 2004; Alberto et al. 2006). Phenolic compounds present in fruits have antioxidant activity and protect the plant from environmental stress and fungal or bacterial infections. Waste products remaining after juice processing (peel, seeds, stems, flesh) are good sources of these ingredients. Plant and agrowaste are considered as economical and eco-friendly source of phenolic compounds.
4.1 Flavonoids
Flavonoids are plant compounds which contain a 2-phenylbenzopyran nucleus consisting of two benzene rings (A and B) linked through a heterocyclic pyrane ring (C). Flavonoids are one of the important secondary metabolites found in various plant species. They protect the plants from UV radiation and other environmental stresses. Flavonoids are found ample in fruits, vegetables, nuts, seeds, stems, and flowers, among others. These compounds are present in photosynthesizing cells (Cushnie and Lamb 2005; Havsteen 1983). Flavonoids present in flowers give it attractive colors for pollinators. Flavonoids in leaves protect them from fungal pathogens and UV radiation (Harborne and Baxter 1999; Harborne and Williams 2000). They have antimicrobial, antifungal, and antiviral properties. Use of various compounds of flavonoids is well known to cure human diseases.
Activity of flavonoids against pathogenic microbes is attributed to cell wall permeability and the porins in the outer membrane present in microorganisms. Activity of some flavonoids may also be due to their ability to complex with extracellular and soluble proteins and then with bacterial cell walls. Main classes of flavonoids are flavonols, flavanones, isoflavones, anthocyanidins, flavones, aurones, flavanon-3-ols flavans, flavan-3,4-diols, chalcones, and flavan-3-ols.
4.2 Essential Oils
Essential oils are secondary metabolites found in plants. Fragrance of the plants is carried in essential oil fraction. Their general chemical structure, called terpenes, is C10H16. Other compounds in these groups are diterpenes, triterpenes, tetraterpenes, hemiterpenes, and sesquiterpenes. Terpenoids are well known as antibacterial, antifungal, and antiviral. As per a report, about 60 % of essential oil derivatives are inhibitory to fungi, while 30 % inhibit bacteria (Saleem et al. 2010). Antibacterial mechanism of terpenes is reported to be due to membrane disruption by the lipophilic compounds.
Essential oils from various plants have shown antimicrobial activity against many pathogenic microbes (Melendez and Capriles 2006; Wannissorna et al. 2005). Essential oils from Citrus spp. are very effective antimicrobial agent against bacteria as well as fungi. Citrus essential oils are used for improving the shelf life and preservation of processed food and fruits (Lanciotti et al. 2004), skim milk, and low-fat milk. Citrus essential oils are present in fruit flavedo in great quantities (Chanthaphon et al. 2008). Antibacterial and antifungal activities of essential oils of Mentha arvensis and Zingiber chrysanthum leaves have been reported by Singh and Negi (1992a, b). There are many reports on antimicrobial activity of essential oils and plant extracts, such as rosemary, peppermint, bay, basil, tea tree, celery seed, and fennel.
4.3 Coumarins
Coumarins belong to benzopyrone group compounds, of which flavonoids are the other main member. Coumarin (1,2-benzopyrone) is available in a wide variety of plants. Coumarins are reported to be formed as a defense mechanism in response to traumatic injury during the wilting process or by plant diseases or through drying. Although coumarins are synthesized mainly in leaves, they accumulate on the surface of the leaves, fruits, and seeds. They are reported to protect the plants from fungal pathogens, beetles, and other terrestrials Coumarins are present in tonka bean, vanilla grass, woodruff, mullein, lavender, strawberries, apricots, cherries, cinnamon, sweet clover, bison grass, cassia, and yellow sweet clover. It was first isolated from tonka beans and has higher presence in some essential oils, particularly cinnamon bark oil and lavender oil, fruits (e.g., bilberry, cloudberry), green tea, etc. (Mirunalini and Krishnaveni 2011; Monga et al. 2012).
Several coumarins have been reported to have antimicrobial properties (Kwon et al. 1997; Kayser and Kolodziej 1997). Methanol extract from Mitracarpus scaber against S. aureus and C. albicans (Bisignano et al. 2000) and water extract from Pelargonium sidoides against E. coli and Klebsiella pneumoniae (Kayser and Kolodziej 1997) have shown antimicrobial effect.
4.4 Quinones
Quinones, naturally occurring pigments, are aromatic rings with two ketone substitutions. Conversion from hydroquinone to quinone and vice versa takes place through oxidation and reduction reactions. This redox potential of the particular quinone–hydroquinone pair is important in many biological systems. A number of quinones, such as anthraquinones, naphthoquinones, and benzoquinones, are widely distributed in nature. Quinone is well known to demonstrate antibacterial, antifungal, antiviral, antimicrobial, and anticancer activities (Beheshti et al. 2012).
4.5 Tannins
Tannins are polyphenolic substances found in bark, wood, leaves, fruits, and roots of plants. They are reported to function as chemical defenses against pathogens and herbivores (Gedir et al. 2005). They are called tannins for their ability of tanning leather. Tannins are grouped in two major structural classes: hydrolyzable tannins and condensed tannins. Condensed tannins are derived from flavonoid monomers, whereas hydrolyzable tannins are gallic acid-based multiple esters with d-glucose. Condensed tannins are more widely distributed in nature. They have been found toxic to filamentous fungi, yeasts, and bacteria. They inhibit the growth of pathogens and herbivory (Gedir et al. 2005). Tannins are natural detergent and suitable substitute for synthetic anthelmintics (Tanner et al. 1995). Mechanism of antimicrobial action of tannins may be related to their ability to inactivate microbial adhesins, enzymes, cell envelope transport proteins, etc. (Yao et al. 1992).
4.6 Alkaloids
Alkaloids are heterocyclic nitrogen compounds. Several alkaloids from natural sources possess potent antimicrobial properties and could be useful as antimicrobial compounds. One of the first medicinally important alkaloid was morphine isolated from Papaver somniferum (Saleem et al. 2010). Diterpenoid from the plants of Ranunculaceae; solamargine, a glycoalkaloid from the berries of Solanum khasianum; and some other alkaloids have been reported to be useful against HIV infection (Mendoza et al. 1997; Cowan 1999).
4.7 Peptides
Peptides are low molecular weight natural compounds and exhibit antimicrobial activity. It contains disulfide bonds. Cationic peptides are the most common peptides. The discovery of non-ribosomally synthesized peptides present in plants and ribosomally synthesized peptides with antimicrobial properties has been very useful in fighting pathogens. Very limited resistance for the bacterial strains has made antibiotic peptides a promising source of antimicrobial drugs (Zasloff 2002). Plant peptides isolated from roots, seeds, flowers, stems, and leaves have shown activities against phytopathogens as well as against human pathogens. Many bioactive peptides have been isolated from milk-based products, eggs, meat, and fish as well as from different plant protein sources, such as soy and wheat, among others. Antimicrobial peptides act against different Gram-positive and Gram-negative bacteria (Escherichia, Helicobacter, Listeria, Salmonella, and Staphylococcus), yeasts, and filamentous fungi. The disruption of normal membrane permeability is understood to be partly responsible for the antibacterial mechanism of lactoferricins (Hartmann and Meisel 2007).
4.8 Lignan
Lignans are diphenolic compounds with a 2,3-dibenzylbutane structure. These are important plant phenolic compounds with known antimicrobial, antitumor, anti-inflammatory, and antiviral properties. Lignans are available in fiber-rich plants, such as wheat, beans, barley, oats, soybeans, lentils, garlic, asparagus, broccoli, and carrots. Flaxseed (Linum usitatissimum) is a very rich source of lignans. Lignans as source of plant phenolic compounds have been reported to reduce the risk of certain cancers. Lignans are one of the major classes of phytoestrogens which act as antioxidants. A lignan, (+)-lyoniresinol-3a-O-β-d-glucopyranoside, isolated from the root bark of Lycium chinense has shown antimicrobial activity against Staphylococcus aureus and three human-pathogenic fungi, Candida albicans, Saccharomyces cerevisiae, and Trichosporon beigelii (Saleem et al. 2010).
4.9 Xanthones
Xanthones (9H-xanthen-9-ones) are heterocyclic compounds with the dibenzo-γ-pyrone framework. Xanthone derivatives are secondary plant metabolites and have been isolated from fungi, lichens, and a few higher plants. Mangosteen (Garcinia mangostana Linn.) tree is a very good source of xanthones. Xanthones have been isolated from peel, whole fruit, bark, and leaves of mangosteen (VishnuPriya et al. 2010). Several studies have reported antioxidant, antitumoral, anti-inflammatory, antiallergy, antibacterial, antifungal, and antiviral activities of xanthones (Suksamrarn et al. 2006; Pedraza et al. 2008).
4.10 Iridoids
Iridoids are monoterpenoids, widely distributed in dicotyledonous plant families, such as the Apocynaceae, Scrophulariaceae, Diervillaceae, Lamiaceae, Loganiaceae, and Rubiaceae. Iridoids exhibit a wide range of bioactivities, such as anti-inflammatory, antioxidant, antibacterial, anticoagulant, antifungal, and antiprotozoal, among others. Aucubin and catalpol are the most spread iridoid glucosides in the Veronica genus. Aucubin has been reported to have shown wide pharmacological activities.
5 Antimicrobial Compounds from Plant and Agrowaste
Substantial amount of reusable substances, such as soluble sugars, proteins, phenols, and fibers, are present in the agro-industrial wastes which can be very valuable for the production of value-added antimicrobial products. Normally, one or two parts of plants, vegetables, fruits, cereals, etc., are used for some useful product and the rest of the parts such as peels, pomace, seed, leaves, hull, bark, and root are dumped as waste. A number of research groups are working to explore various possibilities of utilizing the plant and agrowaste as value-added products (Table 14.1). Some of the important works on exploration of these natural wastes for production of antimicrobial compounds are summed up below.
5.1 Antimicrobial Compounds from Fruit Waste
Huge quantities of fruits are used worldwide but their peel, seed, and pomace remain mostly unutilized, thus generating huge amount of waste. These wastes, though highly perishable and seasonal, are a serious challenge for the processing industries and pollution monitoring agencies. Suitable methods can be adopted to utilize this waste into value-added products, which can improve the overall economics of processing units and can help to reduce environmental pollution. A lot of developments have taken place in the field of utilizing fruit waste and by-products for extraction of antimicrobial compounds. Some of the important studies on utilization of fruit wastes for production of antimicrobial agents are briefed here.
5.1.1 Antimicrobial and Antioxidant Potential of Juice Pressing Waste
Anthocyanins, tannins, starches, saponins, polypeptides, and lectins were found in the water extract, and polyphenols, lactones, flavones, and phenons were additional phytochemicals traced in the extracts of the pomace (peels, seeds, flesh) remaining after pressing the juice of Fragaria ananassa (strawberry), Prunus cerasus (sour cherry), Ribes nigrum (black currant), Ribes rubrum (red currant), Rubus fruticosus (blackberry), and Rubus idaeus (raspberry). Antimicrobial and antioxidant potential of water and methanol extracts was investigated on Bacillus cereus, B. subtilis, Campylobacter jejuni, E. coli, Salmonella typhimurium and Serratia marcescens, C. albicans, C. krusei, C. glabrata, C. pulcherrima, and C. parapsilosis by broth dilution method (Krisch et al. 2009). Both aqueous and methanol extracts inhibited growth of almost all bacteria. Methanol extracts had stronger inhibitory effect than water extracts.
Pomace of beetroot is also disposed as low-value feed or manure, although it is rich in phenols. During juice pressing, most of the secondary plant metabolites and dietary fiber compounds of beetroot are not transferred into the liquid phase and remain in the pomace (Will et al. 2000). Beetroot (Beta vulgaris L. ssp. vulgaris) has very high total phenolic content, i.e., in the range of 50–60 μmol/g dry weight and very good antioxidant property (Vinson et al. 1998; Kahkonen et al. 1999). Beetroot peels are reported to contain l-tryptophan, p-coumaric, and ferulic acids as well as cyclodopa glucoside derivatives (Kujala et al. 2001).
Phenolic content in beetroot is mainly present in the peel (50 %), crown (37 %), and flesh (13 %) (Canadanovic et al. 2011). Canadanovic et al. (2011) reported phenolic content (376.4 mg/g of dry beetroot pomace extract), flavonoid content (269.70 mg/g), and betalain (41.85 mg/g) in the ethanolic extract of the beetroot. They evaluated antibacterial activity of ethanol extract of beetroot pomace by disk diffusion and microdilution method against Staphylococcus aureus, Bacillus cereus, Escherichia coli, and Pseudomonas aeruginosa. Gram-positive bacteria Staphylococcus aureus and Bacillus cereus demonstrated higher susceptibility than Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa against beetroot extract.
5.1.2 Antimicrobial Activity of Citrus Fruit Peels
Citrus is one of the major fruit commercially grown all over the world. Huge quantity of wastes such as peels are generated every year, as juice yield of citrus is not even half of the total fruit mass (Manthey and Grohmann 2001). Citrus peels are rich in nutrients and contain many phytochemicals like flavanones and polymethoxylated flavones (Ahmad et al. 2006), thus can be explored for antimicrobial compounds. Therapeutic value of citrus oil as an antidiabetic, antimicrobial, antifungal, hypotensive agent, antioxidant, antibacterial, and antiviral agent has been studied by many research groups (Kumamoto et al. 1986; Caccioni et al. 1998; Hamendra and Anand 2007; Kanaze et al. 2008).
Phytochemical analysis and antimicrobial activities of peel of Citrus limon (lemon) and Citrus sinensis (sweet orange) were studied by Ashok et al. (2011). They used five different solvent extracts (ethyl acetate, acetone, ethanol, petroleum ether, and water) for both types of peels and screened their extract against five pathogenic bacteria Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, and Salmonella typhi. They carried out phytochemical analysis of powdered plant parts and reported presence of flavonoids, saponins, tannins, alkaloids, and terpenoids in the peel extracts. Solvent extracts of Citrus sinensis peel and Citrus limon showed significant activities and presence of different phytochemicals. Acetone peel extract of Citrus sinensis showed highest antibacterial activity followed by the ethyl acetate peel extract of Citrus limon.
Chanthaphon et al. (2008) studied ethyl acetate extracts and hydrodistillated essential oils of Citrus spp. and kaffir lime peels (Citrus hystrix DC.) for antimicrobial activities against food-related microorganisms. Ethyl acetate extract from kaffir lime contained limonene (31.64 %), citronellal (25.96 %), and β-pinene (6.83 %). Essential oil obtained from hydrodistillation contained β-pinene (30.48 %), sabinene (22.75 %), and citronellal (15.66 %). They evaluated antimicrobial activities of ethyl acetate extracts from fresh peels and dried peels of tropical citrus fruits (lime), kaffir lime, and pomelo peels against pathogenic E. coli. Extracts from both fresh and dried limes, kaffir lime, and pomelo peels showed antibacterial activity against S. aureus, but the ones from fresh peels showed higher activity. Similarly, the extracts from fresh lime and kaffir lime peels showed activity against E. coli but no activity was observed with dried peels. They also observed that ethyl acetate extracts from all citrus peels showed better antimicrobial activities than their essential oils. The ethyl acetate extract of peels inhibited Gram-positive bacteria, yeast, and molds: Staphylococcus aureus, Bacillus cereus, Listeria monocytogenes, Saccharomyces cerevisiae var. sake, and Aspergillus fumigatus.
5.1.3 Antioxidant and Antimicrobial Activity of Pomegranate Peel and Seed
Pomegranate peel extract (PE) also shows excellent antioxidant and antimicrobial activity. Kanatt et al. (2010) studied the antimicrobial and antioxidant properties of pomegranate peel and seed extracts. Antimicrobial activity of pomegranate peel extract was tested against Staphylococcus aureus, Bacillus cereus, Escherichia coli, and S. typhimurium. It inhibited growth of Staphylococcus aureus and Bacillus cereus but Escherichia coli and S. typhimurium were resistant to it. Pomegranate peel extract (PE) was found very effective in scavenging hydroxyl and superoxide anion radicals. Investigators used pomegranate peel extract to enhance shelf life of chicken meat products successfully by 2–3 weeks during chilled storage.
Tehranifara et al. (2011) also investigated the antioxidant properties of peel, seed, and leaf of pomegranate (Punica granatum L.). They used aqueous and methanolic extraction with different concentrations (0, 500, 1,000, and 1,500 ppm) on three fungus Penicillium italicum, Rhizopus stolonifer, and Botrytis cinerea. Methanolic extract showed the highest inhibitory effect on the mycelia growth and spore germination. Peel and seed extracts showed more inhibitory effect than leaf extract. Antioxidant capacities of peel, seed, and leaf extracts of pomegranate were 55.3 %, 35.7 %, and 16.4 %, respectively. The phenolic content was 2.8 times higher in peel extract than leaf extract, which may be the reason for better antimicrobial activity of peel extract. These studies propose pomegranate peel and seed as source of powerful antioxidant and antifungal compounds.
5.1.4 Antimicrobial Effect of Apple Skins
Apples are high on phenolic compounds (Mangas et al. 1999; Podsedek et al. 2000; Shoji et al. 2003). Apple pulp and skins are reported to contain catechin, procyanidin, caffeic acid, and chlorogenic acid among other compounds. Skin of apple also contains flavonoids such as quercetin glycosides and cyanidin glycosides which are not present in pulp (Escarpa and Gonzalez 1998; Vander et al. 2001). Alberto et al. (2006) examined the antimicrobial activity of phenolic compounds extracted from the skin of two apple varieties Royal Gala and Granny Smith using the agar diffusion method. The phenolic compounds were extracted in acetone, methanol, and ethanol solvents. Total phenolic and flavonoid content was obtained with Folin–Ciocalteu reagent (Singleton and Rossi 1965). Granny Smith variety skin was found containing more polyphenols and flavonoid than Royal Gala, whereas maximum phenolics and flavonoids were obtained in acetone extract for both varieties of apples. The highest inhibitory effect of both apple varieties corresponded to extract which contained high phenolic content. Antimicrobial activities of different extracts were evaluated against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Enterococcus faecalis, and Listeria monocytogenes. Extracts of both the apple skins were reported to inhibit these microorganisms; however, extracts of Granny Smith were found more effective demonstrating a direct relationship between the phenolic content of the extracts and the antimicrobial effect. This study established the usefulness of apple skin for its antibacterial properties, thereby adding value to the already known benefits of apple to the human health.
5.1.5 Fruit and Vegetable Peels
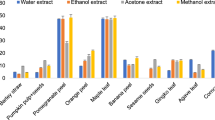
Chanda et al. (2011) evaluated seven fruit and vegetable peels for their antimicrobial properties: Mangifera indica L. (Anacardiaceae), Lagenaria siceraria (Molina) Standl. (Cucurbitaceae), Solanum tuberosum L. (Solanaceae), Ananas comosus (Linnaeus) Merr. (Bromeliaceae), Luffa acutangula (L.) Roxb. (Cucurbitaceae), Momordica charantia L. (Cucurbitaceae), and Moringa oleifera Lam. (Moringaceae). Antimicrobial activities of hexane, chloroform, acetone, and methanol extracts of these samples were evaluated by agar well diffusion method against Staphylococcus aureus, Staphylococcus subflava, Corynebacterium rubrum, Salmonella typhimurium, Enterobacter aerogenes, Klebsiella pneumoniae, Proteus mirabilis, Cryptococcus luteolus, Candida albicans, Candida tropicalis, and Candida glabrata. Mangifera indica peel showed best and promising antimicrobial activity. Polar solvents (acetone and methanol) were found more effective than nonpolar solvents (hexane and chloroform). Activities shown by acetone extracts were the best, followed by methanol extracts. The extracts showed better antifungal activity than antibacterial activity. C. glabrata and K. pneumoniae were the most susceptible organisms. Report suggested that broad spectrum of antibacterial activity by M. indica peels may help to discover new chemical classes of antibiotic substances.
5.1.6 Antibacterial Effect of Grape Seeds
Grape seeds, which are normally a waste of winery or juice pressing, can be effectively utilized for production of antioxidants and antimicrobial compounds. Jayaprakasha et al. (2003) have reported antimicrobial activity of acetone and methanol extracts of grape seed against Bacillus cereus, Bacillus coagulans, Bacillus subtilis, S. aureus, E. coli, and P. aeruginosa. The authors reported that the extracts were free radical inhibitors and primary antioxidants that react with free radicals.
In another report on antimicrobial activity of grape seed extract, Baydar et al. (2004) revealed that the grape seed extracts in acetone/water/acetic acid and ethyl acetate/methanol/water solvents inhibited various test organisms: Aeromonas hydrophila, B. brevis, B. cereus, B. megaterium, B. subtilis, E. faecalis, E. coli, Klebsiella pneumoniae, L. monocytogenes, Mycobacterium smegmatis, Proteus vulgaris, P. aeruginosa, and S. aureus. The grape seed extracts were found to contain high total phenolics, which should be the reason for their strong antimicrobial activity.
5.2 Antimicrobial Compounds from Plant Wastes
5.2.1 Antimicrobial Activity of Leaf Waste
Organic and aqueous extracts of mahagony (S. macrophylla) seeds have been reported to possess antimicrobial, antimalaria (Soediro et al. 1990), antidiabetic, antidiarrheal, anti-inflammatory, and antitumor-promoting (Amelia et al. 1996) properties. Tan et al. (2009) studied the antimicrobial and antioxidant activities of methanol and dichloromethane extracts of S. macrophylla leaves, which are otherwise not considered as a valuable product. They evaluated the antimicrobial activities of the extracts against four bacteria Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Pseudomonas aeruginosa and a fungus Candida albicans. The methanol and the dichloromethane extracts were found more active against Gram-positive bacteria, i.e., Staphylococcus aureus and Bacillus subtilis, but showed very limited activity against Escherichia coli and Pseudomonas aeruginosa. Methanol extract also showed activity against Candida albicans. Considerable amount of phenolic, tannin, and flavonoid contents were found in the mahagony leaf extracts.
A study on the antimicrobial activity of extracts of the leaves and leaf waste of Agave sisalana Perrine, popularly known as sisal, has been carried out by Santos et al. (2009). It is a monocotyledonous plant largely grown in Brazil and Mexico. Only 5 % of the decortications of the leaves of sisal (A. sisalana) are reported to be used to produce useful hard fiber and the remaining 95 % being wasted as solid and juice waste. The study was carried out to evaluate the antimicrobial activity of extracts of the leaves and leaf waste. Investigators determined the antimicrobial activity by the paper disk diffusion method against bacteria and fungus both nonresistant and resistant to antibiotics. The hydroalcoholic extract obtained from leaves and from sisal waste showed significant inhibition of C. albicans. However, HCl and methanol extract of leave waste did not show any activity against S. aureus, E. coli, M. luteus, B. cereus, P. aeruginosa, and S. choleraesuis.
Pereira et al. (2007) used different cultivars of walnut (Juglans regia L.) leaves (Franquette, Mayette, Marbot, Mellanaise, and Parisienne) grown in Portugal, for evaluating their antimicrobial and antioxidant properties. Phenolic analysis of the extracts identified 3- and 5-caffeoylquinic acids, 3- and 4-p-coumaroylquinic acids, p-coumaric acid, quercetin 3-galactoside, quercetin 3-pentoside derivative, quercetin 3-arabinoside, quercetin 3-xyloside, and quercetin 3-rhamnoside in the extract. The extract was found to inhibit the growth of Gram-positive test bacteria (Bacillus cereus, B. subtilis, Staphylococcus aureus). However, Gram-negative test bacteria (Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae) were resistant to the extracts.
Waste tobacco leaves have also been explored for extraction of flavonoids, mainly rutin. Tobacco plant is a rich source of medicinally useful alkaloids and flavonoids. Rutin, well-known flavonoid, is known to reduce capillary fragility, swelling, bruising, hemorrhoids, diabetic vascular disease, diabetic retinopathy, pain, tired legs, night cramps, and restless legs (Grinberg et al. 1994; Beretz and Cazenave 1988). Fathiazada et al. (2006) studied tobacco waste of tobacco factories as a source of flavonoids. They determined the content of rutin in waste and unfermented leaves of tobacco by HPLC and found the content of rutin in waste leaves of tobacco (0.6 %) less than that of unfermented leaves (1.5 %). However, waste leaves can still be an economical source of rutin.
In another report on antibacterial activity of the citrus plant leaf waste, Ekwenye and Edeha (2010) reported inhibitory effect of ethanol and aqueous extract of Citrus sinensis leaf against E. coli, K. pneumoniae, and S. aureus.
5.2.2 Antibacterial Activity of Other Plant Parts
Nascimento et al. (2000) studied antimicrobial activities of eight plant parts and tested antimicrobial activities of their extract against various microorganisms. The plants used for the study, plant parts used for extractions, and compounds obtained are given in Table 14.2.
Staphylococcus aureus, Salmonella choleraesuis, Pseudomonas aeruginosa, Bacillus subtilis, Candida albicans, Proteus spp., K. pneumoniae, Shigella spp., Proteus spp., Pseudomonas aeruginosa, Enterobacter aerogenes, Escherichia, and Staphylococcus aureus were used in the study. The extracts from clove and jambolan were active against nine and eight test microorganisms, respectively. Other extracts were also active against at least one of the tested microorganisms.
Stems and leaves are generated as waste in the production of the cherry liquor from ginja cherry plant. Piccirillo et al. (2010) investigated the antimicrobial properties of these wastes. The ethanol extracts of stems and leaves were tested against Gram-positive and Gram-negative bacteria (B. subtilis, S. aureus MSSA, S. aureus MRSA, Pseudomonas sp., P. aeruginosa, Flavobacterium sp., E. coli, Salmonella) using the disk diffusion and the broth dilution techniques. The ethanol extracts of stems and leaves inhibited growth of Gram-positive as well as Gram-negative bacteria but it showed better antibacterial activity against Gram-positive bacteria than Gram-negative bacteria. The antimicrobial activity of ginja cherry stems and leaves waste is attributed to the presence of valuable compounds like polyphenols in the extract.
5.3 Antimicrobial Potential of Agro-Industrial Wastes
5.3.1 Extraction of Polyphenolic Compounds from Mango Seed Kernels
Huge quantities of peel and kernel by-products are generated during processing of mango. This waste has high content of phenolic compounds and saturated fatty acids. Mango kernels are good source of phospholipids, phenolic compounds, campesterol, β-sitosterols, stigmasterol, and tocopherols (Soong and Barlow 2004). Mango seed kernel enhances oxidative stability and shelf life of fresh cheese and ghee (Parmar and Sharmar 1990). Good antioxidant property of mango seed kernel is attributed to the presence of polyphenols, sesquiterpenoids, phytosterols, and microelements, such as selenium, copper, and zinc (Schiber et al. 2003). Maisuthisakul (2008) found phenolic compounds like phenolic acids and flavonoids in the extract of Thai mango (Mangifera indica Linn.) seed kernels.
5.3.2 Extraction of Tannins from Agricultural By-Products
Huge amount of agricultural wastes of tannin-containing plants are generated. Tannins, a polyphenolic compound, showed antimicrobial (Doughari et al. 2008) and antioxidant activities (Zargham and Zargham 2008). Sung et al. (2012) investigated wastes from green tea processing, acorn, chestnut, and persimmon hulls for extraction of tannin and their antibacterial and antioxidant activities. Tannin content in the ethanol, acetone, and aqueous extracts was determined. They found tannin concentrations in the extracts of chestnut hull, green tea waste, acorn hull, and persimmon hull. Tannin concentration for green tea waste was highest in ethanol extracts, whereas for chestnut hull it was highest in ethanol and acetone extracts. Antibacterial activities of various extracts were screened against Staphylococcus aureus, E. coli, S. flexneri, L. monocytogenes, and B. coagulans. Tannin extracts from green tea waste showed higher antibacterial activity than the extracts of acorn, chestnut, and persimmon hulls.
5.3.3 Biological Activity of Jojoba Hull Extracts
Simmondsia chinensis, better known as jojoba, is commercially cultivated in many countries all over the world. It is grown commercially for its seed oil. It is also used as food by many animals. Jojoba seed oil has medicinal properties useful for treatment of cancer, kidney disorder, obesity, sore heart, warts, and wounds (Leung and Foster 1996). Wagdy and Taha (2012) evaluated antimicrobial activity of phenolic extracts of jojoba hulls using different extracting solvents against five bacterial strains Escherichia coli, Staphylococcus aureus, Bacillus cereus, Listeria monocytogenes, and Salmonella typhimurium. They used five different extracts: methanol, ethanol, acetone, isopropanol, and ethyl acetate. Different extracts of jojoba hulls inhibited growth of the test microorganisms through varying degree. Maximum inhibition of B. cereus and S. typhimurium was exhibited by ethyl acetate extract. Highest inhibition of B. cereus was achieved with ethyl acetate. S. aureus was inhibited most by methanol extract. Highest inhibition of L. monocytogenes and E. coli was obtained with ethanol and acetone extract, respectively. Wagdy and Taha (2012) suggested that jojoba hull is a very promising source of bioactive compounds with very good antioxidant, antimicrobial, and anticancer properties.
5.3.4 Antimicrobial Compounds from Olive Oil Mill Waste
Olive fruit is a rich source of phenolic compounds, such as phenol acids, phenol alcohols, catechol, methylcatechol, phenyl alcohols (tyrosol, hydroxytyrosol), flavonoids (luteolin-7-glucoside, apigenin-7-glucoside, rutin and quercetin), and several anthocyanin pigments (cyaniding-3-glucoside and cyaniding-3-rutinoside) (Ragazzi et al. 1973; Andary et al. 1982; Romero et al. 2002). Hydroxytyrosol is one of major phenolic compound present in olive fruit with remarkable pharmacological and antioxidant activity (Visioli et al. 2004; Fernandez et al. 2006). Oleuropein is another valuable compound with certain antiviral, antibacterial, antifungal, antioxidant, and anti-inflammatory properties (Aziz et al. 1998; Visioli and Galli 2002). Phenolic compounds derived from olives have shown antibacterial activities against pathogenic bacteria, such as Salmonella spp., Staphylococcus aureus, Clostridium botulinum, and Listeria monocytogenes (Payne et al. 1989; Nychas et al. 1990; Tassou and Nychas 1995).
It is a known fact that olive oil mill waste contains polyphenols in considerable amount. Concentration of polyphenols has been reported to be so strong that phytopathogenic bacteria like Pseudomonas syringae and Corynebacterium michiganense fail to grow in it (Capasso et al. 1995). Ciafardini and Zullo (2003) studied the effect of polyphenols present in the olive oil mill wastewater (OMWW) on the crucifer seed-borne phytopathogen Xanthomonas campestris. They reported that polyphenols in contact with the bacterial cultures react with the protein of the bacterial cell walls and disrupt them. OMWW was able to control the seed-borne phytopathogen Xanthomonas campestris completely without damaging the germinability of the crucifer seeds and the metallic greenhouse structures. Therefore, Ciafardini and Zullo (2003) suggested that polyphenols from OMWW are a natural substitute for commercial corrosive chemicals like sodium hypochlorite, which is currently used to disinfect seeds and greenhouses.
5.3.5 Other Agrowastes
Martin et al. (2012) assessed the antimicrobial potential and chemical composition of guava bagasse (Psidium guajava), Cabernet Sauvignon, Pinot Noir (Vitis vinifera) grape marc, Isabella grape marc (Vitis labrusca) wastes, Petit Verdot Verdejo grape marcs, Syrah and Verdejo grape stems, Petit Verdot grape seed and red grape fermentation lees (Vitis vinifera), tomato bagasse (Solanum lycopersicum) wastes, and vegetable wastes, kale (Brassica oleracea), beet (Beta vulgaris), broccoli (Brassica oleracea) and turnip stems (Brassica rapa), carrot (Daucus carota) and radish leaves (Raphanus sativus), pumpkin (Cucurbita sp.) and peanut peel, and passion fruit hulls (Passiflora edulis) against pathogenic microorganisms of importance in food. Samples were immersed in ethanol and methanol solutions to prepare extract. Beet stalk, peanut peel, Pinot Noir grape marc, Petit Verdot grape seed and marc, red grape fermentation lees, and guava bagasse wastes showed antimicrobial activities against Gram-positive bacteria Staphylococcus aureus and Listeria monocytogenes. Methanol extract of peanut peels and ethanol extract of guava bagasse extracts showed the lowest MIC against S. aureus and L. monocytogenes. Guava bagasse extract showed the highest antimicrobial activity against L. monocytogenes.
5.4 Utilization of Agrowaste as Substrate
Agro-industrial wastes, such as wheat bran, rice husk, corn bran fenugreek straw, corn bran, and sugarcane bagasse, can be used as a substrate for production of important antimicrobial agents using various fermentation techniques (Negi and Banerjee 2009) using suitable microbes. This can make the antimicrobial compound very economical and eco-friendly. Microorganisms like endophytic microbes are rich source of novel natural compounds, and these compounds can be used for production of antimicrobial compounds using agrowaste as suitable substrate.
5.4.1 Sugarcane Molasses as the Source of Antimicrobial Products
Sugarcane molasses is the waste generated during sugar production and crystallization from sugarcane. Generally, it is used as a source for animal feed or biomass for ethanol production. Molasses holds the antimicrobial properties because of phenolic compounds present in it. Sugarcane molasses show strong antioxidative and tyrosinase inhibitory activities (Nakasone et al. 1996; Takara et al. 2002, 2003, 2007). Takara et al. (2007) tested the bioactive compounds present in the sugarcane molasses against two pathogenic bacteria S. mutans and S. sobrinus responsible for dental caries in human and animals.
5.4.2 Pine Needles as the Source of Antimicrobial Products
Chir pines, or Pinus roxburghii, are pines growing wild in the Himalayan range. P. roxburghii contains a number of phytochemicals like alkaloids, glycosides, flavonoids, saponins, tannins, and terpenoids in needles, female cones, and bark. Antibacterial and antifungal activities of essential oils of Pinus roxburghii stems were studied by Hassan and Amjid (2009). Major components in essential oil were α-pinene (41.9 %) followed by 3-carene (16.3 %), caryophyllene (12.3 %), p-cymene (1.9 %), terpineol (1.8 %), limonene (1.7 %), borneol acetate (1.1 %), caryophyllene oxide (1.0 %), camphene (0.9 %), and terpinyl acetate (0.8 %). Antibacterial activity of stem essential oil was observed against Staphylococcus aureus and Bacillus subtilis, while no activity was observed against E. coli and Enterobacter aerogenes. Similarly, antifungal activity of Pinus roxburghii essential oil was found inhibiting Aspergillus terreus, Aspergillus flavus, Aspergillus candidus, Aspergillus versicolor, Aspergillus niger, and Trichoderma viride.
5.4.3 Sugarcane Bagasse as the Source of Antimicrobial Products
Sugarcane bagasse is a good source for the production of coumaric acid. Ou et al. (2009) obtained coumaric acid from the sugarcane bagasse. The purified product has shown antimicrobial, anti-inflammatory, antioxidant, and free radical scavenging activities like ferulic acid.
5.4.4 Groundnut Shells as the Source of Antimicrobial Products
Oxytetracycline is one of the most important antibiotics produced from streptomyces, which is considered as the largest antibiotic-producing genus (Walve et al. 2001). Four agricultural wastes, namely, groundnut shells, corncob, corn pomace, and cassava peels, were used by Asagbra et al. (2005) as the substrate for the growth of Streptomyces spp. in solid-state fermentation. Oxytetracycline production started within three days and reached its peak on the sixth day with groundnut shells as substrate.
5.4.5 Production of Oxytetracycline from Cocoyam Peels
Ndubuisi Ezejiofor et al. (2012) used cocoyam peels (household kitchen waste) for production of oxytetracycline by Streptomyces speibonae OXS1 in solid-state fermentation. They used cocoyam peels of species Colocasia esculenta and Xanthosoma esculenta as substrate. Oxytetracycline production started on the first day of fermentation and reached its peak on third day. Higher biomass weight (140.86 g) was found for C. esculenta than X. esculenta (101.62 g) after seven days fermentation indicating higher presence of oxytetracycline in the fermented C. esculenta. Oxytetracycline was present in both species of the cocoyam peels.
6 Recent Advances
Advances in biology and chemistry along with powerful screening and isolation techniques have given the field of natural antimicrobials a big thrust. With the use of latest technologies with particular emphasis on the total synthesis and analog design, it is now possible to determine the mechanism of action of a newly discovered antibiotic and resistance mechanism of bacteria. New techniques and technologies not only help in exploration of new antimicrobial compounds but also ensure their intelligent application. Nanotechnology is one of the important emerging fields that can significantly improve the exploitation and application of antimicrobial compounds. In the recent past, there has been significant research to discover nanoparticles with novel physical, chemical, and biological properties. Nanotechnology is being used for delivery of antimicrobial phenolic compound extracts to effectively inhibit food-borne pathogens. Effectiveness of the antimicrobial compound with nanoparticle delivery compared with conventional delivery system has improved significantly. By using nanoparticle delivery system, phenolic compounds can be used as natural and better replacement of chemicals to control pathogens for commercial food safety applications.
Various nanomolecules, such as silver nanoparticles, carbon nanotubes, magnesium oxide nanoparticles, and zinc oxide nanoparticles, are being used very effectively in tackling human and food pathogens. Silver nanoparticles are nontoxic to the human body at low concentrations but have broad-spectrum antibacterial actions (Lara et al. 2010). Silver particles have strong antimicrobial effect against many drug-resistant organism such as Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, E. coli, and S. pyogenes. The nano-silver materials have immense potential application due to the strong antimicrobial activity of silver against a broad spectrum of bacteria, viruses, and fungi and the low frequency of development of resistance (Egger et al. 2009).
Similarly, carbon nanotubes have applications in drug delivery and as components in medical nanodevices. They also show strong antibacterial activity like silver nanoparticles. Direct interaction of the carbon nanotubes with the bacterial cell membranes causing significant membrane damage is reported to be the reason for their bactericidal effect. Magnesium oxide nanoparticles and zinc oxide nanoparticles also have antimicrobial properties and being explored for therapeutic applications (Matthews et al. 2010).
Ravichandran et al. (2011) demonstrated the efficacy of phenolics on pathogen reduction delivered by nanoparticles by studying their effects on Listeria monocytogenes (L.m.), Escherichia coli O157:H7 (E.c.), and Salmonella typhimurium (S.t.) in brain–heart infusion broth (BHI) and meat system. Encapsulation of benzoic acid in polylactic-co-glycolic acid nanoparticles inhibited both the pathogens much better than the delivery without nanoparticles. With the advent of new nanoparticles and technology, target-specific delivery of therapeutic agents using nanocarriers has become a very promising field. Various delivery vehicles have been designed based on different nanomaterials, such as polymers, dendrimers, liposomes, nanotubes, and nanorods (Matthews et al. 2010).
7 Conclusion
Despite numerous advances in the field of antibiotics, bacterial and fungal infections are still major concern for pharmaceutical as well as food industry. This gets aggravated with the presence of multidrug-resistant strains. Recent advances in biology, chemistry, nanotechnology, and other associated fields, though, have improved the discoveries of novel classes of antibiotics from natural sources. Large numbers of studies are being carried out to explore the various possibilities of utilizing the plant and agro-industrial waste for production of value-added products including antimicrobial agents. There are unlimited possibilities in the field of waste utilization, which requires more push from the industry to take the benefits of the researches in the laboratories to the common man through industry. Utilization of plant and agrowaste for production of antimicrobial agents may significantly reduce the cost of medicines and food preservatives, thereby improving the health and food scenario in the society and at the same time can help to improve the environment. Although extensive research in the field of plants and agro-based antimicrobials has led to the discovery of many new natural antimicrobial agents by pharmaceutical industry, still the area of reusing the plant and agro-industrial by-products or wastes for extraction or production of antimicrobial agents needs more support from industries.
References
Ahmad MM, Salim-ur-Rehman Z, Iqbal-Anjum FM, Sultan JI (2006) Genetic variability to essential oil composition in four citrus fruit species. Pak J Bot 38(2):319–324
Alberto MR, Canavosio MAR, Manca de Nadra MC (2006) Effect of polyphenols from apple skins on human bacterial pathogens. Electron J Biotechnol 9(3)
Amelia P, Guevara AA, Hiromu S, Mutsou K, Harukuni T (1996) Anti-inflammatory, anti-mutagenicity and antitumor-promoting activities of mahogany seeds, Swietenia macrophylla (Meliaceae). Philip J Sci 125(4):271–278
Anastasiadi M, Chorianopoulos NG, Nychas GJE, Haroutounian SA (2008) Antilisterial activities of polyphenol-rich extracts of grapes and vinification byproducts. J Agric Food Chem 57:457–463
Andary C, Wylde R, Laffite C, Privat G, Winternitz F (1982) Structure of verbascoside and orobancoside, caffeic acid, sugar esters from Orobanche rapum-genistae. Phytochemistry 21:1123–1127
Artizzu N, Bonsignore L, Cottiglia F, Loy G (1995) Studies of the diuretic and antimicrobial activity of Cynodon dactylon essential oil. Fitoterapia 66:174–175
Asagbra AE, Oyewole OB, Odunfa SA (2005) Production of oxytetracycline from agricultural wastes using streptomyces spp. Niger Food J 2:174–181
Ashok K, Narayani M, Subanthini A, Jayakumar M (2011) Antimicrobial activity and phytochemical analysis of citrus fruit peels—utilization of fruit waste. Int J Eng Sci Technol 3(6):5414–5421
Aziz NH, Farag SE, Mousa LAA, Abo-Zaidt MA (1998) Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios 93:43–54
Baydar NG, Ozkan G, Sagdic O (2004) Total phenolic contents and antibacterial activities of grapes (Vitis vinifera L.) extracts. Food Control 15(5):335–339
Beheshti A, Norouzi P, Ganjali MR (2012) A simple and robust model for predicting the reduction potential of quinones family electrophilicity index effect. Int J Electrochem Sci 7:4811–4821
Beretz A, Cazenave J (1988) The effect of flavonoids on blood vessel wall interactions. In: Cody V, Middleton E, Harborne JB, Beretz A (eds) Plant flavonoids in biology and medicine II: biochemical, cellular and medicinal properties. Alan R Liss, New York, pp 187–200
Bisignano G, Sanogo R, Marino A, Aquino R, D’angelo V, Germanò MP, De Pasquale R, Pizza C (2000) Antimicrobial activity of Mitracarpus scaber extract and isolated constituents. Lett Appl Microbiol 30:105–108
Borris RP (1996) Natural products research: perspectives from a major pharmaceutical company. J Ethnopharmacol 51:29–38
Caccioni DR, Guizzardi M, Biondi DM, Renda A, Ruberto G (1998) Relationship between volatile components of citrus fruit essential oils and antimicrobial action on Penicillium digitatum and Penicillium italicum. Int J Food Microbiol 43:73–79
Calvo J, Martinez ML (2009) Antimicrobial mechanisms of action. Enferm Infecc Microbiol Clin 27(1):44–52
Canadanovic BJM, Savatovic SS, Cetkovic GS, Vulic JJ, Djilas SM, Markov SL, Cvetkovic DD (2011) Antioxidant and antimicrobial activities of beet root pomace extracts. Czech J Food Sci 29(6):575–585
Capasso R, Evidente A, Schivo L, Orru G, Marcialis MA, Cristinzio G (1995) Antibacterial polyphenols from olive oil mill waste waters. J Appl Bacteriol 79:393–398
Carpenter CF, Chambers HF (2004) Daptomycin: another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin Infect Dis 38:994–1000
Chanda S, Baravalia Y, Kaneria M, Rakholiya K (2010) Fruit and vegetable peels — strong natural source of antimicrobics. In: Mendes-Vilas A (ed) Current research, technology and education topics in applied microbiology and applied biotechnology. Publisher: Formatex Research Center. Vol. 1, pp 444–450
Chanthaphon S, Chanthachum S, Hongpattarakere T (2008) Antimicrobial activities of essential oils and crude extracts from tropical Citrus spp. against food-related microorganisms. Songklanakarin J Sci Technol 30(1):125–131
Ciafardini G, Zullo BA (2003) Antimicrobial activity of oil-mill waste water polyphenols on the phytopathogen Xanthomonas campestris spp. Ann Microbiol 53(3):283–290
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12:564–582
Cushnie TPT, Lamb AJ (2005) Antimicrobial activity of flavonoids. Int J Antimicro Ag 26:343–356
Davidson PM, Branen AL (2005) Food antimicrobials—an introduction. In: Davidson PM, Sofos JN, Branen AL (eds) Antimicrobials in food. CRC Press, Boca Raton, FL, pp 1–10
Doughari JH, El-mahmood AM, Tyoyina I (2008) Antimicrobial activity of leaf extracts of Senna obtusifolia (L). Afr J Pharm Pharmacol 2:7–13
Dzidic S, Suskovic J, Kos B (2008) Antibiotic resistance mechanisms in bacteria: biochemical and genetic aspects. Antibiotic resistance in bacteria. Food Technol Biotechnol 46(1):11–21
Egger S, Lehmann RP, Height MJ, Loessner MJ, Schuppler M (2009) Antimicrobial properties of a novel silver-silica nanocomposite material. Appl Environ Microbiol 75(9):2973–2976
Ekwenye UN, Edeha OV (2010) The antibacterial activity of crude leaf extract of Citrus sinensis (sweet orange). Int J Pharm Bio Sci 1(4):742–750
El-Massry KF, El-Ghorab AH, Shaaban HA, Shibamoto T (2009) Chemical compositions and antioxidant/antimicrobial activities of various samples prepared from Schinus terebinthifolius leaves cultivated in Egypt. J Agric Food Chem 57:5265–5270
Escarpa A, Gonzalez MC (1998) High-performance liquid chromatography with diode-array detection for the determination of phenolic compounds in peel and pulp from different apple varieties. J Chromatogr 823(1–2):331–337
Fathiazada F, Delazara A, Amiria R, Sarker SD (2006) Extraction of flavonoids and quantification of rutin from waste tobacco leaves. Iran J Pharm Res 3:222–227
Fernandez BJ, Rodríguez G, Rodríguez R, Guillen R, Jimenez A (2006) Potential use of olive by products; extraction of interesting organic compounds from olive oil waste. Grasas Y Aceites 57(1):95–106, Enero-Marzo
Gedir JV, Sporns P, Hudson RJ (2005) Extraction of condensed tannins from cervid feed and feces and quantification using a radial diffusion assay. J Chem Ecol 31:2761–2773
Gollnick H, Schramm M (1998) Topical therapy in acne. J Eur Acad Dermatol Venereol 11:8–12
Grinberg LN, Rachmilewitz EA, Newmark H (1994) Protective effects of rutin against hemoglobin oxidation. Biochem Pharmacol 48:643–649
Hamendra SP, Anand K (2007) Antidiabetic potential of Citrus sinensis and Punica granatum peel extracts in alloxan treated male mice. Bio Factors 31:17–24
Harborne JB, Baxter H (1999) The handbook of natural flavonoids, Vol:1 and 2. John Wiley and Sons, Chichester
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55:481–504
Hartmann R, Meisel H (2007) Food-derived peptides with biological activity: from research to food applications. Curr Opin Biotechnol 18:163–169
Hassan A, Amjid I (2009) Gas chromatography–mass spectrometric studies of essential oil of Pinus roxburghii stems and their antibacterial and antifungal activities. J Med Plants Res 3(9):670–673
Havsteen B (1983) Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol 32:1141–1148
Izzo AA, Di Carlo G, Biscardi D, Fusco R, Mascolo N, Borreli F, Capasso F, Fasulo MP, Autore G (1995) Biological screening of Italian medicinal plants for antibacterial activity. Phytother Res 9:281–286
Jayaprakasha GK, Selvi T, Sakaria KK (2003) Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res Int 36:117–122
Kahkonen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M (1999) Antioxidant activity of plant extracts containing phenolic compounds. J Agr Food Chem 47:3954–3962
Kanatt SR, Chander R, Sharma A (2010) Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. Int J Food Sci Technol 45(2):216–222
Kanaze FI, Termentzi A, Gabrieli C, Niopas I, Georgarakis M, Kokkalou E (2008) The phytochemical analysis and antioxidant activity assessment of orange peel (Citrus sinensis) cultivated in Greece-Crete indicates a new commercial source of hesperidin. Biomed Chromatogr 23:239–249
Kayser O, Kolodziej H (1997) Antibacterial activity of extracts and constituents of Pelargonium sidoides and Pelargonium reniforme. Planta Med 63:508–510
Krisch J, Galgoczy L, Papp T, Csaba Vagvolgyi C (2009) Antimicrobial and antioxidant potential of waste products remaining after juice pressing. J Eng tome vii (year. Fascicule 4):131–134
Kubo L, Muroi H, Himejima M (1993) Structure–antibacterial activity relationships of anacardic acids. J Agri Food Chem 41:1016–1019
Kujala T, Loponen J, Pihlaja K (2001) Betalains and phenolics in red beetroot (Beta vulgaris) peel extracts: extraction and characterisation. Zeitschrift fur Naturforschung- C 56:343–348
Kumamoto H, Matsubara Y, Iizuka Y, Okamoto K, Yokoi K (1986) Structure and hypotensive effect of flavonoid glycosides in orange (Citrus sinensis OSBECK) peelings. Agricult Biol Chem 50:781–783
Kwon YS, Kobayashi A, Kajiyama SI, Kawazu K, Kanzaki H, Kim CM (1997) Antimicrobial constituents of Angelica dahurica roots. Phytochemistry 44(5):887–889
Lanciotti R, Gianotti A, Patrignani F, Belletti N, Guerzoni EM, Gardini F (2004) Use of natural aroma compounds to improve shelf-life and safety of minimally processed fruits. Trends Food Sci Tech 15:201–208
Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C (2010) Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J Microbiol Biotechnol 26:615–621
Leung AY, Foster S (1996) Encyclopaedia of common natural ingredients, 2nd edn. Wiley, New York
Maisuthisakul P (2008) Antiradical scavenging activity and polyphenolic compounds extracted from Thai mango seed kernels. As J Food Food Ag-Ind 1(02):87–96
Mangas JJ, Rodriguez R, Suarez B, Picinelli A, Dapena E (1999) Study of the phenolic profile of cider apple cultivars at maturity by multivariate techniques. J Agr Food Chem 47(10):4046–4052
Manthey A, Grohmann K (2001) Phenols in citrus peel byproducts: concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. J Agr Food Chem 49:3268–3273
Martin JGP, Porto E, Correa GB, Matias de Alencar S, Micotti da Gloria E, Cabral ISR, Maria L, de Aquino L (2012) Antimicrobial potential and chemical composition of agro-industrial wastes. J Nat Prod 5:27–36
Matthews L, Kanwar RK, Zhou S, Punj V, Kanwar JR (2010) Applications of nanomedicine in antibacterial medical therapeutics and diagnostics. Open Trop Med J 3:1–9
McManus MC (1997) Mechanisms of bacterial resistance to antimicrobial agents. Am J Health Syst Pharm 54:1420–1433
Melendez PA, Capriles VA (2006) Antibacterial properties of tropical plants from Puerto Rico. Phytomedicine 13:272–276
Mendoza L, Wilkens M, Urzua A (1997) Antimicrobial study of the resinous exudates and of diterpenoids and flavonoids isolated from some Chilean Pseudognaphalium (Asteraceae). J Ethnopharmacol 58:85–88
Mirunalini S, Krishnaveni M (2011) Coumarin: a plant derived polyphenol with wide biomedical applications. Int J PharmTech Res 3(3):1693–1696
Moerman DE (1996) An analysis of the food plants and drug plants of native North America. J Ethnopharmacol 52:1–22
Monga PK, Sharma D, Dubey A (2012) Comparative study of microwave and conventional synthesis and pharmacological activity of coumarins: a review. J Chem Pharm Res 4(1):822–850
Nakasone N, Takara K, Wada K, Tanaka J, Yogi J, Nakatani N (1996) Antioxidative compounds isolated from kokuto, non centrifugal cane sugar. Biosci Biotechnol Biochem 60:1714–1716
Nascimento GGF, Locatelli J, Freitas PC, Silva GL (2000) Antibacterial activity of plant extracts and phytochemicals on antibiotic resistant bacteria. Braz J Microbiol 31:247–256
Ndubuisi Ezejiofor TI, Duru CI, Asagbra AE, Ezejiofor AN, Oisakwe OE, Afonne JO, Obi E (2012) Waste to wealth production of oxytetracycline using streptomyces species from household kitchen wastes of agricultural produce. Afr J Biotechnol 11(43):10115–10124
Negi S, Banerjee R (2009) Optimization of extraction and purification of glucoamylase produced by Aspergillus awamori in solid-state fermentation. Biotechnol Bioproc Eng 14:60–66
Nikaido H, Zgurskaya HI (1999) Antibiotic efflux mechanisms. Curr Opin Infect Dis 12:529–536
Nychas GJE, Tassou CC, Board RG (1990) Phenolic extract from olives inhibition of Staphylococcus aureus. Lett Appl Microbiol 10:217–220
Ou SY, Luo YL, Huang CH, Jackson M (2009) Production of coumaric acid from sugarcane bagasse. Innov Food Sci Emerg 10:253–259
Parmar SS, Sharmar RS (1990) Effect of mango (Mangifera indica L.) seed kernel pre-extract on the oxidative stability of buffalo ghee. Food Chem 35:99–107
Payne K, Rico-Munoz E, Davidson PM (1989) The antimicrobial activity of phenolic compounds against Listeria monocytogenes and their effectiveness in a model milk system. J Food Protect 52:151–153
Pedraza CJ, Cardenas-Rodriguez N, Orozco-Ibarra M, Perez-Rojas JM (2008) Medicinal properties of mangosteen (Garcinia mangostana). Food Chem Toxicol 46:3227–3239
Pereira JA, Oliveira I, Sousa A, Valentão P, Andrade PB, Ferreira IC, Ferreres F, Bento A, Seabra R, Estevinho L (2007) Walnut (Juglans regia L.) leaves: phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem Toxicol 45(11):2287–2295
Piccirillo C, Demiray S, Franco AR, Castro PML, Pintado ME (2010) High added-value compounds with antibacterial properties from ginja cherries by-products. Waste Biomass Valorization 1(2):209–217
Podsedek A, Wilska Jeska J, Anders B, Markowski J (2000) Compositional characterization of some apple varieties. Eur Food Res Technol 210(4):268–272
Pretorius JC, Magama S, Zietsman PC (2003) Growth inhibition of plant pathogenic bacteria and fungi by extracts from selected South African plant species. S Afr J Bot 20:188–192
Ragazzi E, Veronese G, Guitto A (1973) Demethyloleuropein, a new glucoside extracted from ripe olives. Ann Chim 63:13–20
Ravichandran M, Hettiarachchy NS, Ganesh V, Ricke SC, Singh S (2011) Enhancement of antimicrobial activities of naturally occurring phenolic compounds by nanoscale delivery against listeria monocytogenes, escherichia coli O157:H7 and salmonella typhimurium in broth and chicken meat system. J Food Safety 31(4):462–471
Roberts MC (2003) Acquired tetracycline and/or macrolide–lincosamides–streptogramin resistance in anaerobes. Anaerobe 9(2):63–69
Romero C, Garcia P, Brenes M, Garcia A, Garrido A (2002) Phenolic compounds in natural black Spanish olive varieties. Eur Food Res Technol 215:482–496
Saleem M, Nazir M, Ali MS, Hussain H, Lee YS, Riaza N, Jabbara A (2010) Antimicrobial natural products: an update on future antibiotic drug candidates. Nat Prod Rep 27:238–254
Santos JDG, Branco A, Silva AF, Pinheiro CSR, Neto AG, Uetanabaro APT, Queiroz SROD, Osuna JTA (2009) Antimicrobial activity of Agave sisalana. Afr J Biotechnol 8(22):6181–6184
Schiber A, Berardini N, Carle R (2003) Identification of flavonol and xanthol glycosides from mango peels by HPLC. J Agr Food Chem 51:5006–5011
Shoji T, Akazome Y, Kanda T, Ikeda M (2004) The toxicology and safety of apple polyphenol extract. Food Chem Toxicol 42:959–967
Shoji T, Mutsuga M, Nakamura T, Kanda T, Akiyama H, Goda Y (2003) Isolation and structural elucidation of some procyanidins from apple by low-temperature NMR. J Agr Food Chem 51(13):3806–3813
Singh SP, Negi S (1992a) Antibacterial and antifungal activities of Mentha arvensis essential oil. Fitoterapia 63(1):76–78
Singh SP, Negi S (1992b) Antibacterial properties of essential oils from Zingiber chrysanthum leaves and rhizomes. Fitoterapia 63(1):73–75
Singleton VL, Rossi JA Jr (1965) Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am J Enol Viticult 16(1):144–158
Soediro I, Padmawinata K, Wattimena JR, Rekita S (1990) Study of the active antimalarial methanolic extract of Swietenia macrophylla King (Meliaceae). Acta Pharm Indones 15(1):1–13
Soong Y, Barlow P (2004) Antioxidant activity and phenolic content of selected fruit seeds. Food Chem 88:411–417
Spratt BG (1994) Resistance to antibiotics mediated by target alterations. Science 264:388–393
Suksamrarn S, Komutiban O, Ratananukul P, Chimnoi N, Lartpornmatulee N, Suksamrarn A (2006) Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana. Chem Pharm Bull 54:301–305
Sung SH, Kim KH, Jeon BT, Cheong SH, Park JH, Kim DH, Kweon HJ, Moon SH (2012) Antibacterial and antioxidant activities of tannins extracted from agricultural by-products. J Med Plants Res 6(15):3072–3079
Takara K, Matsui D, Wada k, Ichiba T, Chinen L, Nakasone Y (2003) New phenolic compounds from kokuto non centrifuged cane sugar. Biosci Biotechnol Biochem 67:376–379
Takara K, Matsui D, Wada K, Ichiba T, Nakasone Y (2002) New anti-oxidative phenolic glycosides isolated from kokuto non centrifuged cane sugar. Biosci Biotechnol Biochem 60:1714–1716
Takara K, Ushijima K, Wada K, Iwasaki H, Yamashita M (2007) Phenolic compounds from sugarcane molasses possessing antibacterial activity against cariogenic bacteria. J Oleo Sci 56(11):611–614
Tan SK, Osman H, Wong KC, Boey PL, Ibrahim P (2009) Antimicrobial and antioxidant activities of Swietenia macrophylla leaf extracts. As J Food Ag-Ind 2(02):181–188
Tanner GJ, Moate PJ, Davis LH, Laby RH, Yuguang L, Larkin PJ (1995) Proanthocyanidins (condensed tannins) destabilise plant protein foams in a dose-dependent manner. Aust J Agric Res 46:1101–1109
Tassou CC, Nychas GJE (1995) Inhibition of Salmonella enteritidis by oleuropein in broth and in a model food system. Lett Appl Microbiol 20:120–124
Tehranifara A, Selahvarzia Y, Kharrazia M, Bakhshb VJ (2011) High potential of agro-industrial by-products of pomegranate (Punica granatum L.) as the powerful antifungal and antioxidant substances. Ind Crop Prod 34(3):1523–1527
Tenover FC (2006) Mechanisms of antimicrobial resistance in bacteria. Am J Med 119(6A):S3–S10
Vander SAA, Dekker M, De Jager A, Jongen WMF (2001) Activity and concentration of polyphenolic antioxidants in apple: effect of cultivar, harvest year, and storage conditions. J Agr Food Chem 49(8):3606–3613
Vinson JA, Hao Y, Su X, Zubik L (1998) Phenol antioxidant quantity and quality in foods: vegetables. J Agr Food Chem 46:3630–3634
VishnuPriya V, Mallika J, Surapaneni KM, Saraswathi P, Chandra SGSV (2010) Antimicrobial activity of pericarp extract of garcinia mangostana linn. Int J Pharm Sci Res 1(8):278–281
Visioli F, Galli C (2002) Biological properties of olive oil phytochemicals. Crit Rev Food Sci Nut 42:209–221
Visioli F, Grande S, Bogani P, Galli C (2004) The role of antioxidants in the Mediterranean diets: focus on cancer. Eur J Cancer Prev 13:337–343
Wagdy SM, Taha FS (2012) primary assessment of the biological activity of jojoba hull extracts. Life Sci J 9(2):244–253
Walve MG, Trekoo R, Log MM, Bhole BD (2001) How many antibiotics are produced by the genus streptomyces. Arch Microbiol 177(5):86–90
Wannissorna B, Jarikasemb S, Siriwangchaib T, Thubthimthed S (2005) Antibacterial properties of essential oils from Thai medicinal plants. Fitoterapia 76:233–236
Webber MA, Piddock LJ (2003) The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother 51:9–11
WHO (2012) Antimicrobial resistance. http://www.who.int/mediacentre/factsheets/fs194/en/index.html. Accessed 10 Aug 2012
Will F, Bauckhage K, Dietrich H (2000) Apple pomace liquefaction with pectinases and cellulases: analytical data of the corresponding juices. Eur Food Res Technol 211:291–297
Woodford N, Ellington MJ (2007) The emergence of antibiotic resistance by mutation. Eur J Clin Microbiol Infect Dis 13:5–18
Wright GD (2005) Bacterial resistance to antibiotics: enzymatic degradation and modification. Adv Drug Deliver Rev 57:451–1470
Yao XJ, Wainberg MA, Parniak MA (1992) Mechanism of inhibition of HIV-1 infection in vitro by purified extract of Prunella vulgaris. Virology 178:56–62
Zargham H, Zargham R (2008) Tannin extracted from Sumac inhibits vascular smooth muscle cell migration. Mcgill J Med 11:119–123
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415(6870):389–395
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Negi, S. (2014). Exploring Plant and Agro-industrial Wastes for Antimicrobial Biochemicals. In: Brar, S., Dhillon, G., Soccol, C. (eds) Biotransformation of Waste Biomass into High Value Biochemicals. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8005-1_14
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8005-1_14
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8004-4
Online ISBN: 978-1-4614-8005-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)