Abstract
Stearoyl-CoA desaturase 2 (SCD2) is one of four highly similar mouse Δ9 desaturases with close identity to the human Δ9 desaturase, hSCD1. SCD2 functions in skin to maintain palmitoleate and oleate in complex lipids and linoleic acid in acylceramide, a key component in protecting the epidermal barrier. Thus, SCD2 null mice exhibit high lethality, likely due to severe dehydration and skin permeability barrier defects. SCD2 also plays a major role in hepatic lipogenesis in embryonic and newborn mice. However, the decreased hepatic triglyceride and VLDL production observed in newborn SCD2 null mice is rescued by rising levels of SCD1 in adult animals. Interestingly, SCD2 function may be distinct from SCD1 in cultured adipocytes since its depletion by siRNA in preadipocytes uniquely disrupts the differentiation process. This effect is associated with reduced translation of selective proteins, including PPARγ. Here we examine the regulation, and physiological roles in adipose tissue, B and T cells, liver, skin, reproduction, and during development.
Jennifer L. Cantley and Lucas M. O’Neill contributed equally to this work.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Stearoyl-CoA desaturase-2 (SCD2) is a key member of the murine Δ9-desaturase family and is documented to be the major isoform required for the early development of mouse (Miyazaki et al. 2005). Despite this, little is known about its physiological roles. Historically, SCD2 was labeled as “brain SCD,” because at the time, SCD2 was thought to be the only SCD isoform expressed in the brain (Kaestner et al. 1989; DeWillie and Farmer 1992). Despite being highly expressed in the brain, the role of SCD2 in this organ has been largely unexplored. Today, we know SCD2 to be expressed ubiquitously and is the predominate isoform in tissues such as B lymphocytes, epidermis, lung, pancreas, spleen, testis, and ovaries (Sampath and Ntambi 2011a, b; Swick and Lane 1992; Saether et al. 2003). We are also beginning to grasp the temporal expression pattern of SCD2 and its role in adipocytes.
SCD2 is expressed in adult mice, but it is more highly expressed and critical during embryonic development and in newborn mice prior to weaning. Complete loss of SCD2 is embryonic lethal in the C57Bl6 genetic background (B6) (Miyazaki et al. 2005). In the 129SvEv genetic background (129), complete loss of SCD2 is ~70-100 % lethal at birth with few survivors (Miyazaki et al. 2005). In cultured 3T3L1 adipocytes, SCD2 is the only SCD isoform required for adipocyte differentiation (Christianson et al. 2007). The mechanism for this is currently unknown, but it is thought that SCD2 regulates the expression of PPARγ, a key determinant of the adipogenic process. In fact, knockdown of SCD2 is just as powerful at inhibiting adipocyte differentiation as silencing PPARγ itself (Christianson et al. 2007).
There are four known SCD isoforms in mouse (SCD1–4) and two in human (hSCD1 and hSCD5), (Beiragi et al. 2003; Man et al. 2005; Ntambi and Miyazaki 2004; Zhang et al. 1999) but only human SCD1 is considered an orthologue of the mouse SCD isoforms (Evans 2008; Wang et al. 2005). Both mouse SCD1 and SCD2 display approximately 80 % sequence identity to human SCD1 (Fig. 10.1), utilize the same substrates, and share common promoter elements (Fig.10.2) and tissue expression profiles (Hodson and Fielding 2012; Waters et al. 1997; Zhang et al. 2005, 1999). Similar to mouse SCD1 and SCD2, human SCD1 is also ubiquitously expressed with the highest expression in the adipose tissue (Hodson and Fielding 2012; Roongta et al. 2011; Zhang et al. 1999). Human SCD5 is highly expressed in the brain and pancreas, similar to mouse SCD2, despite not sharing functional homology (Wang et al. 2005). Functionally, human SCD1 may encompass the characteristics of both SCD1 and SCD2 in one enzyme: while mouse SCD1 and SCD2 appear to have redundant functions in most tissues, functional differences for these isoforms do appear to exist in adipose tissue, which may be fulfilled solely by SCD1 in humans (Christianson et al. 2007).
Comparison of the amino acid sequences of the mouse SCD2 (mSDC2) and human SDC1 (hSDC1). There is very high sequence similarity between these mouse and human proteins, with highest variability in the N-terminus region. Both the N-terminus and the C-terminus regions of these proteins are thought to be cytoplasmic, while four putative transmembrane regions of these proteins are thought to cross the endoplasmic reticulum membrane. The variable N-terminus region is far removed from the more central segments containing the histidine regions that form the core of the catalytic domain, suggesting they may not be involved in substrate recognition (Zhang et al. 2005)
Location of transcription factor recognition sites in the promoter regions of mouse SCD1 (mSCD1), mouse SCD2 (mSCD2), and human SCD1 (hSCD1). The differences in recognition sites and their locations between mSCD1 and mSCD2 likely explain in part their distinct tissue expression profiles, for example that mSCD2 is more highly expressed in embryonic and newborn liver than mSCD1. NF nuclear factor, C/EBP CCAAT enhancer binding protein, SRE serum response factor (Bene et al. 2001)
The study of SCD2 opens the door to discovering new lipid regulated developmental signaling pathways along with exploring new roles for monounsaturated fatty acids (MUFAs). The legacy of SCD2 is still developing and many questions regarding the physiological roles of this enzyme remain unanswered. Undoubtedly, SCD2 plays a unique and essential role. However, more research is warranted to determine the exact pathways that SCD2 affects and how to use this information in relation to human development.
This chapter will be a cumulative review about SCD2 that will include its regulation and its known physiological roles in different tissues.
Discovery of SCD2
During the initial identification of SCD1, southern blots were performed on 3T3-L1 genomic DNA using a variety of probes against multiple regions of the SCD gene. One probe in particular, containing the entire coding region of SCD1, was hybridized to 3T3-L1 genomic DNA that was collected 5 days after the addition of a differentiation cocktail consisting of methylisobutylxanthine, dexamethasone, and insulin, and digested with EcoR1. Surprisingly, in addition to the three EcoR1 fragments that comprised the complete gene of SCD1, this probe hybridized to a 14- and 2.2-kb genomic DNA EcoRI fragment (Kaestner et al. 1989). The additional hybridizations could be explained by two possibilities; the 14- and 2.2-kb fragments could be a part of an SCD pseudogene, or secondly, the fragments could be representative of another SCD isoform (Ntambi et al. 1988).
It is at this point, in 1986, that Klaus Kaestner, a graduate student working with James Ntambi in M. Daniel Lane’s laboratory at The Johns Hopkins School of Medicine, decided to further explore these unidentified fragments for the possibility that they represented a second SCD gene. Kaestner began his investigation with a clone, designated λE14, which was created during the initial cloning of SCD1 (Kaestner et al. 1989). It contained five exons that were very similar to those in the SCD1 gene, but they were not identical. In order to determine if this clone was a second SCD, Kaestner set out to find its corresponding cDNA. Using a probe containing part of the first exon (nucleotides 205–324), generated by the digestion of λE14 with PstI, he screened a mouse 3T3-L1 cDNA library. The longest clone purified from the cDNA library was 2.7kb fragment called λ32 (Kaestner et al. 1989).
The last 200 bp of λ32 was then used to probe the same mouse 3T3-L1 cDNA library, and from this a clone λ53, containing a sequence containing a poly(A)-track, was isolated (Kaestner et al. 1989). Together λ32 and λ53 represent the complete mRNA of 5 kb. This was subcloned and sequenced using dideoxy chain termination. The sequence encoded for a protein that was >87 % similar to SCD1 and was named SCD2 (Kaestner et al. 1989).
SCD2 Regulation
Unlike Scd1, which is located on the negative strand of chromosome 19, Scd2 and the other two SCD family members, Scd3 and Scd4, are found on the positive strand of chromosome 19 (Man et al. 2005). Scd2 is ~15 kb in length and has six exons, five introns, and a uniquely large 3′ UTR that is indicative of the SCD family (Kaestner et al. 1989). Most often, genes that have a long 3′ UTR, including the other SCD isoforms, have multiple polyadenylation signals, so it is surprising that SCD2 has only one such signal. To date, the function of this long 3′ UTR remains unknown.
Many of the regulatory components of Scd2 were discovered by the differential tissue expression patterns of SCD2 and SCD1 in response to diet. SCD1 and SCD2 are both induced by diets low in unsaturated fatty acids. However, they are induced in different tissues. For example, mice fed a low-fat diet induce SCD1, but not SCD2, expression in the liver; in contrast, this diet induces SCD2 expression in the kidney to a much higher extent than SCD1 (Kaestner et al. 1989). The differences in tissue expression and dietary induction were thought to be differences in the control of gene expression. Kaestner recognized that the promoters of SCD2 and SCD1 are significantly different and that these differences may have an effect on the expression and induction differences between the two isoforms (Kaestner et al. 1989). A detailed examination of the promoters would come in the years that followed his initial work, but at the time, Kaestner was able to determine that SCD2 has two conserved CCAAT boxes found in SCD1 and that SCD2 has a missing cAMP core regulatory sequence that is present in the promoter of SCD1 (Fig. 10.2) (Kaestner et al. 1989).
During the differentiation of 3T3L1 preadipocytes into adipocytes, genes involved in fatty acid uptake, synthesis, and storage are activated (Christianson et al. 2007; Hodson and Fielding 2012). In 1992, Andrew Swick and M. Daniel Lane discovered that SCD2 contains a preadipocyte repressor element (PRE) site in the promoter (between −435 and −410) that is targeted by a ~58 kDa PRE binding protein causing transcription repression (Swick and Lane 1992).
In 1998, David Tabor and Peter Edwards determined that SCD2 is regulated by the nuclear levels of the pro-lipogenic transcription factor, sterol regulatory element binding protein (SREBP) (Tabor et al. 1999). They also identified a novel sterol regulatory element (SRE) located within the proximal promoter of SCD2 that does not comprise of the usual C/TCAC/T repeats that are separated by a single nucleotide (Fig. 10.2) (Tabor et al. 1999). Additionally, this group found that either a mutation in the SRE or the CCAAT boxes could attenuate SCD2 expression in response to sterols (Tabor et al. 1999). This demonstrated that that sterol-induced transcription is both NF-Y- and SREBP-dependent.
SCD2 Function in the Skin
SCD2 appears to be critical for lipid synthesis during neonatal development, since the SCD2 null mice have a severe skin permeability barrier defect and decreased triglyceride and FFA (free fatty acid) levels in the liver, skin, and plasma. This lack in lipid synthesis appears to be critical for survival because while the SCD2 null mice are born with the expected frequency, >70 % of them die within 24 h after birth when the genetic background is 129SvEv and 100 % die during embryonic development when the genetic background is C57Bl6. Most likely the lethality displayed in the 129SvEv genetic backgound is due to severe dehydration from the skin permeability dysfunction.
The SCD2 null mice that do survive are significantly smaller than the wild-type mice and have reduced body weights. The newborn SCD2 null mice have dry, cracked skin due to the skin permeability barrier defect, with a tightly packed and thickened epidermal stratum corneum. Examination of the lamellar membrane system in the skin reveals there are normal numbers of lamellar bodies in the knockout mice, but a decrease in their internal contents and a delay in lamellar membrane formation. While the epidermis has increased saturated FFA content, it has significantly decreased levels of cholesterol esters, triglyceride, acylceramide, and glucosylceramide, which are the primary constituents of lamellar bodies. Therefore, there appears to be decreased lipid delivery to lamellar granules, leading to deficient deposition of lipids in the stratum corneum interstices.
Additionally, while the total content of linoleic acid is not altered, there is a 80 % decrease of linoleic acid in the acylceramide fraction and an increase of this fatty acid in the phospholipid fraction. Since acylceramide is a major lipid involved in epidermal barrier function, decreasing its content or altering its composition will affect membrane barrier integrity. The epidermal lipid composition also showed the expected decrease in palmitoleate and oleate levels in total lipids of SCD2 null mice, with corresponding increases in saturated fatty acid levels. These differences occurred despite a several fold increase in SCD1 expression in both the epidermis and dermis of the SCD2 null mice, presumably a response to the loss of SCD2. Depletion of SCD2 also decreased the expression of keratinocyte differentiation genes transglutaminase-1 and involucrin, indicating that SCD2 products may play a role in gene expression programming during development. Nonetheless two genes that function in modulating the skin permeability barrier, glucosidase beta and diacylglycerol acyltransferase-2, were not altered in their mRNA levels. Together, these studies indicate that SCD2 is crucial for proper epidermal lipid synthesis, lipid channeling into different epidermal cellular compartments, and skin permeability barrier formation in neonate mice.
SCD2 Function in the Liver
While SCD1 regulates hepatic triglyceride synthesis in adult mice, SCD2 controls hepatic lipid metabolism in embryonic and newborn mice. This concept is consistent with the finding that hepatic total SCD catalytic activity is decreased by 70 % in newborn SCD2 null mice compared to wild-type mice, but not different between adult SCD2 null mice and age-matched controls. In the absence of SCD2, there is a near 50 % reduction in hepatic and plasma triglyceride levels and a 27 % reduction in total hepatic FFA in newborn mice. The decrease in triglyceride content appears to be due to decreased triglyceride synthesis, since 3H-glycerol incorporation after IP injection is 54 % reduced in the triglyceride fraction11. However, there are no differences in the plasma glucose concentration, hepatic glycogen content, or hepatic phospholipid and cholesterol ester content. Also, there are no differences in the hepatic phospholipid synthesis rate or in the phospholipid composition as seen in the epidermis of the knockout mice. In the mice that survive past weaning, the defects in lipid synthesis are normalized due to increased SCD1 expression. Therefore, SCD2 is required for appropriate FFA, triglyceride, and VLDL production in the liver of embryos and neonates, but not in adult mice.
SCD2 Function in the Adipose Tissue
In adult mice, SCD1 is highly expressed in white adipose tissue depots and regulates MUFA production (Miyazaki et al. 2001; Ntambi and Miyazaki 2004). However, SCD2 is also expressed in adipose tissue, despite the abundant expression of SCD1. Evidence suggests that SCD1 and SCD2 may have disparate cellular functions in the white adipose tissue, as opposed to the liver and skin, where SCD1 appears to compensate for SCD2 function. It was reported that in mice fed a high-fat diet there is a large fold induction of SCD2 mRNA expression, much larger than effects on SCD1. In fact, out of 26 lipid metabolizing enzymes examined, SCD2 mRNA expression increased in per cent more than any other enzyme. One possible explanation for this observation is that SCD2 may be required to metabolize particular fatty acid species under high-fat feeding conditions. However, since increasing obesity on a high-fat diet also promotes adipogenesis, the process whereby preadipocyte fibroblasts differentiate into fully mature adipocytes, a role for SCD2 in controlling adipocyte development in the mouse is also possible.
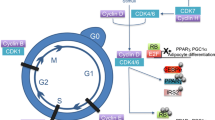
Consistent with this latter hypothesis, studies in 3T3-L1 adipocytes indicate that significant levels of SCD2, but not of SCD1, are required for adipogenesis and for the maintenance of the adipocyte phenotype in fully differentiated adipocytes. The process of adipogenesis occurs through a transcriptional cascade beginning with the rapid and transient expression of C/EBPβ and C/EBPδ. These transcriptional factors then induce the expression of the ligand-activated receptor, PPARγ, and C/EBPα, which are critical for the completion of adipogenesis as well as the maintenance of adipocyte gene expression in fully differentiated cells (Ishida-Yamamoto and Kishibe 2011; Tang and Lane 2012). Recent experiments showed that siRNA-mediated depletion of SCD2 prevented PPARγ mRNA induction during adipogenesis and blocked the conversion of fibroblasts to adipocytes as indicated by oil red O staining of neutral lipid accumulation. Remarkably, silencing SCD2 was as powerful at inhibiting adipocyte differentiation as silencing PPARγ in this cultured preadipocyte system. In line with this interpretation, GLUT4 expression in 3T3-L1 cells was inhibited by about 90 % when either SCD2 or PPARγ were depleted with siRNA prior to the induction of differentiation. PPARγ protein levels were also reduced by about 90 % upon siRNA-mediated silencing of SCD2 under these same conditions. This defective adipogenesis observed upon SCD2 depletion was not restored by the addition of the PPARγ-specific ligand, rosiglitazone, suggesting SCD2 is not controlling the production of a PPARγ ligand.
In fully differentiated adipocytes, SCD2 regulates PPARγ protein expression, rather than transcript levels, which are unchanged after SCD2 depletion. It appears that PPARγ translation is inhibited in the absence of SCD2, while degradation is not affected. This downregulation of PPARγ translation and protein expression then leads to decreased expression of PPARγ target genes and loss of adipocyte function (Fig. 10.3). However, the effect of SCD2 depletion on protein synthesis is not specific to PPARγ since it appears SCD2 is required to maintain general protein synthesis. In fact, [35S] methionine/cysteine labeling shows a small 15 % decrease in newly synthesized protein in 3T3-L1 adipocytes depleted of SCD2 by siRNA silencing. However, unlike PPARγ, many proteins actually decrease at the transcript level, while others increase. Thus, in the mature adipocytes, lack of SCD2 decreases PPARγ protein, which in turn decreases expression of downstream genes important for maintaining the adipocyte phenotype, such as SCD1, fatty acid synthase, and acetyl CoA carboxylase (Fig. 10.3).
Hypothetical model whereby mSCD2 may function directly or indirectly to selectively stimulate protein translation in cultured adipocytes. Such an action of mSCD2 protein or products that it produces increase PPARγ and CEBPα protein-levels (among other proteins), that in turn function to promote expression of genes [e.g., GLUT4 glucose transporter, Fat-specific protein (FSP) 27, Adiponectin (AdipoQ)] required for adipocyte differentiation. See text and Christianson et al. (2007)
The mechanism by which SCD2 regulates PPARγ mRNA expression in preadipocytes and protein translation in fully differentiated adipocytes remains a mystery. It may be through the production of an SCD2-specific unsaturated fatty acid or the proper shuttling of a fatty acid, as seen in the SCD2 knockout mouse, that regulates the translational machinery. Another possibility is that SCD2 is necessary for a protein–protein interaction that regulates translation. Regardless of the mechanism, these studies have described a distinct function for SCD2, apparently divergent from SCD1, in 3T3-L1 adipocytes. This may also be the case in other mouse tissues, explaining why these two isoforms are often simultaneously expressed. Furthermore, it is possible that human SCD1 also controls adipogenesis and adipocyte function, a hypothesis that deserves testing in future experiments.
SCD2 Expression in Immune Cells
Stearic acid potently inhibits the growth and proliferation of T lymphocytes, whereas B lymphocytes display a high degree of immunity (Pourbohloul et al. 1985; Pourbohloul and Buttke 1990). The reason for the differences in T lymphocyte and B lymphocyte susceptibility can be attributed to their Δ9-desaturase activity. B lymphocytes exclusively express SCD2 to a high level, and in contrast T lymphocytes do not have any detectable SCD transcript (Tebby and Buttke 1992). Accordingly, when B and T lymphocytes are treated with stearate in vitro, the B cells are able to desaturate up to 25 % of the stearate into oleate, whereas the T cells are not able to desaturate stearate (Buttke et al. 1989). The inability of T cells to desaturate stearate causes the T cell to display 2–4 times more stearate in distearoylphosphatidylcholine than B cells. This level is equivalent to the amount of distearoylphosphatidylcholine that causes hemolysis of erythrocytes (Kuypers et al. 1984). Our knowledge of the role SCD2 in mouse immunology is limited. However, changes in the levels of dietary fat can profoundly alter immune responses in vivo, and it has been suggested that T cells are the predominate sensitive immune cell population (Buttke et al. 1989).
SCD2 Function in Reproduction
Functional testes and ovaries are in part dependent on the proper balance of fatty acids. This balance is critical for maintaining membrane fluidity and motility of sperm, and for membrane fluidity and the proper lipid reserve in the oocytes (Moreau et al. 2005; Saether et al. 2003). Although these alone can be attributed to the abundance and distribution of 22:6(n-3), functional sperm also require a balanced composition of n-3, n-6, and n-9 fatty acids. SCD2 is the predominate Δ9 desaturase expressed in the testes and ovaries and is responsible for the Δ9-desaturase activity in these organs (Moreau et al. 2005; Saether et al. 2003).
The roles of palmitoleate and oleate in the testis are not clear. However, the expression pattern of SCD1 and SCD2 within the testis has been established. SCD1 and SCD2 are both highly expressed in the epididymis, whereas only SCD2 is predominate in the testis, germ cells, and Sertoli cells (Saether et al. 2003). Sertoli cells are also known as “nurse cells” for their function of providing nutrients, such as PUFAs, to developing sperm cells. They are thought to be the key provider of PUFAs to the germ cells (Saether et al. 2003). Since SCD2 is highly expressed in the Sertoli cells, it is possible that in addition to providing PUFAs, the Sertoli cells also provide the germ cells with MUFAs (Saether et al. 2003).
The regulation of SCD1 and SCD2 within the testis is very interesting. SCD2 expression is up-regulated by insulin and dexamethasone while SCD1 is down-regulated (Saether et al. 2003). This is surprising, given the opposite effect these hormones have on SCD1 in the liver, where SCD1 is up-regulated by insulin and dexamethasone and SCD2 is down-regulated. One such explanation for this difference is that the regulation may reflect the roles that the testis and liver play in fatty acid metabolism. The primary role of SCD1 in the liver is to provide MUFAs for export, whereas the primary role of SCD2 in the testis is to replenish lost MUFAs (Saether et al. 2003).
Another key regulator of desaturase activity in the testis is follical stimulating hormone (FSH). FSH stimulates both SCD2 and SCD1 to very high levels in the Sertoli cells (Saether et al. 2003). It is reported that in the developing testis, FSH enhances the desaturase expression to levels needed for proper spermatogenesis (Saether et al. 2003).
Female oocytes of a variety of vertebrates have a high abundance of MUFAs, mainly palmitoleate and oleate, in the form of a lipid reserve that is critical for reproduction (Moreau et al. 2005). Much of the work analyzing the expression and regulation of SCD2 in the ovaries was by Céline Moreau and Joëlle Dupont (Moreau et al. 2005). In agreement with the work done on rat testis, by the Haugen group, the level of Scd2 mRNA was high and predominate in whole ovary compared to Scd1, while the opposite is true for liver (Moreau et al. 2005; Saether et al. 2003). Both SCD1 and SCD2 are expressed in the granulosa cells of large follicles, corpus luteum, and cumulus oophorus of the ovary, but SCD2 is predominate in all of these tissues (Moreau et al. 2005). The cumulus cells provide nutrition for the oocyte and given expression of SCD2 is absent in the oocyte (Moreau et al. 2005); it can be speculated that SCD2 is the main depositor of MUFAs within that tissue. Also, SCD2 is highly expressed within the granulosa cell of the cumulus and is virtually absent from the oocytes (Moreau et al. 2005), which supports the hypothesis that lipids are transported into the oocytes from the cumulus. In the ovary SCD2 is increased during follicular development and is positively regulated by pregnancy hormones that affect follicular development such as eCG and hCG as well as the hormones FSH and IGF1 (Moreau et al. 2005). Using rat granulosa cells to investigate the molecular mechanisms involving IGF1- and FSH-induced SCD2 expression, it was determined that the MAPK3/MAP1 and AKT signaling are involved in inducing expression, respectively (Moreau et al. 2005). This is in agreement with evidence suggesting that MAP3K/MAP1 and AKT pathways are involved in granulosa cell proliferation and steroidgenesis (Moreau et al. 2005).
Human Relevance
The two known human SCD isoforms are hSCD1 and hSCD5. hSCD1 shares high amino acid sequence similarity to SCD2. However hSCD5, also shares a high amino acid sequence similarity to SCD2, but unlike hSCD1, hSCD5 is expressed in a tissue specific manner similar to SCD2 (Beiragi et al. 2003; Dobrzyn and Ntambi 2005; Miyazaki et al. 2006; Wang et al. 2005). In addition, it was discovered that a pericentric inversion of hSCD5 was found in two generations of a family with cheiloschisis or cleft lip (Beiragi et al. 2003). Although defects in SCD2 and hSCD5 produce different tissue morphologies (Beiragi et al. 2003; Miyazaki et al. 2005), there is a strong connection between MUFA synthesis and tissue development.
Conclusions
In recent years it has become evident that SCD2 and the other isoforms of SCD are playing distinct and nonredundant roles. In this chapter we examined the known function of SCD2, both tissue specifically and temporally. Future research on SCD2 will be exciting as there are many unanswered questions about how exactly MUFAs influence early development, tissue morphogenesis, signaling pathways, and adipocyte differentiation.
References
Beiragi S, Zhou M, Talmadge CB, Went-Sumegi N, Davis JR, Huang D, Saal H, Seemayer T, Sumegi J (2003) Identification and characterization of a novel gene disrupted by a pericentric inversion inv(4)(p13.lq21.1) in a family with cleft lip. Gene 309:11–21
Bene H, Lasky D, Ntambi JM (2001) Cloning and Characterization of the Human Stearoyl CoA Desaturase Gene Promoter: Transcriptional Activation by Sterol Regulatory Element Binding Protein and Repression by Polyunsaturated Fatty Acids and Cholesterol. Biochem Biophys Res Commun 284:1194–1198
Buttke T, Van Cleave S, Steelman L, McCubrey J (1989) Absence of unsaturated fatty acid synthesis in murine T lymphocytes. Proc Natl Acad Sci U S A 86:6133–6137
Christianson JL, Nicoloro S, Straubhaar J, Czech MP (2007) Stearoyl-CoA desaturase 2 is required for peroxisome proliferator-activated receptor γ expression and adipogenesis in cultured 3T3–L1 cells. J Biol Chem 283(5):2906–2916
DeWillie JW, Farmer SJ (1992) Quaking phenotype influences brain lipid-related mRNA levels. Neurosci Lett 141(12):195–198
Dobrzyn A, Ntambi JM (2005) Stearoyl-CoA desaturase as a new drug target for obesity treatment. Obes Rev 6(2):169–174
Evans H (2008) Ancient and modern duplication events and the evolution of stearoyl-CoA desaturases in teleost fishes. Physiol Genomics 35:18–29
Hodson L, Fielding BA (2012) Stearoyl-CoA desaturase: rogue or innocent bystander? Prog Lipid Res 52:15–42
Ishida-Yamamoto A, Kishibe M (2011) Involvement of corneodesmosome degradation and lamellar granule transportation in the desquamation process. Med Mol Morphol 44:1–6
Kaestner KH, Ntambi JM, Kelly TJ, Lane MD (1989) Differentiation-induced gene expression in 3T3-L1 preadipocytes: a second differentially-expressed gene encoding stearoyl-CoA desaturase. J Biol Chem 264:14755–14761
Kuypers FA, Roelofsen B, Berendsen W, Op den Kamp JA, van Deenen LL (1984) Shape changes in human erythrocytes induced by replacement of the native phosphatidylcholine with species containing various fatty acids. J cell Biol 99(6):2260– 2267
Man WC, Miyazaki M, Chu K, Ntambi JM (2005) Membrane topology of mouse stearoyl-CoA desaturase 1. J Biol Chem 281(2):1251–1260
Miyazaki M, Kim YC, Ntambi JM (2001) A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J Lipid Res 42:1018–1024
Miyazaki M, Dobrzyn A, Elias P, Ntambi J (2005) Stearoyl-CoA desaturase-2 gene expression is required for lipid synthesis during early skin and liver development. Proc Natl Acad Sci USA 102(35):12501–12506
Miyazaki M, Bruggink SM, Ntambi JM (2006) Identification of mouse palmitoyl-coenzyme A Δ9-desaturase. J Lipid Res 47:700–704
Moreau C, Froment P, Tosca L, Moreau V, Dupont J (2005) Expression and regulation of the SCD2 desaturase in the rat ovary. Biol Reprod 74:75–87
Ntambi JM, Miyazaki M (2004) Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res 4:91–104
Ntambi JM, Buhrow S, Kaestner KH, Christy RJ, Sibley E, Kelly TJ, Lane MD (1988) Differentiation-induced gene expression in 3T3-L1 preadipocytes: characterization of a differentially-expressed gene encoding stearoyl-CoA desaturase. J Biol Chem 3:17291–17300
Pourbohloul S, Mallett GS, Buttke TM (1985) Inhibition of lymphocyte proliferation by free fatty acids. III. Modulation of thymus-dependent immune responses. Immun. 56(4):659–666
Pourbohloul SC, Buttke TM (1990) Identification of T-helper cells as the target of stearic acid-inhibition in primary antibody responses in vitro. Int J Immunopharmcol 12(7):799–807
Roongta UV, Pabalan JG, Wang X, Ryseck RP, Fargnoli J, Henely BJ, Yang WP, Zhu J, Madireddi MT, Lawrence RM, Wong TW, Rupnow BA (2011) Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy. Mol Cancer Res 9:1551–1561
Saether T, Tran TN, Rootwelt H, Christophersen BO, Haugen TB (2003) Expression and regulation of Δ5-desaturase, Δ6-desaturase, stearoyl-coenzyme A (CoA) desaturase 1, and stearoyl-CoA desaturase 2 in rat testis. Biol Reprod 69(1):117–124
Sampath H, Ntambi JM (2011a) The role of stearoyl-CoA desaturase in obesity, insulin resistance, and inflammation. Ann N Y Acad Sci 1243:47–53
Sampath H, Ntambi JM (2011b) The role of fatty acid desaturases in epidermal metabolism. Dermatoendocrinol 3(2):62–64
Swick AG, Lane MD (1992) Identification of a transcriptional repressor down-regulated during preadipocyte differentiation. Proc Natl Acad Sci U S A 89:7895–7599
Tabor DE, Kim JB, Spiegelman BM, Edwards PA (1999) Identification of conserved cis-elements and transcription factors required for sterol-regulated transcription of stearoyl-CoA desaturase 1 and 2. J Biol Chem 274(29):20603–20610
Tang QQ, Lane MD (2012) Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem 81:715–736
Tebby PW, Buttke TM (1992) Stearoyl-CoA desaturase gene expression in lymphocytes. Biochem Biophys Res Commun 186(1):531–536
Wang J, Yu L, Schmidt RE, Su C, Huang X, Gould K, Cao G (2005) Characterization of HSCD5, a novel human stearoyl-CoA desaturase unique to primates. Biochem Biophys Res Commun 332:735–742
Waters KM, Miller CW, Ntambi JM (1997) Localization of a polyunsaturated fatty acid response region in stearoyl-CoA desaturase gene 1. Biochim Biophys Acta 1349:33–42
Zhang L, Ge L, Parimoo S, Stenn K, Prouty SM (1999) Human stearoyl-CoA desaturase: alternative transcripts generated from a single gene by usage of tandem polyadenylation sites. Biochem J 340(Pt 1):255–264
Zhang S, Yang Y, Shi Y (2005) Characterization of human SCD2, an oligomeric desaturase with improved stability and enzyme activity by cross-linking in intact cells. Biochem J 388:135–142
Acknowledgments
The authors thank members of the Czech and Ntambi laboratory for stimulating discussions on the topics addressed in this chapter. The work in the Czech laboratory related to the issues discussed in this review was funded by grants DK085753 and DK030898 from the National Institutes of Health and from the international Research Alliance at the Novo Nordisk Foundation Center for Metabolic Research. The work in the Ntambi lab related to this review was funded by NIH grant NIDDK-RO162388, AAHA Grant-in aid Wisconsin Affiliate, USDA (Hatch) grant #142–4306, and in part by funds from Xenon Genetics, Inc. Lucas M. O’Neill is a fellow supported by NIH grant 5T32GM007215-37 and SciMed GRS.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Cantley, J.L., O’Neill, L.M., Ntambi, J.M., Czech, M.P. (2013). The Cellular Function of Stearoyl-CoA Desaturase-2 in Development and Differentiation. In: Ntambi, Ph.D., J. (eds) Stearoyl-CoA Desaturase Genes in Lipid Metabolism. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7969-7_10
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7969-7_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7968-0
Online ISBN: 978-1-4614-7969-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)