Abstract
Nailfold videocapillaroscopy (NVC) is a noninvasive imaging technique that is used for the in vivo assessment of the microcirculation. Its principal role in rheumatology and dermatology is to differentiate the primary and secondary forms of Raynaud’s phenomenon (RP) associated with connective tissue disease, including a predictive value for clinical complications (i.e., digital in systemic sclerosis [SSc]). The morphologic changes that characterize the microvasculature in the scleroderma-spectrum diseases at the level of the nailfold include giant capillaries, microhemorrhages, loss of capillaries, angiogenesis, and avascular areas. These alterations have been recently reclassified in SSc into three defined and different NVC patterns: (1) “early pattern” (few giant capillaries, few capillary microhemorrhages, no evident loss of capillaries, and relatively well-preserved capillary distribution), (2) “active pattern” (frequent giant capillaries, frequent capillary microhemorrhages, moderate loss of capillaries, absence or mild ramified capillaries with mild disorganization of the capillary architecture), and (3) “late pattern” (almost absent giant capillaries and microhemorrhages, severe loss of capillaries with extensive avascular areas, ramified/bushy capillaries, and intense disorganization of the normal capillary array). SSc microangiopathy correlates with disease subsets and the severity of peripheral vascular, skin, and lung involvement; in particular, patients with the “late” pattern show an increased risk to have active disease and have moderate/severe skin or visceral involvement compared to patients with “early” and “active” patterns. Skin ulcers are a common vascular complication of SSc and seem now recognized in association with rapidly progressive capillary loss and the “late” NVC pattern. NVC is considered fundamental for differentiating between primary and secondary RP, therefore making possible the early diagnosis of SSc. The validated scoring of the NVC markers of the scleroderma patterns is supported for follow-up of SSc patients. The prognostic and predictive value of capillary density is becoming an index of outcome in patients affected by SSc.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Capillaroscopy
Capillaroscopy is a noninvasive imaging technique that is used for the in vivo assessment of the microcirculation. Its principal role in rheumatology and dermatology is to differentiate the primary and secondary forms of Raynaud’s phenomenon (RP) associated with connective tissue disease [1]. Capillary microscopy can be performed with various optical instruments such as the ophthalmoscope, dermatoscope, photomacrography system, stereomicroscope, conventional optical microscope, and the videocapillaroscope. The nailfold videocapillaroscope (NVC) with handheld device has the advantages that it is easy to learn and is a handheld device (= probe) which can be used in situations in which the use of the traditional microscope may be less convenient (such as in patients with finger flexion contractures) and its relatively high magnification (200−500x) is advantageous compared to the older widefield technique (Fig. 11.1).
Capillaroscopic Detection of the Early Microvascular Alterations That Are Markers of Secondary Raynaud’s Phenomenon
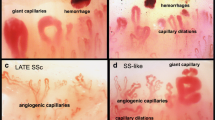
The morphologic changes that characterize the microvasculature in the scleroderma-spectrum diseases at the level of the nailfold have been extensively described – giant capillaries, microhemorrhages, loss of capillaries, neovascularization, and avascular areas (Fig. 11.2) [2].
The microvascular alterations have been recently reclassified in systemic sclerosis (SSc) into three defined and different NVC patterns:
-
1.
An “early pattern” (few giant capillaries, few capillary microhemorrhages, no evident loss of capillaries, and relatively well-preserved capillary distribution)
-
2.
An “active pattern” (frequent giant capillaries, frequent capillary microhemorrhages, moderate loss of capillaries, absence or mild ramified capillaries with mild disorganization of the capillary architecture)
-
3.
A “late pattern” (almost absent giant capillaries and microhemorrhages, severe loss of capillaries with extensive avascular areas, ramified/bushy capillaries, and intense disorganization of the normal capillary array) [3] (Fig. 11.3)
The marked increase in capillary size is the most characteristic feature of the nailfold capillary bed (giant capillary) in early secondary RP. In particular, the detection of enlarged and giant capillaries, together with microextravasation (hemorrhage) of the red blood cells in the nailfold, most likely represents the first morphologic sign of the altered microcirculation in systemic sclerosis.
The shape of the widened capillaries is largely heterogeneous, but giant capillaries with a homogeneous enlargement (diameter over 50 μm) are an absolute marker of the scleroderma capillaroscopic pattern. This picture characterizes the “early” (initial) SSc capillaroscopic pattern (Fig. 11.3) [4]. As the pathophysiological process of SSc progresses into fibrosis, the capillaroscopic analysis most likely reflects the effects of tissue hypoxia/massive capillary destruction, then increased loss of capillaries and avascular areas are observed, together with bushy capillaries indicating neoangiogenesis. This advanced stage of the systemic sclerosis is characterized by the “late” capillaroscopic pattern (Fig. 11.3). We know that patients with the “early” capillaroscopic pattern may have had RP for many years, and a transition from primary to secondary RP may be expected in almost 15 % of subjects in an average time of 29 months [5].
To quantify the microvascular changes, a practical system to score these capillaroscopic alterations in systemic sclerosis patients has been proposed recently and validated [6, 7]. Studies on nailfold capillary changes over time or in response to therapy are currently ongoing.
Learning to Use Capillaroscopy
Recent preliminary experience indicates that technical and operative skills in capillaroscopy, using a videomicroscopy system, can be achieved in approximately five nonconsecutive hours by an untrained specialist, in the context of a self-teaching program under expert supervision [8]. The beginner’s performances show that self-efficacy increases with practical experience, and image quality is reached in up to 70 % of learners by the third session [8].
Scleroderma Capillaroscopic Patterns and Serum Autoantibodies in Systemic Sclerosis
Regarding the anti-endothelial cell antibodies (AECA), these have been detected significantly more frequent in SSc patients with the “late” capillaroscopic pattern compared to the “early” and “active” patterns (p < 0.05) [9]. Concerning the anti-topoisomerase I (anti-Scl70) and anticentromere (ACA) antibodies, anti-Scl70 were found more frequent again in SSc patients showing the “active” and “late” patterns, whereas ACA especially in patients with the “early” pattern [10].
In a large prospective study, enlarged/giant capillaries, capillary loss, and more SSc-specific autoantibodies independently predicted definite SSc [11]. The authors reported associations between certain patterns of capillary abnormality and different specific autoantibodies. Anti-CENP-B and anti-Th/To antibodies predicted enlarged/giant capillaries; these autoantibodies and anti-RNAP III predicted capillary loss. Interestingly, each autoantibody was associated with a distinct time course of microvascular damage, as gauged by capillary enlargement or capillary loss. At follow-up, 79.5 % of patients with one of these autoantibodies and abnormal findings on nailfold capillary microscopy at baseline had developed definite SSc. Patients with both baseline predictors (abnormal capillaroscopy and an SSc-specific autoantibody) were 60 times more likely to develop definite SSc.
Finally, the association of specific antinuclear antibodies positivity and the presence of the NVC scleroderma pattern have been proposed as biomarkers for the very early diagnosis of SSc together with clinical symptoms (i.e., Raynaud’s phenomenon and/or puffy fingers) [12].
Possible Predictive Value of Capillaroscopy for Clinical Outcome in Systemic Sclerosis Patients
Systemic sclerosis microangiopathy correlates with disease subsets and the severity of peripheral vascular, skin, and lung involvement; in particular, patients with the “late” pattern showed an increased risk to have active disease and have moderate/severe skin or visceral involvement compared to patients with “early” and “active” capillaroscopic patterns [13].
Skin ulcers are a common vascular complication of SSc and seem now recognized in association with rapidly progressive capillary loss and the “late” NVC pattern, characterized by progressive avascular areas. An association with trophic lesions and loss of capillaries, as assessed by semiquantitative scoring, has also been reported [14]. Very recently, in 130 SSc patients examined at entry and after 20 months of follow-up, the diffuse cutaneous SSc phenotype with avascular areas on capillaroscopy represented, among other factors (e.g., increased IL-6), the major risk factor for ulcer development [15].
A recent study developed a capillaroscopic skin ulcer risk index (CSURI) that might predict the onset of new digital ulcers by using NVC analysis in patients with SSc [16]. However, this index is complex and time consuming, and the authors found that also the simple decrease of capillary number alone is sufficient as marker. A more recent simple capillaroscopic index (day-to-day DU index), prognostic for digital trophic skin lesions and based just on loss of capillaries alone, has been published showing high sensitivity and specificity [17].
Digital ulcers have been associated with significantly reduced blood flow at fingertips; in addition, SSc patients with the scleroderma “active” and “late” NVC patterns showed decreased blood flow velocity (65.5 % and 66.2 % reduction, respectively), and in particular, the reduced blood flow velocity was significantly associated with capillary ramification and capillary loss [18].
Ideally, future studies examining associations between digital ulceration and nailfold capillaroscopic patterns should consider digital tip ulcers and extensor surface ulcers. Recent studies showed that nailfold capillary density and its decrease are associated with the presence of lung involvement and severity of pulmonary arterial hypertension in systemic sclerosis patients [19, 20].
Conclusions
Videocapillaroscopy is considered fundamental for differentiating between primary and secondary Raynaud’s phenomenon [1–3, 5]. The validated scoring of the capillaroscopic markers of the scleroderma patterns is supported for follow-up of systemic sclerosis patients, at least already in some centers [6, 7]. The prognostic and predictive value of capillary density is becoming an index of outcome in patients affected by systemic sclerosis [21, 22]. As with specific autoantibodies, further larger-scale studies, both cross-sectional and longitudinal, are required and are ongoing to examine further the associations between capillaroscopic findings and other parameters/biomarkers of disease.
References
Cutolo M, Sulli A, Smith V. Assessing microvascular changes in systemic sclerosis diagnosis and management. Nature Rev Rheumatol. 2010;6:578.
Herrick AL, Cutolo M. Clinical implications from capillaroscopic analysis in patients with Raynaud’s phenomenon and systemic sclerosis. Arthritis Rheum. 2010;62:2595.
Cutolo M, Sulli A, Pizzorni C, et al. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol. 2000;27:155.
Cutolo M, Sulli A, Secchi ME, et al. The contribution of capillaroscopy to the differential diagnosis of connective autoimmune diseases. Best Pract Res Clin Rheumatol. 2007;21:1093.
Cutolo M, Pizzorni C, Sulli A. Identification of transition from primary Raynaud’s phenomenon to secondary Raynaud’s phenomenon by nailfold videocapillaroscopy. Arthritis Rheum. 2007;56:2102.
Sulli A, Secchi ME, Pizzorni C, Cutolo M. Scoring the nailfold microvascular changes during the capillaroscopic analysis in systemic sclerosis patients. Ann Rheum Dis. 2008;67:885.
Smith V, Pizzorni C, De Keyser F, et al. Reliability of the qualitative and semiquantitative nailfold videocapillaroscopy assessment in a systemic sclerosis cohort: a two-centre study. Ann Rheum Dis. 2010;69:1092.
De Angelis R, Cutolo M, Salaffi F, Restrepo JP, Grassi W. Quantitative and qualitative assessment of one rheumatology trainee’s experience with a self-teaching programme in videocapillaroscopy. Clin Exp Rheumatol. 2009;27:651.
Riccieri V, Germano V, Alessandri C, et al. More severe nailfold capillaroscopy findings and anti-endothelial cell antibodies. Are they useful tools for prognostic use in systemic sclerosis? Clin Exp Rheumatol. 2008;26:992.
Cutolo M, Pizzorni C, Tuccio M, et al. Nailfold videocapillaroscopic patterns and serum autoantibodies in systemic sclerosis. Rheumatology (Oxford). 2004;43:719.
Koenig M, Joyal F, Fritzler MJ, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 2008;58:3902.
Avouac J, Fransen J, Walker UA, et al. Preliminary criteria for the very early diagnosis of systemic sclerosis: results of a Delphi consensus study from EULAR scleroderma trials and research group. Ann Rheum Dis. 2011;70:476.
Caramaschi P, Canestrini S, Martinelli N, et al. Scleroderma patients nailfold videocapillaroscopic patterns are associated with disease subset and disease severity. Rheumatology (Oxford). 2007;46:1566.
Smith V, Pizzorni C, De Keyser F, et al. Validation of the qualitative and semiquantitative assessment of the scleroderma spectrum patterns by nailfold videocapillaroscopy: preliminary results. Arthritis Rheum. 2009;60:S164.
Alivernini S, De Santis M, Tolusso B, et al. Skin ulcers in systemic sclerosis: determinants of presence and predictive factors of healing. J Am Acad Dermatol. 2009;60:426.
Sebastiani M, Manfredi A, Colaci M, et al. Capillaroscopic skin ulcer risk index: a new prognostic tool for digital skin ulcer development in systemic sclerosis patients. Arthritis Rheum. 2009;61:688.
Smith V, De Keyser F, Pizzorni C, et al. Nailfold capillaroscopy for day-to-day clinical use: construction of a simple scoring modality as a clinical prognostic index for digital trophic lesions. Ann Rheum Dis. 2011;70:180.
Cutolo M, Ferrone C, Pizzorni C, et al. Peripheral blood perfusion correlates with microvascular abnormalities in systemic sclerosis: a laser-Doppler and nailfold videocapillaroscopy study. J Rheumatol. 2010;37:1174.
Ong YY, Nikoloutsopoulos T, Bond CP, et al. Decreased nailfold capillary density in limited scleroderma with pulmonary hypertension. Asian Pac J Allergy Immunol. 1998;16:8.
Hofstee HM, Noordegraaf AV, Voskuyl AE, et al. Nailfold capillary density is associated with the presence and severity of pulmonary arterial hypertension in systemic sclerosis. Ann Rheum Dis. 2009;68:19.
Sulli A, Pizzorni C, Smith V, et al. Timing of transition between capillaroscopic patterns in systemic sclerosis. Arthritis Rheum. 2012;64:821–5.
Smith V, Decuman S, Sulli A, et al. Do worsening scleroderma capillaroscopic patterns predict future severe organ involvement? A pilot study. Ann Rheum Dis. 2012;71(10):1636–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Cutolo, M., Sulli, A., Pizzorni, C., Smith, V. (2014). Capillaroscopy. In: Matucci-Cerinic, M., Furst, D., Fiorentino, D. (eds) Skin Manifestations in Rheumatic Disease. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7849-2_11
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7849-2_11
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7848-5
Online ISBN: 978-1-4614-7849-2
eBook Packages: MedicineMedicine (R0)