Abstract
Neophalloplasty is one of the most difficult surgical procedures in genital reconstructive surgery. It is indicated in men where the penis is missing due to either congenital or acquired reasons, as well in women with gender dysphoria. Many different tissues have been applied such as local vascularized flaps or microvascular free transfer grafts. The main goal of the neophalloplasty is to construct the functional and cosmetically acceptable penis. Urethral reconstruction in neophalloplasty presents a great challenge for surgeons who manage genital reconstruction. Different flaps (penile skin, scrotal skin, abdominal skin, labial skin, vaginal flaps, etc.) or grafts (skin, bladder, buccal mucosa) have been suggested for urethral lengthening. Although serious complications were reported in the past, new techniques and modifications for primary and secondary neophallus urethroplasty seem to be safe in experienced hands.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Summary
Neophalloplasty is one of the most difficult surgical procedures in genital reconstructive surgery. It is indicated in men where the penis is missing due to either congenital or acquired reasons, as well in women with gender dysphoria. Many different tissues have been applied such as local vascularized flaps or microvascular free transfer grafts. The main goal of the neophalloplasty is to construct the functional and cosmetically acceptable penis. Urethral reconstruction in neophalloplasty presents a great challenge for surgeons who manage genital reconstruction. Different flaps (penile skin, scrotal skin, abdominal skin, labial skin, vaginal flaps, etc.) or grafts (skin, bladder, buccal mucosa) have been suggested for urethral lengthening. Although serious complications were reported in the past, new techniques and modifications for primary and secondary neophallus urethroplasty seem to be safe in experienced hands.
Introduction
Many different surgical techniques for neophallic reconstruction have been reported using either available local vascularized tissue or microvascular tissue transfer. However, none of them satisfy all the goals of modern penile construction, i.e., reproducibility, tactile and erogenous sensation, a competent neourethra with a meatus at the top of the neophallus, large size that enables safe insertion of penile implants, satisfactory cosmetic appearance with hairless and normally colored skin. Normal penis has some unique characteristics and restoring its psychosexual function in both the flaccid and erectile state, and the possibility of sexual intercourse with full erogenous sensations, is almost impossible. The indications for neophalloplasty were initially limited to trauma victims who required surgery to restore their male anatomy. Today, surgical indications are expanded to many other disorders such as penile agenesis, micropenis, disorder of sexual development (intersex conditions), failed epispadias or hypospadias repair, penile cancer, as well female transsexuals.
Bogoras first described phallic reconstruction in 1936 [1]. Neophalloplasty options have followed the advances made in genital reconstructive surgery. Usually, it was a complex, time-consuming, multistage procedure with variable and suboptimal results. The development of microsurgical techniques was followed in phallic reconstruction, and Chang completed the first microvascular transfer of radial forearm free flap [2], which today represents the gold standard of this procedure. Other techniques have been also described, but none of them presents the ideal solution with low complication rate. Perovic [3] reported phalloplasty in 24 patients using the extended island groin flap. Senzeger et al. [4] suggested total phallic reconstruction using sensate osteocutaneous free fibula flap with promising results in 18 cases. Bettocchi et al. [5] recommended pedicled pubic phalloplasty in gender dysphoria patients as acceptable solution without disfiguring the donor skin site. Based on favorable experimental and clinical experiences [6], we started to use the musculocutaneous latissimus dorsi flap for total phalloplasty [7, 8]. Due to its workable size, ease to identification, long neurovascular pedicle, and minimal functional loss after removal, the latissimus dorsi flap has been used for a variety of reconstructions.
Since congenital defects constitute one of the indications for neophalloplasty, questions arise about age for reconstruction as well size of the neophallus and neophallic growth during puberty. There are only few data about this surgery in children. Gilbert et al. were the first to describe phallic reconstruction in boys without functioning penis [9]. We recommended this surgery before puberty to ensure optimal psychosexual development. Performing genital reconstruction in this period is important to minimize any psychological impact of this surgery. Since genitals have an important role in creating body image and, without any doubt, determine future mental image, we assumed that phalloplasty with normal-looking external genitalia and physical appearance before the delicate period of puberty is of utmost importance in order to prevent psychological stress related to genital inadequacy. Furthermore, it provides a good basis for stable masculine identity in adolescence and adulthood [7].
Possible disadvantages in children inspired us to find another option for neophalloplasty. It was for this reason that we started to use musculocutaneous latissimus dorsi flap for neophallic reconstruction. It has provided excellent length and circumference, follows somatic growth patterns, and is not influenced by pubescent hormonal effects that make them suitable for all of phalloplasty indications. Phallic retraction with muscle-based grafts seems less likely to occur than with use of fasciocutaneous forearm flap, since denervated well-vascularized muscle is less prone than connective tissue to contract.
Techniques of Phalloplasty
The most widely used flap for total neophalloplasty is the radial forearm flap [2]. However, it has many drawbacks, e.g., an unsightly donor site scar, very frequent urethral complications, and small-sized penis that does not allow the safe insertion of penile prosthesis in majority of cases. This was the main reason that we developed new technique using the musculocutaneous latissimus dorsi free transfer flap, which mostly satisfies the requirements noted above. It has a reliable and suitable anatomy to meet the esthetic and functional needs for phallic reconstruction. It can be also used successfully in children [7].
Radial Forearm Free Flap Phalloplasty
In the classic surgical procedure, three surgical teams operate at the same time: (1) the vascular surgeon prepares recipient blood vessels, (2) the urologist is operating in perineal area and prepares recipient site for neophallus, and (3) the plastic/genital surgeon is dissecting the free vascularized flap of the forearm for tubularization and creation of the neophallus. Neophallus is transferred to the pubic area and fixed at the proper position (Figs. 35.1 and 35.2). The radial artery is miscrosurgically connected to the common femoral artery in an end-to-side fashion. The venous anastomosis is performed between cephalic vein and the greater saphenous vein. One forearm nerve is connected to the ilioinguinal nerve for tactile sensation, and another nerve is anastomosed to the dorsal clitoral nerve for erogenous sensation. The defect of the donor area is covered either by full-thickness or split-thickness skin grafts [10–13].
Musculocutaneous Latissimus Dorsi Free Flap Phalloplasty
A latissimus dorsi musculocutaneous flap of the nondominant side is designed and harvested with thoracodorsal artery, vein, and nerve. The surface of the flap is templated in two parts: (1) a rectangular part for neophallic shaft to be approx. 15 × 14 cm and (2) additional circular or semilunar component for glans reconstruction (Fig. 35.3). The flap is completely elevated except for the neurovascular bundle, which was not transected until the recipient vessels and nerve had been prepared for microanastomosis. The neophallus is created while the flap is still perfusing on its vascular pedicle; the flap is tubularized in the midline and the neoglans formed by folding over and approximating to the penile shaft. The new constructed phallus is detached from the axilla after clamping dividing neurovascular pedicle with aim to achieve maximal pedicle length. The donor site defect is closed by direct skin approximation. If the impossible, remaining donor site defect is grafted with split-thickness skin graft. Simultaneously, another team dissects the recipient area together with femoral artery, saphenous vein, and ilioinguinal nerve. Additional “Y” incision is made in the pubic area, and a wide tunnel is created to place the flap pedicle. The neophallus is transferred to the recipient area, and microsurgical anastomoses are created between thoracodorsal and femoral artery, thoracodorsal and saphenous vein, and thoracodorsal and ilioinguinal nerve. Specially constructed dressing is used to keep the neophallus in an elevated position for approximately 2 weeks [7, 8].
Urethral Reconstruction
Construction of the neourethra in neophalloplasty is necessary to achieve the goal of voiding while standing. In men, it remains a complex problem, particularly in patients who have had previous penile amputation due to tumor or trauma and patients with micropenis or who have undergone gender reassignment. Many different techniques currently in use recreate the urethra but are prone to recurrent complications and fail to achieve voiding with a good stream when standing. Primary reconstruction of the neophallic urethra depends on indication for phalloplasty. Generally, there are some principles on how to create neophalloplasty urethra. It is possible to make in first stage, using a “tube-in-a-tube” technique, or to perform staged urethroplasty. Additionally, the urethral part of the neophallus should be lengthened and tubed more to bridge the gap between the neourethra and normal urethra.
Tube-in-a-Tube Technique
This principle is described in radial forearm flap phalloplasty, modified by Chang [2] and Gilbert [9], and in groin flap phalloplasty described by Perovic [3]. It includes design of additional part of the flap that will be tubularized over the 18 Fr catheter and insert into the new created phallus. The urine is diverted by means of a suprapubic tune, and 14 Fr Foley catheter is left in place in the newly formed urethra. This way, urethra inside the neophallus is created by a tube-in-a-tube technique and is anastomosed to the native urethral stump when applicable (Fig. 35.4). In opposite, additional tissue is necessary to bridge the gap between neophallic and native urethra. Transurethral catheter is left for 2 weeks. After that, the patient is started with voiding (Fig. 35.5). Suprapubic catheter is removed once the patient is able to void without significant residual volume.
Urethral Reconstruction with Radial Forearm Free Flap
This technique is based on original forearm free flap and reserved for cases after penile amputation or who have undergone gender reasignment [14]. Because all patients required a subtotal urethra reconstruction extending from the perineum area to the tip of the glans, a neourethra length up to 22 cm should be planned. A radial fasciocutaneous free flap is raised on the non-hair-bearing part of the forearm. The flap is tubed in situ to form a neourethra. The fascia is sutured separately around the neourethra to provide additional “waterproofing” layer. Penile stump is prepared ensuring that an adequate tunnel was created for insertion of the tubularized urethra while avoiding compression of the flap. The base of the neourethra is placed proximally to anastomose with the native urethral stump. Microsurgical anastomosis is done between donor and recipient blood vessels.
The principle of using radial forearm free flap is potentially applicable but should be used only in a very selected cases in whom the initial phalloplasty has failed with regard to urethroplasty or penile size and in men who do not desire a later penile prosthesis implantation.
Staged Neophallic Urethroplasty
This is the most promising technique for the reconstruction of the neophallus urethra and based on two-staged procedure. This principle introduced by Johanson [15] in the 1950s is still in use today, directly or indirectly. The first stage includes creation of the new “urethral plate” using buccal mucosa graft. The use of buccal mucosa graft that was first described seven decades ago has been the gold standard for urethral reconstruction. It is tough, elastic, simple to harvest, and easy to handle and leaves no noticeable scar at the donor site. It is also attractive when there may be a paucity of available genital skin, such as after multiple failed urethral reconstructions. Using the Johanson principle, buccal mucosa graft presents the optimal option in staged urethroplasty [16].
The principles of the harvesting and transfer are the same as described previously. Buccal mucosa grafts (either pairs or single, depending on the width and length of neourethra needed) are placed on the ventral side of the penis. When the healed grafts are ready for final stage tubularization and closure, it is important to incise the underlying tissue that will support the neourethra and avoid ischemia at the neourethral suture line. It is recommended to create second layer from surrounding tissue to cover and support the new created urethra. The key for successful repair is waiting long enough until the skin is supple. The classic mistake is to perform second stage too early. Second stage should be performed when the “urethral plate” has matured enough to be supple and thus more easily mobilized for a tubularization. If it is necessary, additional buccal mucosa grafts can be used for urethral plate augmentation and easier tubularization (Fig. 35.6).
Reconstruction of neophallus urethra: (a) Urethral plate is dissected from the penile skin. (b) Distal part is incised in the midline, and additional buccal mucosa graft is interposed for its augmentation. (c) Tubularization of the phallic urethral plate is done. (d) Final appearance after urethral reconstruction and glanuloplasty
Neophalloplasty with Urethroplasty in Female-to-Male Transsexual
Creation of the neophallus is one of the most difficult parts in the treatment of female-to-male transsexuals. Although a variety of surgical techniques are available, their results are not similarly acceptable to all patients. Metoidioplasty represents a technique for creating a neophallus from hormonally hypertrophied clitoris in female-to-male transsexuals who do not wish to have sexual intercourse [17–19]. In patients who desire a larger phallus, already described techniques of phalloplasty can also be used. Urethral reconstruction presents the main problem in this type of sex reassignment surgery and includes creation a very long neourethra, since the native urethral meatus in females is positioned too far from the tip of the glans. Lengthening of the native urethra presents a great challenge, especially first part that should be the bridge between native meatus and neophallic urethra.
Urethral Reconstruction in Metoidioplasty
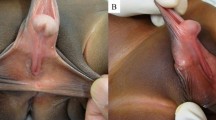
The operative technique starts with straightening and lengthening of the hormonally enlarged clitoris (Fig. 35.7a). Fundiform and suspensory clitoral ligaments are detached from the pubic bone to advance the clitoris. Ventrally, short urethral plate is dissected from the clitoral bodies and divided at the level of glanular corona to correct the ventral curvature. Reconstruction of the neourethra starts with reconstruction of its bulbar part. A vaginal flap is harvested from the anterior vaginal wall with the base close to the female urethral meatus. This flap is joined with the remaining part of the divided urethral plate forming the bulbar part of the neourethra (Fig. 35.7b). Additional urethral lengthening was done using the buccal mucosa graft and vascularized labia minora flap. The buccal mucosa graft is always use to cover the gap after division of short urethral plate. The length of the graft depends on the distance between the tip of the glans and urethral meatus. The graft is fixed and quilted to the corporal bodies ventrally, completing the dorsal aspect of the urethra. The ventral part is created as an onlay flap from the labia minora. The inner surface of the one of the labia minora is dissected to create a flap with dimensions appropriate to cover dorsal part of the neourethra. Outer labial skin is de-epithelialized forming good vascularized and mobile flap. This flap is joined to the buccal mucosa graft by two lateral running sutures. The urethra was calibrated before closure to be not <18 F in diameter. A 14 F silicone tube is placed into the new urethra as a small caliber stent to be used for buccal mucosa moisturizing and to maintain the urethral lumen (Fig. 35.7c, d). The glans is opened by two parallel incisions, and both glans wings are dissected extensively to enable glans approximation without tension after reconstruction of glandial urethra. Using this technique, the neourethra with the meatus placed at the tip of the glans is created. Covering of the penile shaft is achieved using the remaining clitoral and labial skin. Both labia majora are joined in the midline, and silicone testicular implants are inserted finalizing the scrotoplasty (Fig. 35.7e).
Urethral reconstruction in metoidioplasty: (a) Preoperative appearance. Clitoris hormonally enlarged. (b) Bulbar part of new urethra created by joining anterior vaginal wall flap and proximal part of urethral plate. (c) Long flap designed at inner surface of labia minora to be harvested and mobilized by simple de-epithelialization of outer surface of labia minora. Buccal mucosa is placed on the ventral corpora cavernosa and quilted. (d) Labial flap anastomosed with buccal mucosa graft, creating neourethra. Abundant pedicle of the flap completely covering all suture lines. (e) Appearance at the end of surgery. Penile skin reconstruction performed using remaining clitoral and labial skin. Two testicular implants inserted into scrotum created from both labia majora. (f) Outcome 2 months later. Voiding while standing achieved
Postoperative care included elastic dressing around the penis in a stretched position. The buccal mucosa graft should be moistened with saline every 3 h, for the first 2 days following surgery to improve survival of the mucosa. Oral antibiotics and oxybutynin are administered to prevent postoperative infection and bladder irritation, respectively. Two weeks after surgery, anterograde urethrography was performed to exclude leaks, and the suprapubic catheter was removed (Fig. 35.7f).
Urethral Reconstruction in Total Phalloplasty
The principles of the reconstruction of the neophallus urethra are previously described. In female-to-male transsexuals gap between the neourethra and female urethral meatus is always more than 10 cm. The principle of the reconstruction is the same as in metoidioplasty. Recently, we started to use all available vascularized hairless tissue to lengthen the neourethra maximally preventing the postoperative complications. For this reason, both labia minora and available clitoral skin are used for urethral tubularization. This way, new urethral opening is placed in first half of neophallus, minimizing the requests for longer neophallus urethroplasty (Fig. 35.8).
Urethral lengthening in latissimus dorsi phalloplasty. (a) Proximal urethra created using urethral plate and vaginal flap. Very long labia minora flap is dissected on well-vascularized pedicle. (b) Flap is tubularized giving the additional gain in urethral length. (c) Musculocutaneous latissimus dorsi flap is tubularized creating neophallus. (d) Appearance at the end of surgery. Neophallus is placed at the proper position. Microvascular anastomosis is done. New urethral orifice is located at the half of the neophallus. (e) Second stage. Penile prosthesis is inserted using dorsal approach. (f) Buccal mucosa graft is positioned in distal third of the neophallus, forming the new urethral plate. Glans is reconstructed by Norfolk technique
Complications and Secondary Repair
Although the esthetic results of microsurgical phalloplasty have become quite satisfactory, the urethroplasty complication rate remains high. Complications are common after all phalloplasty surgery, and Zielinski [21], using a groin flap in 127 patients with gender dysphoria, reported urethral complications in 16 %. Rohrmann and Jakse [13] reported 58 % incidence of urethral complications in a series of 25 patients having a free radial forearm flap. Fang et al. [22] had a 41 % of fistula and 14 % stricture rate in 22 patients who underwent free radial osteocutaneous flap phalloplasty. In a review of 81 cases underwent radial forearm phalloplasty, Monstrey et al. [10] reported urethral complications in 42 %. The fistula usually formed at the junction of the native and neourethra at perineal region. In our group of 38 patients after metoidioplasty, only 3 fistulas occurred. Similar results were achieved in urethral lengthening in our total phalloplasty [7, 8, 20]. The key of success presents urethral reconstruction using good vascularized flaps.
Most of the urethral problems can be corrected with secondary procedures. It has been our experience that more than half of urethral fistulas and strictures are solved conservatively, while less than half complications need an additional surgical procedure. At least, there is still no ideal technique for phalloplasty resulting in excellent esthetic and functional outcomes. There are still problems with the neourethral reconstruction, but the incidence of complications has been reduced with new refinements of one-stage repair or by using a staged procedure.
Preferred Surgical Instruments of M Djordjevic
General Instruments
-
Weitlaner retractor 3 × 4 prongs sharp or blunt 6½″ (16.5 cm)
-
Ring retractor
-
Satinsky vascular clamp
-
John Hopkins Bulldog Clamp 2 1/4″ (5.7 cm)
Special Instruments
-
Metzenbaum scissors – Power Cut Black, 7˝ (18 cm), curved
-
Ragnell undermining scissors, curved, 5˝ (12.5 cm)
-
Kelly’s vascular scissors straight 6¼″ (16 cm)
-
Micro scissors, round handle, 5 1/2˝ (14 cm), sharp straight
-
Adson tissue forceps 4.75″; 1 × 2 teeth tips
-
Castroviejo suture forceps tying platform, 1 × 2 angled teeth, 4″ (10.5 cm)
-
DeBakey-Adson tissue forceps, 1.5 mm, atraumatic tip, 4 3/4″ (12 cm)
-
Barraquer needle holder, micro jaw, 4 3/4″ (12 cm)
-
Sontec Mayo-Hegar needle holder, TC serrated, 5 1/2″ (14 cm)
Suture Material
-
6/0 and 7/0 Prolene (Polypropylene) suture, synthetic, nonabsorbable, monofilament
-
4/0, 5/0, 6/0 PDS (polydioxanone) suture or Monocryl (poliglecaprone 25) or Monosyn (glyconate) suture, synthetic, absorbable, monofilament
-
1, 0, 2/0, 3/0, 4/0 Vicryl (polyglactin 910) synthetic, absorbable suture, multifilament
-
3/0 and 4/0 Nylon suture, synthetic, nonabsorbable, monofilament
Surgical Pearls and Pitfalls Key Surgical Points
Congenital/Acquired Penile Anomalies
-
Penile remnants are carefully dissected.
-
Corpora cavernosa stumps are used for insertion of penile prosthesis.
-
Urethra is mobilized as much as possible to reach the base of the new created phallus.
-
All vascularized hairless skin from genital region is used for urethral lengthening, as a fasciocutaneous flaps.
-
Neophalloplasty is performed with good defined dimensions.
-
Neophallic urethra is created with buccal mucosa or skin graft in staged procedure (Johanson principle).
-
Subcutaneous vascularized flaps from the scrotal area are used for neourethral covering and fistula prevention.
-
Urinary catheter and suprapubic tube are placed during surgery.
Female Transsexuals
-
A flap from the anterior vaginal wall is created for bulbar urethral reconstruction.
-
Vagina is closed in many layers to cover new created bulbar urethra.
-
Both labia minora and dorsal clitoral skin are harvested with preserved blood supply to be used for urethral lengthening.
-
Neophallus is placed just above the top of the labia majora creating good relationship between phallus and scrotums.
-
Neophallic urethra is reconstructed as a stage procedure with buccal mucosa and skin grafts.
Potential Surgical Problems
-
Multiple failed surgeries result in insufficient vascularized genital tissue that should be used for urethral lengthening: Manage with Staged urethroplasty with buccal mucosa graft.
-
High recurrence of the urethral complications: Prolonged suprapubic urinary drainage (minimum 4 weeks) and postoperative dilation of the neourethra.
Editorial Comment
My surgical experience with neophalloplasty is mainly with repairing complications of urethral strictures. Strictures are typically at the meatus, mid-penile shaft, or at the anastomosis of the native urethral stump to the skin tube of the neophallus. The neophallus skin is fairly noncompliant and rigid and cannot be used as an onlay for reconstruction. Also, as the urethra is just a skin tube, there is no corpus spongiosum for spongioplasty to keep a graft alive. For that reason, I have managed neophallus strictures by a staged technique with a ventral urethrotomy as in a Johanson, and buccal grafts laid along side. I wait at least 6 months to a year and then tubularized the grafts and underlying tissue. If the skin of the neophallus is rigid, the skin edges cannot be mobilized to cover the tubed buccal graft – then I harvest a small split-thickness skin graft to cover and hold it in place with a bolster dressing. If the staged urethroplasty fails, I usually resort to a staged Cecil urethroplasty. Here I incise the scrotal or groin skin on three sides into the subcutaneous fat in rectangular shape to mirror the size of the urethral stricture. A ventral urethrotomy is made on the neophallus and then the penis is sewn to the groin or scrotum. After 3–6 weeks, the remaining skin edge is incised, and the skin freed up and transferred on to the penis. A small STSG is then placed on top. The harvest site from the groin or scrotum is primarily closed. For anastomotic stricture of the native and neourethra, urethrotomy typically fails and fails quickly. Because the neourethra strictures are so difficult to repair, I have a whole cadre of patients who are managed by urethrotomy or dilation and then daily intermittent self-catheterization. The other method that seems to work well is a ventral buccal graft. The always seems to be some subcutaneous tissue available for which can be laid over and quilted to the graft.
—Steven B. Brandes
References
Bogoras N. Plastic construction of penis capable of accomplishing coitus. Zentral Chir. 1936;63:1271–6.
Chang TS, Hwang WY. Forearm flap in one-stage reconstruction of the penis. Plast Reconstr Surg. 1984;74:251–8.
Perovic S. Phalloplasty in children and adolescent using the extended pedicle island groin flap. J Urol. 1995;154:848–53.
Sengezer M, Ozturk S, Deveci M, et al. Long-term follow-up of total penile reconstruction with sensate osteocutaneous free fibula flap in 18 biological male patients. Plast Reconstr Surg. 2004;114:439–50.
Bettocchi C, Ralph DJ, Pryor JP. Pedicled pubic phalloplasty in females with gender dysphoria. BJU Int. 2005;95:120–4.
Stenzl A, Schwabegger A, Bartsch G, Prosser R, Ninkovic M. Free neurovascular transfer of latissimus dorsi muscle for the treatment of bladder acontractility. II. Clinical results. J Urol. 2003;169:1379–83.
Djordjevic ML, Bumbasirevic MZ, Vukovic PM, Sansalone S, Perovic SV. Musculocutaneous latissimus dorsi free transfer flap for total phalloplasty in children. J Pediatr Urol. 2006;2:333–9.
Perovic SV, Djinovic R, Bumbasirevic M, Djordjevic M, Vukovic P. Total phalloplasty using a musculocutaneous latissimus dorsi flap. BJU Int. 2007;100:899–905.
Gilbert DA, Jordan GH, Devine Jr CJ, Winslow BH, Schlossberg SM. Phallic construction in prepubertal and adolescent boy. J Urol. 1993;149:1521–6.
Monstrey S, Hoebeke P, Dhont M, Selvaggi G, Hamdi M, Van Landuyt K, Blondeel P. Radial forearm phalloplasty: a review of 81 cases. Eur J Plast Surg. 2005;28:206–12.
Lumen N, Monstrey S, Selvaggi G, Ceulemans P, De Cuypere G, Van Laecke E, Hoebeke P. Phalloplasty: a valuable treatment for males with penile insufficiency. Urology. 2008;71:272–6.
De Castro R, Merlini E, Rigamonti W, Macedo A. Phalloplasty and urethroplasty in children with penile agenesis: preliminary report. J Urol. 2007;177:1112–7.
Rohrmann D, Jakse G. Urethroplasty in female-to-male transsexuals. Eur Urol. 2003;44:611–4.
Dabernig J, Shelley OP, Cuccia G, Schaff J. Urethral reconstruction using the radial forearm free flap: experience in oncology cases and gender reassignment. Eur Urol. 2007;52:547–54.
Johanson B. Reconstruction of the male urethra in strictures. Application of the buried intact epithelium tube. Acta Chir Scand. 1953;176:1.
Barbagli G, Palminteri E, De Stefani S, Lazzeri M. Penile urethroplasty. Techniques and outcomes using buccal mucosa grafts. Contemp Urol. 2006;18:25–33.
Lebovic GS, Laub DR. Metoidioplasty. In: Ehrlich RM, Alter GJ, editors. Reconstructive and plastic surgery of the external genitalia. Philadelphia: WB Saunders Co.; 1999. p. 355–60.
Hage JJ, Turnhout WM. Long-term outcome of metoidioplasty in 70 female to male transsexuals. Ann Plast Surg. 2006;57:312–6.
Djordjevic M, Stanojevic D, Bizic M, Kojovic V, Majstorovic M, Vujovic S, Milosevic A, Korac G, Perovic SV. Metoidioplasty as a single stage sex reassignment surgery in female transsexuals: Belgrade experience. J Sex Med. 2009;6:1306–13.
Djordjevic ML, Bizic M, Stanojevic D, Bumbasirevic M, Kojovic V, Majstorovic M, Acimovic M, Pandey S, Perovic SV. Urethral lengthening in metoidioplasty (female-to-male sex reassignment surgery) by combined buccal mucosa graft and labia minora flap. Urology. 2009;74:349–53.
Zielinski T. Phalloplasty using a lateral groin flap in female-to-male transsexuals. Acta Chir Plast. 1999;41:15–9.
Fang RH, Kao YS, Ma S, Lin JT. Phalloplasty in female-to-male transsexuals using free radial osteocutaneous flap. A series of 22 cases. Br J Plast Surg. 1999;52:217–22.
Acknowledgments
This paper is supported by Ministry of Science, Republic of Serbia, Project No. 175048.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Djordjevic, M.L. (2014). Primary and Secondary Reconstruction of the Neophallus Urethra. In: Brandes, S., Morey, A. (eds) Advanced Male Urethral and Genital Reconstructive Surgery. Current Clinical Urology. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4614-7708-2_35
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7708-2_35
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4614-7707-5
Online ISBN: 978-1-4614-7708-2
eBook Packages: MedicineMedicine (R0)