Abstract
Reactions to iodinated contrast media range from mild inconvenience to life-threatening emergency. Histamine release can account for many of the symptoms and nonionic agents are tolerated better than ionics. Reactions can be immediate (IR) or delayed (DR). Incidences of the former are 3–4 % (ionics), 0.2–0.7 % (non-ionics), severe reactions 0.1–0.4 % (ionics), and 0.02–0.04 % (non-ionics). Up to 80 % of reactions can be avoided by using nonionic agents. For DRs, there is no difference between the incidences of reactions to each of the agents. Risk factors for IRs are a previous reaction to a contrast medium, bronchial asthma, cardiac disease, and highly allergic subjects; for DRs, a previous reaction, use of β-blockers, treatment with IL-2, history of drug allergy, and contact allergy. Diagnosis of IRs is based largely on skin tests; IgE antibodies have not been convincingly demonstrated. Breakthrough reactions have occurred following corticosteroid and H1 antagonist premedication. Gadolinium-based agents, especially the linear chelates, have been associated with nephrogenic systemic fibrosis. They show an adverse reaction incidence of about 0.48 % and 0.01 % for anaphylaxis. Overall, given the large number of contrast media administered, they are one of the safest drugs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Contrast Medium
- Iodinate Contrast Medium
- Iodine Atom
- Nephrogenic Systemic Fibrosis
- Gadopentetate Dimeglumine
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Along with the development and introduction of powerful investigative techniques and advanced diagnostic equipment, contrast media have helped place clinical diagnostic radiology irreplaceably at the center of diagnostic medicine today. By increasing the contrast of anatomical structures that are otherwise not easily seen and discriminated, contrast media allow the visualization of details of internal tissues such as blood vessels, intestine and the various organs. The agents are now so widely and frequently used that they are said to be the most commonly used drugs in the history of modern medicine. Contrast media are not dyes. For contrast media-aided visualization of the body’s internal structures, X-ray imaging techniques are most often used. Information was originally recorded on X-ray film, but that has now been largely superceded by digitized images using computer-based methods of recording, storage, and display. Instead of passing a single X-ray beam through the body, a computerized tomography scanner takes X-ray images at many different angles. A computer is used to work out the relative density of the emerging X-rays and ultimately a 3D image can be constructed. Sometimes structures cannot be visualized by X-rays alone, even with the aid of contrast media, for example, the cord of nerve roots. Here, specific contrast media can be employed and visualized directly using magnetic resonance imaging (MRI).
In an X-ray examination of a patient some of the X-rays are scattered in all directions and some are absorbed by the different tissues: this is known as attenuation of the rays. The amount of X-rays absorbed depends on the thickness and density of the material (for example, gas in the lungs verse lung tissue) in the path of the rays and, importantly, its chemical composition. Since the absorption of X-rays increases with the number of electrons, the chemical composition of a tissue can be thought of as the average or effective atomic numbers of all the atoms involved. This is so since the atomic number (Z) of an atom is the number of protons in the nucleus of an atom and, in an electrically neutral atom, Z is equal to the number of electrons in the atom. In addition to the contribution of density, being able to distinguish, for example, soft tissue from bone on a radiograph, is a consequence of the low average atomic number of the soft tissue contrasted with the significantly higher average atomic number of the calcium-containing bone. Effective atomic numbers and densities (expressed as g/cm3) are 7.42 and 1 for water, 7.46 and 1 for muscle, 5.92 and 0.91 for fat, and 20 and 1.55 for calcium. If two tissues have similar densities, thickness, and average atomic numbers, visualizing and distinguishing the tissues are more difficult and may not be possible. Although this situation occurs commonly in diagnostic radiology, visualization and contrast can be artificially altered by increasing the average atomic number of a structure. This is often achieved by administering a liquid of high average atomic number, for example, to the blood to visualize blood vessels.
10.1 Iodinated Contrast Media
Contrast media are substances that affect the attenuation of X-rays, thus changing the contrast seen in X-ray images. Introduction of gases, as in the examination of the gastrointestinal tract, is an example of the application of negative contrast media where there is a reduction in the attenuation of X-rays, but most contrast media are positive or radio-opaque, that is, they increase the attenuation of X-rays. Since X-ray absorption increases with the number of electrons (that is the atomic number), the presence of atoms of high atomic number will absorb more X-rays than atoms of low atomic number such as hydrogen (Z = 1), carbon (6), nitrogen (7), and oxygen (8). In one of the most successful examples of improvement in clinical diagnostics, the sciences of pharmacology and synthetic medicinal chemistry cooperated to increase the water solubility and attenuation of contrast media while at the same time reducing toxicity. This was achieved by the introduction of iodinated compounds in an evolving program of increasing effectiveness. The use of iodine in a water-soluble form for contrast imaging began in the 1920s with sodium iodide and then monoiodinated pyridine derivatives, but toxicity and poor contrast results led on to the second-generation di-iodinated pyridines. The first big breakthrough came in the 1950s with the lower toxicity but still very hyperosmolar sodium and meglumine (an amino sugar derived from sorbitol) salts of triiodinated benzoic acid. Since, for better tolerance, the osmolalities of the injected contrast medium and body fluids should be as close as possible, another significant advance was the introduction in the 1970s of nonionic iodinated contrast media. By converting the carboxyl group of triiodobenzoic acid to the amide, dissociation in solution could no longer occur and the iodine:ions/particle ratio was changed from 1.5:1 (three iodine atoms:two ions) for high-osmolality contrast media for, example, diatrizoate, to 3:1, a contrast medium of lower osmolality, for example, iohexol (Tables 10.1 and 10.2). In addition to the low-osmolar nonionic compounds, low osmolality was also achieved by preparing ionic dimers such as ioxaglate (Tables 10.1 and 10.2) where only one of the carboxyls of the two linked triiodinated aromatic rings was converted to an amide, thus producing two ions in solution with a total of six iodine atoms and a ratio of 3:1. The osmolality of this ionic dimer is a little less than the osmolality of, for example, the nonionic monomer iohexol, but both are still twice as osmolar as human blood. Contrast media with approximately the same osmolality of blood were finally produced in the 1980s with the introduction of nonionic dimers containing six iodine atoms for each non-dissociating molecule, for example, iodixanol (Tables 10.1 and 10.2).
The iodine atom has an atomic radius of 140 pm (1.4 Å) (empirically measured) which is significantly larger than the atomic radii from, for example, carbon (70 pm), hydrogen (25 pm), oxygen (60 pm), nitrogen (65 pm), sulfur (100 pm), and chlorine (100 pm). To obtain a visual image of how the presence of iodine atoms dominates the volume in space of iodinated contrast media molecules, the two-dimensional structures of the four contrast agents, containing either three or six iodine atoms and shown in Table 10.1, are represented three-dimensionally as CPK models in Fig. 10.1. The presence of the bulky iodine atoms significantly influences the physical and chemical properties of the contrast media and produces structures that, as potential immunogens (antigens and allergens), are relatively unique.
10.2 Usage and Safety of Contrast Media
Iodinated contrast media are one of the most often administered and safest pharmaceutical products used today not only in radiology but in all areas of medicine. In 2005 it was estimated that worldwide administrations of contrast media exceeded 75 million per year and it is safe to assume that this figure grows annually. Unlike most drugs, they are not designed to have a specific therapeutic action; in fact, the more pharmacologically inert they are the better. With normal renal function, the iodinated agents are cleared almost completely by the kidneys with a half-life of only 1–2 h. Although most often used intravenously, contrast media can be given into arteries, the abdomen, and intrathecally, and numerous surveys in hospitals throughout the Western world attest to their heavy and widespread usage. As diagnostic technologies increase in effectiveness and sophistication, new agents and procedures become accepted as standard practice, and costs become more affordable, contrast media usage will inevitably increase. A trend that will almost certainly continue is the preference for the better-tolerated nonionic compounds and this will be driven by demand from both doctor and patient. For example, even in the late 1990s, surveys revealed that this preference was already clear in many areas of the USA. In one report, 43 % of hospitals surveyed in the southeast of the country used nonionic contrast media 100 % of the time while 71 % used them more than 75 % of the time. To reduce the usage of the nonionic agents, hospitals often introduced selective protocols.
As with every administered pharmaceutical agent, adverse reactions to iodinated contrast media do occur, but the incidence is low. Reactions can be unrelated to the dose or concentration of the solution administered (see below) or they can be dose dependent. An important contributor to the latter category is the osmolality of the administered agent which is responsible for the feelings of discomfort, heat, and pain and which may also provoke disturbance of the electrolyte balance in small children, renal problems, and damage to the blood–brain barrier. As already indicated, the tolerance of contrast media increases as the osmolality approaches the osmolality of serum. Also related to the concentration used are the viscosity, the hydrophilicity/lipophilicity balance, protein-binding capacity, and histamine-releasing properties of the contrast medium. Viscosity increases with molecular weight so the viscosities of the dimer solutions are higher than the solutions of monomers. The practical implications of a more viscous solution are the greater force required for injection, especially through thin catheters, and slow flow influencing the visualization of tissues. Some additional adjustments made to reduce toxicity include adjusting the pH to neutral, adding calcium ions to reduce cardiac toxicity, and altering the number of hydroxyl groups to decrease neural toxicity.
10.3 Adverse Reactions
10.3.1 Classification and Symptoms
10.3.1.1 Acute (Immediate) Reactions
Iodinated contrast media used today have been carefully developed with the aim of maximizing their effectiveness for tissue visualization while at the same time minimizing toxic effects. With the relatively safe agents used today, of more concern than dose-related intolerance and toxicity outlined above are adverse reactions covering a range of severities that are mostly independent of dose or concentration. Severity is, in fact, a convenient and useful way of categorizing these reactions since this approach is clinically relevant and provides a guide for subsequent treatment. As with the so-called toxic reactions, the adverse reactions are small in number relative to the millions of doses administered each year. A number of professional bodies including, for example, The American College of Radiology and the European Society of Urogenital Radiology, have issued classifications and guidelines based on a primary division of acute (or immediate) and late reactions with the former division subdivided further into mild, moderate, or severe reactions. Acute reactions are those that occur within about 1 h of the administration of contrast media. The cutoff time of 1 h for a reaction to be classified as acute is, of course, somewhat arbitrary and it is a figure disputed by some who argue that findings with some patients show that the cutoff point should be extended to 2 or even 3 h. A similar problem of deciding the time when the designations “immediate” ends and “delayed” begins is seen in other drug allergies. The question is probably best resolved by the patient’s symptomatology.
Signs and symptoms commonly listed for the three different acute reaction categories include mild reactions (generally self-limiting without evidence of progression)—nausea, vomiting, cough, headache, itching, pallor, flushing, and chills; moderate reactions (require treatment but not immediately life-threatening)—tachycardia/bradycardia, bronchospasm, laryngeal edema, marked urticaria; and severe reactions (life-threatening)—hypotensive shock, cardiac and respiratory arrest, severe laryngeal edema, and convulsions. Table 10.3 sets out the classification of acute non-renal adverse reactions to contrast media and the associated symptoms listed by the European Society of Urogenital Radiology in their ESUR Guidelines on Contrast Media.
10.3.1.2 Late Reactions
Late reactions become apparent more than 1 h and up to about 1 week after contrast media exposure. Excluding contrast media-induced nephropathy, the symptoms most commonly seen include nausea, vomiting, headache, and cutaneous reactions (Table 10.3) which tend to be self-limiting and include maculopapular rash in over 50 % of affected patients, xanthema, urticaria, and usually pruritus. In rare cases, cutaneous reactions may progress to a cutaneous vasculitis or even a Stevens–Johnson-like syndrome. Late reactions may often be missed since patients generally leave the department sooner than an hour after administration of the contrast preparation, and because the delayed reactions are so often self-limiting, the radiologist may remain unaware of them. Recent reports, however, of delayed skin reactions and a few cases of serious delayed reactions involving hypotension, shock, and angioedema following intravascular injection of nonionic iodinated dimers highlight the potential dangers of late reactions (see below) occurring in the absence of direct medical awareness and supervision. Gadolinium-based contrast media are referred to in Table 10.3 for the sake of completeness. These agents are discussed later in this chapter.
10.3.2 Incidence of Reactions
10.3.2.1 Acute Reactions
The largest study so far of the incidences of adverse reactions to different contrast media was reported by the Japanese Committee on the Safety of Contrast Media in 1990. In this prospective study of 337,647 cases, 169,284 cases (50.1 %) received ionic contrast media and 168,363 (49.9 %) received nonionic contrast media. Adverse drug reactions occurred in 12.66 % of the ionic contrast media group and 3.13 % of the nonionic group. For severe adverse reactions the corresponding figures were 0.22 % and 0.04 %, respectively, with one death occurred in each group. The authors of the study concluded that “non-ionic contrast media significantly reduce the frequency of severe and potentially life-threatening adverse drug reactions to contrast media at all levels of risk and that use of these media represents the most effective means of increasing the safety of contrast media examinations.” While the incidence of reactions to high-osmolar ionic contrast media in the Japanese survey is higher than most estimates, it is clear that reactions to these agents occur within the range of about 2–8 % with a figure of around 3 or 4 % perhaps being most likely. The estimated reaction frequency for the low-osmolar nonionic agents ranges up to a maximum of about 3 %, but figures of 0.2–0.7 % have been deduced in a number of studies. Figures for the incidences of severe reactions are much more settled being 0.1–0.4 % for ionic and 0.02–0.04 % for nonionic agents. For reactions judged to be severe, the corresponding figures are 0.04 % and 0.004 %. Fatal reactions occur rarely and do not differ between the low- and high-osmolality agents. The mortality rate has been estimated to be in the range 1 in 100,000 to 1 in 170,000.
In summary then, it can be said that as well as provoking a higher incidence of adverse reactions, high-osmolar ionic contrast media cause reactions that are more severe than the low-osmolar nonionic contrast media-induced reactions and the nonionic preparations are less distressing for the patient overall. Although severe reactions with high-osmolar ionic contrast media are still rare, they are more frequent than severe reactions to low-osmolar nonionic media. Up to 80 % of the reactions to the ionic agents can be avoided by substituting a nonionic medium.
10.3.2.2 Late Reactions
Obtaining reliable and relevant information on the frequency of late reactions to contrast media is not easy for a number of the usual reasons related to data collection but particularly because of the relatively larger time interval between the injection of the agent and the appearance of symptoms. And, of course, the bigger the time interval, the more difficult it is to be sure that the symptoms were caused by the contrast medium. Most studies show that there is no significant difference in the incidences of late reactions between ionic and nonionic media or between the different nonionic preparations. Although figures as low as 0.52 % and as high as 23 % have been reported, the incidence of reactions in the first 24 h appears to be about 4 % settling to about 1–3 % over a 7-day period. The nonionic compound iopamidol showed an incidence of 5.5 % of late skin rashes in a survey of 1,381 patients.
A curious seasonal variation in the occurrence of late adverse skin reactions has been reported from Finland. In a study of a possible relationship between sun exposure and late reactions in 4,875 adults who had received an iodinated contrast medium, a 3-month (April to June) peak in the incidence of reactions was seen. This period included 35 % of all events and most of the reactions occurred on sun-exposed areas of the body, leading the authors to conclude that a possible explanation for the observations was the photosensitizing effect of the contrast media.
10.3.3 Risk Factors
10.3.3.1 Acute Reactions
Risk factors for acute reactions to contrast media are summarized in Table 10.4. The most significant risk is for patients who have experienced a previous immediate reaction to an iodinated contrast medium. Reexposure to the same or structurally similar ionic preparation is said to carry with it a 21–60 % risk of a repeat reaction. This risk is one-tenth as great if a nonionic contrast medium is substituted for the repeat injection. Comparable figures for nonionic media used for the initial and the repeat administrations do not seem to be available. Other important risks are bronchial asthma, the use of β-blockers, cardiac disease, and subjects who are highly allergic. Procedures and efforts to reduce the risks of an acute reaction are set out in Table 10.4. Some of the points, for example, use of nonionic media, substituting a different contrast medium, and keeping patients under surveillance longer, have been considered above. Physicians using contrast media should be trained to recognize, test for, and treat anaphylaxis. Drugs and instruments that should be close at hand for acute reaction emergencies following administration of contrast media are listed in Table 10.5.
10.3.3.2 Late Reactions
Risk factors for a late skin reaction following administration of an iodinated contrast medium include current and up to 2 years past treatment with interleukin-2 (IL-2), a history of drug allergy or contact hypersensitivity, and a history of reaction to a previous contrast medium. Late reactions are more common in patients who reacted previously, especially if the same contrast medium is administered (see below). The latter fact is interesting since it suggests that the mechanism of the late reaction with its demonstration of memory may be immunologically and, in particular, T cell mediated.
10.3.4 Biphasic Reactions
About 20 % of adverse reactions to iodinated contrast media are biphasic in nature and although severe biphasic reactions are rare they are of concern since they can be life-threatening. The second or late phase usually occurs after an asymptomatic period of from about 1 h up to 3 days or more (see Sect. 10.5.1) and it can be less, equal, or more severe than the immediate reaction. A recently reported case summarized in Table 10.6 dramatically illustrates why, after an anaphylactic reaction to a contrast medium, a physician should be wary of a second-phase response and patients should be made aware of the risk on discharge. Although there appears to be no clinical features that can indicate the possibility of a biphasic acute reaction, it seems that patients who experience the delayed response require higher doses of epinephrine to control their initial reaction.
10.4 Mechanisms of Adverse Reactions to Iodinated Contrast Media
The range and diversity of adverse effects provoked by contrast media remain poorly understood and hence difficult to categorize. For the allergist and clinical immunologist used to thinking of immediate reactions as type I allergic responses mediated by IgE antibodies and delayed reactions as type IV hypersensitivity reactions mediated by antigen-specific effector T cells, adverse reactions to contrast media, divided as they often are into acute and late reactions, do not fit neatly into the conventional mechanism-based classification (refer to chapter 2).
10.4.1 Anaphylactoid and Anaphylactic Reactions
The serious severe reactions induced by contrast media show close similarity to other drug-induced anaphylactoid and anaphylactic reactions discussed at length in earlier chapters, but, as with drugs such as neuromuscular blocking agents, the opioids and other histamine-releasing agents (Chaps. 7 and 8), distinguishing the true, immunologically based anaphylactic reactions from pseudoallergic or anaphylactoid reactions caused by release of inflammatory mediators is generally difficult.
10.4.1.1 Histamine Release
Preformed histamine when newly and rapidly released by degranulation of mast cells and basophils accounts for most of the primary manifestations seen in an anaphylactic reaction (Sect. 3.2.5.1), but the usefulness of assessing histamine release in vitro has not led to general application of the strategy for the diagnosis of drug allergies (see Sect. 4.5.2). Contrast media of high-, low-, and iso-osmolality are well-known releasers of histamine, a property demonstrated in many studies over a period of more than 40 years, so it is not surprising that the experimental findings have led to the suggestion that released histamine may be the mechanism of severe anaphylactic-like reactions to these agents. High- and low-osmolality contrast media have been shown to produce a rise in plasma histamine that peaks and falls back to baseline over a period of about 10 min, but there seems to be no direct relationship between the magnitude of the rise and the severity of the reaction. Even so, a relationship to moderately severe symptoms such as vomiting and rash was suggested and this raised the question of the probability of histamine’s contribution to the more severe reactions to contrast media. The fact that high-osmolar contrast media are responsible for more severe acute reactions than the low-osmolar compounds seems to fit with research findings showing that the high-osmolar compounds release more histamine than the low- and iso-osmolar contrast media, although some studies have concluded that hypertonicity is not an absolute requirement for contrast media-induced histamine release from human basophils in vitro. Another significant conclusion from in vitro experiments was the finding that bloods from previous reactors release a larger percentage of histamine than bloods from nonreactors. Just as the opioid drugs show differences in the amount of histamine they release and in the sites where release occurs (Sect. 8.4), contrast media show similar anatomical selectivity releasing histamine and tryptase from human lung and heart mast cells but not from skin mast cells. Depending on the particular contrast medium injected, this selectivity may influence the symptoms and severity of any subsequent reaction to the drug. Among the ionic agents, meglumine salts are more potent releasers of histamine than the corresponding sodium salts.
Of course, with any case of drug-induced histamine release, the key question to consider is the mechanism underlying the release. Release might proceed in an immediate reaction by direct action of the drug on the mast cells and/or basophils or it might be immunologically mediated as in anaphylaxis mediated by drug-reactive IgE antibodies. However, no cell membrane receptors for any iodinated contrast medium have been identified so far and proving an immunological basis even for the most serious acute reactions has been difficult. For the large majority of patients with contrast medium-induced symptoms appearing within 1 h, IgE antibodies complementary to the culprit drug cannot be demonstrated (see below for a further discussion of IgE antibodies and contrast media).
Activation of complement by contrast media to produce the anaphylatoxins C3a and C5a has also been proposed as the mechanism for contrast media-induced histamine release. These pro-inflammatory complement fragments act via specific receptors on endothelial and mast cells and can induce a shock-like reaction similar to that seen in type I allergic responses. As yet, however, with apparently only a single study showing no differences in anaphylatoxin levels between patients and controls, there appears to be no compelling evidence either way to accept or refute this proposal.
There are many obvious problems to confront in any study designed to examine the role of histamine (and other inflammatory mediators) in adverse reactions to contrast media. Plasma histamine levels peak within 1–8 min following direct treatment with most histamine-liberating drugs (see Sect. 8.4.3) and within 5–15 min after antigen challenge, returning to baseline about 30 min and 60 min later, respectively. There are obvious difficulties in being able to select and study the right patients at the right time and within the required short time frame. Obtaining results, even from small numbers of patients experiencing a severe immediate reaction, involves many difficulties plus an element of luck on the investigator’s part. The rarity of severe reactions emphasizes the paucity of suitable subjects available for contrast media-induced histamine release studies and probably results in the examination of too many patients undergoing minor reactions. Ideally, one would like to be able to perform specific skin tests, specific IgE antibody assays (both with the appropriate controls which include skin testing normal subjects with contrast media and IgE-contrast media inhibition studies), tryptase sampling at suitable times, and quantitation of released histamine.
10.4.1.2 The Question of the Involvement of Contrast Media-Reactive IgE Antibodies
In true type I immediate allergic responses to drugs, just as with immediate reactions to common inhalant, food, and venom allergens, IgE antibodies mediate the reactions and one would therefore anticipate the presence of contrast media-reactive IgE antibodies in the sera of subjects showing immediate reactions, especially severe ones, following injection of contrast media.
Intriguingly, however, positive tests for serum antibodies have been extremely rare and when found they have been in patients with severe acute reactions (see Sect. 10.5.2 below). The subject has been bedeviled by the inconsistencies of results obtained from investigations of fatal reactions and from a broad group of mild to severe reactors suffering an acute (immediate) adverse reaction. There are also the questions of who is to be investigated and when investigations should be pursued. Despite the fact that many drug reactions occur on first exposure to the drug (for example with neuromuscular blockers, quinolones, and a wide range of different drugs in some individuals), a belief persists that without prior sensitization to the drug no immunological response, and in particular an IgE antibody response, can occur. Much the same attitude can sometimes be found toward breakthrough reactions to contrast media after premedication with antihistamine and cortisone. The problem with these poorly informed approaches is that some patients with genuine acute reactions that may be antibody mediated, and even potentially anaphylactic, remain unstudied and undetected.
The question of whether or not iodinated contrast media elicit antibody formation, and in particular IgE antibodies, has been considered from the viewpoint of whether the drugs can stimulate antibody formation in the first place. Using what was described as “highly favorable conditions for the production of antibodies” that included Nippostrongylus brasiliensis-infected Hooded Lister rats that are said to be better at producing antibodies than other strains and the proven adjuvant Bordetella pertussis, meglumine ioglycamate and sodium/meglumine diatrizoate conjugated to carrier proteins were employed as immunogens and any subsequent homocytotropic antibody formation was monitored by the passive cutaneous anaphylaxis (PCA) technique. No evidence for the formation of reaginic antibodies was found leading to the conclusion that “it therefore seems unlikely that the majority of adverse reactions to radiographic contrast media are allergic in nature”. Apart from the obvious doubts about extrapolating results in rodents to humans, a number of different drugs in their original unbound state may allergically sensitize and elicit antibody responses by direct interaction with immune cells and, as with the neuromuscular blocking drugs for example, the sensitizing agent may not be the drug in question. Perhaps another study that should be considered is the injection of a large number of rats or mice with contrast media in an attempt to mimic the situation with human patients and then look for reaginic antibody responses. Considering the human situation, one might expect that for this experiment to be informative, a large number of animals would have to be examined. A clue to what might be a possible immunological function of contrast media in vivo is the finding that iopamidol had a marked adjuvant effect on the production of anti-hapten IgE and IgG1 antibodies in mice and enhancement of antibody production was associated with IL-4 release. Antibodies to the contrast medium were not detected.
In the absence of easy-to-perform and reliable assays for the detection of contrast media-reactive IgE antibodies in patients’ sera, some other less direct but arguably more biologically and clinically relevant test procedures have occasionally been employed. With both the basophil activation and Prausnitz–Kustner (P–K) tests, the interaction between allergen (contrast medium) and IgE antibodies takes place at the basophil (or mast cell) surface, thus mimicking the in vivo situation. However, for different reasons, neither test has become, nor is likely to soon become, a routinely and widely applied procedure for the diagnosis of adverse reactions to contrast media or any other drug (see below).
An interesting finding with the low-osmolar dimer ioxaglate may have some relevance to the question of whether or not contrast media-reactive IgE antibodies are part of the mechanism underlying some reactions to contrast media. Some (but not all) study comparisons have reported more reactions to the dimers than to higher osmolar ionic media, a similar incidence of severe reactions by the two, and a lower incidence of monomer-induced fatal reactions. This has prompted the speculation that the dimers may be antigenically divalent, thus allowing them to bridge adjacent antibody combining sites of mast cell-bound IgE molecules (see Sect. 3.1.2). The prediction is that if this is so, dimeric iodinated contrast media might be more likely to induce mediator release and anaphylaxis than univalent monomers. A similar prediction was advanced for the neuromuscular blocking drugs (Sect. 7.4.2.3).
10.4.1.3 Activation of the Kinin System and Bradykinin
See Sect. 3.2.8.5.2 and Fig. 3.13) for a summary of the kallikrein–kinin system.
Bradykinin, a potent vasoactive nonapeptide, is formed by interaction of factor XII (Hageman factor), high molecular weight kininogens and prekallikrein on negatively charged surfaces (for example, silicates), on macromolecular surfaces such as collagen of connective tissue, heparin and mucopolysaccharides, and on the surfaces of cells together with some specialized proteins including complement component C1q. Bradykinin is also produced by a mechanism bypassing factor XII that involves protease activation of prekallikrein. Cell activation during inflammation and heparin release is thought to activate the plasma cascade leading to bradykinin release and, via its inflammatory and hypotensive effects and capacity to induce tissue hyperresponsiveness, its detrimental role in asthma, anaphylaxis, and other allergic conditions. Bradykinin is degraded by carboxypeptidase N and angiotensin-converting enzyme and it has been claimed that the latter enzyme is inhibited in asthmatics with active bronchospasm and by ionic contrast media at concentrations attainable in the circulation. Such an action reducing or preventing the hydrolysis of bradykinin and therefore limiting its effects might help to explain the increased susceptibility of asthmatics to contrast media. More findings advanced to support the role of the kinin system in elucidating the mechanism(s) of contrast media-induced adverse effects are the reported increases in the plasma of negatively charged heparin-like contact activators and so-called cryptic soluble negatively charged surfaces in subjects who react to contrast media and in asthmatics. An indirect action of bradykinin also contributes to its pro-inflammatory effects. By activating phospholipase A2, the peptide stimulates the release of arachidonic acid from phospholipids leading to the production of prostaglandins and leukotrienes via the cyclooxygenase (Sect. 9.4.1) and lipoxygenase (Sect. 3.2.5.2.1) pathways. Currently it is difficult to judge the importance of these proposed mechanisms to explain contrast media reactivity since other supporting and follow-up evidence has not been forthcoming.
10.4.2 Delayed Reactions
Delayed-type hypersensitivity reactions to iodinated contrast media are said to be rare, but there are some claims that 1–3 % of patients injected intravenously with the agents experience such reactions. Symptoms include persistent pain at the injection site, nausea, vomiting, flu-like symptoms, angioedema, dyspnea, fixed drug eruption, and maculopapular exanthema. Only a small number of cases showing documented evidence supporting a diagnosis of delayed hypersensitivity reactions with positive delayed skin tests have been reported. Maculopapular exanthema is the most commonly seen reaction, accounting for over 50 % of patients with a delayed reaction to a contrast medium. In one well-documented case, a 61-year-old patient with no history of allergy or prior exposure to contrast media developed generalized maculopapular exanthema 7 days after injection of the nonionic agent iopamidol. Three months after the reaction, iopamidol was again administered. Despite premedication with prednisone and cetirizine commencing 3 days before injection, the patient reacted 1 day later with generalized, confluent macular exanthema accompanied by severe itching and enanthema of the oral mucosa. Patch testing showed a positive allergic reaction to iopamidol. In follow-up patch tests, iohexol and ioversol as well as iopamidol gave positive reactions, but the ionic agent sodium amidotrizoate proved negative. Although the ionic compound shares a 2,4,6-triiodobenzene core nucleus with the three nonionic drugs, it is structurally different in the groups attached at positions 1 and 3 of the ring where the latter compounds each have acetamido groups. This structural difference accounts for cross-reactivity of the nonionic agents and the absence of it for the ionic drug.
Some investigators believe that cell-mediated hypersensitivities are responsible for most of the non-immediate reactions to iodinated contrast media but a number of different cellular and cytokine-driven processes may be involved, making this a difficult and complex problem to study. Positive patch and delayed intradermal tests, the presence of T cells at skin test sites, positive responses to provocation testing, immunohistological findings, and contrast media-induced proliferation of T cells from patients with delayed reactions to the agents all give weight to the belief that these late reactions are mediated by T cells. Results from a recent investigation of possible pathways for recognition of iodinated contrast media by T cells suggested that two mechanisms of T cell stimulation were operative. Contrast media-specific T cell clones (TCC) were generated from contrast media-allergic patients and a specific T cell receptor (TCR) was transfected into a mouse T cell hybridoma. Proliferation and IL-2 and Ca2+ assays were performed using HLA-DR-matched or mismatched antigen-presenting cells (APC). An increase in intracellular Ca2+ within seconds of the addition of drug, cell proliferation, and IL-2 secretion in the presence of glutaraldehyde-fixed APCs suggested that stimulation occurred by direct binding to the major histocompatibility complex (MHC)–TCR complex. With other TCCs, abrogation of presentation by glutaraldehyde-fixed APCs, failure to wash away drug from APCs preincubated with contrast media, and an optimal pulsing time of 10–20 h suggested processing by APCs.
The precise mediators of contrast media-induced allergic reaction are also largely unknown, but cytokines are known to participate in both immediate and late reactions. Investigations so far of possible cytokine involvement following contrast media injection revealed an early increase (after 1 h) in IL-2 followed by a delayed increase of IL-4 and IL-6, indicating a Th1 to Th2 shift in late adverse reactions. The highest histamine levels were seen in late reactors 24 h after injection of the contrast medium. TNF-α did not show any significant change. T cell studies, still in their early days and so far on small numbers of patients, have generated iodinated contrast media-specific T cell clones and demonstrated cross-reactivity with some other contrast media by some of the CD4(+) clones. Iomeprol-specific peripheral T cells for example were shown to occur with a frequency of 0.6 %.
10.5 Tests for the Diagnosis and Study of Adverse Reactions to Contrast Media
As always, a meticulously recorded and studied history is desirable, but additional clinical and laboratory tests can contribute greatly to an accurate diagnosis and help to identify the mechanism(s) of the reaction. Skin tests, detection, and quantitation of released mediators and serum IgE antibodies may all contribute to establishing a more precise diagnosis of an adverse response to an iodinated contrast medium.
10.5.1 Skin Tests
For a general discussion of skin testing, see Sect. 4.2.
Although there is no long-established and widespread diagnostic practice of skin testing patients with contrast media, results from recent studies indicate that skin testing with iodinated contrast media is a useful tool in efforts to improve the diagnosis of allergy to these agents. Some small skin test studies and tests on individuals have been carried out intermittently over the years, but a recent European multicenter prospective study carried out under the auspices of the European Network of Drug Allergy and the European Academy of Allergy and Clinical Immunology Interest Group on Drug Hypersensitivity set out, for the first time, to determine in a large study the specificity and sensitivity of skin tests in patients who experienced reactions to iodinated contrast media. Skin prick tests followed by intradermal tests and patch tests were performed on 220 patients with a reported previous hypersensitivity reaction, either immediate or delayed, to contrast media using the dilutions, procedures, and controls summarized in Table 10.7. Overall, positive skin tests were seen in 32 of 122 patients (26 %) with immediate reactions. Intradermal tests were positive in 30 patients and prick tests in only 4. An early and obvious finding was the relationship between the skin test result and the time between reaction and testing. Within 2–6 months of the adverse reaction, the percentage of positive reactors increased from the overall figure of 26 to 50 % (14/28). For patients tested at other times, that is, earlier than 2 months and later than 6 months, the figure was only 18 % (17/92).
For non-immediate reactors, a combination of intradermal and patch tests were needed to identify the maximum number of reactants. Delayed skin tests were positive in 37 of 98 patients (38 %) with only three positive in the skin prick test. Of 31 patients positive in the intradermal test, 9 delayed reactions were detected on day 1, 16 on day 2, and 6 on day 3. Patch tests required times of up to 3 days to detect all of the 22 positive patients out of 79 tested (28 %). Some patients were positive in the intradermal but not in the patch test while with some others it was the reverse. As with the immediate reactors, most reactors (29 of 62; 47 %) were detected when the tests were performed within 6 months of the reactions to contrast media; only 8 of 36 (22 %) were positive at later times. Cross-reactions between some contrast media were detected especially between those of similar structure, for example, iophexol, iomeprol, iopentol, ioversol, and the nonionic dimer iodixanol. From the wider perspective of the mystery of how some patients become allergically sensitized to some drugs (see for example, Sect. 3.1), it is interesting to note that one-third of the patients with a positive delayed skin test in this study reacted to contrast media on their first exposure to the agent. In what may turn out to be a useful observation, patients with a delayed positive skin test showed a higher number with maculopapular exanthema and a lower number with urticaria-like exanthema than the patients who were skin test negative.
The authors of the European multicenter study pointed out some limitations in their report. These included lack of provocation testing, the need for more control subjects exposed to a contrast medium but without clinical signs of a hypersensitivity reaction, the culprit drug was not always identified, a possible lack of test sensitivity, and the fact that the negative predictive value of the skin tests had not been determined. In relation to the last point, a negative predictive value of 96.6 % has recently been claimed for tests with iodinated contrast media (see Sect. 10.5.4), but, to be confident of this figure, studies with larger numbers of patients need to be done. Despite these limitations and some others, most of which are not necessarily easy to overcome, the main and most important conclusions from the first 4 years of this prospective study are that about half of the hypersensitivity reactions to iodinated contrast media have an immunological basis and skin testing, especially intradermal and patch testing, is a useful diagnostic tool that may aid the selection of a safe contrast agent for those patients who have experienced a previous reaction.
10.5.2 IgE Antibody Tests
10.5.2.1 Prausnitz–Kustner Test
This test relies on the capacity of homocytotropic antibodies or reagins to fix to human skin and be detected by subsequent injection of the suspected allergenic drug. Although a positive reaction is not immediately indicative of the involvement of IgE antibodies, their presence is often inferred if skin sensitization is prevented by prior heating of the serum to 56 °C. Prior to the realization of the possibility of the transfer of blood-borne viruses, and the introduction of easier-to-carry-out tests that specifically identify IgE, the P–K test was used in studies aimed at determining whether or not the antibody was implicated in some adverse reactions to iodinated contrast media. In one case study, a positive P–K test was taken as evidence that an immediate reaction to ioglycamic acid was mediated by IgE antibodies.
10.5.2.2 Serum Tests
For details of the detection of drug-reactive IgE antibodies in sera, see Sect. 4.3. As already outlined, IgE antibodies to iodinated contrast media have not been convincingly and consistently demonstrated and the current consensus is that true type I IgE antibody-mediated reactions to the drugs are rare, but they do occur, generally in the most severe immediate cases. This conclusion, or impression, may be correct, based as it is on the antibody detection methodologies applied to date, but consistent failures to detect the antibodies may be due to the inadequacies or inappropriateness of those methodologies. Even with the small number of positive sera detected in some studies, reactions are often weak, quantitative inhibition results to demonstrate specificity are not shown, and details of the materials and methods used are not provided. The average association constant of IgE for contrast media was low in what appears to be the only study where the affinity of the reaction was looked at. The first more convincing demonstration of the detection of contrast media-reactive IgE antibodies involved activation of the hemisuccinate of ioxaglic acid to form the N-hydroxysuccinimide ester before linking to amino groups on human serum albumin as carrier protein (Fig. 10.2) and using the drug–carrier complex in immunoassays with patients’ sera. Although binding uptakes were weak, specificity of the reaction with the contrast medium was demonstrated by dose-dependent inhibition in the range 25–80 % and IgE antibody was detected in 16 of 34 patients (47 %) with a history of adverse reactions to ioxaglate and in 14 of 68 patients whose sera were collected at the time of the adverse reaction to the contrast agent. The frequency of 47 % seems consistent with a previous claim of 42 % for the presence of IgE antibodies in patients with an adverse reaction to a contrast medium.
10.5.2.3 Basophil Activation Test
Unlike in vitro methodologies that detect binding of serum IgE antibodies, usually to an allergen in solid phase form, the basophil activation test (see Sect. 4.6) is an in vitro activation of a patient’s basophils and as such the test mimics the interaction between the allergen and circulating basophils in the patient’s body. The test is not, however, a primary diagnostic tool and is essentially complementary to skin tests and quantitation of allergen-reactive IgE antibodies. Despite references to its application to the investigation of contrast media adverse reactions, there currently appears to be very few published studies where it has been applied in this way. In a 2008 study the contrast media iomeprol and iopromide were diluted and used over a broad range up to 1 μg/ml and a minimum of 500 basophils per sample were activated (CD63+, IgE++) and assessed by flow cytometry. Because drugs give lower activation percentages than inhalant and venom allergens, activation was considered positive if data analysis showed more than 5 % activated basophils. In three patients the test revealed 15 % maximum activation of basophils at 1 μg/ml. Two patients showed positive results only with iomeprol while the third was positive to both contrast media. In the control samples, activation remained negative at all contrast media concentrations. A recent study in Thailand on 26 patients with diagnosed immediate reactions to contrast media and 43 healthy volunteers found significantly higher percentage activations at drug dilutions of 1:10 and 1:100 with the patients’ than the volunteers’ cells. Once again the conclusion was reached that the basophil activation test has potential as a diagnostic tool, especially as a confirmatory test.
10.5.3 Tests to Detect the Release of Mediators
From at least the early 1980s, studies relevant to the possibility of monitoring contrast media-induced histamine release either directly or indirectly via measurement of urinary methylhistamine have been pursued. Although concentrations of histamine and its metabolite have been shown to increase in some patients who had an adverse reaction to a contrast medium, diagnostic tests for these mediators have not often been used and it was not until an assay for tryptase (see Sect. 4.5.1) became widely available that routine measurement of a mast cell mediator became almost standard practice. In one of the most informative studies, released histamine and tryptase levels correlated significantly with the severity of symptoms to contrast media and it was suggested that less clear results from many previous investigations may have been due to the recruitment of patients with minor or only moderate reactions. The relative half-lives of histamine (about 2 min) and tryptase (about 90 min) give another indication of why the latter mediator, the most abundant protein produced by the human mast cell, is preferred in diagnostic investigations of severe immediate but not delayed allergic reactions.
10.5.4 Challenge Tests
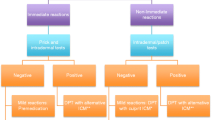
Although considered to be the “gold standard” in the diagnosis of drug hypersensitivity, challenge tests (Sect. 4.4) are time-consuming, are potentially dangerous, and tend to be reserved for use when the information gathered from other tests yields inconclusive results or contradictions. Challenge tests with contrast media have so far also been employed rarely but appear to be valuable to identify contrast media that are tolerated by challenging intravenously skin test-positive patients with skin test-negative agents. In addressing the paucity of data on the negative predictive value for skin tests with iodinated contrast media, 29 skin test-negative patients needing a new contrast medium were rechallenged without premedication. Mild reactions resulted in 1 of 24 patients with a history of an immediate reaction and one of four with a history of a non-immediate reaction, giving a negative predictive value of 96 %. Two patients with a positive skin test to an iodinated contrast medium tolerated an alternative drug without experiencing a reaction. A protocol for challenge with contrast media is set out in Table 10.8.
10.6 Premedication for the Prevention of Anaphylactoid/Anaphylactic Reactions to Iodinated Contrast Media
From the results of studies in the USA dating back to the mid-1970s, it was recommended that lower osmolality contrast media should be given to patients who had previously experienced what was called an “immediate generalized reaction.” In addition, the prophylactic use of prednisone and diphenhydramine was recommended to reduce the chance of a reaction in high-risk patients. Such premedication is often given, but opinion of its effectiveness is divided and it could probably be said that the practice has not received wide support. There are many reports of breakthrough reactions—some detailing that hypersensitivity responses were not prevented in a number of patients; some reporting a significantly higher recurrence rate in those who had a previous mild reaction but prevention of a reaction in those who had a severe previous reaction; and a frequent finding that breakthrough reactions were of similar severity to the patients’ initial reactions.
The theory behind the inclusion of histamine H1 antagonists in the premedication is obvious but the mode of action of corticosteroids is not completely understood so some believe its inclusion cannot be explained and justified. Corticosteroids ultimately inhibit kallikrein, a peptide that lowers blood pressure and liberates bradykinin. Corticosteroids also act in the arachidonic acid cascade to inhibit the production of prostaglandins and leukotrienes, so there does seem to be some rationale for their use. There are some indications that premedication prevents the recurrence of many minor reactions. Some, or even many, of these reactions may not be immune mediated, proceeding instead via a nonspecific and low-level histamine release. In the case of severe immediate reactions, IgE antibody-mediated explosive histamine release from mast cells may overwhelm the potential effectiveness of premedication.
Despite calls to discontinue the prophylactic use of corticosteroids and antihistamines for contrast medium-induced anaphylactoid reactions in the USA, the recommendation for their use is still unaltered. This situation is in contrast to the attitude in some other countries, for example, France, but one wonders what the attitude of the critics of premedication would be if they faced injection of an iodinated contrast medium for angiography after experiencing a life-threatening reaction knowing they were allergic to both ionic and nonionic media of all osmolalities. Given our knowledge of the pharmacological effects of histamine H1 antagonists and the steroids, could there be anything to lose in opting for premedication in such a situation? A suitable premedication regime is set out in the legend of Table 10.4.
Of course, for high-risk patients when administration of an iodinated contrast medium is regarded as essential, a nonionic agent would be the first choice, but if that choice is also potentially dangerous and cannot be avoided, the patient should be informed of the risks, patient approval should be obtained, and resuscitation arrangements should be fully in place. Gadolinium-based contrast media may also be considered, but with these agents some extra factors need to be considered.
10.7 Gadolinium-Based Contrast Agents
Gadolinium, a rare earth metal, forms trivalent ions with paramagnetic properties that make solutions of chelated gadolinium complexes with large organic molecules useful as intravenously administered contrast agents detected by magnetic resonance imaging (MRI). Gadolinium-based contrast agents are approved by the FDA (and many other licensing authorities) for use with MRI as a contrast agent, but, although they can be used for magnetic resonance angiography (MRA), there is no approval for this use. The usual dose for many MRI applications is 0.1 mmol/kg up to a maximum approved dose of 0.3 mmol/kg for intravenous use. Above that figure the agents may induce potentially fatal nephrogenic systemic fibrosis (NSF), a scleroderma- and eosinophilic fasciitis-like disease of the joints, skin, eyes, and organs particularly in patients with kidney failure.
10.7.1 Molecular Structures of Gadolinium-Based Contrast Agents
These agents may be acyclic (linear) or macrocyclic and ionic or nonionic. Examples from each category are acyclic, ionic—gadopentetate (Gd-DTPA) dimeglumine; acyclic, nonionic—gadodiamide (Gd-DTPA-BMA); macrocyclic, ionic—gadoterate (Gd-DOTA) meglumine; and macrocyclic, nonionic—gadoteridol (Gd-HP-DO3A). The structures of the acyclic agent gadopentetic acid (gadopentetate dimeglumine is the salt) are shown in Fig. 10.3 and structures of three macrocyclics, gadoterate meglumine, gadoteridol, and the nonionic gadobutrol (Gd-BT-DO3A) are set out in Fig. 10.4. Stability of gadolinium chelates is a major concern and great emphasis has been placed on this because of the possibility of transmetallation, that is, exchange or release of free Gd3+. With the Gd3+ caged within the chelate complex, the macrocyclic compounds are generally more stable than their acyclic counterparts.
2D structure (a, b), ball-and-stick (c), and CPK (d) models of gadopentetic acid, the first gadolinium-based magnetic resonance imaging contrast agent introduced in 1987. This linear, ionic agent is usually administered as the meglumine salt gadopentetate dimeglumine, a complex of Gd3+ with diethylenetriaminepentacetate (DTPA5−) (Gd-DTPA) with the nine-coordinate Gd ion surrounded by three nitrogens and five oxygens and the ninth site occupied by a water molecule (a, b)
10.7.2 Nephrogenic Systemic Fibrosis
Identified in 1997 and reported in 2000, nephrogenic systemic fibrosis (NSF) was first associated with gadolinium contrast agents in 2006. Chronic kidney disease, hepato-renal syndrome with renal insufficiency, and acute kidney injury were described as the clinical settings in early case reports and it soon became apparent that the disease affects multiple organs including the lungs, heart, liver, and muscles. In patients with reduced renal function, the prevalence of NSF after exposure to gadodiamide is reported to be 3–7 %. Of 589 patients who developed NSF associated with gadolinium contrast agents between 1997 and 2007, all were to linear chelates—68 % with gadodiamide, 26 % with gadopentetate, and 5 % with gadoversetamide. Subsequent to 2006, of 1,603 cases reported to the FDA, 93 % were from 60 hospitals in the USA and 4 % from 2 hospitals in Denmark. Gadodiamide was revealed to be a key factor in the relatively high incidence of NSF found in Denmark. The macrocyclic agents are regarded as low-risk compounds. By 2009, there had been no cases of NSF after exposure to a macrocyclic, but three cases were reported in Denmark in 2011.
10.7.3 Other Adverse Reactions
Risk factors for acute reactions to gadolinium-based contrast media and procedures and strategies to reduce those risks are summarized in Table 10.9. Note that the risk of an adverse reaction to a gadolinium-based contrast medium is eight times higher in patients who have experienced a previous reaction to a gadolinium agent. Gadolinium chelates have been used parenterally for over 30 years and are well tolerated in the vast majority of patients. Adverse reactions to gadolinium contrast media appear to be less frequent than reactions to the iodinated media. The American College of Radiology (ACR) Committee on Drugs and Contrast Media has reported that the frequency of all adverse events after injection of 0.1–0.2 mmol/kg of gadolinium chelate is in the range 0.07–2.4 % with the vast majority of these reactions being mild, for example, nausea, vomiting, headache, paresthesia, dizziness, itching, and coldness at the injection site. “Allergic-type” reactions have an even lower frequency of 0.004–0.7 % with symptoms of rash, urticaria, and rarely bronchospasm. Anaphylactic/anaphylactoid reactions are extremely rare (0.001–0.01 %). In one study, an adverse reaction rate of 0.48 % and an incidence of 0.01 % for severe anaphylactoid reactions were reported for gadolinium chelates. Of the 45 patients with 46 adverse reactions, 96 % were mild reactions, 2 % were moderate, and 2 % severe. Three (6.7 %) of the 45 patients had prior reactions to iodinated contrast media. In an assessment of the clinical safety and diagnostic value of the gadolinium chelate gadoterate meglumine in 24,308 patients injected with the agent, adverse reactions were seen in 0.4 % of the examinations and were mostly rated as minor, that is, feelings of warmth or taste alterations. Only one serious adverse reaction was seen. A review of 21,000 patients administered gadolinium contrast media in a Michigan hospital revealed 36 adverse reactions (0.17 %) classified into four groups: mild, nonallergic reactions (nausea, vomiting) 15 patients; mild reactions resembling allergy (hives, erythema, skin irritation) 12 patients; moderate reactions resembling allergy (respiratory symptoms) 7 patients; and life-threatening reactions resembling allergy (chest tightness, respiratory distress, periorbital edema) 2 patients (0.01 %). Reactions resembling allergy therefore occurred in 21 patients. Four of the patients had previous reactions to iodinated contrast media which is consistent with a previous conclusion that the risk of adverse reactions to gadopentetate is 3.7 times higher in patients with a history of reaction to iodinated contrast media. Gadopentetate dimeglumine was the agent most often implicated being the administered drug in 29 of the 36 patients (0.138 %) including both of the life-threatening reactions (0.01 %). These 2 % for gadopentetate differ significantly from an earlier investigation of the safety of the same agent where the overall incidence of adverse events was higher (1–2 %) while the incidence for anaphylactoid reactions was only 0.0003 %. For comparison, incidences of life-threatening reactions to iodinated contrast media are said to be 0.031 % for low-osmolarity agents and 0.157 % for high-osmolality agents.
A recent survey of the incidences of immediate reactions to gadolinium contrast media revealed rates of 0.2, 0.5, 1.2, and 3.3 per 1,000 injections of gadodiamide, gadopentetate dimeglumine, gadobenate dimeglumine, and gadoteridol, respectively. For the period 2004–2009, the FDA received reports of 40 NSF-unrelated deaths resulting from 51 million administrations of gadolinium contrast agents with incidences of 0.15, 0.19, 0.7, 0.97, and 2.7 per million for gadodiamide, gadoversetamide, gadoteridol, gadopentetate dimeglumine, and gadobenate dimeglumine, respectively. It has been pointed out that these figures represent a similar risk of death from traveling 86 miles by car! With gadoterate meglumine, positive skin tests, sometimes with a positive leukocyte histamine release test, indicated that the reactions were almost certainly IgE antibody mediated. Positive skin tests and a positive tryptase finding were also found in a case of anaphylaxis to gadobenate (Gd-BOPTA) dimeglumine. Skin testing with other gadolinium chelates generally revealed mono-sensitization with none of the other agents showing cross-reactions. In some of the investigations, skin test-negative findings with high concentrations of meglumine ruled it out as the provoking agent and direct and explosive mast cell degranulation by gadolinium chelates in the reported cases seems unlikely since in vitro experiments have shown that the concentrations needed for direct histamine release are about 100–400 times the normal serum concentrations found in patients. The skin test findings so far with the gadolinium chelates suggest that this simple and easy-to-carry-out test might be useful for identifying alternative MRI contrast agents, but more extensive testing with many more patients is needed to validate the procedures before the predictive value of skin testing can be established. As with the iodinated contrast media, breakthrough reactions to gadolinium contrast agents, sometimes described as “allergic-like,” have occurred after corticosteroid and antihistamine premedication.
In some cases where the history was reliable enough and where questioning about exposure to products containing gadolinium (for example, in CDs, electronic components, nuclear materials, alloys, phosphors, optical glass, ceramics, and manufacturing plants where the element is used) was undertaken, it became apparent that reactions to gadolinium chelates occurred on first exposure. As discussed before in this volume, this is not unusual in drug allergy and while a number of different speculative explanations have been offered for the mechanism of sensitization for some other drugs such as neuromuscular blockers, the advancement of any sort of plausible speculation accounting for allergic sensitization to complexes containing a rare earth metal seems even more difficult.
10. Summary
Iodinated Contrast Media
-
Considering the very large number of administrations worldwide, iodinated contrast media are one of the safest of all drugs.
-
Reactions to iodinated contrast media can be dose-dependent (toxic reaction) or unrelated to the dose (for example, an immunological reaction).
-
Reactions range from a mild inconvenience such as heat sensation and nausea to a life-threatening emergency.
-
In the great majority of cases reactions are mild and direct histamine release can account for the symptoms.
-
Contrast media are better tolerated when the osmolalities of the injected media and body fluids are as close as possible. Nonionic media are better tolerated than the ionics.
-
Adverse reactions are divided into acute or immediate and late or delayed. The former occur within an hour and the latter from about 1 h up to a week but usually 1–3 days.
-
Acute reactions are conveniently divided into mild (with symptoms including nausea, vomiting, and headache), moderate (tachycardia/brachycardia, marked urticaria, severe vomiting), and severe (hypotensive shock, cardiac and respiratory arrest, laryngeal edema). Late reactions include nausea, vomiting, and especially (and usually self-limiting) maculopapular rash, exanthema, urticaria, and pruritus.
-
Severe biphasic reactions to iodinated contrast media, that is, a life-threatening late reaction after an initial acute immediate reaction, are rare but can occur. After discharge following an acute immediate reaction to a contrast medium, patients should be made aware of the risk of a second-phase response.
-
The incidence of acute (immediate) reactions to the ionic media is about 3–4 % with up to about 12 % reported. For the low-osmolar nonionic agents, the figure is 0.2–0.7 % (up to about 3 %). For severe immediate reactions (mainly anaphylactic), incidences are 0.1–0.4 % for ionic and 0.02–0.04 % for nonionic media. For very severe reactions the percentages drop to 0.04–0.004 %.
-
Fatal reactions (1 in 100,000 to 1 in 170,000) are extremely rare and show no differences between low- and high-osmolar agents.
-
Up to 80 % of reactions to an ionic agent can be avoided by substituting a nonionic medium.
-
For delayed reactions, there is no difference between the incidences of reactions to ionic and nonionic media or between the different nonionic agents. Incidences of delayed reactions occurring in the first 24 h and over a 7-day period are approximately 4 % and 1–3 %, respectively.
-
Risk factors: for immediate reactors—a previous immediate reaction to an iodinated contrast medium; bronchial asthma; use of β-blockers; cardiac disease; highly allergic subject. For late reactors–a previous reaction; treatment with IL-2; a history of drug allergy; contact allergy.
-
Evidence that immediate reactions to a contrast medium is IgE antibody mediated is based largely on skin test results with or without tryptase measurements and very occasionally serum IgE and basophil activation tests.
-
Delayed reactions mainly manifest as exanthematous skin eruptions. They are mediated by antigen-specific effector T cells with as yet poorly defined cytokine involvement.
-
Contrast media-induced hypersensitivity has traditionally been regarded as nonallergic in nature with skin testing not relevant. Skin tests with iodinated contrast media are, however, positive in a subgroup of reactors.
-
In a large multicenter study, 50 % of immediate reactors tested within 2–6 months of the reaction showed a positive skin test. The prick test was only rarely positive. The intradermal test was clearly more informative.
-
For delayed reactors, a combination of intradermal and patch testing identified the maximum number of positive reactions (47 %). The highest number of positives was detected when tests were performed within 6 months of the reaction.
-
Cross-reactions between iodinated contrast media were detected in skin tests on delayed reactors making intradermal and patch tests useful tools for selecting a safe contrast medium.
-
IgE antibodies to iodinated contrast media have not been consistently and convincingly demonstrated. This raises doubts about patient selection (that is, the degree of severity of patient reactions) and the adequacy and appropriateness of the present IgE test methodologies.
-
Challenge tests with contrast media, rarely employed and refused by most patients, are valuable for the identification of tolerated skin test-negative agents.
-
Opinion on the effectiveness of premedication with corticosteroids and a histamine H1-antagonist is divided since many breakthrough reactions have been reported.
-
A significant number of immediate and delayed reactors with positive skin tests to contrast media reacted on first exposure to the agents.
Gadolinium-Based Contrast Agents
-
Gadolinium, a rare earth metal, forms ions with paramagnetic properties making the toxic metal in chelate form a useful contrast agent that can be detected by magnetic resonance imaging.
-
Gadolinium-based contrast agents were associated with nephrogenic systemic fibrosis in 2006. The linear chelates, and especially gadodiamide, are most commonly implicated. The macrocyclic agents are regarded as relatively safe.
-
Gadolinium chelates show an adverse reaction incidence of about 0.48 % and an incidence of about 0.01 % for anaphylactoid reactions. This is lower than the corresponding figures for iodinated contrast media.
-
There are a number of reports of anaphylaxis to gadolinium-based contrast media with the diagnosis supported in some cases by positive skin tests to the agents.
-
Some adverse reactions occurred on first exposure to the contrast agents.
-
Gadolinium contrast media are well tolerated by the vast majority of patients and are regarded as remarkably safe drugs.
Further Reading
ACR manual on contrast media, version 7. ACR committee on drugs and contrast media. American College of Radiology; 2010
Brockow K, Romano A, Aberer W, et al. Skin testing in patients with hypersensitivity reactions to iodinated contrast media – a European multicenter study. Allergy. 2009;64:234–41.
Caravan P, Ellison JJ, McMurry TJ, et al. Gadolinium (III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev. 1999;99:2293–352.
ESUR guidelines on contrast media. European society of urogenital radiology, version 7.0 view. http://www.esur.org/Contrast-media.51.0.html
Morcos SK. Acute serious and fatal reactions to contrast media: our current understanding. Br J Radiol. 2005;78:686–93.
Murphy KJ, Brunberg JA, Cohan RH. Adverse reactions to gadolinium contrast media: a review of 36 cases. AJR Am J Roentgenol. 1996;167:847–9.
Prince MR, Zhang H, Zou Z, et al. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol. 2011;196:W138–43.
Thomsen HS. ESUR guideline: gadolinium-based contrast media and nephrogenic systemic fibrosis. Eur Radiol. 2007;17:2692–6.
Thomsen HS. Nephrogenic systemic fibrosis: history and epidemiology. Radiol Clin North Am. 2009;47:827–31.
Trcka J, Schmidt C, Seitz CS, et al. Anaphylaxis to iodinated contrast material: nonallergic hypersensitivity or IgE-mediated allergy? AJR Am J Roentgenol. 2008; 190:666–70.
Webb JAW, Stacul F, Thomsen HS, et al. Late adverse reactions to intravascular iodinated contrast media. Eur Radiol. 2003;13:181–4.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Baldo, B.A., Pham, N.H. (2013). Contrast Media. In: Drug Allergy. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7261-2_10
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7261-2_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7260-5
Online ISBN: 978-1-4614-7261-2
eBook Packages: MedicineMedicine (R0)