Abstract
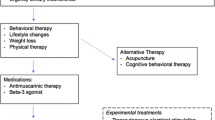
Primary goals in diagnosis and treatment of functional bladder disorders:
-
1.
Stop urinary incontinence.
-
2.
Reduce associated UTI.
A secondary aim is to reduce behavior dysfunction associated with incontinence.
Summary of evidence for these goals:
-
Several studies indicate placebo results in cure or improvement of symptoms in approximately 40 % of children with voiding dysfunction.
-
There is little evidence that biofeedback, AC, or alpha-blockers are more effective than urotherapy or placebo to reduce diurnal incontinence. One trial demonstrated an alarm watch is better than timed voiding.
-
Several small series indicate botulinum A injection in patients with refractory overactive bladder (OAB) stops incontinence in approximately 50 %, with an average response duration of 6 months.
-
Two small series report transcutaneous electrical nerve stimulation (TENS) or Interstim devices improve symptoms in children refractory to other treatments.
-
While urinary symptoms improve during treatment for constipation, one randomized controlled trial (RCT) found no added benefit to polyethylene glycol versus placebo, and another study reported improvement in diurnal incontinence in patients both with and without improved stooling.
-
Our review found no evidence that the voiding dysfunction treatment reduces UTI. Two studies reported recurrent UTI within 1 year in approximately 40 % of females despite various treatments (urotherapy, biofeedback, AC).
-
While children with voiding dysfunction also have increased behavioral problems, our review found only one study that reports impact of therapy, finding decreased problems in those with dysfunctional voiding but not urge syndrome. One case–control study found improved self-esteem in patients after incontinence therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Lower Urinary Tract Symptom
- Detrusor Overactivity
- Tethered Cord
- Dysfunctional Voiding
- Daytime Urinary Incontinence
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Primary goals in diagnosis and treatment of functional bladder disorders:
-
1.
Stop urinary incontinence.
-
2.
Reduce associated UTI.
A secondary aim is to reduce behavior dysfunction associated with incontinence.
Summary of evidence for these goals:
-
Several studies indicate placebo results in cure or improvement of symptoms in approximately 40 % of children with voiding dysfunction.
-
There is little evidence that biofeedback, AC, or alpha-blockers are more effective than urotherapy or placebo to reduce diurnal incontinence. One trial demonstrated an alarm watch is better than timed voiding.
-
Several small series indicate botulinum A injection in patients with refractory overactive bladder (OAB) stops incontinence in approximately 50 %, with an average response duration of 6 months.
-
Two small series report transcutaneous electrical nerve stimulation (TENS) or Interstim devices improve symptoms in children refractory to other treatments.
-
While urinary symptoms improve during treatment for constipation, one RCT found no added benefit to polyethylene glycol versus placebo, and another study reported improvement in diurnal incontinence in patients both with and without improved stooling.
-
Our review found no evidence that the voiding dysfunction treatment reduces UTI. Two studies reported recurrent UTI within 1 year in approximately 40 % of females despite various treatments (urotherapy, biofeedback, AC).
-
While children with voiding dysfunction also have increased behavioral problems, our review found only one study that reports impact of therapy, finding decreased problems in those with dysfunctional voiding but not urge syndrome. One case–control study found improved self-esteem in patients after incontinence therapy.
ICCS Definitions
Subgroups listed below apply to children who have attained bladder control or are ≥5 years of age. The general phrase for voiding symptoms in children is now similar to that in adults, “lower urinary tract symptoms” (LUTS), which replaces the phrase “dysfunctional voiding.” The ICCS defines urinary frequency as ≥8 voids/day and decreased frequency as ≤3 voids/day (Neveus et al. 2006).
Overactive bladder (OAB). urinary urgency, defined as a sudden and unexpected need to void. Concomitant urinary frequency and/or incontinence may be present but are not prerequisites. Children with OAB should not be diagnosed with detrusor overactivity without cystometric confirmation (Neveus et al. 2006).
Urge syndrome. frequent attacks of urinary urgency with squatting or other holding maneuvers, voiding frequency >7×/day, and urge incontinence (Bael et al. 2008a). While the ICCS has adopted adult terminology in using “OAB” to describe children with urgency, others continue to use “urge syndrome” to define children with a characteristic pattern of urinary frequency and urgency ± incontinence.
Extraordinary daytime urinary frequency. daytime voiding ≥1×/h with small volumes ≤50 % estimated capacity for age, usually without incontinence and with normal nocturnal bladder behavior.
Underactive bladder. low voiding frequency and straining to initiate, maintain, or complete voiding.
Voiding postponement. daytime incontinence associated with low micturition frequency and holding maneuvers.
Giggle incontinence. complete voiding occurring during or immediately after laughing in a child with normal bladder function when not laughing.
Dysfunctional voiding. habitual contraction of the urethral sphincter during voiding verified with repeated uroflows demonstrating a staccato pattern on UD.
Hinman’s syndrome. constellation of findings including diurnal urinary ± fecal incontinence, bladder trabeculation in the absence of outlet obstruction or neurologic disease, and upper tract changes including VUR and/or hydronephrosis. The term was used by the editors of the Journal of Urology to replace “nonneurogenic neurogenic bladder” and was not included in 2006 ICCS terminology (Hinman 1986).
Constipation. the ICCS notes “there are no good definitions of constipation.” Table 3.1 lists criteria for diagnoses commonly referenced (Rome II, Rome III and PACCT). The Bristol Stool Form Scale has not been validated for the diagnosis of constipation in children. It was originally developed as a measure of gastrointestinal transit time in adults with various bowel pathologies.
Retrospective chart review of 336 children mean age 6 ± 3.5 years referred for defecation disorder compared Rome II and Rome III criteria; 34 % versus 87 % (p < 0.001) met criteria for functional constipation using these diagnostic recommendations, respectively (Burgers et al. 2012).
A prospective longitudinal study of 128 consecutive children mean age 67 ± 46 months referred to a tertiary constipation clinic compared Rome II to PACCT criteria. Functional defecation disorders (functional constipation, fecal retention, or fecal soiling without retention) were diagnosed in 48 % versus 89 %, p = 0.001. There was poor agreement (Kappa = 0.173), implying these two sets of criteria identify different patient groups. Among those diagnosed with constipation, 80 % had straining and pebble-like stools, 66 % had painful defecation, and 63 % had large-diameter stools (Boccia et al. 2007).
Our review found no report concerning prevalence of the various categories of voiding dysfunction or constipation in unselected children referred for urologic evaluation.
Prevalence
Population-based surveys found that reported LUTS and, specifically, OAB, decreases with age, with <20 % of children 7 years old having daytime incontinence, which occurred daily in <1 %.
Prevalence of the various clinical conditions that together comprise functional bladder disorders has not been reported in consecutive (unselected) patients.
Constipation was reported in ≤20 % of children with LUTS.
The Avon Longitudinal Study of Parents and Children, a prospective population-based study with 14,000 children followed since birth, queried parents about daytime urinary incontinence (DUI) in children at ages 4.5, 5.5, 6.5, 7.5, and 9.5 years. Data were available for 10,819 children (Swithinbank et al. 2010):
-
DUI was present in 15.5 % at 4.5 years and decreased to 4.5 % at 9.5 years, with girls exhibiting slightly higher rates at all time points; daytime wetting >2×/week was present in 1.9, 1.5, 1.0, 1.0, and 0.5 % at the above-listed ages.
-
At age 7.5 years, DUI frequency was <1 episode per week in 9 %, 2–5 episodes per week in 0.5 %, and nearly daily in 0.3 %.
-
Voiding frequency was significantly less in those without DUI, <5×/daily in 43 % and 5–9×/daily in 56 % versus 36 % and 61.5 % in incontinent children. Boys and those with DUI > 5 episodes/week had higher voiding frequency.
-
Both constipation and soiling were significantly more likely in those with DUI versus no incontinence (13 and 29 % vs. 10 and 5 %).
A validated questionnaire about bowel and bladder habits was completed by the parents of 2,856 children (mean 7 ± 1 year) in a cross-sectional, population-based study. Daytime incontinence (>1×/prior 6 months) was reported in 17 % of children, constipation (Rome II criteria) in 6 %, and encopresis in 10 % (Sureshkumar et al. 2009).
A survey was done in 1,982 among 3,627 children entering school at age 7 years, with school nurses asking about voiding dysfunctions. Positive answers prompted an additional phone call to parents from the study team. Incontinence was defined as one or more episodes of wetting in 3 months. Daytime incontinence was reported by 5 %, which was less than 1×/week in 2 %, and 3–7×/week in 0.7 % (Hellstrom et al. 1990).
OAB (urgency ± urge incontinence during the past 3 months) was present in 2,740 (17 %) Korean children in a population-based, cross-sectional parental survey of 16,516 children. Of children with urgency, 739/2,740 (27 %) had urge incontinence. Prevalence of urgency decreased with age (23 % at age 5 decreasing to 12 % at age 13, p = 0.0001), and was evenly split between boys and girls (1,435 [52 %] boys and 1,305 [48 %] girls) (Chung et al. 2010).
Similar OAB prevalence of 18 % was reported in Japan despite older mean age of 9 ± 2 years. Among 5,282 children in this cross-sectional, population-based survey, 19 % boys and 17 % girls experienced OAB, defined as increased daytime frequency (>1 SD beyond the average number voids/day for age) ± urge in the past month (i.e., urge was not a prerequisite). Daytime frequency decreased with increasing age. Constipation (<3×/week) was present in 971 (18.5 %) children, of which symptoms of OAB were equally present in those with and without constipation (20 % vs. 17.5 %, p > 0.05) (Kajiwara et al. 2006).
Diagnostic Instruments
History
The ICCS recommends that a detailed history be taken from the child when possible, but stated “literature on this topic is sparse” (Hoebeke et al. 2010).
Questionnaires
Four validated questionnaires have been developed—two answered by children, one by their parents, one by either, depending on patient age—that distinguish children with voiding dysfunctions from normal children.
A scoring system was validated comparing parent responses in 86 children (1.5:1 females to males, mean age 8 years, range 4–10) with “various wetting and daytime voiding problems” to 265 controls of similar age without urinary symptoms. The questionnaire had 13 questions with potential scores ranging from 0 to 35. Median scores in patients versus controls were 18.6 versus 2.9. Receiver operating characteristic plotting showed the optimal cutoff score of 8.5 distinguished children with “voiding abnormalities” from normal children with 90 % sensitivity and specificity (Akbal et al. 2005).
The dysfunctional voiding symptom score was adapted from the International Prostate Symptom Score to include ten questions (potential scores 0–30) relevant to children and designed to be answered by children. Validation compared 104 consecutive patients (4:1 female to male) between ages 3 and 10 years with a history of diurnal incontinence, “abnormal voiding habits,” or UTI to 54 age-matched children (1.3:1 female to male) without urologic complaints. Using receiver operating characteristics, the optimal cutoff score for “dysfunctional voiding” was 6 in females (93 % sensitivity, 87 % specificity) and 9 in males (81 % sensitivity, 91 % specificity) (Farhat et al. 2000). Among a subset of 48 patients who underwent a bladder behavioral modification program for “dysfunctional voiding,” follow-up DVSS scores improved from 14.5 to >6.0 among the 28 patients whose parents indicated they were compliant with the program versus no significant change (14.5 to >11.0) among the 20 patients who were reportedly not compliant (Farhat et al. 2001).
The Incontinence Symptom Index-Pediatric is a validated 11-item instrument with subdomains for stress incontinence, urge incontinence, insensate incontinence, enuresis, and pad use designed for children 11–17 years of age to answer independently. Scores were significantly different in 19 patients (with incontinence) versus controls (well-child exams) for the full instrument and subdomains (Nelson et al. 2007).
Evaluation of these three instruments was done, with 37 children 4–10 years of age using the DVSS (answered by patients) and Akbar questionnaire (scored by parents), and 35 children 11–17 years old answering the Nelson survey. These scores were compared to blinded physician rating using a scoring sheet. Physician rating correlated with all three instruments, but was best with patient reporting using either the DVSS or Nelson surveys (Schneider et al. 2011).
A fourth instrument uses 14 questions to be answered by children ≥9 years old, or by parents of younger patients, concerning bowel and bladder dysfunctions. A Likert scale scores each from 0 to 4, for a maximum score of 56. It was validated in 62 patients median age 8 years (4–16) and 50 healthy controls, with a median 14 in patients and 6 in controls, p = 0.001. ROC curve indicated that a score of 11 was the optimum threshold with AUC 0.9 (95 % CI 0.8–0.9) (Afshar et al. 2009).
Voiding Diary/Pad Tests
One study reported parent responses to the questionnaire underestimated incontinence and overestimated frequency when compared to a 72-h voiding diary.
Two studies report pad tests did not record wetting in approximately one-third of subjects reporting incontinence.
Two hundred two children ages 6–12 enrolled in the EBDS had pretreatment questionnaires regarding voiding frequency, volume, and diurnal incontinence completed by their parents. A 72-h voiding diary was then obtained, and a 12-h pad test was used to measure urinary loss at home. Parents were found to underestimate incontinence and to overestimate frequency when answering the questionnaire. The 12-h pad test provided quantitative data but had poor sensitivity (64 %) in those with incontinence, because it missed wetting in 36 % of children. Specificity for those without incontinence was 91 % (Bael et al. 2007).
Fifty children (39 females, mean age 9 years, range 6–14) with urinary incontinence had pad testing done in a 2-h clinic setting and at home. For clinic testing, an oral fluid load 13 mL/kg was given over 15 min and then various exercises were done. The 12-h pad testing was used for home, changing pads every hour, with or without a similar fluid provocation. An increase in pad weight of 0.5 g was considered “significant urine leak.” Results were as folows: 35/50 (70 %) had a positive clinic test, 34 (68 %) a positive home test, and 16/20 (80 %) a positive provocative fluid intake home test, with eight positive only in the clinic and eight positive only at home. The authors concluded clinic testing was not needed (Hellstrom et al. 1986).
Uroflowometry
One prospective trial reported uroflow patterns did not correlate with clinical diagnosis of either urge syndrome or dysfunctional voiding.
Several studies report uroflow parameter ( Q max , curve shape) improved with treatment, but did not consistently correlate with clinical outcomes.
One study found uroflow pattern (bell-shaped vs. other) did not predict past history or future development of UTI.
The EBDS included 97 children with clinically diagnosed urge syndrome and 105 with clinically diagnosed dysfunctional voiding. Uroflowometry was described by patterns as “normal bell-shaped,” “steep tower-shaped,” or “staccato or fractionated.” Voided volumes were not reported, nor was the minimum volume for an acceptable test. Among 66 patients with urge syndrome, 29 % had bell, 20 % had tower, and 51 % had staccato or fractionated patterns. Patterns for 78 patients with dysfunctional voiding were 18 % bell, 6 % tower, and 76 % staccato or fractionated. Patterns for neither condition predicted clinical results of therapy (Bael et al. 2008b).
A prospective trial included 86 children referred for dysfunctional voiding after failure of timed voiding and constipation management for 3 months. Inclusion criteria were symptoms (incontinence, straining, intermittent and/or weak stream) and three consecutive staccato and/or interrupted uroflows with positive EMG activity during voiding. Following therapy, urinary incontinence stopped in 22 and persisted in 20 who had post-treatment uroflowometry; 18/22 (82 %) cured had a bell-shaped curve versus 5/20 (25 %) not cured, p < 0.001. Staccato/interrupted curve persisted in 3/22 (14 %) cured versus 7/20 (35 %) still incontinent, p = 0.15 (Vesna et al. 2010).
A retrospective review was done in 81 consecutive patients, 80 % female, mean age 8 years (4–17) treated with biofeedback for voiding dysfunction with incontinence and/or recurrent UTI. Baseline uroflows were obtained, but timing for uroflow during or after treatment was not stated. There was significant improvement in Q max, decrease in PVR, and normalization of uroflow curves, but no significant differences when patients were stratified as cured or unchanged (Nelson et al. 2004).
A retrospective review of 23 children with dysfunctional voiding treated with tamsulosin obtained baseline uroflowometry with two-thirds bladder capacity and repeated the study at 1 month on therapy. Mean Q max, mean voided volume, and mean PVR all showed significant improvement, while the proportion with a non-bell-shaped curve decreased from 100 to 50 %. Results were only presented as means for the study group, without dividing them according to responders versus nonresponders (Vanderbrink et al. 2009).
A review of 148 consecutive toilet-trained patients, 69 % female, mean age 9 years (4–18) with voiding dysfunction analyzed uroflow patterns (bell-shaped vs. other) and recurrent UTI. Of 78 baseline studies, 61 (78 %) were abnormal. No correlation was seen in abnormal pattern and the number of prior or subsequent UTIs (Shaikh et al. 2005).
Bladder Wall Thickness/Post-void Residual
There is no consensus regarding the ultrasound (US) method to measure bladder wall thickness, for example, with the bladder nearly empty versus filled. Clinical usefulness was limited in one study by overlap in normal and abnormal patients.
One study found that post-void residual (PVR) >10 % bladder capacity for age (1998 ICCS definition of abnormal) increased risk for recurrent UTI, but was not strongly predictive, as 50 % of patients with residuals did not have infection.
Nomograms were developed from observations in 3,376 consecutive children undergoing US for non-urologic indications, from which bladder volume wall thickness index values were calculated. Bladder wall thickness was measured in the transverse plane with the bladder nearly empty (after voiding), averaging anteriorlateral, posteriorlateral, and lateral measurements. A trend of increasing thickness for increasing age was noted (Leung et al. 2007).
A retrospective study evaluated 139 children (69 % girls, 7 months to 16 years) who underwent UD for various indications and compared US measurements of bladder wall thickness at maximum UD-determined capacity. Bladder wall thickness (mm) varied according to diagnostic category defined by 1998 ICCS criteria: normal UD 1.3 ± 0.5 in 46 children; urge syndrome 2.0 ± 0.7 in 52 children; dysfunctional voiding 2.6 ± 0.5 in 33 children; and lazy bladder (bladder underactivity) 0.9 ± 0.1 in 4 children, p < 0.05 for all mean comparisons except normal versus underactive bladder. However, there was wide overlap in the bladder wall thickness between groups such that a cutoff value was not possible (Cvitkovic-Kuzmic et al. 2002).
The retrospective review by Shaikh et al. (2005) mentioned above analyzed elevated PVR (>10 % bladder capacity for age) with number of UTIs. Fifteen percent had elevated PVR, with 9/18 having subsequent UTI during follow-up at a mean of 19 months (7–30). There was a positive correlation between PVR and the number of subsequent UTIs (r = 0.3, p < 0.002), even when controlling for female gender, VUR, and UTIs prior to study. However, the risk for children to develop UTI could not be predicted by elevated PVR, as 50 % of those with PVR did not have recurrent infection.
Urodynamics
Cochrane review found no trial comparing clinical versus UD-based diagnosis or treatment in children with incontinence.
The EBDS reported 28 % of children with clinical urge syndrome had either no or “slight” detrusor overactivity by UD, and 7 % of children with a clinical diagnosis of dysfunctional voiding had normal UD patch EMG activity during voiding.
One study reported poor agreement between blinded versus unblinded UD interpretation for detrusor overactivity.
Two studies performing UD in selected referred patients with voiding dysfunctions both reported detrusor overactivity in over 50 % of cases, followed by dysfunctional voiding in ≥25 %, “lazy bladder” in 4 %, and normal in 6–17 %. Neither correlated UD findings to clinical diagnoses.
Cochrane Review was done to determine if treatment for incontinence according to UD-based diagnosis versus clinical diagnosis is more effective. Seven trials were included, with the following findings:
-
No trial included children.
-
There was no evidence that UD improved treatment of adult females; the proportion with persistent incontinence after 1 year, 70 %, was with versus 62 % without UD, RR 1.23 (95 % CI 0.6–2.6).
-
There was discordance regarding usefulness of UD in clinical management. Two studies reported that a patient with UD was more likely to receive drugs, but in three studies they were not more likely to have surgery (Glazener and Lapitan 2012).
The EBDS described above also performed UD in patients clinically diagnosed with urge syndrome and dysfunctional voiding. Detrusor overactivity expected in children with urge syndrome was absent or “slight” in 25/88 (28 %), only occurred “before voiding” in 35 (40 %), and occurred throughout filling in 28 (32 %). Pelvic floor relaxation determined by patch electrodes was considered normal in 6/91 (7 %) children with a clinical diagnosis of voiding dysfunction and reported as incompletely relaxed in another 22 (24 %). The remainder had increased pelvic floor activity during voiding. Inclusion in the trial was based on clinical history rather than UD results (Bael et al. 2008b).
The EBDS also reported agreement in UD interpretation between the unblinded reviewer who performed the study versus a blinded committee of three other reviewers. All UD were performed in the same manner following a consensus 3-day workshop before the study began, and a standardized scoring sheet was used for interpretations. Among 247 UD tracings, concordance for detrusor overactivity during filling was 37 %. Concordance for pelvic floor relaxation was greater at 81 %. The authors concluded investigator bias in UD interpretation could only be overcome with blinded reviews (Bael et al. 2009).
UD was done in 366 of more than 500 referred patients with dysfunctional voiding symptoms, excluding those “too young, those who refused, and those with infrequent voiding responding to behavioral modification.” Patients had diurnal incontinence ± enuresis and/or recurrent UTI. UD diagnoses were detrusor overactivity in 52 %, voiding dysfunction (bladder/sphincter dysfunction) in 25 %, normal in 17 %, and lazy bladder in 4 % (despite exclusion of these). The authors did not correlate UD findings to clinical symptoms (Schulman et al. 1999).
A subsequent study performed UD in 1,000 children, 48 % female, with “history of UTI, small bladder capacity despite urotherapy, repeated dysfunctional uroflow, US abnormalities, or resistance to therapy,” from a population of 3,500 patients seen for incontinence. UD diagnosis was urge syndrome (detrusor overactivity) in 58 %, dysfunctional voiding in 32 %, lazy bladder (voiding postponement) in 4 %, and normal in 6 %, which also were not correlated to clinical symptoms (Hoebeke et al. 2000).
KUB
Systematic review did not support use of KUB to assist in the diagnosis of constipation.
One study reported poor interrater reliability using three fecal loading scoring systems (Barr, Blethyn, Leech).
Another trial found Blethyn and Leech scores did not correlate to clinical symptoms before or after polyethylene glycol treatment.
Systematic review was done to determine the association between KUB and signs and symptoms of constipation, limited to controlled observational studies in children 1–18 years old. Six articles were included, of which only one was designed to determine whether clinical variables identified patients with radiologically diagnosed constipation. Findings included the following:
-
One study found a clinical diagnosis existed 1.2× more often than the radiologic diagnosis, LR 1.2 (95 % CI 1.0–1.4).
-
Four studies examined whether KUB could discriminate children with versus without clinical symptoms of constipation, with only one reporting significant discriminative value, LR 3.0 (95 % CI 1.6–4.3).
-
All but one study reported moderate to excellent interobserver reliability (K 0.63–0.95); intraobserver reliability reported by three studies was moderate to excellent (K 0.52–0.85).
-
There was conflicting evidence for the correlation of radiologic versus clinical diagnosis of constipation, meaning there was no evidence supporting use of KUB in the case of doubt for constipation in a child (Reuchlin-Vroklage et al. 2005).
Another study had two pediatric urologists, a pediatric radiologist, and three pediatric nurse practitioners score KUBs from children 4–12 years of age who had “lower urinary tract dysfunction symptoms” and age-matched controls with radiographs after foreign-body ingestion, using three methods: Barr, Blethyn, and Leech. Interrater reliability was poor to marginally good using all three rating systems (kappa range Barr, 0.049–0.481; Blethyn, 0.045–0.451; Leech, 0.119–0.273; with kappa values of >0.75 indicating excellent, 0.4–0.75 good, and <0.4 poor reliability). Similarly, intraclass correlations (which describe the reproducibility of measurements made by different observers rating the same image) were poor for all three rating systems (Barr, 0.026; Blethyn, 0.201; and Leech, 0.331). None of the scoring systems provided reliable results between observers in grading constipation (Moylan et al. 2010).
One RCT used KUB scored using Leech and Blethyn systems in 138 children mean age 7 years, 62 % female, with OAB. Baseline urinary and bowel symptom questionnaires were used, with minimal bowel symptoms reported, yet 61(47 %) had KUB-defined constipation using either scale. There was no correlation of urinary urge and bowel symptoms. Patients were then randomized to placebo versus polyethylene glycol for 1 month. Complete response to urge symptoms occurred in 11/71(15 %) completers, and overall 45 % had improvement (>20 % decrease in urge symptoms) in both cohorts, but KUB scores did not change (Bush et al. 2012).
Ultrasound-Determined Rectal Diameter
Three studies reported constipated children have a larger transverse rectal ampulla diameter on pelvic US than normal controls.
Two suggesting a 3 cm diameter as the threshold for constipation stated that 25–44 % of constipated and 4–25 % of normal children would be misdiagnosed by this criteria.
One study compared rectal diameter behind the bladder in 82 normal children (no history of constipation), median age 5.5 years, and 95 patients, median age 6.5 years, with chronic constipation for at least 6 months. Median rectal diameter in controls was 2.4 cm (1.3–4.2) versus 3.4 cm (2.1–7) in patients, p < 0.001. Age was a significant confounder; the older the child, the larger the rectal diameter, but no adjustment values were described. The authors recommended 3 cm as the threshold to diagnose rectal distention, although use of that cutoff would result in mis-classification of 25 % of both controls and patients (Singh et al. 2005).
A second study compared 120 children, mean age 6 years (1.5–18) with constipation by Rome II criteria to 105 controls, mean age 8 years, with “a normal defecation pattern.” Results were reported as the ratio between the diameter of the rectal ampulla and pelvic width (distance between the anterior superior iliac spines). There were significantly greater ratios in patients than controls when analyzed by age groups <3, 3–6, 6–12, and >12 years. To compare to other publications, the authors added that patients have a mean 4.3 cm (3–8.2 cm) rectal diameter (Bijos et al. 2007).
Another study included 51 children ages 4–12 years, 27 having constipation according to Rome III criteria and the other 24 considered normal. US was done using a 7.5-MHz probe to measure the rectal diameter. Constipated children had a larger diameter than controls, 4 ± 1 cm versus 2 ± 0.6 cm, p < 0.001. When normal was considered the mean +2 SD of controls, a cutoff value for constipation was 3 cm, the authors noting that this would misdiagnose 12/27 (44 %) patients and 1/24 (4 %) controls. Disimpaction and 4 weeks of polyethylene glycol significantly reduced rectal diameter in patients to mean 3 ± 0.5 cm, p < 0.001 (Joensson et al. 2008).
Spinal Cord MRI
Less than 10 % of imaged patients with normal neurologic examinations, no sacral skin lesions, and persistent incontinence have abnormal MRI findings (tethered cord, thick filum, syrinx, lipoma).
A prospective trial that enrolled 176 children with functional urinary and bowel problems (enuresis, diurnal incontinence, urinary frequency, UTI, VUR, chronic constipation, encopresis) referred to a center after unsuccessful management—88 with spina bifida occulta on KUB and the other 88 age- and gender-matched controls. None had known neurologic conditions. MRI was abnormal in 12 (7 %) with tethered cord (n = 5), syrinx (n = 4), club-shaped conus (n = 2), and thick filum (n = 1); there was no difference in abnormal findings in those with versus those without spina bifida occulta (Nejat et al. 2008).
Another prospective study included 114 children, median age 9 years (5–14), with UTI and/or voiding dysfunction symptoms. All had 6 months of therapy, mostly AC, with resolution of voiding symptoms in 46 %, none of which had abnormal sacral skin lesions. The other 61 who did not respond had MRI, and included 19 (31 %) with sacral skin lesions, including “macula, lipoma, dimple, or hyertrichosis”; 7/19 (36 %) with skin lesions and 2/42 (5 %) without skin lesions had abnormal MRI with tethered cord (n = 8), lipoma (n = 2), and diastematomyelia (n = 1) (Tarcan et al. 2012).
A retrospective study included 23 children, 3–17 years of age, with persistent incontinence despite treatment a mean of 29 months with timed voiding, anticholinergics, and constipation management. Six also had intermittent back or leg pain, but all had normal neurologic examinations. MRI was normal in 21 (91 %), showed a thoracic spine syrinx in one, and spinal cord tethering in one (Ritchey et al. 1994).
A retrospective study of 456 patients with day and night incontinence revealed spina bifida occulta in 48 children among the subset of 127 (28 %) who underwent X-rays of the spine. The outcomes of those with known spina bifida occulta were compared to the 79 children with normal spine X-rays during mean follow-up of 3 years in both groups. Most patients resolved the incontinence with timed voiding ± AC, with similar results between groups. Of the 48 with spina bifida occulta, MRI or US of the spine was performed in ten, and lipoma with tethered cord was found in one (Ritchey et al. 1994).
Associated Conditions
One study reported that a history of fUTI at age <2 years did not increase likelihood for dysfunctional elimination after toilet training, which occurred in 22 %. There was no difference in prevalence of dysfunctional elimination in those with versus those without VUR.
One study using UD-based categorization of voiding dysfunction reported 34 % of all patients had a history of UTI and 14 % had VUR.
UTI/VUR (See Also Chap. 2)
Dysfunctional elimination, variously defined, was reported in from 14 to 51 % of children with VUR and 34–43 % with UTI.
One study found no difference in prevalence of dysfunctional voiding in patients with versus those without a prior history of UTI before age 2.
Children enrolled in the European branch of the International Reflux Study in Children underwent a questionnaire of voiding symptoms. Among these 310 children, all of whom had grade III or IV VUR, 255 (82 %) were considered to have normal bladder/sphincter function, with 26 (8 %) exhibiting symptoms of OAB, 4 (1 %) staccato voiding, 14 (5 %) incomplete voiding, 3 (1 %) voiding postponement, and 8 (3 %) “unclassifiable” voiding dysfunction by blinded review of the questionnaires by two experienced pediatric nephrologists. However, exclusion criteria for the study included “overt dysfunctional voiding” (van Gool et al. 1992).
One study collected prospective data for 2,759 consecutive children who underwent VCUG. “Dysfunctional elimination syndrome” (DES) was defined as daytime wetting beyond 3.5 years for girls/4.0 years for boys, or three of six criteria: thick-walled bladder on US, trabeculated bladder on VCUG, >30 % bladder capacity for age on VCUG, spinning top deformity on VCUG, Vincent’s curtsy, and/or voiding <3×/day. They reported the following:
-
DES among 32 % of children with VUR, indicating most VUR patients did not have DES.
-
DES occurred in 43 % of children with UTI (not further defined), also indicating most UTI patients did not have DES (Chen et al., 2004).
The study of 1,000 UD by Hoebeke et al. (2000) described the above reported history of UTI (not defined) in 34 %, and VUR in 14 %. Among subgroups defined by ICCS criteria, VUR was identified in 88/582 (15 %) patients with OAB, 46/316 (15 %) with dysfunctional voiding, and 4/40 (10 %) with underactive bladder.
A prospective study evaluated 143 children, 73 % female, age not stated, who had VUR for “dysfunctional elimination syndrome” (DES): bladder instability (urge ± incontinence), infrequent voiding (<4×/day), and/or constipation (≤3×/week, encopresis, or stool on X-ray). Sixty-six (46 %) had DES, including OAB in 18, infrequent voiding in 15, and constipation in 33 (Koff et al. 1998).
A retrospective study evaluated 128 children, 84 % female, 3–10 years old, with VUR in whom history for voiding dysfunction was systematically obtained. Symptoms of frequency, urgency, holding maneuvers, and/or diurnal incontinence for at least 6 months were diagnosed with voiding dysfunction. Of females with a history of UTI (characterized as 1–2 or ≥3 in the preceding 12 months; febrile vs. non-febrile not stated), 38/94 (40 %) had voiding dysfunction. Of 128 children with VUR, 66 (51 %) had voiding dysfunction (Snodgrass 1998).
The dysfunctional voiding symptom score questionnaire (described above) was administered to 123 children with a history of fUTI before age 2 years and 125 controls without UTI, with no difference in the two cohorts based on gender, age (mean 7 years), or ethnicity. Dysfunctional elimination was diagnosed by a score >6 in females and >9 in males. Symptoms of dysfunctional voiding did not differ between the two groups; they were present in 22 % with and 21 % without UTI. Among patients with UTI history, there was no difference in dysfunctional elimination in those with versus those without VUR (18 % with vs. 25 %, p = 0.52) (Shaikh et al. 2003).
Therapy
Placebo/Timed Voiding/Urotherapy
One study reported that 45 % of children with OAB given placebo had significant symptom reduction. Cochrane meta-analysis found approximately 40 % symptom improvement among adults with OAB taking placebo.
One RCT found children using a daytime alarm watch to have significantly greater resolution of diurnal incontinence than those with timed voiding.
Another trial reported no benefit (to reduce recurrent UTI or incontinence) in urotherapy plus uroflowmetry to produce bell-shaped voiding patterns over urotherapy alone.
Cochrane meta-analysis of adults assigned to placebo versus anticholinergics for symptoms of OAB found that 41 % of patients assigned placebo reported cure or improvement in symptoms (Nabi et al. 2006).
One double-blinded RCT randomized children with OAB to 1 month of placebo versus polyethylene glycol with no discussion of voiding modification. Urinary symptom questionnaires modified from DVSS for OAB were administered at baseline and repeated at 1 month, with improvement defined as ≥20 % decrease in score. Symptom improvement occurred in 17/38 (45 %) given placebo, including complete symptom resolution in five (Bush et al. 2012).
Our review found no RCT comparing no treatment to timed voiding.
A RCT enrolled 60 children at mean age 7 years with diurnal urge incontinence and OAB without constipation (Rome III criteria) to first undergo “standard urotherapy” for 1 month (>1,200 mL fluid equally distributed through the day, timed voiding every 2 h). Forty-eight hour voiding diaries confirmed frequency and incontinence. Ninety-five percent of patients had previously failed urotherapy (not described). Two had a complete response (no diurnal incontinence); the remainder were randomized to continue standard urotherapy versus standard urotherapy using an alarm watch for timed voiding for 12 additional weeks. Using the alarm watch, 9/30 (30 %) had a complete response versus 0/28 using standard time voiding, p = 0.002 (Hagstroem et al. 2010).
Another trial included 143 children at mean age 8 years with dysfunctional voiding who had “clear peaks and declines” in two uroflows with ≥100 cm3, PVR ≥10 %, and recurrent UTI (not defined). These were allocated to three treatment groups for 2 months: “standard treatment” (education, “proper voiding pattern,” “good toilet positioning”) standard treatment with video instructions (reemphasizing voiding pattern and toilet positioning, watched daily), and standard treatment with uroflowometry (and instructions to produce a bell-shaped curve 4× daily). All received antibiotic prophylaxis for 24 weeks. The endpoint was no infections and no incontinence at 52 weeks. There was no difference in results between the groups; there were no further UTIs in 71/130 (55 %) and no incontinence in 66/95 (69 %) (Klijn et al. 2006).
Biofeedback
One RCT reported no benefit to biofeedback using pelvic floor EMG during voiding versus pelvic floor contraction/relaxation exercises alone.
Another found no benefit to urotherapy plus biofeedback over urotherapy plus placebo.
A RCT recruited patients from a dysfunctional voiding clinic with incontinence who failed prior treatment in their clinic with timed voiding, “hydration, proper hygiene and constipation management,” and, in some cases, AC. Fifty-six children, 66 % female, ages 6–15 years (mean 10), were allocated to pelvic floor contraction/relaxation exercises versus biofeedback plus pelvic floor exercises (generating an EMG tracing). Patients were evaluated at 1, 6, and 12 months for reduction in incontinent episodes, cure being defined as no incontinence over 4 weeks. There was no difference in outcomes between the two groups, with cures noted at 1 and 12 months in 43 % and 71 % with exercises alone versus 30 % and 75 % with additional biofeedback (Vasconcelos et al. 2006).
The EBDS described above identified 97 children with clinically diagnosed urge syndrome and incontinence who all then received urotherapy (education, adequate fluid intake, voiding diaries, proper voiding posture, and personal hygiene). In addition, patients were randomized to biofeedback (12 sessions of uroflow/EMG) versus double blinded pharmacotherapy using placebo or oxybutynin (0.3 mg/kg/day). There were outcomes data at 6 months for 64 patients, reported before medication cohorts were unblinded. Cure was defined as no incontinence, and did not significantly vary by therapy. Nine of 23 (39 %) with biofeedback, and 14/20 (70 %) and 9/21 (43 %) with either placebo or oxybutynin were cured, indicating a placebo effect (or spontaneous resolution) of at least 43 % and no additional benefit to biofeedback (Misselwitz et al. 1999).
Anticholinergics
One placebo-controlled trial in children with urge syndrome reported cure in 43 % only receiving urotherapy.
Cochrane review of OAB in adults also reported a 42 % placebo response for cure or improvement.
One trial comparing urotherapy, placebo, and tolterodine found significant decrease in DVSS scores with both tolterodine and placebo.
The study by Misselwitz et al. (1999) described above had not unblinded placebo versus AC treatment groups at time of reporting (both receiving urotherapy). Fourteen of 20 (70 %) and 9/21 (43 %) with either placebo or oxybutynin were cured at 6-month follow-up.
Another trial included 71 children (49 % females), ages 4–12 years (mean 8), with voiding dysfunction defined as “incontinence, frequency, urgency or obstructive symptoms ± non-febrile UTI, with PVR < 20 mL” who scored >6 (females) or >9 (males) on the DVSS (described above). All were trained in timed voiding, double voiding, and pelvic floor relaxation during voiding. Patients were then randomized to no additional treatment, placebo, or tolterodine 1 mg 2× daily with repeated DVSS scoring at 1 and 3 months. Outcomes were determined using only 4 of the 10 DVSS questions that the authors considered relevant to OAB, and reported the decrease in mean scores was significantly greater with tolterodine (9 vs. 3.5, p < 0.001) than placebo (9 vs. 5, p < 0.05) or behavioral modification (8 vs. 7). There was no change from baseline in those with urotherapy, but a significant reduction also occurred with placebo (Ayan et al. 2005).
Cochrane review of AC for OAB treatment in adults analyzed 13 studies and 1,770 patients typically treated for 3–12 weeks. Symptomatic improvement during treatment was greater with AC versus bladder training (gradual increase in intervals between voiding, voiding diaries), RR 0.73 (95 % CI 0.59–0.90), and with AC + bladder training versus bladder training alone, RR 0.55 (95 % CI 0.32–0.93) (Alhasso et al. 2006).
Another Cochrane review considered AC versus placebo for OAB in adults, analyzing 61 trials and 11,956 patients. Cure or improvement, difference in 24-h incontinent episodes and number of voids in 24 h all favored AC. However, placebo response was 42 % (cure or improvement), with AC giving an additional 15 % response with number needed to treat of seven (Nabi et al. 2006).
Alpha-Adrenergic Blockers
One retrospective study reported decreased frequency and incontinence with tamsulosin, while one RCT reported a nonsignificant reduction in incontinence versus placebo.
A RCT evaluated doxazosin versus placebo for 1 month as primary therapy for voiding dysfunction (diurnal incontinence with frequency and urgency) in 28 children 5–16 years old. There was a nonsignificant decrease in median incontinent episodes/week with doxazosin (18–4 vs. 14 in placebo, p = 0.13) (Kramer et al. 2005).
A retrospective study reviewed 23 children mean age 9 years (5–16), 52 % females, with dysfunctional voiding (not defined) refractory to 3-h timed voiding and “bowel hygiene” who received tamsulosin (0.2–0.4 mg nightly). All had a non-bell-shaped uroflow pattern and pelvic floor EMG without “excessive” activity during voiding. Three-day voiding diaries were completed before therapy and at ≤4 weeks follow-up while on medication. Mean number of voids and incontinent episodes decreased from baseline 11.5 ± 7.6 and 5.6 ± 1.9 to 7 ± 1 and 0.8 ± 1, p < 0.05 (Vanderbrink et al. 2009).
Nerve Stimulation
One RCT using TENS reported no cure and partial response in 60 % of children with refractory urge incontinence.
One trial using Interstim reported complete resolution of diurnal incontinence in 75 %, but complete relief of all symptoms without medication in 25 %.
A RCT enrolled 25 children, 60 % females, 5–14 years old (mean 9), with diurnal urge incontinence at least 2×/week refractory to urotherapy (hydration and timed voiding), alarm timed voiding, and AC, and randomized them to sacral TENS versus sham for 4 weeks. All had pretreatment UD demonstrating detrusor overactivity. No child had complete cure of incontinence. Partial response occurred in 61 % of TENS versus 17 % of sham TENS patients, p < 0.05 (Hagstroem et al. 2009).
A cohort of 20 patients, 75 % female, mean age 11 years (8–17) had sacral nerve stimulation (Interstim) after failure of prior medical therapies. Of these, 16 had diurnal incontinence and at follow-up at a median of 27 months, 12 (75 %) reported complete resolution. Urgency in 13 children completely resolved in 9 (69 %). However, complete resolution of all symptoms (day and night incontinence, urgency, frequency, constipation) only occurred in five patients (25 %), and only six were able to stop all other medications. Two patients had deactivation without recurrent symptoms, while three others had prompt recurrent symptoms and 18 continued with the device (Roth et al. 2008).
Botulinum A Toxin Injection
Complete resolution of OAB in three trials using botulinum A injections occurred in approximately 40–60 %, durable to at least 6 months.
A prospective study identified 21 children, 52 % females, mean age 11 years (8–14), with OAB and UD-demonstrated detrusor overactivity refractory to AC and urotherapy. These were injected with 100 μ Botox (Allergan, Irvine, CA, USA) at 15 sites sparing the trigone. There was follow-up in 15 at 6 months, with 9 (60 %) having a complete response (no incontinence or urgency). Eight had continued complete response at 12 months. Side effects included urinary retention for 10 days in one, flank pain with voiding for 2 weeks in one, and UTI in two (Hoebeke et al. 2006).
Another prospective trial included 13 children (8 females) at median age 11 years (7–19) with urge incontinence refractory to timed voiding and AC. Twelve had detrusor overactivity on UD pretreatment. Primary injections used 50–100 IU botulinum A at 20–30 sites, sparing the trigone. Complete response was no incontinence reported at follow-up at a mean of 1.5 months later (0.3–2.8), which occurred in five (38 %) and persisted at 6 months in 30 % and 12 months in 33 % (Lahdes-Vasama et al. 2011).
A retrospective review included 57 children, 39 % female, with OAB who failed AC or had side effects precluding further treatment. Injection was done using 12 IU/kg Dysport (Ipsen, Paris, France) to maximum of 480 IU. Complete response (no voiding symptoms and no incontinence) was achieved in 38 (67 %) with mean duration of 6 months (2–18). No side effects were reported (McDowell et al. 2012).
Constipation Management
One RCT reported 45 % of patients had improvement in urge syndrome symptoms with placebo, and no added benefit to polyethylene glycol.
One study of patients referred to an encopresis clinic, mostly males, reported a significant decrease in diurnal incontinence with successful stool management (≥3 stools/week without soiling), but a significant reduction in urinary symptoms also occurred in those with persistent constipation.
Prospective assessment was done for voiding symptoms in 234 consecutive children, mean age 9 years (5–18), 25 % female, with constipation and encopresis referred to a university encopresis clinic. Day incontinence was diagnosed if occurring ≥1 episode/week, and was found in 46 %. Treatment included education, disimpaction, high-fiber diet, timed toilet sitting, daily laxatives (mostly milk of magnesia), and stooling diaries continued for “several months” and then decreased to maintain daily stooling without soiling. Follow-up evaluation was at ≥12 months (mean 15), mostly by a questionnaire mailed to families that included questions regarding urinary incontinence. Constipation was rated successfully treated if a patient had ≥3 stools weekly without soiling. Daytime incontinence significantly decreased in those with successful constipation treatment from 28/121 (23 %) to 3/121 (2 %) and in those without successful bowel management from 36/113 (32 %) to 14/113 (12 %) (Loening-Baucke 1997).
A RCT assumed constipation diagnosis might be inaccurate in children presenting with urge syndrome, and so randomized all patients to initial therapy by polyethylene glycol versus placebo for 1 month. The endpoint was ≥20 % reduction in symptom score using a modified DVSS (for urge syndrome). Among 71 completers, improvement occurred in 45 % assigned to placebo and 48.5 % receiving medication, demonstrating no added benefit to polyethylene glycol in either intention to treat (p = 0.77) or per-protocol (p = 0.84) analysis (Bush et al. 2012).
Impact of Voiding Dysfunction on Behavior
Children with urge syndrome, dysfunctional voiding, and voiding postponement have been found to have increased behavioral problems.
Two studies found that severity of bladder or bowel dysfunction did not correlate with quality of life in one study.
One quality-of-life assessment reported children do not report less self-esteem or quality of life versus controls, but their parents score them lower.
One study reported that treatment for dysfunctional voiding, but not urge syndrome, resulted in improved behavior. Presence of behavior problems did not reduce treatment efficacy.
Another study found improved self-esteem in children following treatment for day and/or night urinary incontinence.
A 2011 ICCS consensus statement recommends screening all incontinent children for psychological symptoms with validated questionnaires such as the Child Behavior Checklist or the Short Screening Instrument for Psychological Problems in Enuresis (von Gontard et al. 2011). No data are available whether such widespread screening would improve either psychological or urological outcomes.
Prospective evaluation was done in 94 incontinent children clinically diagnosed with urge syndrome (n = 42) and with voiding postponement (<5 voids/day, incontinence while watching television or playing) (n = 52) recruited at presentation from pediatric and psychiatric clinics in Germany. Parents answered the Achenback Child Behavioral Checklist and a structured interview was done with the child regarding body image and interpretation of wetting. There were significantly more females with urge syndrome (62 %) and more boys with voiding postponement (65 %), with mean age in both 7 years. Parents scored significantly more behavioral problems in those with voiding postponement (37 vs. 14 %). There were no differences in child responses based on clinical diagnosis, with only 11 % responding they had an illness, although 70 % knew the reason for their evaluation. Thirty percent did not know the origin of urine (von Gontard et al. 1998).
In a subsequent study, consecutive children, 5–13 years old, with urge syndrome (n = 22) or voiding postponement (n = 27) and 32 age-matched controls had health-related quality-of-life and self-esteem assessments using standardized and validated instruments. Thirty percent of children with both conditions also had encopresis, versus none in controls. Patients had significantly more behavioral problems, but there was no difference in quality of life or self-esteem versus controls. However, parents rated quality of life lower in patients than controls, especially those with voiding postponement (Natale et al. 2009).
A study of 138 children with incontinence of various etiologies who were referred to a tertiary clinic completed the PINQ (quality of life) and the Akbal (urinary symptom) questionnaire. Children with non-white race, older age, and female gender had lower quality of life scores on the PINQ; severity of urinary symptoms based on the Akbal score (r = 0.15, p = 0.09) did not correlate with worse quality of life (Deshpande et al. 2011).
Another study in 103 children referred for incontinence using a validated health-related quality-of-life questionnaire showed type and severity of urinary or fecal incontinence was not correlated with total scores, and that overall quality of life among children referred for incontinence were similar to reference samples of children with other chronic conditions such as asthma, cystic fibrosis, and epilepsy (Bachmann et al. 2009).
The EBDS included the Achenbach’s Child Behavioral Checklist (a validated checklist documenting social competence and problem behavior) at enrollment and 12 months after treatment, answered by parents. Of 202 patients, 188 completed forms at entry and 111 at 1 year. Overall, 19 % had abnormal behavioral scores, which after treatment decreased to 11 %, the authors commenting that this approximates prevalence in a normal population. However, this improvement occurred in patients with dysfunctional voiding, whereas those with urge syndrome did not change. Similarly, externalizing problems found in 12 % of children decreased significantly after treatment, but only in those with dysfunctional voiding. Internalizing problems occurred in 16 % overall, with no significant change in either subgroup with therapy. The presence of behavioral problems at entry did not influence treatment success for either urge syndrome or dysfunctional voiding (Bael et al. 2008a).
Sixty-six children identified through school-based questionnaire to have day and/or night wetting scored lower than age-matched controls on a Swedish validated self-esteem scale (12.5 vs. 19.4, p < 0.001). Treatment of the incontinence increased self-esteem levels to that of controls at 6 months (19.5), with scores higher in those who achieved dryness versus those still wetting (23.1 vs. 17.3, p < 0.001) (Hagglof et al. 1998).
References
Afshar K, Mirbagheri A, Scott H, MacNeily AE. Development of a symptom score for dysfunctional elimination syndrome. J Urol. 2009;182(4 Suppl):1939–43.
Akbal C, Genc Y, Burgu B, Ozden E, Tekgul S. Dysfunctional voiding and incontinence scoring system: quantitative evaluation of incontinence symptoms in pediatric population. J Urol. 2005;173(3):969–73.
Alhasso AA, McKinlay J, Patrick K, Stewart L. Anticholinergic drugs versus non-drug active therapies for overactive bladder syndrome in adults. Cochrane Database Syst Rev. 2006;18(4):CD003193.
Ayan S, Kaya K, Topsakal K, Kilicarslan H, Gokce G, Gultekin Y. Efficacy of tolterodine as a first-line treatment for non-neurogenic voiding dysfunction in children. BJU Int. 2005;96(3):411–4.
Bachmann C, Lehr D, Janhsen E, Sambach H, Muehlan H, von Gontard A, et al. Health related quality of life of a tertiary referral center population with urinary incontinence using the DCGM-10 questionnaire. J Urol. 2009;182(4 Suppl):2000–6.
Bael AM, Lax H, Hirche H, Gabel E, Winkler P, Hellstrom AL, et al. Self-reported urinary incontinence, voiding frequency, voided volume and pad-test results: variables in a prospective study in children. BJU Int. 2007;100(3):651–6.
Bael A, Winkler P, Lax H, Hirche H, Gabel E, Vijverberg M, et al. Behavior profiles in children with functional urinary incontinence before and after incontinence treatment. Pediatrics. 2008a;121(5):e1196–200.
Bael A, Lax H, de Jong TP, Hoebeke P, Nijman RJ, Sixt R, et al. The relevance of urodynamic studies for Urge syndrome and dysfunctional voiding: a multicenter controlled trial in children. J Urol. 2008b;180(4):1486–93.
Bael A, Verhulst J, Lax H, Hirche H, van Gool JD. Investigator bias in urodynamic studies for functional urinary incontinence. J Urol. 2009;182(4 Suppl):1949–52.
Benninga M, Candy DC, Catto-Smith AG, Clayden G, Loening-Baucke V, Di Lorenzo C, et al. The Paris consensus on childhood constipation terminology (PACCT) group. J Pediatr Gastroenterol Nutr. 2005;40(3):273–5.
Bijos A, Czerwionka-Szaflarska M, Mazur A, Romanczuk W. The usefulness of ultrasound examination of the bowel as a method of assessment of functional chronic constipation in children. Pediatr Radiol. 2007;37(12):1247–52.
Boccia G, Manguso F, Coccorullo P, Masi P, Pensabene L, Staiano A. Functional defecation disorders in children: PACCT criteria versus Rome II criteria. J Pediatr. 2007;151(4):394–8. 8 e1.
Burgers R, Levin AD, Di Lorenzo C, Dijkgraaf MG, Benninga MA. Functional defecation disorders in children: comparing the Rome II with the Rome III criteria. J Pediatr. 2012;161(4):615–20.e1.
Bush NC, Shah A, Barber T, Yang M, Bernstein I, Snodgrass W. Randomized, double-blind, placebo-controlled trial of polyethylene glycol (MiraLAX((R))) for urinary urge symptoms. J Pediatr Urol. 2012,http://dx.doi.org/10.1016/j.jpurol.2012.10.011.
Chen JJ, Mao W, Homayoon K, Steinhardt GF. A multivariate analysis of dysfunctional elimination syndrome, and its relationships with gender, urinary tract infection and vesicoureteral reflux in children. J Urol. 2004;171(5):1907–10.
Chung JM, Lee SD, Kang DI, Kwon DD, Kim KS, Kim SY, et al. An epidemiologic study of voiding and bowel habits in Korean children: a nationwide multicenter study. Urology. 2010;76(1):215–9.
Cvitkovic-Kuzmic A, Brkljacic B, Ivankovic D, Grga A. Ultrasound assessment of detrusor muscle thickness in children with non-neuropathic bladder/sphincter dysfunction. Eur Urol. 2002;41(2):214–8.
Deshpande AV, Craig JC, Smith GH, Caldwell PH. Factors influencing quality of life in children with urinary incontinence. J Urol. 2011;186(3):1048–52.
Farhat W, Bagli DJ, Capolicchio G, O’Reilly S, Merguerian PA, Khoury A, et al. The dysfunctional voiding scoring system: quantitative standardization of dysfunctional voiding symptoms in children. J Urol. 2000;164(3 Pt 2):1011–5.
Farhat W, McLorie GA, O’Reilly S, Khoury A, Bagli DJ. Reliability of the pediatric dysfunctional voiding symptom score in monitoring response to behavioral modification. Can J Urol. 2001;8(6):1401–5.
Glazener CM, Lapitan MC. Urodynamic studies for management of urinary incontinence in children and adults. Cochrane Database Syst Rev. 2012;1:CD003195.
Hagglof B, Andren O, Bergstrom E, Marklund L, Wendelius M. Self-esteem in children with nocturnal enuresis and urinary incontinence: improvement of self-esteem after treatment. Eur Urol. 1998;33 Suppl 3Suppl 3:16–9.
Hagstroem S, Mahler B, Madsen B, Djurhuus JC, Rittig S. Transcutaneous electrical nerve stimulation for refractory daytime urinary urge incontinence. J Urol. 2009;182(4 Suppl):2072–8.
Hagstroem S, Rittig S, Kamperis K, Djurhuus JC. Timer watch assisted urotherapy in children: a randomized controlled trial. J Urol. 2010;184(4):1482–8.
Hellstrom AL, Andersson K, Hjalmas K, Jodal U. Pad tests in children with incontinence. Scand J Urol Nephrol. 1986;20(1):47–50.
Hellstrom AL, Hanson E, Hansson S, Hjalmas K, Jodal U. Micturition habits and incontinence in 7-year-old Swedish school entrants. Eur J Pediatr. 1990;149(6):434–7.
Hinman Jr F. Nonneurogenic neurogenic bladder (the Hinman syndrome)–15 years later. J Urol. 1986;136(4):769–77.
Hoebeke P, De Kuyper P, Goeminne H, Van Laecke E, Everaert K. Bladder neck closure for treating pediatric incontinence. Eur Urol. 2000;38(4):453–6.
Hoebeke P, De Caestecker K, Vande Walle J, Dehoorne J, Raes A, Verleyen P, et al. The effect of botulinum-A toxin in incontinent children with therapy resistant overactive detrusor. J Urol. 2006;176(1):328–30.
Hoebeke P, Bower W, Combs A, De Jong T, Yang S. Diagnostic evaluation of children with daytime incontinence. J Urol. 2010;183(2):699–703.
Joensson IM, Siggaard C, Rittig S, Hagstroem S, Djurhuus JC. Transabdominal ultrasound of rectum as a diagnostic tool in childhood constipation. J Urol. 2008;179(5):1997–2002.
Kajiwara M, Inoue K, Kato M, Usui A, Kurihara M, Usui T. Nocturnal enuresis and overactive bladder in children: an epidemiological study. Int J Urol. 2006;13(1):36–41.
Klijn AJ, Uiterwaal CS, Vijverberg MA, Winkler PL, Dik P, de Jong TP. Home uroflowmetry biofeedback in behavioral training for dysfunctional voiding in school-age children: a randomized controlled study. J Urol. 2006;175(6):2263–8.
Koff SA, Wagner TT, Jayanthi VR. The relationship among dysfunctional elimination syndromes, primary vesicoureteral reflux and urinary tract infections in children. J Urol. 1998;160(3 Pt 2):1019–22.
Kramer SA, Rathbun SR, Elkins D, Karnes RJ, Husmann DA. Double-blind placebo controlled study of alpha-adrenergic receptor antagonists (doxazosin) for treatment of voiding dysfunction in the pediatric population. J Urol. 2005;173(6):2121–4.
Lahdes-Vasama TT, Anttila A, Wahl E, Taskinen S. Urodynamic assessment of children treated with botulinum toxin A injections for urge incontinence: a pilot study. Scand J Urol Nephrol. 2011;45(6):397–400.
Leung VY, Chu WC, Yeung CK, Sreedhar B, Liu JX, Wong EM, et al. Nomograms of total renal volume, urinary bladder volume and bladder wall thickness index in 3,376 children with a normal urinary tract. Pediatr Radiol. 2007;37(2):181–8.
Loening-Baucke V. Urinary incontinence and urinary tract infection and their resolution with treatment of chronic constipation of childhood. Pediatrics. 1997;100(2 Pt 1):228–32.
McDowell DT, Noone D, Tareen F, Waldron M, Quinn F. Urinary incontinence in children: botulinum toxin is a safe and effective treatment option. Pediatr Surg Int. 2012;28(3):315–20.
Misselwitz J, Ulrike J, Tamminen-Mobius T, Lax-Gross H, Hirche H, Van Gool J, et al., editors. Oxybutynine-HCI versus placebo in children with urodynamically proven urge syndrome—a placebo-controlled study. Denver, CO: International Continence Society; 1999.
Moylan S, Armstrong J, Diaz-Saldano D, Saker M, Yerkes EB, Lindgren BW. Are abdominal x-rays a reliable way to assess for constipation? J Urol. 2010;184(4 Suppl):1692–8.
Nabi G, Cody JD, Ellis G, Herbison P, Hay-Smith J. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Cochrane Database Syst Rev. 2006;18(4):CD003781.
Natale N, Kuhn S, Siemer S, Stockle M, von Gontard A. Quality of life and self-esteem for children with urinary urge incontinence and voiding postponement. J Urol. 2009;182(2):692–8.
Nejat F, Radmanesh F, Ansari S, Tajik P, Kajbafzadeh A, El KM. Spina bifida occulta: is it a predictor of underlying spinal cord abnormality in patients with lower urinary tract dysfunction? J Neurosurg Pediatr. 2008;1(2):114–7.
Nelson JD, Cooper CS, Boyt MA, Hawtrey CE, Austin JC. Improved uroflow parameters and post-void residual following biofeedback therapy in pediatric patients with dysfunctional voiding does not correspond to outcome. J Urol. 2004;172(4 Pt 2):1653–6.
Nelson CP, Park JM, Bloom DA, Wan J, Dunn RL, Wei JT. Incontinence Symptom Index-Pediatric: development and initial validation of a urinary incontinence instrument for the older pediatric population. J Urol. 2007;178(4 Pt 2):1763–7.
Neveus T, von Gontard A, Hoebeke P, Hjalmas K, Bauer S, Bower W, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children’s Continence Society. J Urol. 2006;176(1):314–24.
Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130(5):1527–37.
Rasquin-Weber A, Hyman PE, Cucchiara S, Fleisher DR, Hyams JS, Milla PJ, et al. Childhood functional gastrointestinal disorders. Gut. 1999;45 Suppl 2Suppl 2:II60–8.
Reuchlin-Vroklage LM, Bierma-Zeinstra S, Benninga MA, Berger MY. Diagnostic value of abdominal radiography in constipated children: a systematic review. Arch Pediatr Adolesc Med. 2005;159(7):671–8.
Ritchey ML, Sinha A, DiPietro MA, Huang C, Flood H, Bloom DA. Significance of spina bifida occulta in children with diurnal enuresis. J Urol. 1994;152(2 Pt 2):815–8.
Roth TJ, Vandersteen DR, Hollatz P, Inman BA, Reinberg YE. Sacral neuromodulation for the dysfunctional elimination syndrome: a single center experience with 20 children. J Urol. 2008;180(1):306–11.
Schneider D, Yamamoto A, Barone JG. Evaluation of consistency between physician clinical impression and 3 validated survey instruments for measuring lower urinary tract symptoms in children. J Urol. 2011;186(1):261–5.
Schulman SL, Quinn CK, Plachter N, Kodman-Jones C. Comprehensive management of dysfunctional voiding. Pediatrics. 1999;103(3):E31.
Shaikh N, Hoberman A, Wise B, Kurs-Lasky M, Kearney D, Naylor S, et al. Dysfunctional elimination syndrome: is it related to urinary tract infection or vesicoureteral reflux diagnosed early in life? Pediatrics. 2003;112(5):1134–7.
Shaikh N, Abedin S, Docimo SG. Can ultrasonography or uroflowmetry predict which children with voiding dysfunction will have recurrent urinary tract infections? J Urol. 2005;174(4 Pt 2):1620–2.
Singh SJ, Gibbons NJ, Vincent MV, Sithole J, Nwokoma NJ, Alagarswami KV. Use of pelvic ultrasound in the diagnosis of megarectum in children with constipation. J Pediatr Surg. 2005;40(12):1941–4.
Snodgrass W. The impact of treated dysfunctional voiding on the nonsurgical management of vesicoureteral reflux. J Urol. 1998;160(5):1823–5.
Sureshkumar P, Jones M, Cumming R, Craig J. A population based study of 2,856 school-age children with urinary incontinence. J Urol. 2009;181(2):808–15.
Swithinbank LV, Heron J, von Gontard A, Abrams P. The natural history of daytime urinary incontinence in children: a large British cohort. Acta Paediatr. 2010;99(7):1031–6.
Tarcan T, Tinay I, Temiz Y, Alpay H, Ozek M, Simsek F. The value of sacral skin lesions in predicting occult spinal dysraphism in children with voiding dysfunction and normal neurological examination. J Pediatr Urol. 2012;8(1):55–8.
van Gool JD, Hjalmas K, Tamminen-Mobius T, Olbing H. Historical clues to the complex of dysfunctional voiding, urinary tract infection and vesicoureteral reflux. The International Reflux Study in Children. J Urol. 1992;148(5 Pt 2):1699–702.
Vanderbrink BA, Gitlin J, Toro S, Palmer LS. Effect of tamsulosin on systemic blood pressure and nonneurogenic dysfunctional voiding in children. J Urol. 2009;181(2):817–22.
Vasconcelos M, Lima E, Caiafa L, Noronha A, Cangussu R, Gomes S, et al. Voiding dysfunction in children. Pelvic-floor exercises or biofeedback therapy: a randomized study. Pediatr Nephrol. 2006;21(12):1858–64.
Vesna Z, Milica L, Marina V, Andjelka S, Lidija D. Correlation between uroflowmetry parameters and treatment outcome in children with dysfunctional voiding. J Pediatr Urol. 2010;6(4):396–402.
von Gontard A, Lettgen B, Olbing H, Heiken-Lowenau C, Gaebel E, Schmitz I. Behavioural problems in children with urge incontinence and voiding postponement: a comparison of a paediatric and child psychiatric sample. Br J Urol. 1998;81 Suppl 3Suppl 3:100–6.
von Gontard A, Baeyens D, Van Hoecke E, Warzak WJ, Bachmann C. Psychological and psychiatric issues in urinary and fecal incontinence. J Urol. 2011;185(4):1432–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Bush, N.C. (2013). Bladder and Bowel Dysfunction. In: Snodgrass, W. (eds) Pediatric Urology. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6910-0_3
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6910-0_3
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6909-4
Online ISBN: 978-1-4614-6910-0
eBook Packages: MedicineMedicine (R0)