Abstract
There is a complex interplay between congenital heart disease and lymphatic obstruction as evidenced by the literature where associations have been reported between lymphatic impairment and congenital heart disease. Turner syndrome is a classic example of a condition where lymphatic impairment might be the cause of some congenital heart diseases. At the same time there are congenital heart diseases which impair the lymphatic flow, leading to significant morbidity and mortality; these include left-to-right shunt lesions and protein-losing enteropathy. Congenital cardiac surgery which involves extensive mediastinal dissection can also cause injury to the lymphatic system. Hence, the lymphatic system must be given importance while managing patients with congenital heart disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Congenital heart disease
- Lymphatic obstruction
- Turner syndrome
- Cardiac surgery
- Protein-losing enteropathy
Introduction
The lymphatic system maintains homeostasis by receiving proteins and excess fluid from the interstitial spaces and returning them to the venous system [1]. Large lymphatic channels travel with the major coronary arteries in the epicardium and small lymphatics can be found in the endocardium [2]. Impairment of cardiac lymphatic flow has been known to be associated with ventricular fibrillation, increased superior vena cava pressures, and pulmonary arterial hypertension [3]. Many congenital heart surgeries involve excision or destruction of the intrathoracic and mediastinal lymphatics, primarily because it is assumed that the mediastinal lymphatic system is surgically expendable. However, obstruction of the cardiac lymphatic obstruction may lead to cardiac dysfunction and cardiac lymph edema [4]. Similarly, pulmonary lymphatic obstruction can cause pulmonary perivascular lymphedema, endothelial injury, and pulmonary arterial obstruction. In this chapter we will address the issue of cardiac lymphatic obstruction and its impact on congenital heart disease and vice versa.
Link Between Lymphatic Obstruction and Congenital Heart Disease
Increased Nuchal Translucency and Congenital Heart Disease
Increased nuchal translucency (NT) has been strongly associated with congenital heart disease [5]. This finding, as often happens, was a by-product of other studies that were primarily concerned with screening for chromosomal abnormalities. While risks for chromosomal abnormalities are adjusted for maternal age and serum biochemistry, risks for congenital heart disease appear to be solely dependent on the degree of NT itself.
Initially increased NT was thought to be associated with chromosomal abnormalities. Later it was found out that in euploid fetuses with increased NT, there is increased incidence of congenital heart diseases, the most common being the narrowing of the aortic isthmus [6]. The incidence of congenital heart disease in fetuses with increased NT and normal karyotype varies with the degree of NT, and approximately one third of fetuses with major congenital heart disease can potentially be identified by NT screening. Increased NT however does not predict the type of cardiac abnormality that may be encountered [5].
The etiology of increased NT has been widely debated with etiologies including cardiac heart disease itself, cardiac failure, and lymphatic system abnormalities. A mesenchyme-lined fluid-filled cavity (edema) was found in the posterior nuchal region together with a bilaterally enlarged jugular lymphatic system (JLS) in mutant mouse models (trisomy 16, equivalent to human trisomy 21) (Fig. 3.1) [7]. The persistent JLS were also seen by ultrasound in a large proportion of human fetuses with increased nuchal thickness. A possible delay in the development of the lymphatic vessels in the neck has been suggested to cause increased NT [8, 9]. The JLS is also the first part of the lymphatic system to develop, and a delay in such development of these vessels would lead to fluid accumulation in the neck region. As the process is only delayed, the fluid is eventually drained away when the JLS is finally able to reconnect to the venous system.
Embryonic mouse. (a) Wild-type mouse embryo, day 14 of development. (b) Trisomy 16 embryo, day 14 of development with increased NT (Reprinted from Haak et al. [7] and used with permission of Elsevier)
Lymphatic Obstruction and Chromosomal Abnormalities
Disruption of the normal lymphatic system has been seen in patients with congenital heart diseases associated with various syndromes such as Turner and Noonan [10–12]. There is increased incidence of congenital heart disease in individuals with Turner syndrome, as well as neck webbing [13], which is formed from the postnatal residua of nuchal cystic hygromas caused by obstructed jugular lymphatics in utero. On the basis of this observation, Clark [13] proposed that centrally localized distended lymphatics compress the developing aortic root, resulting in specific left-sided defects, including hypoplastic left heart, bicuspid aortic valve, and coarctation of the aorta as a result of low flow. There are also specific right-sided defects that can occur, such as persistent left superior vena cava, anomalous pulmonary venous return, and dilated right atrium as a result of back pressure in response to obstructions in forward flow. This view was supported by further epidemiologic observations in a study of 120 infants with neck webbing reported in the Iowa Birth Defects registry, among which 66% were found to have flow-related defects [14]. These observations came from pathology studies which were focused on the most severely affected fetuses, raising the possibility that the association between congenital heart diseases and neck webbing simply reflects the most severe phenotype in 45, X individuals rather than a specific connection between these two phenotypic features of X-chromosome deletion [15].
However, in an observational study of Turner syndrome patients who were not selected for cardiovascular disease, a significant association was made between central fetal lymphedema, signaled by neck webbing, and defects such as bicuspid aortic valve and coarctation of the aorta [15]. The anatomic defects associated with fetal lymphedema in Turner syndrome are decreased numbers of lymphatics and dilated lymphatic channels that end in distended sacs, which lack connections with the venous system. Severe lymphatic obstruction early in fetal development may cause heart failure from compression and/or impaired filling of developing cardiovascular structures, leading to fetal hydrops and demise [16]. Similarly, de Mooij et al. [17] demonstrated that Noonan syndrome fetuses of gestational age 16 + 0 weeks demonstrated nuchal edema and distended JLS with less tissue compared to the control fetuses.
Congenital Heart Disease and Its Effect on Lymphatic System
Congenital and acquired malformations of lymphatic circulation are well known in patients with congenital heart disease [15, 18–20]. Lymphangiectasis has been observed in infants and children with obstructive left-sided lesions, such as hypoplastic left heart syndrome with restrictive atrial septum [21, 22] or total anomalous pulmonary venous return [23]. Patients with lymphatic hypoplasia usually present with lymphoedema. Congenital heart disease is rare in these patients (a 1–4% incidence was described in one series [18]) and no particular cardiac lesion predominates. Similarly, lymphatic hyperplasia was not associated with any particular congenital heart disease, despite the incidence of 9.7% being greater than expected in the general population (0.9%) [19].
Congenital Heart Diseases Associated with Increased Pulmonary Blood Flow
Congenital heart diseases with increased pulmonary blood flow mainly include ventricular septal defects, atrial septal defects, atrioventricular canal defects, and patent ductus arteriosus. These are the most common types of congenital heart diseases. These patients often have significant morbidity which can be attributed to increased lung water, impairment of normal respiratory function, and increased metabolic burden on an already compromised cardiovascular system.
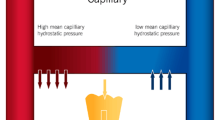
Increased pulmonary blood flow leads to increased capillary filtration of protein-poor fluid into the interstitial space and increased clearance of lymphatic fluid [24]. Reddy et al. [25] created an ovine model of chronically increased pulmonary blood flow by placing a large vascular graft between the aorta and pulmonary artery of a fetal lamb. Following spontaneous delivery, these lambs had increased pulmonary blood flow and demonstrated hemodynamic and morphologic features that mimicked the human disease. Acute increase in pulmonary blood flow can result in alterations in pulmonary vascular endothelial function including disruption in endothelium-dependent nitric oxide (NO) signaling [26].
Chronic increase in pulmonary blood flow leads to lymphatic alterations, including endothelial dysfunction, resulting in decreased lymphatic flow. There is decrease in protein-poor lymph flow which is less than expected for the increased pulmonary blood flow in such lesions [26]. These changes were seen in the ovine model, and they mimic the symptomatology of tachypnea and the failure to thrive seen in children with left-to-right shunt lesions. There is an increase in pulmonary capillary hydrostatic pressure in acute and chronic shunt due to an increase in pulmonary blood flow [26]. There is a decrease in lymphatic nitric oxide production, which could play a role in the perturbation of lymphatic function as well as the postnatal development of lymphatic network [27].
Lymphatic Obstruction and Protein-Losing Enteropathy
Protein-losing enteropathy (PLE) is a relatively uncommon complication of surgical procedures used for palliation of complex congenital heart disease. The relevant lymphatic circulation converges variably, but predictably, upon a discrete location in the central venous system (Fig. 3.2) [28, 29]. Hence, obstruction of the lymphatic system could be considered as one of the etiologies of PLE.
Variations in termination of the thoracic duct. (a) Preterminal branching of thoracic duct and opening near the internal jugular vein. (b) A typical example of termination of thoracic duct into the internal jugular vein. (c) A bifid termination into the vertebral vein. (d) A complicated trifurcated termination draining into the internal jugular vein, subclavian vein, and lateral venous angle. The bronchomediastinal duct is also demonstrated (Redrawn from Langford et al. [28] and Reprinted with permission from Elsevier)
There have been studies demonstrating a link between lymphatic obstruction and congenital heart disease, especially in cases of PLE in patients undergoing Fontan operation [30]. In their retrospective case control study, Meadows et al. [30] found a relatively high prevalence (25%) of lymphatic disruption or central venous obstruction at the site of usual lymphatic drainage in patients with PLE when compared to controls (3%). Lymphatic obstruction was evident by MRI, angiography, or documented surgical thoracic duct ligation. Central venous catheter was not shown to be associated with PLE. This suggested that physical lymphatic obstruction may play an important, and previously unrecognized, role in the development of PLE in patients with complex congenital heart disease.
The lymphatic system in patients with Fontan physiology operates at, or near, its physiologic limit [31]. Elevated central venous pressure coherent to passive circulation in Fontan patients is transmitted to the hepatic and intestinal venous circulation, leading to increased lymph production [32]. At the same time, increased central venous pressure decreased the lymphatic return to central circulation [33, 34]. As a result, the lymphatic system operated at or near its physiological limit. There is subclinical enteric protein loss with rare decompensation to clinical PLE during unpredictable physiologic insults [35].
Lymphatic Obstruction and Left Heart Lesions
Hypoplastic left heart syndrome is one of the most extensively studied lesions, in relation to its effects on the lymphatic system. Data concerning other obstructive lesions is relatively sparse. Luciani et al. [36], in a report of a single patient with congenital pulmonary lymphagiectasis and hypoplastic left heart syndrome with a restrictive atrial septal defect, described the most severe spectrum of the lymphatic abnormalities. Graziono et al. [21] showed that four of the five patients with a restrictive ASD and hypoplastic left heart syndrome demonstrated moderate lymphatic dilatation and 1 patient had severe dilatation. In contrast, four of the five patients with nonrestrictive defects had normally lymphatics and one patient had mildly dilated lymphatics. The physiological explanation involves increased left atrial pressure in the fetal life which transmits to the pulmonary veins and the lymphatics, thus leading to the changes described above.
Cardiac Surgeries and Lymphatic Obstruction
Patients undergoing cardiac surgery often have morbidity and mortality related to pulmonary edema, impairment of normal respiratory function, and increased metabolic demands. Chylothorax is an additional cause of morbidity in patient undergoing cardiac surgery [30]. It may be caused either by injury of the thoracic duct, increased pressure in the systemic veins exceeding that in the thoracic duct, or a central vein thrombosis.
Mehlhorn et al. [37] found that myocardial contraction is the major determinant of myocardial lymph flow and that impairment of such flow during cardioplegic arrest may contribute to postoperative myocardial edema and left ventricular dysfunction. As depicted in Fig. 3.3, cardiopulmonary bypass in children undergoing surgery for congenital heart disease leads to increased microvascular permeability and decreased intravascular colloid osmotic pressure. This results in increased myocardial filtration of the plasma thereby leading to myocardial edema. Myocardial edema thus causes systolic and diastolic dysfunction, further decreasing myocardial lymph flow and resulting in more myocardial edema [38]. As reliable means to detect myocardial edema in the clinical setting are not readily available at the bedside, many clinicians do not include this entity in their differential diagnosis of cardiac dysfunction. Knowledge of the factors involved in myocardial fluid homeostasis may help to develop techniques minimizing myocardial edema formation and may lead to better therapeutic interventions [39].
Role of myocardial edema after cardiopulmonary bypass (Adapted from Nakamura and Rockson [38] by permission of Oxford University Press)
Conduction Disturbances from Cardiac Lymph Flow Impairment
Anatomical studies have demonstrated the intimate relationship between lymphatic vessels and conduction structures in which a single longitudinal lymphatic pathway always runs parallel to the right bundle in animal studies. This suggests that cardiac lymph flow impairment could contribute to conduction disturbances and arrhythmia. Clinical studies have shown that lymphedema is associated with arrhythmia [40]. It has also been proposed that higher resistance in mediastinal lymphatics could be the cause of supraventricular tachycardia as these lymphatics are responsible for draining the atria.
Summary
In conclusion, the cardiac lymphatic system plays an important role in the pathophysiology and management of patients with congenital heart disease. Also there are significant lymphatic alterations in patients who have congenital heart disease, including left-to-right shunt lesions and left heart obstructive lesions. More efforts and research should be designed to utilize this knowledge of the cardiac lymphatic system for better healthcare management of children with congenital heart disease.
References
Casley-Smith JR (1968) How the lymphatic system works. Lymphology 1(3):77–80
Loukas M, Abel N, Tubbs RS, Grabska J, Birungi J, Anderson RH (2011) The cardiac lymphatic system. Clin Anat 24(6):684–691. doi:10.1002/ca.21104;10.1002/ca.21104
Lupinski RW (2009) Aortic fat pad and atrial fibrillation: Cardiac lymphatics revisited. ANZ J Surg 79(1–2):70–74. doi:10.1111/j.1445-2197.2008.04802.x
Cui Y, Urschel JD, Petrelli NJ (2001) The effect of cardiopulmonary lymphatic obstruction on heart and lung function. Thorac Cardiovasc Surg 49(1):35–40. doi:10.1055/s-2001-9917
Carvalho JS (2005) The fetal heart or the lymphatic system or …? the quest for the etiology of increased nuchal translucency. Ultrasound Obstet Gynecol 25(3):215–220. doi:10.1002/uog.1865
Hyett J, Moscoso G, Papapanagiotou G, Perdu M, Nicolaides KH (1996) Abnormalities of the heart and great arteries in chromosomally normal fetuses with increased nuchal translucency thickness at 11–13 weeks of gestation. Ultrasound Obstet Gynecol 7(4):245–250. doi:10.1046/j.1469-0705.1996.07040245.x
Haak MC, Bartelings MM, Jackson DG, Webb S, van Vugt JM, Gittenberger-de Groot AC (2002) Increased nuchal translucency is associated with jugular lymphatic distension. Hum Reprod 17(4):1086–1092
Bekker MN, Haak MC, Rekoert-Hollander M, Twisk J, Van Vugt JM (2005) Increased nuchal translucency and distended jugular lymphatic sacs on first-trimester ultrasound. Ultrasound Obstet Gynecol 25(3):239–245. doi:10.1002/uog.1831
Castelli E, Todros T, Mattutino G, Torre C, Panattoni G (2003) Light and scanning electron microscope study of nuchal translucency in a normal fetus. Ultrasound Obstet Gynecol 21(5):514–516. doi:10.1002/uog.95
Allanson JE (1987) Noonan syndrome. J Med Genet 24(1):9–13
Davenport ML (2010) Approach to the patient with turner syndrome. J Clin Endocrinol Metab 95(4):1487–1495. doi:10.1210/jc.2009-0926
Ferrell RE (2002) Research perspectives in inherited lymphatic disease. Ann N Y Acad Sci 979:39–51, discussion 76–9
Clark EB (1984) Neck web and congenital heart defects: A pathogenic association in 45 X-O turner syndrome? Teratology 29(3):355–361. doi:10.1002/tera.1420290305
Berdahl LD, Wenstrom KD, Hanson JW (1995) Web neck anomaly and its association with congenital heart disease. Am J Med Genet 56(3):304–307. doi:10.1002/ajmg.1320560318
Loscalzo ML, Van PL, Ho VB et al (2005) Association between fetal lymphedema and congenital cardiovascular defects in turner syndrome. Pediatrics 115(3):732–735. doi:10.1542/peds.2004-1369
Barr M Jr, Oman-Ganes L (2002) Turner syndrome morphology and morphometrics: Cardiac hypoplasia as a cause of midgestation death. Teratology 66(2):65–72. doi:10.1002/tera.10064
de Mooij YM, van den Akker NM, Bekker MN, Bartelings MM, van Vugt JM, Gittenberger-de Groot AC (2011) Aberrant lymphatic development in euploid fetuses with increased nuchal translucency including Noonan syndrome. Prenat Diagn 31(2):159–166. doi:10.1002/pd.2666;10.1002/pd.2666
Corbett CR, Dale RF, Coltart DJ, Kinmonth JB (1982) Congenital heart disease in patients with primary lymphedemas. Lymphology 15(3):85–90
Dutka DP, Cousins C, Manhire AR (1991) Lymphatic abnormalities in Alagille’s syndrome. Br Heart J 65(3):168–170
Noonan JA, Walters LR, Reeves JT (1970) Congenital pulmonary lymphangiectasis. Am J Dis Child 120(4):314–319
Graziano JN, Heidelberger KP, Ensing GJ, Gomez CA, Ludomirsky A (2002) The influence of a restrictive atrial septal defect on pulmonary vascular morphology in patients with hypoplastic left heart syndrome. Pediatr Cardiol 23(2):146–151. doi:10.1007/s00246-001-0038-7
Rychik J, Rome JJ, Collins MH, DeCampli WM, Spray TL (1999) The hypoplastic left heart syndrome with intact atrial septum: Atrial morphology, pulmonary vascular histopathology and outcome. J Am Coll Cardiol 34(2):554–560
Bellini C, Boccardo F, Campisi C, Bonioli E (2006) Congenital pulmonary lymphangiectasia. Orphanet J Rare Dis 1:43. doi:10.1186/1750-1172-1-43
Feltes TF, Hansen TN (1989) Effects of an aorticopulmonary shunt on lung fluid balance in the young lamb. Pediatr Res 26(2):94–97. doi:10.1203/00006450-198908000-00004
Reddy VM, Meyrick B, Wong J et al (1995) In utero placement of aortopulmonary shunts. A model of postnatal pulmonary hypertension with increased pulmonary blood flow in lambs. Circulation 92(3):606–613
Datar SA, Johnson EG, Oishi PE et al (2012) Altered lymphatics in an ovine model of congenital heart disease with increased pulmonary blood flow. Am J Physiol Lung Cell Mol Physiol 302(6):L530–L540. doi:10.1152/ajplung.00324.2011
Hagendoorn J, Padera TP, Fukumura D, Jain RK (2005) Molecular regulation of microlymphatic formation and function: Role of nitric oxide. Trends Cardiovasc Med 15(5):169–173. doi:10.1016/j.tcm.2005.06.003
Langford RJ, Daudia AT, Malins TJ (1999) A morphological study of the thoracic duct at the jugulo-subclavian junction. J Craniomaxillofac Surg 27(2):100–104
Zorzetto NL, Ripari W, De Freitas V, Seullner G (1977) Anatomical observations on the ending of the human thoracic duct. J Morphol 153(3):363–369. doi:10.1002/jmor.1051530303
Meadows J, Gauvreau K, Jenkins K (2008) Lymphatic obstruction and protein-losing enteropathy in patients with congenital heart disease. Congenit Heart Dis 3(4):269–276. doi:10.1111/j.1747-0803.2008.00201.x
Mertens L, Hagler DJ, Sauer U, Somerville J, Gewillig M (1998) Protein-losing enteropathy after the Fontan operation: An international multicenter study. PLE study group. J Thorac Cardiovasc Surg 115(5):1063–1073
Witte MH, Dumont AE, Clauss RH, Rader B, Levine N, Breed ES (1969) Lymph circulation in congestive heart failure: Effect of external thoracic duct drainage. Circulation 39(6):723–733
Szabo G, Magyar Z (1967) Effect of increased systemic venous pressure on lymph pressure and flow. Am J Physiol 212(6):1469–1474
Wegria R, Zekert H, Walter KE et al (1963) Effect of systemic venous pressure on drainage of lymph from thoracic duct. Am J Physiol 204:284–288
Lenz D, Hambsch J, Schneider P et al (2003) Protein-losing enteropathy in patients with Fontan circulation: is it triggered by infection? Crit Care 7(2):185–190
Luciani GB, Pessotto R, Mombello A, Mazzucco A (1999) Hypoplastic left heart syndrome with restrictive atrial septal defect and congenital pulmonary lymphangiectasis. Cardiovasc Pathol 8(1):49–51
Mehlhorn U, Davis KL, Burke EJ, Adams D, Laine GA, Allen SJ (1995) Impact of cardiopulmonary bypass and cardioplegic arrest on myocardial lymphatic function. Am J Physiol 268(1 Pt 2):H178–H183
Nakamura K, Rockson SG (2008) The role of the lymphatic circulation in the natural history and expression of cardiovascular disease. Int J Cardiol 129(3):309–317. doi:10.1016/j.ijcard.2008.02.007
Mehlhorn U, Geissler HJ, Laine GA, Allen SJ (2001) Myocardial fluid balance. Eur J Cardiothorac Surg 20(6):1220–1230
Cui Y (2010) Impact of lymphatic vessels on the heart. Thorac Cardiovasc Surg 58(1):1–7. doi:10.1055/s-0029-1240553
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Bansal, M. (2013). The Link Between Lymphatic Obstruction and Congenital Heart Disease. In: Karunamuni, G. (eds) The Cardiac Lymphatic System. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6774-8_3
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6774-8_3
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6773-1
Online ISBN: 978-1-4614-6774-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)